Abstract
Introduction:
Kawasaki disease is an acute, self-limiting childhood systemic vasculitis with unknown etiology. Because there is no diagnostic test for Kawasaki disease, the diagnosis is based on clinical criteria. An important clinical sign that is not included in the classical clinical criteria for Kawasaki disease is a reaction at the Bacille Calmette-Guérin inoculation site that are present in about 30-50% of Kawasaki disease patients.
The aim:
of this review was to highlight the usefulness of the inflammation at the Bacille Calmette-Guérin inoculation site for early diagnosis of Kawasaki disease, we conducted a literature review on Medline in PubMed area, Google scholar, Magiran and Scientific Information Database using the search terms “Kawasaki disease, Erythema, BCG, inoculation site, children, cardiac complications, coronary artery lesion, aneurysm, incomplete Kawasaki in 2013.
Results and discussion:
A total of 15 articles had been found. Erythema at the Bacille Calmette-Guérin inoculation site was found in 49.87% of Kawasaki disease patients. Coronary artery abnormalities were found in 10.3% of cases. According to this review, BCGitis is more prevalent than cervical lymphadenopathy and rash and it can be a useful criterion in the diagnosis of incomplete Kawasaki disease in cases not fulfills the classic criteria of at least four of the five findings.
Keywords: Erythema, BCG, BCGitis, Kawasaki disease
1. INTRODUCTION
Kawasaki disease (KD) was first described in Japan by Tomisaku Kawasaki in 1967. Tomisaku Kawasaki was also the first that reported KD cases with erythema at the Bacillus Callmette-Guerin (BCG) inoculation site (1).
KD or mucocutaneous lymph node syndrome is one of the most common acquired and acute onset cardiovascular diseases in childhood with unknown etiology generally affecting children aged 5 years or younger (2-5). The incidence of KD varies worldwide, and the highest rates was reported in nationwide investigations in Japan; in 2005-2006 with an incidence equal to 184 per 100 000 children less than 5 years old annually (6).
A major obstacle to diagnosing KD is absence of ‘gold Standard’ for defining the condition. No specific diagnostic tests are currently available (2). Clinical diagnosis criteria as determined by the American Heart Association (AHA) and established by the Japanese Ministry of Health, associated with laboratory criteria may be used to help establish the diagnosis (7). The clinical diagnostic criteria are included: fever lasting 5 days or more without other reason, associated with at least four out of five of the following principal features:changes in extremities including indurative edema of palms and soles followed by desquamation, polymorphous rash, bilateral non-exudative conjunctivitis, oral mucosal edema and erythema, including pharyngeal injection, dry fissured lips, and/or strawberry tongue and acute non-purulent cervical lymphadenopathy (>1.5 cm diameter) (7). If less than four of the principal features are present associated with two-dimensional echocardiography detecting coronary artery abnormalities, patients are diagnosed with incomplete Kawasaki disease (IKD) (7).
Supplementary laboratory criteria to diagnosing KD are also included: Albumin less than 3 g/dL, C-reactive protein more than 3 mg, Erythrocyte sedimentation rate more than 40 mm/h, Elevated alanine aminotransferase, Leukocytosis: white cell count more than 15,000/mm, Normochromic, normocytic anemia, Sterile pyuria more than 10 white blood cell/mm3 (7).
Early diagnosis of KD is very important, because coronary artery aneurysms occur as a result of vasculitis in 15% to 25% of untreated children (8). Coronary artery lesions (CAL) or coronary artery abnormalities (CAA), myocardial infarction, coronary artery fistula, coronary artery dilatation (CAD), and coronary artery aneurysm (CAA) are the most serious side effects of KD (2).
Unfortunately the current definition of KD based on the diagnostic criteria overlaps with other diseases. In atypical cases, it causes diagnostic dilemmas, which do not completely fulfill the diagnostic criteria of KD, but are associated with the development of coronary artery abnormalities (9).
An important clinical sign that is not included in the diagnostic criteria, but can be helpful in the diagnosis of KD is BCGitis or involvement of the BCG scar erythema and induration (Figure 1 910). Acute inflammation at the site of a previous BCG inoculation is one of the unique features of KD among infants in some countries with vaccination policy against tuberculosis (6, 11). BCGitis was reported to have been observed in more than 50% of KD patients 1 to 12 months following BCG inoculation (12). Erythema and induration of a previous BCG vaccination site is a specific and early manifestation of KD (13,14), which was first highlighted in the Japanese literature as a specific and an early sign of KD (15). Several KD cases with BCG reactivation, inflammation, or induration were also reported from other countries (14,16,17).
Figure 1.
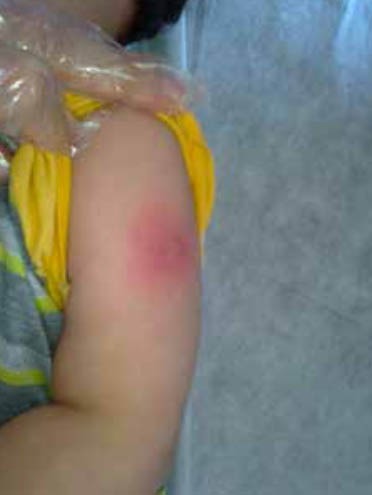
Erythema at BCG inoculation site
Figure 2.
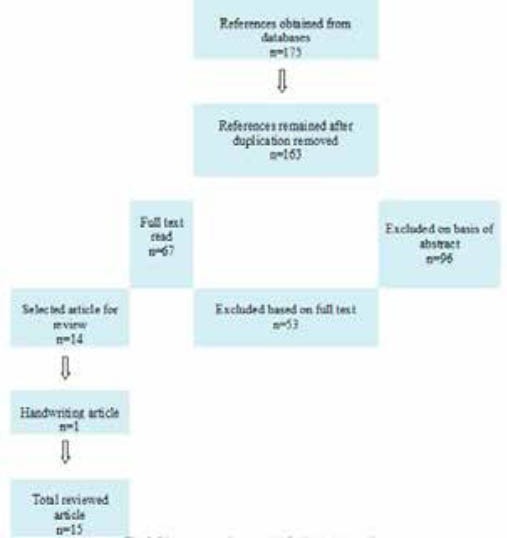
Flow diagram of literature review results for the included studies
Besides, elevated levels of cytokines such as interleukin-1β (IL- β) and tumor necrosis factor-α (TNF- α) were detected at the BCG inoculation site. Redness or crust formation at the BCG inoculation site in KD patients was hypothetically predicted to cross-reaction between mycobacterium HSP65 and human homolog HSP63 (11,18,19). Kai-Sheng Hsieh et al., in a review on records of 28 KD patients, stated that although erythema at a BCG inoculation site is a useful diagnostic sign in KD, it may not be a good predictor of KD-CAE (20).
The aim of this review was:
To reveal the diagnostic accuracy of erythema at BCG inoculation site in KD and also its role on early detection of CAA.
On the other hand, conducting similar studies in Iran and other countries and determining correlation between erythema around the BCG inoculation site using diagnostic criteria for KD is useful to early diagnosis and prevention of cardiac complications in these patients.
2. METHODOLOGY
A literature review was conducted on Medline in PubMed area, Google scholar, Magiran and SID databases using the search terms “Kawasaki disease, Erythema, BCG, inoculation site, children, cardiac complications, coronary artery lesion, aneurysm, incomplete Kawasaki in 2013. All English and Persian written articles related to KD patients with erythema at BCG inoculation site and one handwriting unpublished article by the authors were included. Duplicates were removed. The total data of KD patients with erythema at BCG inoculation site were extracted from all included papers. The searching strategy of the included studies was summarized in Table 1.
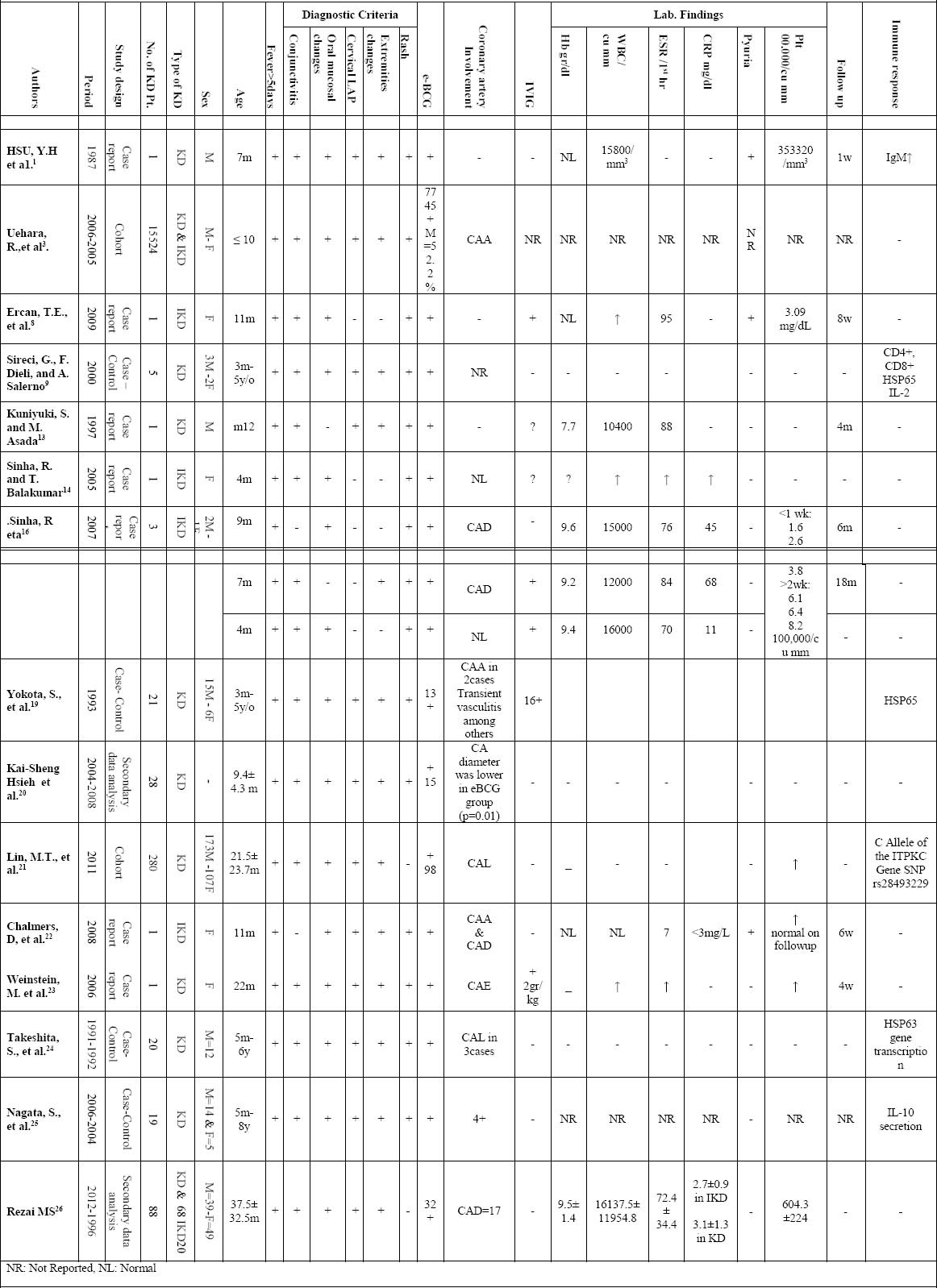
Table 1.
Characteristics and results of the 15 studies related to Erythema at the BCG inoculation site in Kawasaki disease patients
3. RESULTS
A total of 15 articles met the criteria of the study. Characteristics and results of the studied papers have shown in Table 2.
A total of 15,955 KD patients with a history of BCG vaccination were identified and 52.3 % were male patients. The age of the patients ranged from 0 to 120 months, and the mean age was ≤ 10years. Of the 15,955 patients, 13,186 (82.6%) had the criteria for the diagnosis of KD and 2769 (17.4%) diagnosed IKD. All patients excepted two had fever persisting for five days or more. Conjunctivitis, erythema of lips and changing of oral mucosa, changes of extremities, rash, and cervical lymphadenopathy were other clinical findings in KD patients. The most clinical manifestations in IKD patients were fever (100%), Conjunctivitis (99.9%), erythema of lips and changing of oral mucosa (99.9%), rash (99.3%) and erythema at the BCG inoculation site (100%). Coronary artery abnormalities (CAA) were found in 1625 (10.3%) cases. Erythema at the BCG inoculation site was found in 7956 (49.87%) including all of IKD patients and 5196 (32.57%) of KD patients. Of total, we found 20 cases reported to receive intravenous immunoglobulin (IVIG) 2gr/kg. Mean Hb was 9.3gr/dl. Excepted in one case report, WBC was more than 15000 in most all reported cases. Mean ESR was 67.4mm/h. The lower and upper limit of ESR was 7mm/h and 95mm/h respectively and the both were belonged to IKD patients. The mean value of Platelet count was 342.66×103/mm3. Pyuria was reported in two IKD and one KD patients. The mean follow up period was 6.5 months. The most common immune responses to KD were expression of CD4+, CD8+, HSP65, C Allele of the ITPKC Gene SNP rs28493229, IL12 deficiency, and increase IgM, IgE and IgA.
4. DISCUSSION
In this review we indented to investigate how erythema at the BCG inoculation site (BCGitis) can be a useful diagnostic criterion of KD.
BCGitis was reported to be a positive sign in the diagnostic guideline of KD (1,3,12,14). Besides, the Kawasaki Disease Research Committee in Japan and the AHA have listed erythema, induration, or crust at the BCG inoculation site as a significant finding among the diagnostic guidelines for KD (27-29). In some Eastern Asian countries with national BCG vaccination program, such as Japan, Korea, and Taiwan, the erythema at the BCG inoculation site was also a strong supporting evidence for the diagnosis of KD (21). In this review, we also found BCGitis in 49.87% of KD patients. This finding was similar to Uehara et al. study in 2010 in Japan (3). They had studied the epidemiologic feature of KD patients with similar changes at the BCG inoculation site to evaluate the specificity of this sign in KD patients. They found BCGitis in 49.9% of the cases. They have also reported that more than 70% of these patients were aged 3 to 20 months. In our review, the age range of the patients was from 0 to 120 months. Similarly, they showed that the proportion of male patients with erythema at the BCG inoculation site was higher than that of female patients. They reported that among the complete KD patients aged 2 years or younger, redness or crust formation at the BCG inoculation site was more prevalent than cervical lymphadenopathy. The results of our review also showed that among all the IKD patients, BCGitis was more prevalent than cervical lymphadenopathy and rash. It seems, BCGitis can be a useful criterion in the diagnosis of IKD in cases not fulfills the classic criteria of at least four of the five findings. Uehara et al had also investigated the relationship between the day of hospitalization and the onset of the disease. They showed that this sign appears in the early stage of the disease, between 1 and 4 days after the onset of the illness by fever (3). Similar finding was reported by Hsu et al. in 1986 and others (1,8,16).
Coronary artery lesions (CAL) may be observed in 15-25% of untreated patients with KD or in 5%-10% of those treated with intravenous immunoglobulin therapy (21). In this review, we found coronary arteries involvement in 10.3% of the KD patients with BCGitis (3,16,19-26). Conversely, Uehara et al. had found no association redness or crust formation at the inoculation site and the development of CAA among patients aged 3 to 20 months, and they suggested these changes are not useful for predicting the presence of CAA(3). In a study by Newburger et al., IKD had been suggested as an independent risk factor for subsequent coronary artery ectasia and early treatment with IVIG should be decreased its risk (30). Also, Hsieh et al., in a study investigated the correlation between BCGitis in KD with coronary artery ectasia (CAE). They had concluded although BCGitis was a useful diagnostic sign in KD, it cannot be a good predictor of KD-CAE (20). Although the cause of the KD is unknown, the central aspects of disease are immune activation and cytokine- mediated generalized vasculitis and epidemiologic features suggest that the mechanism involves an immune response to a precipitating infection in genetically predisposed individuals. Immunologically, a highly increased level of several cytokines and both the activation of immunocompetent cells including activated CD4+ and CD8+ T cells, as well as monocyte/macrophages has been demonstrated(9,11,19,23-25). Sireci et al., in 2000 showed that T Cells recognize an immunodominant Epitope of Heat Shock Protein 65(HSP65) in KD. They determined at a clonal level the presence of T lymphocytes recognizing HSP65 to analyze the possible role of HSP during the acute phase of KD. They concluded that cross-reactivity between specific epitopes of mycobacterial and human HSP could play a role in the development of the tissue-damage characteristic of KD. Furthermore, acute KD is associated with increased production of IL-1, TNF-γ, IL-2, IL-6, and IFN-γ. The production of these cytokines by T cells and monocytes is thought to have an important role in the pathogenesis of KD, especially in vascular endothelial cell injury (9,31). Lin et al., in 2011 investigated the clinical implication of the C Allele of the ITPKC Gene single nucleotide polymorphism (SNP) rs28493229 in 280 classic KD. They found that the GC and CC genotypes of ITPKC gene SNP rs28493229 were overrepresented in KD and these patients were more likely to have reactivation at the BCG inoculation site. Such association was particularly strong in patients aged <20 months. They reported coronary arterial lesions in 57.1% of the KD patients; however the development and the severity of coronary arterial lesion were also not associated with this SNP. They also compared the differences in the clinical manifestations and the development of CAL between KD patients with and without BCG reactivation, and they found that the only difference was noted in the onset of KD (21). In a study by Rezai et al., coronary arteries involvement was reported 34.1% and was also prominent in boys than girls (26).
Treatment with a single dose of 2 g/kg of IVIG infused over 12 hours plus Aspirin at a dose of 80 to 100 mg/kg per day in 4 divided doses was recommended by the AHA in children suspected to have KD. Treatment has to start within the first 10 days of disease. However, IVIG should also be given to patients who present after the 10th day of illness (32). In this review, 20 patients were reported to receive IVIG therapy (8,16,19,23). In 2009, Ercan et al. from Turkey reported an 11-month-old girl with high fever associated with other manifestations such as conjunctivitis, erythematous lips, and a maculopapular rash. Her fever continued despite antibiotic treatment, suggestive of KD appeared, in addition to the inflammation of the BCG scar. Thus, a diagnosis of incomplete Kawasaki disease was made and IVIG, 2 gr/kg over 12 hours and aspirin 80 mg/kg on the sixth day of fever were started. After IVIG therapy, fever subsided dramatically and erythema around the BCG scar disappeared. They concluded that inflammatory reactivation of a BCG vaccination site is an early and specific sign of Kawasaki disease. Besides, BCG vaccination was as a part of the national immunization schedule in Turkey. So that, they tried to emphasize the usefulness of BCG site reactivation in establishing a diagnosis of Kawasaki/incomplete Kawasaki syndrome that clinicians should be aware of this clinical manifestation especially in countries where BCG vaccination is still a part of the immunization schedule (8). Similarly, Sinha et al., in 2007 presented three cases of IKD to show the usefulness of BCG reactivation in formulating a diagnosis of Incomplete Kawasaki. None of the cases showed more than three classical criteria though they did have fever not responding to antibiotics and increased inflammatory markers. Two of the three children received IVIG therapy. Fever was subsided and BCGitis was also disappeared following IVIG therapy (16). Hypothetically, BCGitis in KD has been ascribed to cross-reactivity between mycobacterial Heat Shock Protein 65 (HSP65) and Human Homologue HSP63. The recognition of strong antibody and cellular reactivity against synthetic peptides of mycobacterial HSP65 and its human homologue in the blood of children with KD confirms this hypothesis (19, 33).
Although, laboratory examinations in the acute phase KD are nonspecific, however, in children who do not complete diagnostic criteria, these may support the diagnosis. Thrombocytosis as a characteristic feature of KD usually occurs during the 2 weeks of the disease. Also, thrombocytopenia is unusual in KD, but it is considered as a risk factor for the development of aneurism(33). In this review, among supplementary laboratory findings to diagnosing KD in patients with BCGitis we found elevated CRP more than 3 mg (8,14,16,26), increased ESR more than 40 mm/h (8,13,16,26), elevated alanine aminotransferase (1,14), Leukocytosis (13,14), normochromic, normocytic anaemia (13,16,26), sterile pyuria more than 10 white blood cell/mm3 (1,8,22), thrombocytosis and thrombocytopenia (1,8,16,21-24,26).
In addition, the most common immune responses to KD were expression of CD4+, CD8+, HSP65, C Allele of the ITPKC Gene SNP rs28493229, IL12 deficiency, and increase IgM, IgE and IgA (1,9,19,21,24,25).
5. CONCLUSION
This review provided evidences to show the importance of reactivation at BCG inoculation site as an early sign suggestive of KD especially IKD among children younger than 5 years. Verification of this sign may facilitate prompt diagnosis, prevention of CAA as the most serious side effects of the disease and treatment of KD, finally yielding enhanced outcomes among children emigrating from countries where BCG vaccination is still a part of the immunization schedule. In addition, in this review, BCGitis is shown to be more prevalent than cervical lymphadenopathy and rash and it can be a useful criterion in the diagnosis of KD or even IKD in cases not fulfills the classic criteria of at least four of the five findings.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Hsu YH, Wang YH, Hsu WY, Lee YP. Kawasaki disease characterized by erythema and induration at the Bacillus Calmette-Guerin and purified protein derivative inoculation sites. Pediatr Infect Dis J. 1987;6:576–578. [PubMed] [Google Scholar]
- 2.Cimaz R, Sundel R. Atypical and incomplete Kawasaki disease. Best Pract Res Clin Rheumatol. 2009;23(5):689–697. doi: 10.1016/j.berh.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Uehara R, Igarashi H, Yashiro M, Nakamura Y, Yanagawa H. Kawasaki disease patients with redness or crust formation at the Bacille Calmette–Guérin inoculation site. Pediatr Infect Dis J. 2010;29:430–433. doi: 10.1097/INF.0b013e3181cacede. [DOI] [PubMed] [Google Scholar]
- 4.Rezai MS, Shokohi L, Saffar MJ, Zeinali A, Abaskhanian A. Investigating the Relationship between Eosinophilia and Coronary Artery Disease in Patients with Kawasaki Disease. J Mazandaran Univ Med Sci. 2012;22(88):10–16. [Google Scholar]
- 5.Rezai MS, Siadati S, Khotaei G, Mamishi S, Sabuni F, Poorakbari B, et al. Isolation of Kawasaki disease-associated with bacterial sequence from peripheral blood leukocytes. J Mazandaran Univ Med Sci. 2008;18(64):22–28. [Google Scholar]
- 6.Harnden A, Takahashi M, Burgner D. Kawasaki disease. BMJ. 2009;338:b1514. doi: 10.1136/bmj.b1514. [DOI] [PubMed] [Google Scholar]
- 7.Jamieson N, Singh-Grewal D. “Kawasaki Disease: A Clinician's Update,”. International Journal of Pediatrics 2013. 2013:1–7. doi: 10.1155/2013/645391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ercan TE, Ercan G, Arpaozu M. An 11 – month-old girl with high fever. Pediatr Ann. 2009;38(6):305–310. doi: 10.3928/00904481-20090508-02. [DOI] [PubMed] [Google Scholar]
- 9.Sireci G, Dieli F, Salerno A. T cells recognize an immunodominant epitope of heat shock protein 65 in Kawasaki disease. Mol Med. 2000;6:581–590. [PMC free article] [PubMed] [Google Scholar]
- 10.Antony D, Jessy PL. Involvement of BCG scar in Kawasaki disease. Indian Pediatr. 2005;42:83–84. [PubMed] [Google Scholar]
- 11.Shahmohammadi S, Saffar M, Rezai M. BCG-osis after BCG vaccination in immunocompromised children: Case series and review. J Pediatr Rev. 2014;2(1):62–74. [Google Scholar]
- 12.Seo Ji Hye, Jeong Jin Yu, Hong Ki Ko, Hyung Soon Choi, Young-Hwue Kim, Jae-Kon Ko. Diagnosis of Incomplete Kawasaki Disease in Infants Based on an Inflammation at the Bacille Calmette-Guérin Inoculation Site. Korean circulation journal. 2012;42(12):823–829. doi: 10.4070/kcj.2012.42.12.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuniyuki S, Asada M. An ulcerated lesion at the BCG vaccination site during the course of Kawasaki disease. J Am Acad Dermatol. 1997;37:303–304. [PubMed] [Google Scholar]
- 14.Sinha R, Balakumar T. BCG reactivation: a useful diagnostic tool even for incomplete Kawasaki disease. Archives of disease in childhood. 2005;90(9):891. doi: 10.1136/adc.2004.071332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takayama J, Yanase Y, Kawasaki T. Study of the changes of the site of the BCG inoculation in MCLS. JPN J Pediatr. 1982;86:567–572. [Google Scholar]
- 16.Sinha R, Balakumar T, Sinha S. Usefulness of BCG reactivation in Incomplete Kawasaki Disease: A Case Series. The Internet Journal of Rheumatology. 2007;3(1):891. [Google Scholar]
- 17.Suliman OSM, Abdelnasser M. Incomplete Kawasaki disease: the usefulness of BCG reactivation as a diagnostic tool. Sudan J Paediatr. 2012;12(1):84–88. [PMC free article] [PubMed] [Google Scholar]
- 18.Yin Ji X, Kang MR, Choi JS, Jeon HS, Han HS, Kim JY, Son BR, Lee YM, Hahn YS. Levels of intra-and extracellular heat shock protein 60 in Kawasaki disease patients treated with intravenous immunoglobulin. Clin immunol. 2007;124:304–310. doi: 10.1016/j.clim.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Yokota S, Tsubaki K, Kuriyama T, Shimizu H, Ibe M, Mitsuda T, et al. Presence in Kawasaki disease of antibodies to mycobacterial heat shock protein hsp65 and autoantibodies to epitopes of human hsp65 cognate antigen. Immunol. Immunopathol. 1993;2:163–170. doi: 10.1006/clin.1993.1060. [DOI] [PubMed] [Google Scholar]
- 20.Kai-Sheng Hsieh, Chin-Fu Wang, Ken-Peng Wong, Chu-Chuan Lin. Erythema at a BCG inoculation site in Kawasaki disease with coronary artery ectasia. Poster Program. Pediatrics International. 2012;54:20–35. doi:10.1111/j.1442-200X.2012.03538.x. [Google Scholar]
- 21.Lin MT, Wang JK, Yeh JI, Sun LC, Chen PL, Wu JF, et al. Clinical Implication of the C Allele of the ITPKC Gene SNP rs28493229 in Kawasaki Disease: Association With Disease Susceptibility and BCG Scar Reactivation. Pediatr Infect Dis J. 2011 Feb;30(2):148–152. doi: 10.1097/INF.0b013e3181f43a4e. [DOI] [PubMed] [Google Scholar]
- 22.Chalmers D, Corban JG, Moore PP. BCG site inflammation: a useful diagnostic sign in incomplete Kawasaki disease. J Paediatr Child Health. 2008;44:525–526. doi: 10.1111/j.1440-1754.2008.01364.x. [DOI] [PubMed] [Google Scholar]
- 23.Weinstein M. Inflammation at a previous inoculation site: an unusual presentation of Kawasaki disease. CMAJ. 2006;174:459–460. doi: 10.1503/cmaj.051211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeshita S, Kawase H, Yamamoto M, Fujisawa T, Sekine I, Yoshioka S. Increased expression of human 63-kD heat shock protein gene in Kawasaki disease determined by quantitative reverse transcription-polymerase chain reaction. Pediatr Res. 1994;35:179–183. doi: 10.1203/00006450-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Nagata S, Yamashiro Y, Ohtsuka Y, Shimizu T, Sakurai Y, Misawa S. Heat shock proteins and superantigenic properties of bacteria from the gastrointestinal tract of patients with Kawasaki disease. Immunology. 2009;128(4):511–520. doi: 10.1111/j.1365-2567.2009.03135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rezai MS, Shokohi L. Erythema at the BCG vaccination site in Kawasaki disease patients . unpublished. doi: 10.5455/msm.2014.26.256-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta-Malhotra M, Rao PS. Current perspectives on Kawasaki disease. Indian J Pediatr. 2005;72:621–629. doi: 10.1007/BF02724189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayusawa M, Sonobe T, Uemura S, et al. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition) Pediatr Int. 2005;47:232–234. doi: 10.1111/j.1442-200x.2005.02033.x. [DOI] [PubMed] [Google Scholar]
- 29.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 30.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, et al. Diagnosis, treatment, and long-term management of Kawasaki Disease. Circulation. 2004;110:2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani A, Defana EE. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989;10:370–375. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- 32.Shiari R. Kawasaki Disease; A Review Article. Archives of Pediatric Infectious Diseases. 2013 Jul;1(4):154–159. Published Online 2013 July 13. [Google Scholar]
- 33.Yokota S, Tsubaki K, Kuriyama T, Shimizu H, Ibe M, Mitsuda T, Aihara Y, Kosuge K, Nomaguchi H. Presence in Kawasaki Disease of antibodies to mycobacterial heat-shock protein HSP65 and autoantibodies to epitopes human HSP65 cognate antigen. Clin Immunol Immunopathol. 1993;67:163–170. doi: 10.1006/clin.1993.1060. [DOI] [PubMed] [Google Scholar]


