In yeast, the oxysterol-binding proteins Osh1–Osh7 are collectively needed to maintain the normal distribution of PI4P. Osh4p is recruited to secretory vesicles in a PI4P-dependent manner and plays an important role in vesicle maturation.
Abstract
Phosphatidylinositol-4-phosphate (PI4P) is produced on both the Golgi and the plasma membrane. Despite extensive vesicular traffic between these compartments, genetic analysis suggests that the two pools of PI4P do not efficiently mix with one another. Several lines of evidence indicate that the PI4P produced on the Golgi is normally incorporated into secretory vesicles, but the fate of that pool has been unclear. We show here that in yeast the oxysterol-binding proteins Osh1–Osh7 are collectively needed to maintain the normal distribution of PI4P and that Osh4p is critical in this function. Osh4p associates with secretory vesicles at least in part through its interaction with PI4P and is needed, together with lipid phosphatases, to reduce the level of PI4P as vesicles approach sites of exocytosis. This reduction in PI4P is necessary for a switch in the regulation of the Sec4p exchange protein, Sec2p, from an interaction with the upstream Rab, Ypt31/32, to an interaction with a downstream Sec4p effector, Sec15p. Spatial regulation of PI4P levels thereby plays an important role in vesicle maturation.
INTRODUCTION
The various organelles of the exocytic and endocytic pathways all have unique functions that are dictated by their unique compositions. These distinct identities are maintained despite high rates of bidirectional vesicular traffic along and between these pathways. Maintenance of organelle identity requires both tightly regulated cargo selection during vesicle budding and accurate vesicle targeting to the appropriate compartment before fusion. Peripherally associated coat proteins direct cargo selection, whereas targeting requires several different regulatory mechanisms that work in concert. Soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) complexes, involving integral membrane proteins on the vesicle and target membranes, provide one level of specificity to the membrane fusion reaction (McNew et al., 2000); however, because vesicle SNAREs must recycle back to the donor compartment after fusion, they are necessarily found on both anterograde and retrograde vesicles and therefore cannot specify the direction of vesicular traffic. Molecular tags that can be removed from the membrane after fusion are better suited to this aspect of vesicle targeting (Behnia and Munro, 2005).
Rab GTPases act as transient tags because they are activated by guanine nucleotide exchange factors (GEFs) and inactivated by GTPase-activating proteins (GAPs; Hutagalung and Novick, 2011). By localizing a GEF on one compartment and a GAP on another compartment, an asymmetric distribution of activated Rab protein can be established that provides directional information to vesicular traffic. Once activated, Rabs recruit a variety of effectors, such as molecular motors, tethering complexes, and regulators of SNARE complex assembly, which together define where a vesicle is delivered, tethered, and fused (Guo et al., 1999b; Grosshans et al., 2006; Jin et al., 2011; Mizuno-Yamasaki et al., 2012).
Phosphoinositides can also serve as transient tags in membrane traffic pathways. Phosphorylation of the inositol head group of phosphatidylinositol at different positions generates distinct tags. By localizing a lipid kinase on one compartment and the competing lipid phosphatase on another, a gradient can be established that provides spatial information for vectorial traffic (Behnia and Munro, 2005; Balla, 2013). These two types of tags can be exploited in a combinatorial manner by proteins that act as coincidence detectors, binding only to membrane domains carrying both the appropriate Rab and phosphoinositide (Carlton and Cullen, 2005).
The yeast exocytic pathway is regulated by a series of Rabs. Ypt1p controls endoplasmic reticulum (ER)-to-Golgi transport, as well as transport through the Golgi (Bacon et al., 1989; Jedd et al., 1995; Sclafani et al., 2010). Ypt31p and Ypt32p are redundant Rabs that control exit from the Golgi (Benli et al., 1996; Jedd et al., 1997). Sec4p controls the delivery of secretory vesicles along polarized actin cables, vesicle tethering by the exocyst complex to exocytic sites specified by polarity establishment machinery, and SNARE-mediated fusion to the plasma membrane (Guo et al., 1999b; Grosshans et al., 2006; Shen et al., 2013). Sec4p is activated by its GEF, Sec2p, which is in turn regulated by several different mechanisms (Walch-Solimena et al., 1997). Sec2p is recruited to membranes by the combinatorial signals of Ypt31/32-GTP and the phosphoinositide phosphatidylinositol-4-phosphate (PI4P; Ortiz et al., 2002; Mizuno-Yamasaki et al., 2010). Sec2p also binds to Sec15p, a subunit of the exocyst complex and a direct downstream effector of Sec4-GTP (Guo et al., 1999b; Medkova et al., 2006). The interaction of Sec2p, a Sec4p GEF, with Sec15p, a Sec4p effector, could generate a positive feedback loop leading to formation of a domain of highly activated Sec4p and highly concentrated exocyst. The binding sites for Ypt31/32-GTP and Sec15p overlap, such that Sec2p can bind to only one or the other (Medkova et al., 2006). This choice is dictated in part by the local PI4P concentration, since high levels of PI4P prevent binding of Sec15p to Sec2p but do not interfere with binding to Ypt31/32 (Mizuno-Yamasaki et al., 2010).
Two PI4 kinases generate most of the PI4P in yeast; Pik1p is found in the Golgi (Walch-Solimena and Novick, 1999) and generates the Golgi-associated pool of PI4P, whereas Stt4p is found on the plasma membrane and generates a similar amount of PI4P at this site (Audhya et al., 2000; Audhya and Emr, 2002). Although Golgi-derived vesicles constantly deliver new membrane to the cell surface, the Golgi pool of PI4P is not efficiently transferred to the plasma membrane, as indicated by the observation that even overexpression of Pik1p fails to complement the loss of Stt4p (Audhya et al., 2000). This finding suggests that the Golgi pool of PI4P either fails to be incorporated into secretory vesicles or the vesicular pool of PI4P is reduced by one of several possible mechanisms. Here we explore the role of the Osh proteins in the regulation of the secretory vesicle pool of PI4P.
RESULTS
Osh proteins are key regulators of PI4P distribution in cells
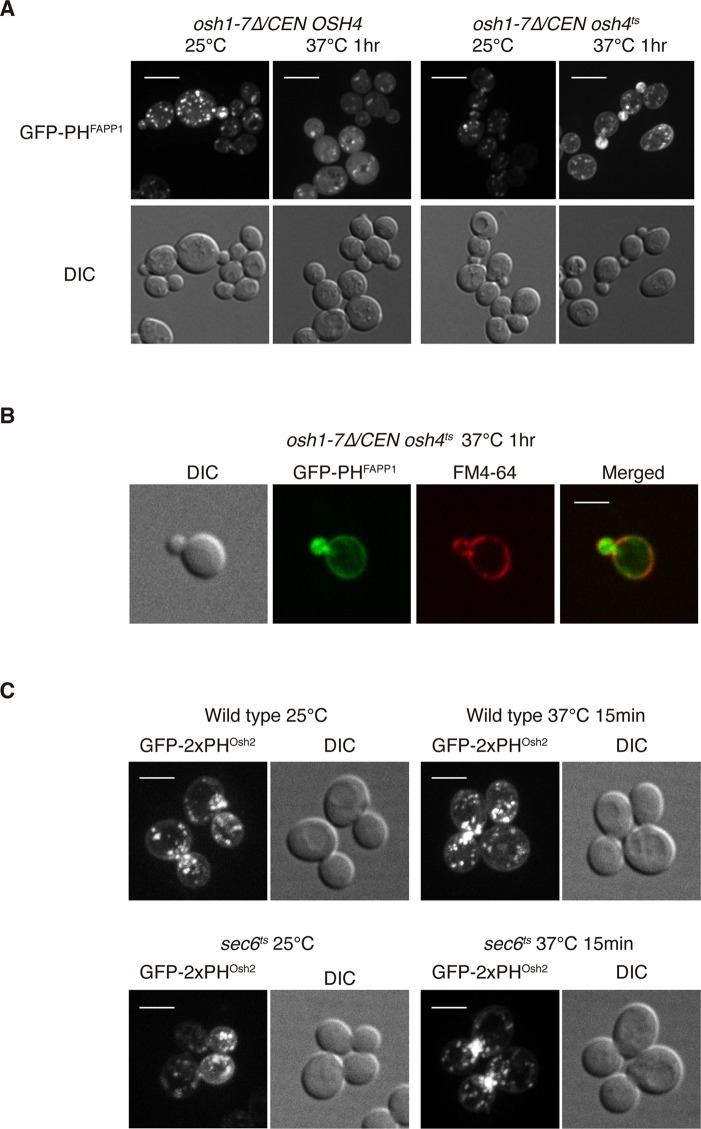
The phosphoinositide PI4P is critical for the localization of a number of proteins involved in polarized secretion, including Sec2p (Mizuno-Yamasaki et al., 2010). Here we investigate how the level of PI4P is regulated on secretory vesicles. Studies from several groups have demonstrated that the oxysterol-binding proteins (Osh proteins) control the level and intracellular distribution of PI4P in the budding yeast Saccharomyces cerevisiae (LeBlanc and McMaster, 2010; Stefan et al., 2011). To begin our analysis of the role of Osh proteins in PI4P distribution, we used cells lacking all endogenous Osh proteins and solely expressing a temperature-sensitive osh4ts allele. Consistent with a prior report (Stefan et al., 2011), we observed that the PI4P probe GFP-PHFAPP1, consisting of the PH domain from the FAPP1 protein fused to green fluorescent protein (GFP), shifted from the Golgi apparatus to the plasma membrane in the osh1-7Δ osh4ts cells after a temperature shift to 37°C (Figure 1A). In addition, there was significantly more GFP-PHFAPP1 at polarized secretory sites, such as the tips of small buds and the necks of large-budded cells, in osh1-7Δ osh4ts cells at the nonpermissive temperature (Figure 1A). These GFP-PHFAPP1–positive structures could represent either secretory vesicles containing abnormally high levels of PI4P or an accumulation of PI4P produced at the plasma membrane. To differentiate these two possibilities, we labeled the plasma membrane with the lipophilic dye FM4-64. We noticed that the GFP-PHFAPP1 positive structures accumulated in the small buds of osh1-7Δ osh4ts cells were clearly internal to the FM4-64–labeled plasma membrane (Figure 1B). These results suggest that one or more Osh proteins are needed to reduce the level of PI4P on secretory vesicles.
FIGURE 1:
Osh proteins regulate PI4P distribution in cells. (A) Localization of GFP-PHFAPP1 in osh1-7Δ /CEN OSH4 cells or osh1-7Δ /CEN osh4ts cells at indicated temperatures. Cells were grown overnight at 25°C in a synthetic medium containing 2% glucose and then shifted to 37°C for 1 h. Cells shown are representative of >100 cells observed. Note that 6 and 55% of osh1-7Δ/CEN osh4ts cells showed polarized localization of GFP-PHFAPP1 at 25 and 37°C, respectively, and 6 and 0% of osh1-7Δ/CEN OSH4 cells showed polarized localization of GFP-PHFAPP1 at 25 and 37°C, respectively. Scale bar, 5 μm. (B) Localization of GFP-PHFAPP1 and FM4-64 in osh1-7Δ/CEN osh4ts cells at 37°C. Cells were grown overnight at 25°C in a synthetic medium containing 2% glucose and then shifted to 37°C for 1 h. FM4-64 was added to cells and kept on ice to label the plasma membrane. Scale bar, 5 μm. (C) The PI4P probe GFP-2xPHOsh2 accumulates at polarized growth sites in sec6ts cells at the nonpermissive temperature. Wild-type and sec6ts cells were grown overnight at 25°C in a synthetic medium containing 2% glucose and then shifted to 37°C for 15 min. Cells shown are representative of >100 cells observed. Scale bar, 5 μm.
To investigate whether PI4P is present on secretory vesicles in a wild-type OSH background, we examined the localization of another PI4P probe, consisting of the PH domain from Osh2p fused to GFP (Roy and Levine, 2004), in a sec6ts mutant allele. We used a second probe for PI4P because the PH domain of FAPP1 has affinity not only for PI4P, but also Arf1p, and may preferentially label membranes carrying both ligands (Roy and Levine, 2004). Sec6 is an essential component of the exocyst complex that is required for tethering secretory vesicles to the plasma membrane, and loss of Sec6 function leads to the accumulation of secretory vesicles (TerBush et al., 1996). Vesicle accumulation is initially confined to regions of active secretion and surface expansion, but after a prolonged shift to the restrictive temperature, vesicles accumulate throughout the cell (Govindan et al., 1995). If PI4P is present on secretory vesicles, we would expect accumulation of the GFP-2xPHOsh2 PI4P probe at polarized growth sites in sec6ts mutant cells after short incubations at the nonpermissive temperature. At the permissive temperature, we noticed that GFP-2xPHOsh2 was present on both the plasma membrane and Golgi apparatus, as previously reported (Roy and Levine, 2004). Intriguingly, upon shifting the sec6ts mutant cells to 37°C for only 15 min, we observed a notable increase of GFP-2xPHOsh2–positive structures at the necks of large-budded cells (Figure 1C). We observed only a slight increase of GFP-2xPHOsh2–positive structures near the cell necks in wild-type cells upon a temperature shift (Figure 1C). These results provide additional evidence that PI4P is present in the membrane of secretory vesicles.
Osh proteins are key regulators of secretion in yeast
PI4P is a major regulator of Golgi-to-plasma membrane (PM) vesicle transport, and loss of Osh protein function affects the secretion of a number of cargo proteins, including Bgl2, in yeast cells (Kozminski et al., 2006). To examine whether Osh proteins affect the fusion of Golgi-derived vesicles to the plasma membrane, we examined osh1-7Δ osh4ts cells and osh1-7Δ OSH4 cells at 37°C using thin-section electron microscopy. Consistent with prior reports (Beh and Rine, 2004), we found that there was a large increase in the number of vesicles present in the cytoplasm of the osh1-7Δ osh4ts cells (Supplemental Figure 1A; 1.72 vesicles/μm2) compared with cells containing wild-type OSH4 (Supplemental Figure 1A; 0.25 vesicle/μm2) under this condition.
Osh proteins have been implicated in regulation of assembly of the exocyst, a protein complex required for tethering secretory vesicles to the plasma membrane (TerBush et al., 1996; Alfaro et al., 2011). We examined the localizations of two subunits of the exocyst complex in the osh1-7Δ osh4ts cells—Sec15p, an exocyst subunit that associates with secretory vesicles as they are delivered to sites of secretion, and Sec3p, an exocyst subunit that can directly recognize exocytic sites on the plasma membrane. Both proteins were mislocalized from polarized secretion sites in >90% of the osh1-7Δ osh4ts cells when they were shifted from the permissive temperature to the nonpermissive temperature (Supplemental Figure 1B). Meanwhile, Sec15p and Sec3p still localized normally in the osh1-7Δ OSH4 cells upon the temperature shift (Supplemental Figure 1B). Together these results suggest that Osh protein function is key to the tethering and/or fusion of secretory vesicles in vivo, presumably by controlling the level and intracellular distribution of PI4P.
Osh4p localizes to Sec4p-positive vesicles
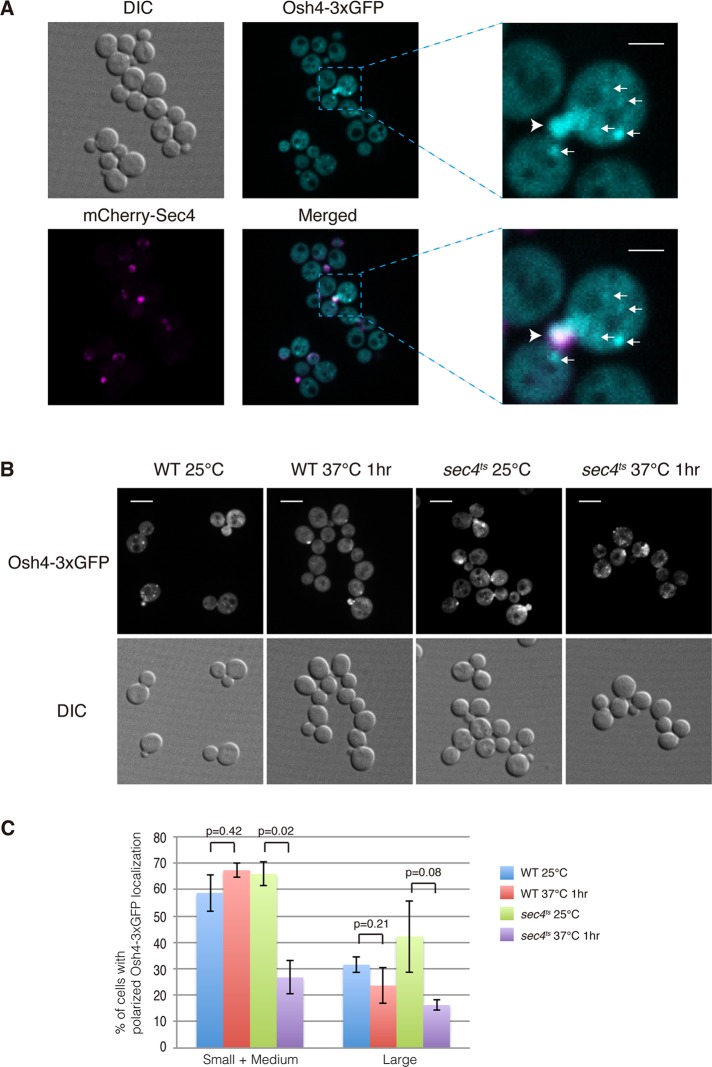
There are seven different Osh proteins in the budding yeast S. cerevisiae. They share redundant yet essential roles in vivo, as any one is sufficient for cell growth, but collectively they are required for cell viability (Beh et al., 2001). Osh1p localizes to Golgi and nuclear–vacuolar junction sites (Levine and Munro, 2001), whereas Osh2p, Osh3p, Osh6p, and Osh7p were previously localized to ER-PM contact sites (Levine and Munro, 2001; Schulz et al., 2009; Stefan et al., 2011). To investigate which Osh protein is the master regulator of PI4P on secretory vesicles, we tagged Osh4p and Osh5p with GFP and examined their in vivo localization using fluorescence microscopy. Only Osh4-GFP displayed a good signal by fluorescence microscopy (unpublished data). To enhance the signal, we constructed a 3xGFP fusion at the C-terminus of Osh4p. We observed three major pools of Osh4-3xGFP present in wild-type cells. One pool was present on punctate structures in cytoplasm, whereas another pool was concentrated at sites of polarized secretion, including the tips of small buds and mother–daughter necks. However, the majority of Osh4-3xGFP was cytoplasmic (Figure 2A). The pool of Osh4-3xGFP found in small bud tips colocalized well with mCherry-tagged Sec4 (Figure 2A), suggesting that it is present on secretory vesicles and therefore a good candidate for the major Osh protein regulating the final stage of the secretory pathway.
FIGURE 2:
Osh4 is present on Sec4 positive structures in cells. (A) Localization of mCherry-Sec4 and Osh4-3xGFP in cells. Cells were grown overnight at 25°C in a synthetic medium containing 2% glucose. The boxed area shows a region magnified fourfold. Arrows indicate that Osh4-3xGFP is present on cytoplasmic punctate structures. Arrowheads indicate that Osh4-3xGFP colocalizes with mCherry-Sec4 in small buds. Scale bar, 2 μm. (B) The polarized localization of Osh4-3xGFP is dependent on Sec4. Osh4-3xGFP localization in wild-type and sec4ts cells at indicated temperatures. Cells were grown overnight at 25°C in a synthetic medium containing 2% glucose and then shifted to 37°C for 1 h. Scale bar, 5 μm. (C) Quantification of wild-type cells and sec4ts cells with polarized localized Osh4-3xGFP at 25 or 37°C for 1 h. A total of 200 cells was counted for each experiment. Values indicate the percentage of cells showing bud or mother–daughter neck localization of Osh4-3xGFP. Mean and SD of three experiments.
Secretory vesicles are normally delivered to sites of polarized growth by the type V myosin motor Myo2p (Govindan et al., 1995; Schott et al., 1999, 2002). Myo2p is recruited to vesicles by Sec4-GTP, and therefore in mutants defective in Sec4p activation, vesicles accumulate in a depolarized manner (Walch-Solimena et al., 1997; Wagner et al., 2002; Donovan and Bretscher, 2012). To determine whether Osh4p is associated with secretory vesicles, we examined Osh4-3xGFP localization in sec4ts mutant cells. At the permissive temperatures, Osh4-3xGFP was present, in part, at polarized secretion sites, including small bud tips and mother–daughter cell necks (Figure 2B). However, upon shifting the cells to the nonpermissive temperature for 1 h, the polarized localization of Osh4-3xGFP was greatly reduced, and we observed some abnormal punctate structures at the cell perimeter (Figure 2, B and C). Osh4-3xGFP still localized normally in wild-type cells under the same condition (Figure 2, B and C). To further investigate whether Sec4p controls Osh4p localization in vivo, we performed a subcellular fractionation experiment. In sec4ts cells, ∼83% of Osh4p was in the S100 fraction at the permissive temperature. However, upon shifting the cells to the nonpermissive temperature for 1 h, the percentage of Osh4p in the soluble fraction increased, with 91% in the S100 fraction (Supplemental Figure 2, A and B). These results suggest that Sec4p contributes to Osh4p's association with secretory vesicles.
To test the possibility that Osh4p is recruited to secretory vesicles directly by Sec4p, we overexpressed Sec4p severalfold in vivo. However, we did not observe a detectable increase of polarized Osh4-3xGFP (Supplemental Figure 3A). In addition, we tested whether Sec4p directly binds Osh4p. Although we cannot rule out that there might be some weak interaction between Sec4p and Osh4p in vivo, we did not observe a direct interaction of these two proteins in vitro, regardless of the nucleotide-loading status of Sec4p (Supplemental Figure 3B). We also tested Osh4p's binding to the upstream Rab Ypt32p, yet did not observe any interaction (Supplemental Figure 3C). These results support the proposal that Osh4p is associated with secretory vesicles, and, although Sec4p contributes to the recruitment of Osh4p, it is not the only factor or switch controlling Osh4p localization in vivo.
The polarized localization of Osh4p is dependent on binding to PI4P
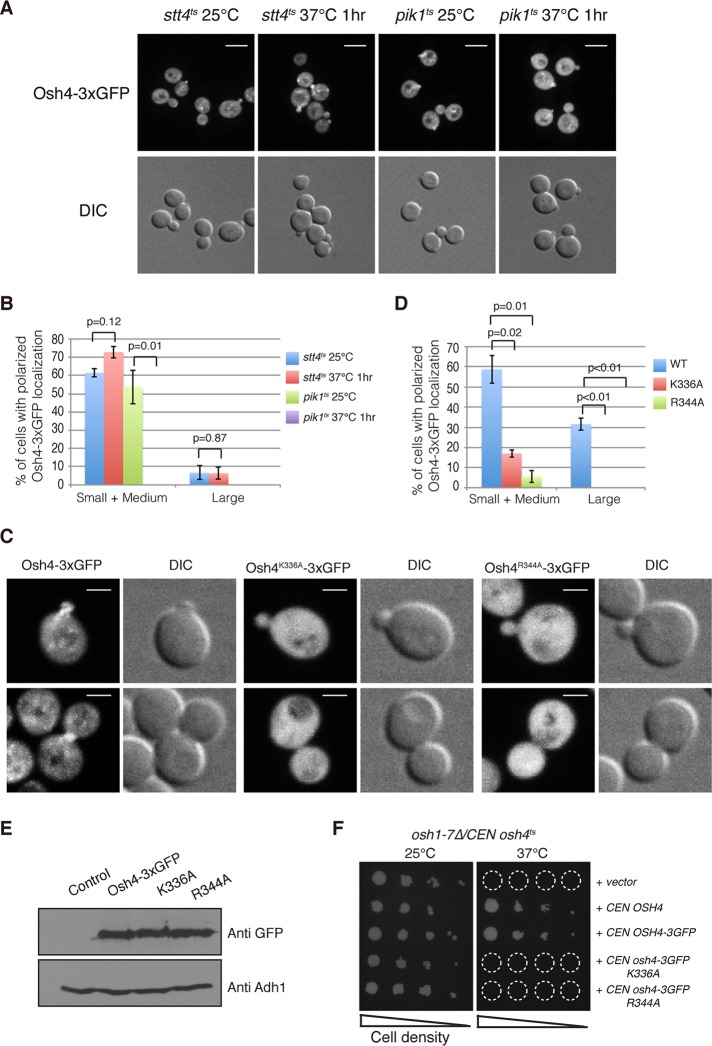
Osh4p binds to both ergosterol and PI4P (Im et al., 2005; de Saint-Jean et al., 2011). A prior study demonstrated that ergosterol is important for Osh4p localization (Alfaro et al., 2011). We speculated that the polarized localization of Osh4p might also be dependent on binding to PI4P, based on PI4P's cellular distribution. To test this hypothesis, we first determined whether the localization of Osh4 is affected by the loss of enzymatic activities that synthesize PI4P. In budding yeast, there are two major PI 4-kinases responsible for PI4P synthesis. Pik1 is a Golgi-localized lipid kinase, whereas Stt4 functions at the plasma membrane (Audhya et al., 2000). Each of these two PI 4-kinases synthesizes ∼50% of the total PI4P in cells. We examined the localization of Osh4-3xGFP in pik1ts and stt4ts cells, respectively. At the permissive temperature, Osh4-3xGFP localized normally in both pik1ts cells and stt4ts cells. Of interest, upon shifting to the nonpermissive temperature for 1 h, Osh4-3xGFP appeared predominantly cytoplasmic in pik1ts cells, whereas its localization was normal in stt4ts cells (Figure 3, A and B). Subcellular fractionation results further support the proposal that Pik1p function is important for Osh4p membrane association (Supplemental Figure 2, A and B). Together these results suggest that the localization of Osh4p is controlled by the Pik1-generated pool of PI4P.
FIGURE 3:
The polarized localization of Osh4 is dependent on PI4P. (A) Localization of Osh4-3xGFP in stt4ts cells and pik1ts cells at indicated temperatures. Osh4-3xGFP is mislocalized in pik1ts cells at the nonpermissive temperature. Cells were grown overnight at 25°C in a synthetic medium containing 2% glucose and then shifted to 37°C for 1 h. Scale bar, 5 μm. (B) Quantification of stt4ts cells and pik1ts cells with regard to polarized localization of Osh4-3xGFP at 25 or 37°C for 1 h. A total of 200 cells was counted for each experiment. Values indicate the percentage of cells showing bud or mother–daughter neck localization of Osh4-3xGFP. Mean and SD of three experiments. Note that there were no pik1ts cells observed with polarized Osh4-3xGFP at 37°C. (C) Localization of Osh4-3xGFP point mutants defective in PI4P binding in cells. Scale bar, 2 μm. (D) Quantification of cells with polarized localized Osh4-3xGFP point mutant proteins. A total of 200 cells was counted for each experiment. Values indicate the percentage of cells showing bud or mother–daughter neck localization of Osh4-3xGFP. Mean and SD of three experiments. Note that there were no large-budded cells observed with polarized Osh4 K396A-3xGFP or Osh4 R344A-3xGFP. (E) Steady-state expression levels of Osh4-3xGFP, Osh4K336A-3xGFP, and Osh4R344A-3xGFP. Yeast whole-cell lysates were prepared from strains expressing different Osh4 mutant proteins as described in Materials and Methods. Adh1p was followed as a loading control. (F) Complementation assays of Osh4p point mutants. The osh1-7Δ /CEN osh4ts cells were transformed with plasmids expressing various mutant Osh4p proteins as indicated. Serial dilutions of yeast cells were grown on –Ura plates to retain plasmids at 25 or 37°C for 3 d; only cells harboring functional OSH4 plasmids grew on plates.
To confirm that PI4P binding is required for normal Osh4p localization, we generated two osh4 alleles harboring point mutations within the PI4P binding pocket, K336A and R344A. Both mutants display defects in PI4P binding but not sterol binding in vitro (de Saint-Jean et al., 2011). Consistent with our expectation, neither Osh4 K336A nor R344A mutant was able to localize to polarized secretory sites in cells (Figure 3, C and D). Western blot analysis revealed that both Osh4p mutant proteins express normally (Figure 3E); thus the microscopy experimental results were not due to the lack of stability of these proteins in vivo. Of interest, the Osh4p mutants defective in PI4P binding were not able to complement the growth of osh1-7Δ osh4ts cells at the nonpermissive temperature (Figure 3F), indicating that the ability to bind PI4P is essential for Osh4 function.
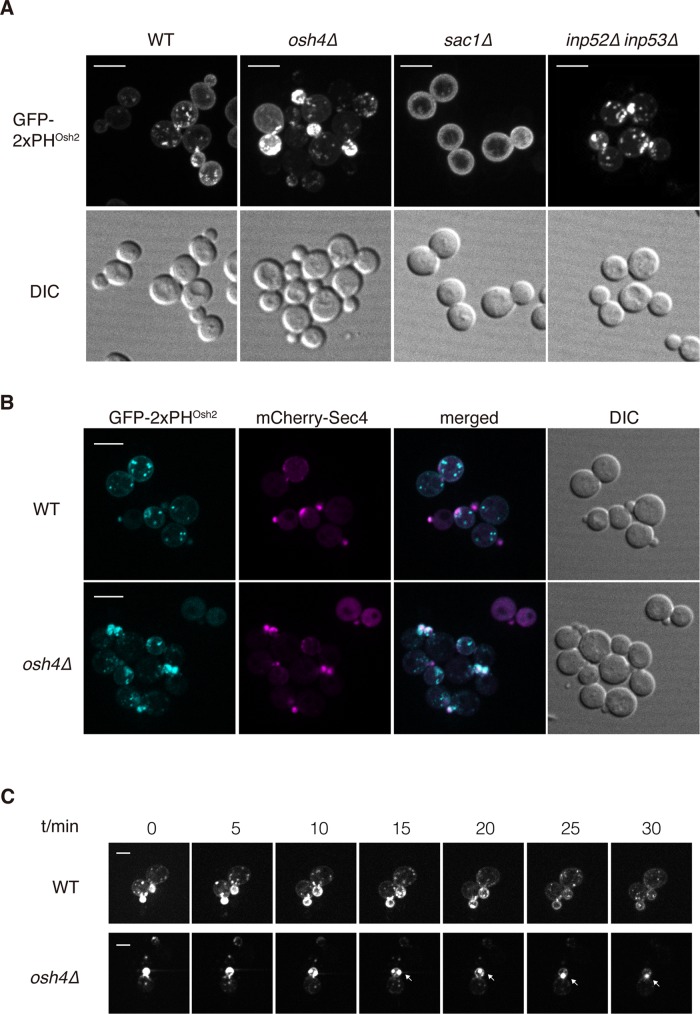
To test whether Osh4p changes localization in response to change of PI4P distribution in cells, we examined the localization of Osh4-3xGFP in wild-type, sac1Δ, and inp52Δ inp53Δ cells, which lack the different phosphatase activities that can dephosphorylate PI4P (Guo et al., 1999a; Hughes et al., 2000; Foti et al., 2001). There was a dramatic increase in the PM-localized PI4P pool in sac1Δ cells and an increase in the localization of the PI4P probe to the cytoplasmic punctate structures in the inp52Δ inp53Δ cells (Figure 4A). Of interest, we did not see Osh4p shift to the plasma membrane in sac1Δ cells. However, we did observe a dramatic increase of large Osh4p-positive puncta in the cytoplasm of sac1Δ cells and a significant increase of small Osh4p-positive puncta in the cytoplasm of inp52Δ inp53Δ cells (Supplemental Figure 4), suggesting that Osh4p's localization was responding to the changes of PI4P distribution distinctly in sac1Δ and inp52Δ inp53Δ cells. In total, our results indicate that PI4P, possibly in concert with additional factors, recruits Osh4p to secretory vesicles.
FIGURE 4:
Osh4 regulates PI4P distribution and dynamics in cells. (A) Localization of the PI4P probe GFP-2xPHOsh2 in wild-type, osh4Δ, sac1Δ, and inp52Δ inp53Δ cells. Cells shown are representative of >100 cells observed. Scale bar, 5 μm. (B) Localization of the PI4P probe GFP-2xPHOsh2 and mCherry-Sec4 in wild-type and osh4Δ cells. Cells shown are representative of >100 cells observed. Scale bar, 5 μm. (C) PI4P dynamics in wild-type and osh4Δ cells. Cells expressing GFP-2xPHOsh2 were examined by time-lapse fluorescence microscopy. Images are shown at indicated time points. Arrows indicate that GFP-2xPHOsh2 accumulated at polarized growth sites. Scale bar, 2 μm.
PI4P accumulates on Sec4p-positive vesicles in osh4Δ cells
Previous studies, as well as those presented here, support the proposal that PI4P is present on secretory vesicles, at least when they are initially formed (Santiago-Tirado et al., 2011). Furthermore, our data indicate that Osh4 is recruited to secretory vesicles by PI4P. Once it has been recruited, Osh4p could act to reduce the PI4P pool present on secretory vesicles. To test whether loss of Osh4p alone can affect the PI4P distribution in yeast cells, we examined the localization of GFP-2xPHOsh2 in wild-type and osh4Δ cells. In wild-type cells, the GFP-2xPHOsh2 was present both on the plasma membrane and Golgi apparatus, as previously reported (Roy and Levine, 2004). Strikingly, we observed in the osh4Δ cells a dramatic shift in the PI4P probe to the budding daughter cells, which have high concentrations of Sec4p in comparison to wild-type cells (Figure 4, A and B). We observed similar results when we assessed the localization of the PHFAPP1 PI4P probe. In osh4Δ cells, PHFAPP1 shifted to structures that are positive for the secretory vesicle marker Sec4p but not the late Golgi marker Sec7p in the budding daughter cells (Supplemental Figure 5, A and B). In contrast, we did not observe any localization change for the phosphatidylinositol (4,5)-bisphosphate (PI(4,5)P2) probe GFP-2xPHPLC in osh4Δ cells (Supplemental Figure 5C). These results suggest that Osh4p plays a role in facilitating either the dephosphorylation or redistribution of PI4P from the secretory vesicles before they become concentrated at exocytic sites.
To better understand how Osh4 affects intracellular PI4P distribution, we captured time-lapse movies of the GFP-2xPHOsh2 probe in wild-type and osh4Δ cells. We observed that the GFP-2xPHOsh2 probe was concentrated in the tips of small buds of both wild-type and osh4Δ cells. However, as daughter cells grew bigger, the polarized localization of the GFP-2xPHOsh2 probe was gradually lost in wild-type cells (Figure 4C and Supplemental Movie S1). Of interest, this process was delayed in the osh4Δ cells, as we can still observe large GFP-2xPHOsh2–positive structures as the cells grow (Figure 4, A and C, and Supplemental Movie S2). This result suggests that high levels of PI4P accumulate on secretory vesicles in osh4Δ cells, and, as a result, the high level of PI4P may inhibit docking or fusion of these vesicles to the plasma membrane.
The localization patterns of Ypt32p and Sec15p are altered in osh4Δ cells
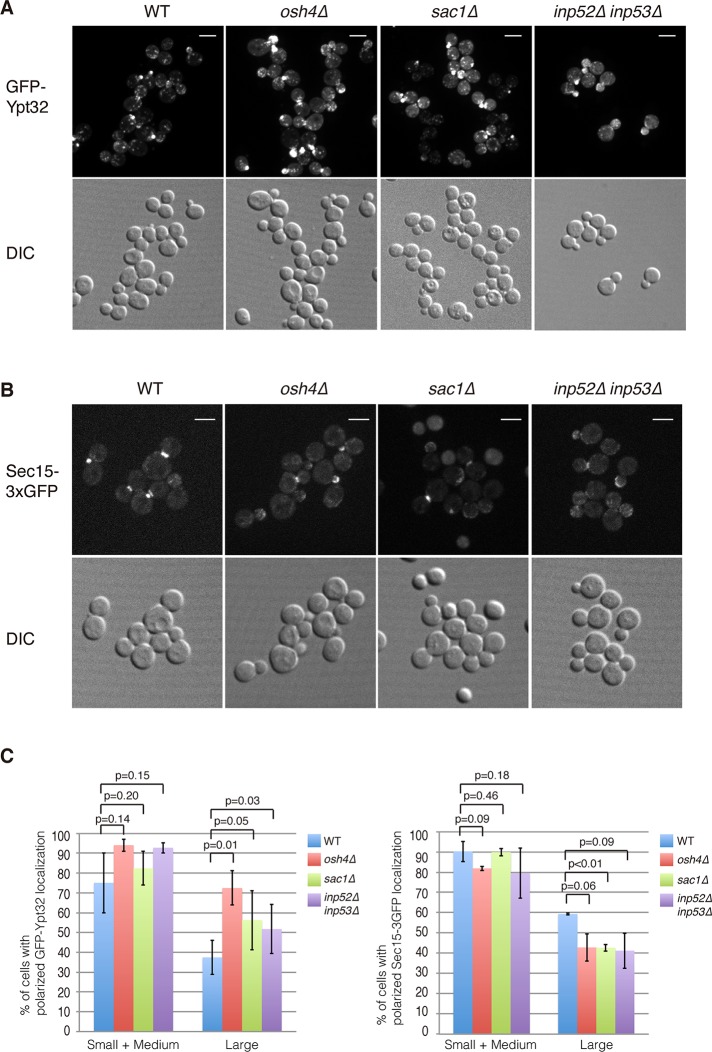
PI4P inhibits the interaction of Sec15p with Sec2p and thereby promotes the interaction of Sec2p with the competing ligand, Ypt32p (Mizuno-Yamasaki et al., 2010). We carried out fluorescence microscopy experiments to ascertain whether Osh4p functions as an upstream factor regulating the localization of Ypt32p and Sec15p, perhaps through its effects on the PI4P levels of secretory vesicles. First, we examined the localization of GFP-Ypt32 in wild-type and osh4Δ cells. As a control, we also included sac1Δ and inp52Δ inp53Δ cells, which are defective in PI4P turnover and may have increased PI4P levels on secretory vesicles. In wild-type cells, the majority of Ypt32p localized to the Golgi, with a pool of Ypt32p observed at the tips of small-budded cells; however, it was rarely observed at polarized secretory sites in large-budded cells (Figure 5, A and C). Strikingly, we observed a dramatic increase of GFP-Ypt32 at polarized secretory sites in large buds of osh4Δ cells (Figure 5A). Similar results were observed in the sac1Δ cells and inp52Δ inp53Δ cells (Figure 5A), suggesting that Osh4p may regulate the localization of Ypt32p by controlling PI4P turnover on secretory vesicles.
FIGURE 5:
Osh4 regulates the distribution of Ypt32 and Sec15 in cells. (A) Localization of GFP-Ypt32 in wild-type, osh4Δ, sac1Δ, and inp52Δ inp53Δ cells. Scale bar, 5 μm. (B) Localization of Sec15-3xGFP in wild-type, osh4Δ, sac1Δ, and inp52Δ inp53Δ cells. Scale bar, 5 μm. (C) Quantification of wild-type, osh4Δ, sac1Δ, and inp52Δ inp53Δ cells with polarized localized GFP-Ypt32 or Sec15-3xGFP. A total of 200 cells was counted for each experiment. Values indicate the percentage of cells showing bud or mother–daughter neck localization of GFP-Ypt32 or Sec15-3xGFP. Mean and SD of three experiments.
Previous results revealed that elevated levels of PI4P inhibit the interaction of Sec15p with Sec2p (Mizuno-Yamasaki et al., 2010), and osh1-7Δ osh4ts cells, which have increased levels of PI4P, exhibit Sec15p mislocalization (Supplemental Figure S1B). To test whether loss of Osh4p function or PI4P phosphatase activity affects Sec15p localization in vivo, we examined the localization of Sec15-3xGFP in wild-type, osh4Δ, sac1Δ, and inp52Δ inp53Δ cells. Unlike the osh1-7Δ osh4ts cells, we can still observe Sec15-3xGFP at both small-budding tips and mother–daughter cell necks in osh4Δ cells (Figure 5B). However, after careful quantification, we observed a significant decrease of Sec15p at polarized secretory sites in osh4Δ, sac1Δ, and inp52Δ inp53Δ cells compared with wild-type cells (Figure 5C). These results are consistent with the idea that PI4P helps to retain Ypt32p on secretory vesicles while inhibiting the recruitment of Sec15p and subsequent exocyst assembly on secretory vesicles.
Osh4p regulates the Sec2p–Sec15p interaction through PI4P
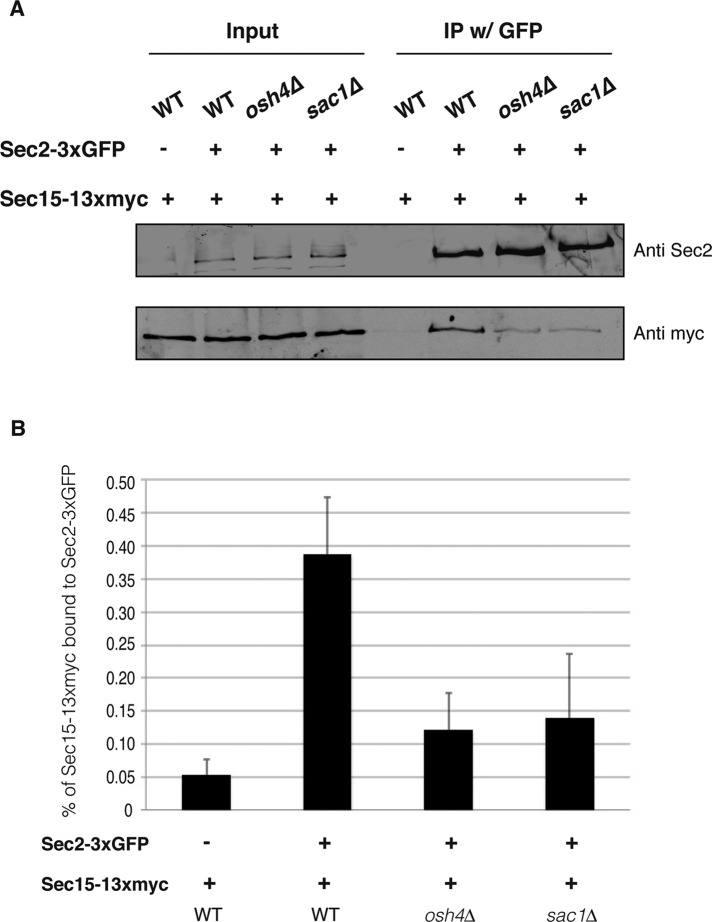
PI4P inhibits the interaction between Sec2p and Sec15p both in vivo and in vitro (Mizuno-Yamasaki et al., 2010); therefore we investigated whether this interaction is also controlled by Osh4p in vivo. We tagged Sec2p with 3xGFP and Sec15p with 13myc in wild-type, osh4Δ, and sac1Δ cells. We performed coimmunoprecipitation experiments, pulling down Sec2-3xGFP with the anti-GFP antibody and then probing for Sec15p with anti-myc antibody. Consistent with the microscopy data, we observed that in both osh4Δ and sac1Δ cells, less Sec15-13myc was coimmunoprecipitated than with wild-type cells (Figure 6A). Quantification of the coprecipitation experiment revealed that the interaction between Sec2p and Sec15p was roughly threefold lower on average in osh4Δ and sac1Δ cells than with wild-type cells (Figure 6B). These data further support the model that Osh4p negatively regulates PI4P levels of secretory vesicles and high levels of PI4P in osh4 Δ cells inhibits the interaction between Sec2p and Sec15p.
FIGURE 6:
Osh4 positively regulates the Sec2–Sec15 interaction. (A) The Sec2–Sec15 interaction is inhibited in osh4Δ cells and sac1Δ cells. Sec2-3xGFP was immunoprecipitated with anti-GFP antibody from wild-type, osh4Δ, or sac1Δ cell lysates. As a negative control, wild-type cells expressing nontagged Sec2 were included in the coimmunoprecipitation experiments. We loaded 0.5% of whole-cell lysates as input and processed the rest of the samples for coimmunoprecipitation. Sec2-3xGFP in the immunoprecipitates was detected with anti-Sec2 antibody. Coprecipitated Sec15-13xmyc was detected with anti-myc antibody. (B) Quantification of the Sec2–Sec15 interaction in wild-type, osh4Δ, and sac1Δ cells. Three independent experiments were performed. The intensity of the bands was quantified using ImageJ. The percentage of Sec15 in the immunoprecipitate was calculated and is indicated. Mean and SD of three experiments.
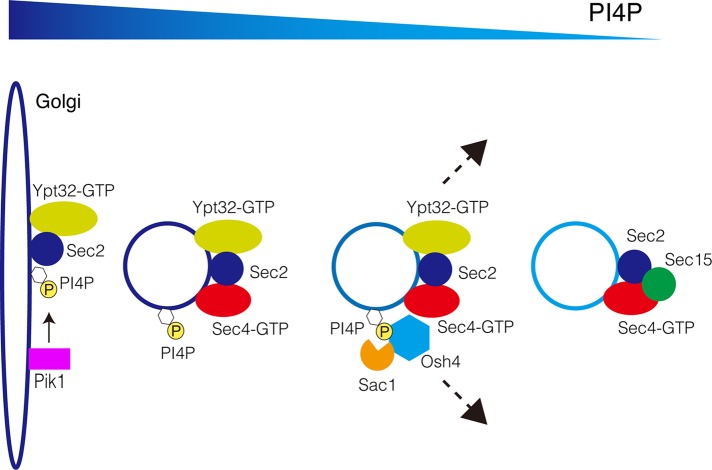
In total, we propose that Osh4p is the principal Osh protein controlling the PI4P level of secretory vesicles. As the secretory vesicles move from Golgi to the plasma membrane, PI4P is turned over and/or extracted under the control of Osh4p (Figure 7). The decrease in the level of PI4P may facilitate the release of Ypt32p and recruitment of Sec15p to secretory vesicles. This mechanism is important for the subsequent assembly of exocyst and tethering of secretory vesicles in preparation for fusion to the plasma membrane.
FIGURE 7:
A model for Osh4's role in regulating PI4P during secretion. Osh4 is recruited to the secretory vesicles through PI4P binding (and possibly through protein–protein interactions as well). Osh4 negatively regulates PI4P levels on the secretory vesicles and thereby enhances the Sec2–Sec15 interaction during this process. Finally, Osh4 leaves the secretory vesicles once PI4P levels are reduced below a certain threshold.
DISCUSSION
The Golgi and plasma membrane represent the two major sites of PI4P synthesis. Several studies, including this one, indicate that PI4P can also be found in Golgi-derived secretory vesicles, and, although these vesicles go on to fuse with the plasma membrane, genetic findings imply that the Golgi pool of PI4P is not efficiently delivered to the plasma membrane (Audhya et al., 2000; Santiago-Tirado et al., 2011). Both the Golgi PI 4-kinase Pik1p and the plasma membrane PI 4-kinase Stt4p are essential for viability, and overexpression of Pik1p fails to complement stt4 mutants (Audhya et al., 2000). This raises the question of the fate of the PI4P that is incorporated into secretory vesicles as they bud from the Golgi. The findings presented here support the proposal that the vesicular pool of PI4P is normally reduced before vesicle fusion with the plasma membrane and that this process involves the oxysterol-binding protein Osh4p (Figure 7). Dephosphorylation by the PI4P phosphatase Sac1p as well as possibly Inp52p and Inp53p, proteins that contain Sac1 lipid phosphatase domains, appears to be a likely mechanism, although we must also consider alternative mechanisms.
Our results show that Osh4p is recruited to secretory vesicles in a PI4P-dependent manner. The Osh4p K336A and R344A mutants are defective in PI4P binding but not in ergosterol binding in vitro (de Saint-Jean et al., 2011), and they exhibit a loss of localization in vivo. Therefore the association of Osh4p with specific membranes is likely regulated by the local PI4P concentration. However, PI4P is probably not sufficient for Osh4p recruitment, since in a sac1Δ mutant strain the level of PI4P on the plasma membrane is greatly increased without a concomitant shift in Osh4p localization to the cell cortex. The membrane curvature–sensing ALPS domain of Osh4p (Drin et al., 2007) could play an important role in the recruitment of Osh4p to 100-nm secretory vesicles in preference to the relatively flat plasma membrane. Sec4p is also required for Osh4p recruitment, although apparently not via a direct interaction.
Osh proteins have been shown to stimulate Sac1p phosphatase activity in vitro, and loss of either Osh protein function or Sac1p function leads to a dramatic increase in PI4P levels in vivo (Foti et al., 2001; Stefan et al., 2011). Nonetheless, Sac1p is an integral membrane protein found predominantly on the ER membrane, with a smaller pool associated with the Golgi, and there is no evidence that Sac1p is incorporated into Golgi-derived secretory vesicles (Faulhammer et al., 2007). This raises the question of how Sac1p on the ER can affect PI4P levels on secretory vesicles. Osh4p can extract PI4P from one set of liposomes and exchange it for ergosterol in another set of liposomes in vitro (de Saint-Jean et al., 2011). This observation prompted the hypothesis that Osh proteins, such as Osh4p, could extract PI4P from cellular membranes, such as secretory vesicles, and present it to enzymes that metabolize it on another membrane, such as Sac1p on the ER. Another possibility is that Osh proteins could present PI4P to the phosphatase at organelle contact sites. The PM-bound pool of PI4P is dephosphorylated by Sac1p at ER-PM contact sites in a reaction that requires Osh proteins (Stefan et al., 2011; Manford et al., 2012). Osh4p on secretory vesicles could present PI4P to the Sac1p phosphatase in-trans as vesicles pass alongside the ER membrane on their way to exocytic sites. Although secretory vesicles do not bind in a stable manner to the ER, most of the cell cortex is lined with a mix of ER sheets and tubules, so vesicles could transiently associate with the cortical ER before fusion with the plasma membrane. The PI4P on secretory vesicles might also be presented by Osh4p to the PI4P 5-kinase Mss4 at the plasma membrane and be converted to PI(4,5)P2 if the vesicles were in proximity to the Mss4 PIK patches (Ling et al., 2012). This could explain why the PI(4,5)P2 level is surprisingly low in osh1-7Δ osh4ts cells at the nonpermissive temperature even though the PI4P level is highly elevated (Stefan et al., 2011). Further studies on the Osh proteins will be needed to clarify these important questions.
The Osh4p-dependent reduction in the level of PI4P on secretory vesicles represents an important vesicle maturation event that helps to direct a switch in the binding partners of Sec2p from an initial interaction with Ypt31/32-GTP to a competing interaction with Sec15p (Figure 7; Mizuno-Yamasaki et al., 2010). Supporting this model, in osh4 or sac1 mutants, there is a decrease in the coprecipitation of Sec15p with Sec2p, and a shift is seen in the distribution of Ypt32p toward exocytic sites and in the distribution of Sec15p away from exocytic sites. This switch in Sec2p's binding partners is also under regulation by phosphorylation (Stalder et al., 2013). Sec2p is phosphorylated within the region responsible for binding both Ypt31/32-GTP and Sec15p, and the phosphorylated form has a strong preference for binding Sec15p, whereas the nonphosphorylated form preferentially binds Ypt32-GTP (Stalder et al., 2013). These two vesicle maturation events—PI4P reduction and Sec2p phosphorylation—may help to prepare the vesicles for tethering and fusion to the plasma membrane.
MATERIALS AND METHODS
Yeast strains and plasmid construction
Lists of S. cerevisiae strains and plasmids used in this study and their genotypes are given in Tables 1 and 2, respectively. Homologous recombination was used to tag or delete genes in yeast (Longtine et al., 1998). All integrations and deletions were verified by PCR analysis, and expression of fusion proteins was confirmed by Western blot analysis. The yeast shuttle vectors used in this study have been previously described (Sikorski and Hieter, 1989).
TABLE 1:
Yeast strains used in this study.
| Strain | Genotype | Source/ reference |
|---|---|---|
| NY1211 | MATα his3-Δ200 leu2-3,112 ura3-52 GAL+ | Lab strain |
| CBY924 | MATα ura3-52 his3-Δ200 leu2-3, 112 trp1-Δ901 suc2-Δ9 lys2-801 osh1Δ::kan-MX4 osh2Δ::kan-MX4 osh3Δ::LYS2 osh4Δ::HIS3 osh5Δ::LEU2 osh6Δ::LEU2 osh7Δ::HIS/OSH4, CEN-TRP | Beh and Rine (2004) |
| CBY926 | MATα ura3-52 his3-Δ200 leu2-3, 112 trp1-Δ901 suc2-Δ9 lys2-801 osh1Δ::kan-MX4 osh2 Δ::kan-MX4 osh3Δ::LYS2 osh4Δ::HIS3 osh5Δ::LEU2 osh6Δ::LEU2 osh7Δ::HIS3/osh4-1, CEN-TRP | Beh and Rine (2004) |
| NY3076 | NY1211 osh4::[OSH4-3GFP HIS3] | This study |
| NY3077 | NY1211 osh4::[OSH4-3GFP HIS3] leu2-3,112::[mcherry-SEC4 LEU2] | This study |
| NY408 | MATα ura3-52 his4-619 sec4-8 | Lab strain |
| NY3078 | NY1211 osh4::[OSH4-3GFP URA3] | This study |
| NY3079 | NY408 osh4::[OSH4-3GFP URA3] | This study |
| AAY102 | MATα ura3-52 his3-Δ200 leu2-3,112 trp1-Δ901 suc2-Δ9 lys2-801 stt4Δ::HIS3 pRS415 stt4-4 | Audhya et al. (2000) |
| NY2189 | MATa leu2-3,112 ura3-52 pik1-101 GAL+ | Lab strain |
| NY3080 | AAY102 osh4::[OSH4-3GFP URA3] | This study |
| NY3081 | NY2189 osh4::[OSH4-3GFP URA3] | This study |
| BY4741 | MATa his3∆1 leu2∆0 met15∆0 ura3∆0 | Lab strain |
| NY3082 | BY4741 inp52Δ::HIS3 inp53Δ | This study |
| NY3083 | BY4741 ura3-52::[GFP-YPT32 URA3] | This study |
| NY3084 | BY4741 sac1Δ ura3-52::[GFP-YPT32 URA3] | This study |
| NY3085 | BY4741 osh4Δ ura3-52::[GFP-YPT32 URA3] | This study |
| NY3086 | BY4741 inp52Δ::HIS3 inp53Δ ura3-52::[GFP-YPT32 URA3] | This study |
| NY3087 | BY4741 SEC15::[SEC15-3GFP URA3] | This study |
| NY3088 | BY4741 sac1Δ SEC15::[SEC15-3GFP URA3] | This study |
| NY3089 | BY4741 osh4Δ SEC15::[SEC15-3GFP URA3] | This study |
| NY3090 | BY4741 inp52Δ::HIS3 inp53Δ SEC15::[SEC15-3GFP URA3] | This study |
| NY3091 | BY4741 SEC15::[SEC15-13myc URA3] | This study |
| NY3092 | BY4741 sac1Δ SEC15::[SEC15-13myc URA3] | This study |
| NY3093 | BY4741 osh4Δ SEC15::[SEC15-13myc URA3] | This study |
| NY3094 | BY4741 SEC15::[SEC15-13myc URA3] sec2::[SEC2-3GFP LEU2] | This study |
| NY3095 | BY4741 sac1Δ SEC15::[SEC15-13myc URA3] sec2::[SEC2-3GFP LEU2] | This study |
| NY3096 | BY4741 osh4Δ SEC15::[SEC15-13myc URA3] sec2::[SEC2-3GFP LEU2] | This study |
| NY3097 | NY1211 osh4::[osh4-3GFP K336A URA3] | This study |
| NY3098 | NY1211 osh4::[osh4-3GFP R344A URA3] | This study |
TABLE 2:
Plasmids used in this study.
| Plasmid | Description | Source/reference |
|---|---|---|
| NRB1445 | pRS306-pADH-GFP-FAPP1-PH | Lab strain |
| NRB1444 | pRS306-pADH-mCherry-FAPP1-PH | Lab strain |
| SFNB797 | pUSE-URA3-SEC7-EGFP | Susan Ferro-Novick lab |
| NRB1584 | pRS303-OSH4(bp 730-1302)-3GFP-tADH | This study |
| NRB1585 | pRS306-OSH4(bp 730-1302)-3GFP-tADH | This study |
| NRB139 | YCp50-URA3-SEC4 | Lab strain |
| NRB170 | Ycp50-URA3-SEC4 (Overexpression) | Lab strain |
| pTL511 | pRS416-2x(GFP-Osh2-PH) | Roy and Levine (2004) |
| NRB1347 | Yiplac128-pADH-mCherry-SEC4-tCYC1 | Lab strain |
| NRB1325 | pRS306-pADH-GFP-YPT32- tCYC1 | Lab strain |
| NRB1308 | pRS306-SEC15-3GFP | Lab strain |
| NRB1586 | pRS306-SEC15-13myc | This study |
| NRB1416 | pRS305-SEC2(bp 122-2277)-3GFP-tADH | Lab strain |
| NRB1587 | pRS306-OSH4(bp 730-1302) K336A-3GFP-tADH | This study |
| NRB1588 | pRS306-OSH4(bp 730-1302) R344A-3GFP-tADH | This study |
| NRB1589 | pET27-10xHis-OSH4 | This study |
| NRB1590 | pGEX4T1-GST-SEC4 | This study |
| NRB1591 | pGEX4T1-GST-sec4(S34N) | This study |
| NRB1592 | pGEX4T1-GST-sec4(Q79L) | This study |
| NRB1440 | pGEX4T1-GST-YPT32 | Lab strain |
| NRB1593 | pRS316-pOSH4-OSH4 | This study |
| NRB1594 | pRS316-pOSH4-OSH4-3GFP-tADH | This study |
| NRB1595 | pRS316-pOSH4-osh4(K336A)-3GFP-tADH | This study |
| NRB1596 | pRS316-pOSH4-osh4(R344A)-3GFP-tADH | This study |
| NRB1597 | pRS426-GFP-2xPH(PLC1delta) | Seth Field lab |
Fluorescence microscopy and analysis of images
Yeast cells were grown overnight to mid log phase (OD600 of 0.3–0.5) in a synthetic medium containing 2% glucose at 25°C. For temperature-sensitive strains, the cell culture was shifted to 37°C for 60 min or as described. Fluorescence images were acquired with a 63× or 100× oil immersion objective (Carl Zeiss, Oberkochen, Germany) on a spinning disk confocal microscopy system (Yokagawa Corporation of America, Sugar Land, TX), which included a microscope (Observer Z1; Carl Zeiss) equipped with an electron-multiplying charge-coupled device camera (QuantEM 512SC; Photometrics). Excitation of GFP, mCherry, or DsRed was achieved using 488-nm argon and 568-nm argon/krypton lasers. For most of the samples, a z-stack of 12 slices with a 270-nm slice distance was generated. For samples in which the localizations of two proteins were examined, only images from one z-section are shown. Images were analyzed using AxioVision software 4.8 (Carl Zeiss). Levels of the fluorescence images and differential interference contrast images were adjusted with Photoshop (Adobe, San Jose, CA), and the same parameters were applied to all images within the same figure. Osh4-3xGFP, GFP-Ypt32, and Sec15-3xGFP localizations were scored based on localization at the tips of small buds or the tips/necks of large buds. From 100 to 200 cells were examined for each condition, and three independent experiments were used to calculate the SD.
Labeling of plasma membrane with FM4-64
We harvested five OD600 equivalents of mid log cells and resuspended them in 100 μl of double-distilled H2O on ice. We mixed 5 μl of cells with 5 μl of FM4-64 (Molecular Probes Invitrogen, Eugene, OR) solution (20 μg/ml FM4-64, 20 mM Tris-HCl, pH 7.5, 20 mM NaN3, 20 mM NaF) on ice. Cells were immediately used for microscopy experiments.
Analysis of cellular protein expression levels
We harvested five OD600 equivalents of mid log cells by precipitation in 10% trichloroacidic acid. Precipitates were washed in acetone, aspirated, resuspended in lysis buffer (150 mM NaCl, 50 mM Tris pH 7.5, 1 mM EDTA, 1% SDS), and mechanically lysed with silica beads. Protein sample buffer (150 mM Tris, pH 6.8, 6 M Urea, 6% SDS, 10% β-mercaptoethanol, 20% glycerol) was added, and extracts were analyzed by SDS–PAGE and immunoblotting with anti-GFP (Santa Cruz Biotechnology, Dallas, TX) or anti-Adh1 antibodies.
Coimmunoprecipitation assay
Yeast cells expressing epitope-tagged proteins were grown to an OD600 of 0.5–0.8. Cells were harvested and spheroplasted with lyticase (Scott and Schekman, 1980). Spheroplasts were lysed mechanically in the lysis buffer (1× phosphate-buffered saline [PBS], 5 mM EDTA, pH 8.0, with protease inhibitors added) by using a homogenizer (Wheaton, Millville, NJ). Cell lysates were treated with 0.5% Tween-20 and cleared by centrifugation at 13,000 × g for 10 min. Immunoprecipitations were performed by adding anti-GFP antibody (Ferro-Novick lab) to the supernatant at 4°C and incubated over night. The protein A/G resin (Thermo Scientific, Waltham, MA) was added to the samples, which were further incubated for 2 h at 4°C the next morning. After washing of the affinity resin with PBS immunoprecipitation buffer four times, immunoprecipitated proteins were eluted and reduced with sample buffer (6 M urea, 150 mM Tris-HCl, pH 6.8, 6% SDS, 10% β-mercaptoethanol, bromophenol blue) and analyzed by SDS–PAGE and immunoblotting. The intensities of Western blot bands were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD).
Supplementary Material
Acknowledgments
We thank S. Ferro-Novick for anti-GFP antibody, B. Antonny and G. Drin for the osh4 K336A and R344A mutants, and S. Field for the GFP-2xPH(PLC1delta) plasmid. We thank Y. Jones from the lab of M. Farquhar for preparation of samples for electron microscopy. This study was supported by National Institutes of Health Grants GM082861 and GM35370 to P.N.
Abbreviations used:
- ER
endoplasmic reticulum
- GAP
GTPase-activating protein
- GEF
guanine nucleotide exchange factor
- GFP
green fluorescent protein
- PH domain
pleckstrin homology domain
- PI4P
phosphatidylinositol-4-phosphate
- PI(4,5)P2
phosphatidylinositol-4,5-bisphosphate
- PM
plasma membrane
- SNARE
soluble N-ethylmaleimide–sensitive factor attachment protein receptor
Footnotes
This article was published online ahead of print in MBoC in Press (http://www .molbiolcell.org/cgi/doi/10.1091/mbc.E14-06-1087) on August 27, 2014.
REFERENCES
- Alfaro G, Johansen J, Dighe SA, Duamel G, Kozminski KG, Beh CT. The sterol-binding protein Kes1/Osh4p is a regulator of polarized exocytosis. Traffic. 2011;12:1521–1536. doi: 10.1111/j.1600-0854.2011.01265.x. [DOI] [PubMed] [Google Scholar]
- Audhya A, Emr SD. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev Cell. 2002;2:593–605. doi: 10.1016/s1534-5807(02)00168-5. [DOI] [PubMed] [Google Scholar]
- Audhya A, Foti M, Emr SD. Distinct roles for the yeast phosphatidylinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics. Mol Biol Cell. 2000;11:2673–2689. doi: 10.1091/mbc.11.8.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon RA, Salminen A, Ruohola H, Novick P, Ferro-Novick S. The GTP-binding protein Ypt1 is required for transport in vitro: the Golgi apparatus is defective in ypt1 mutants. J Cell Biol. 1989;109:1015–1022. doi: 10.1083/jcb.109.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev. 2013;93:1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beh CT, Cool L, Phillips J, Rine J. Overlapping functions of the yeast oxysterol-binding protein homologues. Genetics. 2001;157:1117–1140. doi: 10.1093/genetics/157.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnia R, Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. doi: 10.1038/nature04397. [DOI] [PubMed] [Google Scholar]
- Beh CT, Rine J. A role for yeast oxysterol-binding protein homologs in endocytosis and in the maintenance of intracellular sterol-lipid distribution. J Cell Sci. 2004;117:2983–2996. doi: 10.1242/jcs.01157. [DOI] [PubMed] [Google Scholar]
- Benli M, Doring F, Robinson DG, Yang X, Gallwitz D. Two GTPase isoforms, Ypt31p and Ypt32p, are essential for Golgi function in yeast. EMBO J. 1996;15:6460–6475. [PMC free article] [PubMed] [Google Scholar]
- Carlton JG, Cullen PJ. Coincidence detection in phosphoinositide signaling. Trends Cell Biol. 2005;15:540–547. doi: 10.1016/j.tcb.2005.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Saint-Jean M, Delfosse V, Douguet D, Chicanne G, Payrastre B, Bourguet W, Antonny B, Drin G. Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. J Cell Biol. 2011;195:965–978. doi: 10.1083/jcb.201104062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan KW, Bretscher A. Myosin-V is activated by binding secretory cargo and released in coordination with Rab/exocyst function. Dev Cell. 2012;23:769–781. doi: 10.1016/j.devcel.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drin G, Casella JF, Gautier R, Boehmer T, Schwartz TU, Antonny B. A general amphipathic alpha-helical motif for sensing membrane curvature. Nat Struct Mol Biol. 2007;14:138–146. doi: 10.1038/nsmb1194. [DOI] [PubMed] [Google Scholar]
- Faulhammer F, Kanjilal-Kolar S, Knodler A, Lo J, Lee Y, Konrad G, Mayinger P. Growth control of Golgi phosphoinositides by reciprocal localization of sac1 lipid phosphatase and pik1 4-kinase. Traffic. 2007;8:1554–1567. doi: 10.1111/j.1600-0854.2007.00632.x. [DOI] [PubMed] [Google Scholar]
- Foti M, Audhya A, Emr SD. Sac1 lipid phosphatase and Stt4 phosphatidylinositol 4-kinase regulate a pool of phosphatidylinositol 4-phosphate that functions in the control of the actin cytoskeleton and vacuole morphology. Mol Biol Cell. 2001;12:2396–2411. doi: 10.1091/mbc.12.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan B, Bowser R, Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J Cell Biol. 1995;128:1055–1068. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans BL, Andreeva A, Gangar A, Niessen S, Yates JR, 3rd, Brennwald P, Novick P. The yeast lgl family member Sro7p is an effector of the secretory Rab GTPase Sec4p. J Cell Biol. 2006;172:55–66. doi: 10.1083/jcb.200510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Roth D, Walch-Solimena C, Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999b;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Stolz LE, Lemrow SM, York JD. SAC1-like domains of yeast SAC1, INP52, and INP53 and of human synaptojanin encode polyphosphoinositide phosphatases. J Biol Chem. 1999a;274:12990–12995. doi: 10.1074/jbc.274.19.12990. [DOI] [PubMed] [Google Scholar]
- Hughes WE, Woscholski R, Cooke FT, Patrick RS, Dove SK, McDonald NQ, Parker PJ. SAC1 encodes a regulated lipid phosphoinositide phosphatase, defects in which can be suppressed by the homologous Inp52p and Inp53p phosphatases. J Biol Chem. 2000;275:801–808. doi: 10.1074/jbc.275.2.801. [DOI] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im YJ, Raychaudhuri S, Prinz WA, Hurley JH. Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature. 2005;437:154–158. doi: 10.1038/nature03923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G, Mulholland J, Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J Cell Biol. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G, Richardson C, Litt R, Segev N. The Ypt1 GTPase is essential for the first two steps of the yeast secretory pathway. J Cell Biol. 1995;131:583–590. doi: 10.1083/jcb.131.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Sultana A, Gandhi P, Franklin E, Hamamoto S, Khan AR, Munson M, Schekman R, Weisman LS. Myosin V transports secretory vesicles via a Rab GTPase cascade and interaction with the exocyst complex. Dev Cell. 2011;21:1156–1170. doi: 10.1016/j.devcel.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Alfaro G, Dighe S, Beh CT. Homologues of oxysterol-binding proteins affect Cdc42p- and Rho1p-mediated cell polarization in Saccharomyces cerevisiae. Traffic. 2006;7:1224–1242. doi: 10.1111/j.1600-0854.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- LeBlanc MA, McMaster CR. Lipid binding requirements for oxysterol-binding protein Kes1 inhibition of autophagy and endosome-trans-Golgi trafficking pathways. J Biol Chem. 2010;285:33875–33884. doi: 10.1074/jbc.M110.147264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine TP, Munro S. Dual targeting of Osh1p, a yeast homologue of oxysterol-binding protein, to both the Golgi and the nucleus-vacuole junction. Mol Biol Cell. 2001;12:1633–1644. doi: 10.1091/mbc.12.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y, Stefan CJ, Macgurn JA, Audhya A, Emr SD. The dual PH domain protein Opy1 functions as a sensor and modulator of PtdIns(4,5)P(2) synthesis. EMBO J. 2012;31:2882–2894. doi: 10.1038/emboj.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Manford AG, Stefan CJ, Yuan HL, Macgurn JA, Emr SD. ER-to-plasma membrane tethering proteins regulate cell signaling and ER morphology. Dev Cell. 2012;23:1129–1140. doi: 10.1016/j.devcel.2012.11.004. [DOI] [PubMed] [Google Scholar]
- McNew JA, Parlati F, Fukuda R, Johnston RJ, Paz K, Paumet F, Sollner TH, Rothman JE. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature. 2000;407:153–159. doi: 10.1038/35025000. [DOI] [PubMed] [Google Scholar]
- Medkova M, France YE, Coleman J, Novick P. The rab exchange factor Sec2p reversibly associates with the exocyst. Mol Biol Cell. 2006;17:2757–2769. doi: 10.1091/mbc.E05-10-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno-Yamasaki E, Medkova M, Coleman J, Novick P. Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev Cell. 2010;18:828–840. doi: 10.1016/j.devcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem. 2012;81:637–659. doi: 10.1146/annurev-biochem-052810-093700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz D, Medkova M, Walch-Solimena C, Novick P. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol. 2002;157:1005–1015. doi: 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Levine TP. Multiple pools of phosphatidylinositol 4-phosphate detected using the pleckstrin homology domain of Osh2p. J Biol Chem. 2004;279:44683–44689. doi: 10.1074/jbc.M401583200. [DOI] [PubMed] [Google Scholar]
- Santiago-Tirado FH, Legesse-Miller A, Schott D, Bretscher A. PI4P and Rab inputs collaborate in myosin-V-dependent transport of secretory compartments in yeast. Dev Cell. 2011;20:47–59. doi: 10.1016/j.devcel.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott DH, Collins RN, Bretscher A. Secretory vesicle transport velocity in living cells depends on the myosin-V lever arm length. J Cell Biol. 2002;156:35–39. doi: 10.1083/jcb.200110086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott D, Ho J, Pruyne D, Bretscher A. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J Cell Biol. 1999;147:791–808. doi: 10.1083/jcb.147.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TA, Choi MG, Raychaudhuri S, Mears JA, Ghirlando R, Hinshaw JE, Prinz WA. Lipid-regulated sterol transfer between closely apposed membranes by oxysterol-binding protein homologues. J Cell Biol. 2009;187:889–903. doi: 10.1083/jcb.200905007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Chen S, Rivera-Molina F, Reinisch K, Novick P, Ferro-Novick S. Establishing a role for the GTPase Ypt1p at the late Golgi. Traffic. 2010;11:520–532. doi: 10.1111/j.1600-0854.2010.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JH, Schekman R. Lyticase: endoglucanase and protease activities that act together in yeast cell lysis. J Bacteriol. 1980;142:414–423. doi: 10.1128/jb.142.2.414-423.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Yuan H, Hutagalung A, Verma A, Kummel D, Wu X, Reinisch K, McNew JA, Novick P. The synaptobrevin homologue Snc2p recruits the exocyst to secretory vesicles by binding to Sec6p. J Cell Biol. 2013;202:509–526. doi: 10.1083/jcb.201211148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder D, Mizuno-Yamasaki E, Ghassemian M, Novick PJ. Phosphorylation of the Rab exchange factor Sec2p directs a switch in regulatory binding partners. Proc Natl Acad Sci USA. 2013;110:19995–20002. doi: 10.1073/pnas.1320029110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y, Emr SD. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell. 2011;144:389–401. doi: 10.1016/j.cell.2010.12.034. [DOI] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Wagner W, Bielli P, Wacha S, Ragnini-Wilson A. Mlc1p promotes septum closure during cytokinesis via the IQ motifs of the vesicle motor Myo2p. EMBO J. 2002;21:6397–6408. doi: 10.1093/emboj/cdf650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C, Collins RN, Novick PJ. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J Cell Biol. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C, Novick P. The yeast phosphatidylinositol-4-OH kinase pik1 regulates secretion at the Golgi. Nat Cell Biol. 1999;1:523–525. doi: 10.1038/70319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.