Abstract
The kidney is a key target organ for bioactive components of the renin-angiotensin system (RAS); however, various renal cells such as the tubular epithelium contain an intrinsic RAS. The renal RAS can be functionally divided into ANG II-AT1 receptor and ANG-(1–7)-AT7/Mas receptor arms that functionally oppose one another. The current review considers both extracellular and intracellular pathways that potentially govern the formation and metabolism of angiotensin peptides within the renal proximal tubules.
Keywords: angiotensin II, angiotensin-(1–7), kidney, metabolism, peptidase, fetal programming
the kidney is a key target for the diverse components of the renin-angiotensin-aldosterone system that include prorenin/renin, angiotensin II (ANG II), ANG-(2–8) (ANG III), ANG-(1–7), ANG-(3–8), ANG-(1–9), and aldosterone (2, 3, 6, 10). The kidney also comprises an intrinsic renin-angiotensin system (RAS) particularly within the proximal tubule epithelium capable of producing bioactive peptides to activate their respective receptors (R) in a paracrine or autocrine manner (2, 6, 10). Currently, the renal RAS can be functionally partitioned into at least two arms based on the distinct processing enzymes and receptors that comprise the ANG II-AT1R and the ANG-(1–7)-AT7/MasR axis (2, 3, 7, 10, 14). In general, these two pathways exhibit opposing effects in the kidney and may antagonize the actions of one another (2, 3). Within the tubular system of the kidney, ANG II stimulates the AT1 receptor to enhance the activity of various transport mechanisms to maintain the efficient reabsorption of sodium (10). In contrast, ANG-(1–7) evokes natriuretic and diuretic effects, stimulates nitric oxide release, induces scavenging enzymes to attenuate oxidative stress, and stimulates cellular phosphatases to inhibit mitogen-activated kinase kinase pathways (2, 3). Apart from the distinct angiotensin peptide receptors, evidence to date suggests a complex array of peptidases involved in the synthesis and metabolism of ANG II and ANG-(1–7) (13, 14). Both peptides ultimately arise from the same precursor protein angiotensinogen that may be internalized by the proximal tubular cells and/or locally synthesized within the kidney (2) (Fig. 1). Angiotensin-converting enzyme (ACE) is well recognized as the key ANG II-forming enzyme in the kidney, circulation, and other peripheral and central tissues. In contrast, the ACE homolog ACE2 efficiently metabolizes ANG II to ANG-(1–7) and may markedly alter the functional signature of the RAS (2). ACE2 is a monocarboxypeptidase that does not continue to metabolize ANG-(1–7) due to the COOH-terminal proline; however, ACE hydrolyzes the Ile5-His6 bond of ANG-(1–7) to form ANG-(1–5) (2). ACE inhibitors increase circulating levels of ANG-(1–7) by preventing the rapid metabolism of the peptide, as well as shifting the processing of ANG I to ANG-(1–7) by the endopeptidase neprilysin (NEP) (2, 13, 14). All three enzymes are classified as metallopeptidases with membrane-anchoring domains that orient their active sites on the extracellular cell surface to process substrates within the glomerular filtrate, interstitial fluid, cerebrospinal fluid (CSF), or the blood (Fig. 1). These peptidases comprise the extracellular pathway for the formation of ANG II and ANG-(1–7) to subsequently bind to AT1R or AT7R on the cell surface and activate various signaling pathways (Fig. 1). ACE, ACE2, and NEP also contribute to the metabolism of both peptides to either inactive forms or, in the case of ANG-(1–7), a metabolite that functionally opposes the ANG II-AT1R axis (2, 13).
Fig. 1.
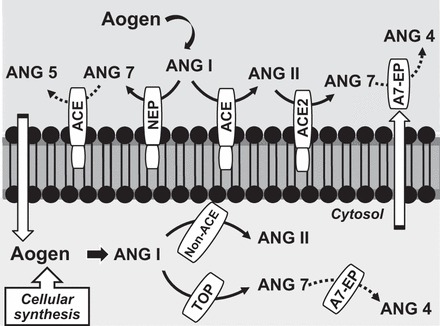
Proposed scheme for the extracellular and intracellular processing of angiotensin peptides within renal proximal tubules. Extracellular metallopeptidases, angiotensin-converting enzyme (ACE), ACE2, and neprilysin (NEP) process ANG I to ANG II or ANG-(1–7) (ANG 7) that subsequently bind to receptors on the cell surface. Intracellular peptidases include thimet oligopeptidase (TOP) to process ANG I to ANG-(1–7) or ACE-independent (non-ACE) pathways such as chymase to form ANG II. An ANG-(1–7) endopeptidase (A7-EP) hydrolyzes the peptide to ANG-(1–4) (ANG 4); the peptidase may be secreted for extracellular metabolism of ANG-(1–7) in addition to ACE-dependent metabolism of ANG-(1–7) to ANG-(1–5) (ANG 5). The precursor angiotensinogen (Aogen) may be internalized by the tubules from extracellular sources or arise from intracellular synthesis.
In addition to the extracellular processing of peptides, there is compelling evidence for the intracellular expression of both ANG II and ANG-(1–7) in the kidney and other tissues (1–8). The tissue expression of angiotensins may indeed lead to their subsequent release into the extracellular space; however, evidence of intracellular AT1R, AT2R, and AT7R may portend for processing pathways that provide an intracellular source of ANG II or ANG-(1–7) as potential receptor ligands (1–8). In glycemic or diabetic conditions, ANG II content is increased in various cell types and appears to reflect the contribution of non-ACE-dependent pathways such as chymase or cathepsins (5, 8, 11). Elucidation of the intracellular pathways for ANG-(1–7) formation and metabolism within the intracellular compartments of the kidney and other tissues is less clear. Costa-Neto and colleagues (12) reported that the soluble enzyme thimet oligopeptidase (TOP) processed ANG I predominantly to ANG-(1–7) in the rat hippocampus independent of ANG II. TOP was also identified as the primary activity that directly converted ANG I to ANG-(1–7) without the prerequisite formation of ANG II in nuclei isolated from the NRK-52E renal epithelial cells (1). In regard to ANG-(1–7) metabolism, our recent studies reveal a soluble endopeptidase that degrades ANG-(1–7) to the inactive metabolite ANG-(1–4) in the CSF and brain medulla of sheep (3, 9). The purified peptidase exhibits a preferred specificity for ANG-(1–7) compared with other angiotensins; the rate of ANG-(1–7) processing to ANG-(1–4) was 10- to 20-fold higher than for ANG II and ANG I, whereas other peptides including bradykinin, neurotensin, and apelin were not hydrolyzed by the enzyme following a 24-h incubation (9). The ANG-(1–7) endopeptidase also exhibits subnanomolar affinity (IC50 = 0.8 nM) against the inhibitor JMV-390 that is markedly lower than that reported for other metallopeptidases such as NEP, neurolysin, TOP, and ACE (9). Preliminary studies now identify the ANG-(1–7) endopeptidase in the soluble fraction of isolated proximal tubules of the sheep kidney, as well as in the human proximal tubule HK-2 cell line (15). Both peptidase activities in the tubules and HK-2 cells exhibit a similar high affinity for the JMV inhibitor to block the conversion of ANG-(1–7) to ANG-(1–4) (15). Moreover, endopeptidase activity was evident in the serum-free media of the HK-2 cells, suggesting that the enzyme is released to participate in the extracellular metabolism of ANG-(1–7) within the filtrate or the interstitial compartment of the kidney (15).
Perspectives and Significance
Functional partitioning of the RAS is facilitated in part through multiple peptidase pathways that occur downstream from the initial processing of angiotensinogen by renin and likely reflects their discrete cellular localization and relative affinities for peptides. Fetal glucocorticoid exposure is one example of in utero programming events that remarkably influence the RAS in adults through altered expression of distinct peptidase components in the kidney, circulation, and brain (3). Although additional characterization of the ANG-(1–7) endopeptidase regarding the enzyme's specificity and regulation is warranted, therapeutic approaches that target both intracellular and extracellular pathways to enhance the “ANG-(1–7) to ANG II tone” that include reduced metabolism of ANG-(1–7) may provide additional renoprotection in diabetes, hypertension, and fetal programming events.
GRANTS
This work was supported by the National Institutes of Health Grants HL-56973, HL-52972, HL-112237, and HD-047584; American Heart Association GRNT20480131.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.A.W., A.C.M., and M.C.C. conception and design of research; B.A.W. performed experiments; B.A.W., A.C.M., E.M.A., and M.C.C. analyzed data; B.A.W., E.M.A., and M.C.C. interpreted results of experiments; B.A.W., A.C.M., E.M.A., and M.C.C. drafted manuscript; B.A.W., A.C.M., and M.C.C. edited and revised manuscript; B.A.W., A.C.M., E.M.A., and M.C.C. approved final version of manuscript; A.C.M. and M.C.C. prepared figures.
REFERENCES
- 1.Alzayadneh EM, Chappell MC. Nuclear expression of renin-angiotensin system components in the NRK-52E renal epithelial cells. J Renin Angiotensin Aldosterone Syst, 2014, Jun 24. pii: 1470320313515039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chappell MC. Nonclassical renin-angiotensin system and renal function. Compr Physiol 2: 2733–2752, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chappell MC, Marshall AC, Alzayadneh EM, Shaltout HA, Diz DI. Update on the ACE2-Angiotensin-(1–7)-Mas receptor axis: intracellular actions, fetal programming and sex differences. Frontiers Endocrinol (Lausanne) 4: 1–13, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook JL, Re R. Lessons from in vitro studies and a related intracellular angiotensin II. Am J Physiol Regul Integr Comp Physiol 302: R482–R493, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durvasula RV, Shankland SJ. Activation of a local renin angiotensin system in podocytes by glucose. Am J Physiol Renal Physiol 294: F830–F839, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Ellis B, Li XC, Miguel-Qin E, Gu V, Zhuo JL. Evidence for a functional intracellular angiotensin system in the proximal tubule of the kidney. Am J Physiol Regul Integr Comp Physiol 302: R494–R509, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gwathmey T, Alzayadneh E, Pendergrass KD, Chappell MC. Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am J Physiol Regul Integr Comp Physiol 302: R518–R530, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar R, Yong QC, Thomas CM, Baker KM. Intracardiac intracellular angiotensin system in diabetes. Am J Physiol Regul Integr Comp Physiol 302: R510–R517, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall AC, Pirro NT, Rose JC, Diz DI, Chappell MC. Evidence for an angiotensin-(1–7) neuropeptidase in the CSF and brain medulla of sheep. J Neurochem, March 25, 2014, 10.1111/jnc.12720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intrarenal renin-angiotensin system in hypertension. Hypertension 57: 355–362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park S, Bivona BJ, Kobori H, Seth DM, Chappell MC, Lazartigues E, Harrison-Bernard LM. Major role for ACE-independent intrarenal ANG II formation in type II diabetes. Am J Physiol Renal Physiol 298: F37–F48, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira MG, Souza LL, Becari C, Duarte DA, Camacho FR, Oliveira JA, Gomes MD, Oliveira EB, Salgado MC, Garcia-Cairasco N, Costa-Neto CM. Angiotensin II-independent angiotensin-(1–7) formation in rat hippocampus: involvement of thimet oligopeptidase. Hypertension 62: 879–885, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Schwacke JH, Spainhour JC, Ierardi JL, Chaves JM, Arthur JM, Janech MG, Velez JC. Network modeling reveals steps in angiotensin peptide processing. Hypertension 61: 690–700, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaltout HA, Westwood B, Averill DB, Ferrario CM, Figueroa J, Diz DI, Rose JC, Chappell MC. Angiotensin metabolism in renal proximal tubules, urine and serum of sheep: ACE2-dependent processing of angiotensin II. Am J Physiol Renal Physiol 292: F82–F91, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Wilson BA, Marsall AC, Pirro NT, Su Y, Rose JC, Chappell MC. Expression of an angiotensin-(1–7) endopeptidase in proximal tubules of sheep and human kidney (Abstract). FASEB J 28: 1088–1014, 2014 [Google Scholar]


