Abstract
The kidney of marine teleosts is the major site of Mg2+ excretion and produces urine with a high Mg2+ concentration. However, the transporters involved in Mg2+ excretion are poorly understood. The cyclin M (Cnnm; also known as ancient conserved domain protein) family comprises membrane proteins homologous to the bacterial Mg2+ and Co2+ efflux protein, CorC. To understand the molecular mechanism of Mg2+ homeostasis in marine teleosts, we analyzed the expression of the Cnnm family genes in the seawater (SW) pufferfish, torafugu (Takifugu rubripes), and the closely related euryhaline species, mefugu (Takifugu obscurus). Database mining and phylogenetic analysis indicated that the Takifugu genome contains six members of the Cnnm family: two orthologs of Cnnm1, one of Cnnm2, one of Cnnm3, and two of Cnnm4. RT-PCR analyses indicated that Cnnm2, Cnnm3, and Cnnm4a are expressed in the kidney, whereas other members are mainly expressed in the brain. Renal expression of Cnnm3 was upregulated in SW mefugu, whereas renal expression of Cnnm2 was upregulated in freshwater (FW) mefugu. No significant difference was observed in renal expression of Cnnm4a between SW and FW mefugu. In situ hybridization and immunohistochemical analyses of the SW mefugu kidney revealed that Cnnm3 is expressed in the proximal tubule, and its product localizes to the lateral membrane. When Cnnm3 was expressed in Xenopus laevis oocytes, whole cellular Mg2+ content and free intracellular Mg2+ activity significantly decreased. These results suggest that Cnnm3 is involved in body fluid Mg2+ homeostasis in marine teleosts.
Keywords: Cnnm3, proximal tubule, marine teleost, magnesium homeostasis, lateral membrane
seawater (sw) contains ∼53 mM Mg2+, a concentration ∼50 times higher than that in the plasma of marine teleosts. The bladder urine Mg2+ concentration of marine teleosts is 57–167 mM (4, 5, 29, 36), and urine-to-plasma ratios of Mg2+ can exceed 100. Renal tubular fluid secretion accounts for much or all initial urine production in glomerular or aglomerular marine teleosts, and the renal proximal tubule is the major site of fluid secretion and Mg2+ excretion (4, 5, 18, 33). In SW-acclimated rainbow trout (Oncorhynchus mykiss), Mg2+ load from glomerular filtration is ∼3.2 μmol·kg−1·h−1; however, excreted Mg2+ rises to ∼100 μmol·kg−1·h−1 as a result of tubular secretion (5). This is in contrast to teleosts in ion-poor freshwater (FW), in which the kidney produces hypo-osmotic urine for water elimination by reabsorbing ions, including Mg2+ (5, 8). In FW-acclimated rainbow trout, Mg2+ load from glomerular filtration is ∼11 μmol·kg−1·h−1, which is reduced to ∼2.9 μmol·kg−1·h−1 in final urine, indicating net Mg2+ reabsorption in the renal tubule (5).
Proximal tubules isolated from the winter flounder (Pseudopleuronectes americanus) generate a Mg2+ concentration of 27 mM in the tubule lumen when the medium contains 1 mM Mg2+ (3, 11). Mg2+ is secreted from the peritubular bath into the tubule lumen against electrochemical potentials; therefore, the mechanism of active Mg2+ transport must be powerful (5). In the urine of marine teleosts, urinary Mg2+ and Na+ concentrations are negatively correlated, suggesting that Mg2+ excretion is coupled with Na+ reabsorption (32). X-ray microanalysis of freeze-dried cryosections of the kidneys of the dogfish shark (Squalus acanthias) demonstrated the presence of small apical vacuoles containing high concentrations of Mg (over 200 mM) in the proximal tubular cells (17). Ion microscopy analysis of the kidneys of the SW killifish (Fundulus heteroclitus) demonstrated that Mg distribution in epithelial cells of the proximal tubule is diffuse and punctate (10). These observations suggest that transepithelial Mg2+ transport involves sequestration of Mg2+ in small apical vacuoles and its subsequent exocytosis at the apical membrane (5, 33). Studies of brush-border membrane vesicles isolated from the proximal tubules of the Mozambique tilapia (Oreochromis mossambicus; FW and SW) (7) and rainbow trout (FW; 14) indicate the presence of electrodiffusive Mg2+ transport consistent with Mg2+ channel-like activity. Mg2+ channel-like activity can facilitate tubular reabsorption, suggesting that the apical membrane is not the site of active Mg2+ secretion. Recently, a pufferfish homolog of a Mg2+ transporter, solute carrier family 41 member 1 (Slc41a1), was discovered to be localized in intracellular vacuoles of the proximal tubule (21) and implicated in vesicular Mg2+ accumulation and excretion; however, the molecular mechanisms of Mg2+ excretion remain poorly understood.
The cyclin M (Cnnm) family, also known as the ancient conserved domain protein (Acdp) family, consists of four proteins (Cnnm1–4 or ACDP1–4) in mammals (44, 45). The Cnnm family was first characterized as comprising mammalian homologs of the bacterial protein, CorC. CorC was initially identified as a Mg2+/Co2+ transport system in bacteria through genetic analysis of Salmonella typhimurium strains resistant to Co2+ (15). In Saccharomyces cerevisiae, MAM3 encodes a homolog of the Cnnm family whose expression levels directly correlate with the degree of Mn toxicity (47). Mammalian Cnnm2 is a plasma membrane protein comprising the following: an N-terminal signal peptide (cleaved by signal peptidases); an extracellular NH2 terminus; three transmembrane domains; and an intracellular COOH terminus containing two cystathionine β-synthase (CBS) domains, a cyclin box motif, and a cyclic nucleotide (cNMP)-binding domain (12, 44, 45). In an investigation of Mg2+-dependent current in Xenopus laevis oocytes (16) and a complementary study of the Mg2+-deficient S. typhimurium strain MM281 (37), Cnnm2 was shown to be involved in Mg2+ transport. A later study on mammalian cells expressing Cnnm2 demonstrated Mg2+-sensitive Na+ current instead of Mg2+ current, suggesting that Cnnm2 is involved in Mg2+ sensing during the regulation of renal Mg2+ uptake (38). In a recent study, Cnnm4 expressed in human embryonic kidney (HEK) 293 cells exhibited an electroneutral Na+/Mg2+-exchange activity, which was involved in Mg2+ extrusion by the cells (46); similar Mg2+ efflux activity was also observed in HEK293 cells expressing Cnnm2 (19).
To elucidate the role of the Cnnm family in Mg2+ homeostasis in marine teleosts, we searched for a Cnnm family member with increased renal expression after SW acclimation. Two closely related Takifugu species, the SW tiger pufferfish (Takifugu rubripes; torafugu) and the euryhaline river pufferfish (Takifugu obscurus; mefugu) were used as animal models, and the renal expressions of Cnnm family members were compared between torafugu, SW-acclimated mefugu, and FW-acclimated mefugu (21, 24). The genome sequence of T. rubripes is available (1), enabling us to thoroughly identify members of the Cnnm family by genome database mining. In this paper, the expression of Cnnm family members was analyzed in torafugu and mefugu, and Cnnm3 was identified as a gene with upregulated renal expression after SW acclimation. Cnnm3 was highly localized to the lateral membrane of the proximal tubule and was associated with decreased cellular Mg2+ content when expressed in X. laevis oocytes. These results suggest that Cnnm3 is involved in Mg2+ efflux in the lateral membrane, and it stimulates paracellular Mg2+ excretion by the proximal tubule in marine teleosts.
MATERIALS AND METHODS
Animals.
Mefugu (T. obscurus) were purchased from a local dealer and held in 150-liter indoor tanks containing brackish water (3%-14% diluted SW) until use, as previously described (21, 24). For FW samples, mefugu were transferred to FW tanks and acclimated for 7–9 days before sample collection. For SW samples, mefugu in FW tanks were transferred to SW tanks and acclimated for 7–9 days. Torafugu (T. rubripes) were purchased from a local dealer and held in SW until use, as previously described (24, 31). The experimental animals were anesthetized by immersion in 0.1% ethyl m-aminobenzoate (MS-222; Tricaine; Nacalai Tesque, Kyoto, Japan) before death. The animal protocols and procedures were approved by the Institutional Animal Care and Use Committee of the Tokyo Institute of Technology, and the investigation conforms with the American Physiological Society (APS) Guiding Principles for the Care and Use of Vertebrate Animals in Research and Training.
Database analyses and construction of phylogenetic trees.
We obtained the sequences of torafugu genes from genome databases (http://genome.jgi-psf.org/ and http://www.ensembl.org/). Collected protein sequences were aligned using the CLUSTALW phylogenetic analysis service (http://www.ddbj.nig.ac.jp/) (42), and phylogenetic trees were constructed using MEGA5 software (http://www.megasoftware.net/) (40) based on the neighbor-joining method with 1,000 bootstrap replicates. Synteny analysis was performed using the Ensembl genome browser (http://www.ensembl.org/).
Semiquantitative reverse transcription polymerase chain reaction.
RNA was isolated using Isogen (Nippon Gene, Tokyo, Japan), and total RNA (5 μg) from each tissue was used for cDNA synthesis using SuperScript III (Invitrogen, Carlsbad, CA) to obtain 20 μl of cDNA solution, as previously described (27, 31). Synthesized cDNA was diluted to 160 μl with distilled water. For primer design, full-length or partial cDNAs of torafugu and mefugu Cnnms were isolated by RT-PCR from the brain (Cnnm1a, Cnnm1b, Cnnm2, Cnnm4a) and kidneys (Cnnm3, Cnnm4a) using the primers listed in Table 1 (DNA Data Bank of Japan/GenBank/European Molecular Biology Laboratory accession numbers: AB871954-AB871965).
Table 1.
List of primers used for polymerase chain reaction amplification
| Gene | Species | Sequence | Remarks |
|---|---|---|---|
| Cnnm1a | Mefugu/Torafugu | TCCAGGAGCTCAAGTTCAACC | RT-PCR (S) |
| Mefugu/Torafugu | GATGCGGTTCCTCTCGTT | RT-PCR (AS) | |
| Torafugu | CTCCCGTGTCCTTCAATAGTTCAGTCAG | Full-length cloning (S) | |
| Torafugu | TGCGTCAGTGCTCTTCCTCTCAGCCACAAT | Full-length cloning (AS) | |
| Mefugu | GCAGCAGGACTTCTCCATCTTC | Partial cloning (S) | |
| Mefugu | TGCGTCAGTGCTCTTCCTCTCAGCCACAAT | Partial cloning (AS) | |
| Cnnm1b | Mefugu/Torafugu | TGACGCGTCTCCTAATGGTG | RT-PCR (S) |
| Mefugu/Torafugu | CCAGTTTGGTGTCGCTGAAG | RT-PCR (AS) | |
| Torafugu | CTGAGCCTCACACGCATC | Full-length cloning (S) | |
| Torafugu | CGCGCACACACAGACTCTG | Full-length cloning (AS) | |
| Mefugu | CACGGAAACTACGTCCTGTGT | Partial cloning (S) | |
| Mefugu | CGCGCACACACAGACTCTG | Partial cloning (AS) | |
| Cnnm2 | Mefugu/Torafugu | ATCCACAGCACCCACCACTG | RT-PCR (S) |
| Mefugu/Torafugu | ATGGGGTCCAGAGCCATGAG | RT-PCR (AS) | |
| Mefugu | TGTCACTGGCTGGTGTGTTAC | qPCR (S) | |
| Mefugu | CCGCAAAAAGCCCCTATCCAT | qPCR (AS) | |
| Mefugu/Torafugu | TCCATTCAGGTTCGCTGCCGTGCGGTTATC | Partial cloning (S) | |
| Mefugu/Torafugu | GGTCCACGAAAGCCAGGTCT | Partial cloning (AS) | |
| Cnnm3 | Mefugu/Torafugu | TCCTGTCCAGAGAAGTGGAAC | RT-PCR (S) |
| Mefugu/Torafugu | AGGATCTGGACTCTGACCAAC | RT-PCR (AS) | |
| Mefugu/Torafugu | CGCAGCAGCCTGACCAAATCCAAT | Full-length cloning (S) | |
| Mefugu/Torafugu | CTATGATCCCTACGGGGTTTTTGTCACAG | Full-length cloning (AS) | |
| Mefugu | AGTGGAACACTTCAGCCCTC | qPCR (S) | |
| Mefugu | GTGGTTCCGCGTGTACAGAT | qPCR (AS) | |
| Torafugu | CCGTTAGAAACAACACGATCAG | 5′ RACE, outer (AS) | |
| Torafugu | AGGTGGTAGAAGGTCTGCTC | 5′ RACE, innner (AS) | |
| Torafugu | TCTGCCCGCTGACTATATCCC | 3′ RACE, outer (S) | |
| Torafugu | TTCAAGCCTCCCGAAGGAGAAC | 3′ RACE, innner (S) | |
| Mefugu/Torafugu | ATCCAATATGGTGGCCAGCTTGGCAG | Cloning (pGEMHE, pcDNA3) (S) | |
| Mefugu/Torafugu | TGCTACCCATGAGCCTCCTCTTCTGTG | Cloning (pGEMHE, pcDNA3) (AS) | |
| Mefugu/Torafugu | CAATATGGTGGCCAGCTTGGC | Cloning (p3xFLAG-CMV-14) (S) | |
| Mefugu/Torafugu | CAGAAGAGGAGGCTCATGGGAT | Cloning (p3xFLAG-CMV-14) (AS) | |
| Cnnm4a | Mefugu/Torafugu | GGTCTTGGGTTTGGTAACGCT | RT-PCR (S) |
| Mefugu/Torafugu | CGCGTGTGATCTTGACGAACT | RT-PCR (AS) | |
| Mefugu/Torafugu | CCCGAACTCAGTGAGCGAAACAAAC | Full-length cloning (S) | |
| Mefugu/Torafugu | CTTTATTTATAGGTGTGTTAATATGCGAGG | Full-length cloning (AS) | |
| Mefugu | AAGGTGTCCCCACAACTGCT | qPCR (S) | |
| Mefugu | AGGATCCGCAGCAGCACTT | qPCR (AS) | |
| Torafugu | GCACAAGCAGAAAAGAGATGAGG | 5′ RACE, outer (AS) | |
| Torafugu | AGGGACTTCTTCTCCTCCAC | 5′ RACE, innner (AS) | |
| Torafugu | CGCCCGATAAGAATAAGAGAGAC | 3′ RACE, outer (S) | |
| Torafugu | TGCAGTTCGTCAAGATCACACG | 3′ RACE, innner (S) | |
| Cnnm4b | Mefugu/Torafugu | ACGCGTATCCCAGTGTACGA | RT-PCR (S) |
| Mefugu/Torafugu | CCGCGCTGGTAAACGTAGTG | RT-PCR (AS) | |
| Mefugu/Torafugu | CATGCGGCTGGAGAAGAGCAACAAG | Partial cloning (S) | |
| Mefugu/Torafugu | CCGCGCTGGTAAACGTAGTG | Partial cloning (AS) | |
| β-Actin | Mefugu/Torafugu | AGCGTGGGTACTCCTTCACTAC | RT-PCR (S) |
| Mefugu/Torafugu | TCGTACTCCTGCTTGCTGATCC | RT-PCR (AS) | |
| Gapdh | Mefugu/Torafugu | GGCCCAATGAAAGGCATTCT | qPCR (S) |
| Mefugu/Torafugu | TGGGTGTCGCCGTTGAA | qPCR (AS) |
S, sense primer; AS, antisense primer.
Semiquantitative RT-PCR was performed by GoTaq Green Master Mix (Promega, Madison, WI) using the primers listed in Table 1. Each reaction mix contained 6.25 μl of GoTaq Green Master Mix, 1.5 μl of each primer (10 μM), 0.5 μl of tissue cDNA solution, and 3.25 μl of nuclease-free water. The PCR conditions comprised 27 cycles of denaturation (94°C, 30 s), annealing (55°C, 30 s), and extension (72°C, 40 s). After PCR, 5 μl from each reaction mix was electrophoresed on a 1.5% agarose gel in Tris·HCl/acetic acid/ethylenediaminetetraacetic acid buffer. The gel was stained with 0.5 μg/ml ethidium bromide for 30 min, and the fluorescent image was analyzed using a Kodak Image Station 2000R (Eastman Kodak, Rochester, NY).
Quantitative real-time PCR.
For the quantification of mRNA levels by real-time PCR, total RNAs were extracted from the kidneys of mefugu acclimated to SW and FW (n = 3–4 for each group). RNA (5 μg) was used as a template for reverse transcription using oligo(dT) primers and the SuperScript III First-Strand Synthesis System (Invitrogen). After reverse transcription, the cDNAs were amplified using primers for Cnnm2, Cnnm3, Cnnm4a, and glyceraldehyde-3-phosphate dehydrogenase (Gapdh) (21). Reactions were performed via the SYBR Green method using a SYBR Premix Ex Taq II Kit (Takara Bio, Otsu, Japan) on a Thermal Cycler Dice Real Time System (Takara Bio). The optimized 25-μl PCR mixture contained SYBR Premix Ex Taq II (12.5 μl), 900 nM forward and reverse primers (Table 1), and template DNA (1 μl), and the reactions were performed in a 96-well plate (Takara Bio). The thermal cycling conditions comprised predenaturation for 5 min at 95°C, 40 cycles of denaturation at 95°C for 15 s, annealing at 57°C for 30 s, and final extension at 95°C for 30 s. Melting curve analysis by raising the temperature from 65 to 95°C at a rate of 0.1°C/s was used to verify specificity. For each assay, the threshold cycle value, defined as the PCR cycle at which the fluorescence signal increased above the background threshold, was determined to quantify each mRNA product. The reference gene gapdh was stably expressed. The experiments were performed in duplicate. Quantitative data were presented as means ± SE. Relative values for the expression levels of each mRNA were compared between FW and SW mefugu, and statistical significances (P values) were calculated by unpaired two-sided Student's t-test using GraphPad Prism software (GraphPad Software, San Diego, CA).
In situ hybridization.
The kidneys of SW mefugu were perfused and fixed with 10% neutral-buffered formalin, embedded in paraffin, and sectioned to a thickness of 6 μm. In situ hybridization histochemistry was performed as previously described (21). In brief, a 339-base pair fragment of mefugu Cnnm3 cDNA (nucleotides 67–405) was subcloned into the pGEM-T Easy Vector (Promega) and used as a template for the preparation of digoxigenin (DIG)-labeled cRNA probes (Roche Diagnostics Deutschland, Mannheim, Germany). Sections were hybridized using DIG-labeled cRNA probes at 60°C for 16 h, and washed several times at 60°C. The bound label was detected using nitro-blue tetrazolium/5-bromo-4-chloro-3′-indolyl phosphate solution, and the sample was counterstained with Kernechtrot (Nuclear Fast Red; Muto Pure Chemicals, Tokyo, Japan). Serial sections were stained with hematoxylin and eosin (Muto Pure Chemicals) or anti-eel Na+/K+-ATPase α-subunit rabbit serum, as previously described (25). Images were observed using a microscope (model Axioskop; Carl Zeiss AG, Oberkochen, Germany) and an AxioCam HRc camera (Carl Zeiss AG) and TOCO automatic virtual slide system (Claro, Hirosaki, Japan).
Antibody production.
Polyclonal antisera were prepared in rabbits immunized with BSA-conjugated synthetic peptides corresponding to parts of mefugu Cnnm3 (amino acid residues: CGSEEEKRVAKRLEP and EFSLFKPPEGEPKI).
Expression of cyclin M3 in Madin-Darby canine kidney cells.
For expression in mammalian cells, cDNA corresponding to the open reading frame of mefugu Cnnm3 was obtained by RT-PCR from the kidneys of SW-acclimated mefugu using KOD plus DNA polymerase (Toyobo, Tokyo, Japan) and the primers listed in Table 1, and subcloned into the pcDNA3 vector (Invitrogen). Madin-Darby canine kidney (MDCK) cells were cultured and transfected with plasmids encoding Cnnm3 (pcDNA3-Cnnm3) using FuGENE 6 transfection reagent (Roche Diagnostics, Mannheim, Germany), according to the manufacturer's protocol. For immunofluorescence experiments, 36 h after transfection, the cells were fixed and reacted with the antisera (1:1,000) for 1 h at room temperature. Subsequently, the cells were treated with Alexa Fluor 488-labeled anti-rabbit secondary antibody (1:2,000; Invitrogen) and Hoechst 33342 (100 ng/ml; Invitrogen) for 1 h at room temperature. In MDCK cells, anti-zona occludens-1 mouse monoclonal antibody (1:200, clone ZO1–1A12; Invitrogen) was used to visualize tight junctions. Preimmune serum was used for negative controls. Fluorescence images were acquired using a laser confocal microscope (model TCS SPE; Leica Microsystems Vertrieb, Wetzlar, Germany) and processed using Leica Application Suite Advanced Fluorescence software (Leica Microsystems Vertrieb).
Expression of cyclin M3 in HEK293 cells and Western blot analysis.
An expression vector for Cnnm3 with a C-terminal 3xFlag epitope tag was obtained by subcloning a PCR product using the primers listed in Table 1 into the p3xFLAG-CMV-14 Vector (Sigma-Aldrich, St. Louis, MO). HEK293 cells were cultured and transfected with plasmids encoding Cnnm3 or FLAG-tagged Cnnm3 using FuGENE 6 transfection reagent, according to the manufacturer's protocol. Two days after transfection, the cells were washed in PBS and lysed in PBS containing 1% Triton X-100 and protease inhibitor mixture at 4°C. Protein concentrations in the samples were determined using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Rockford, IL). The proteins (10 μg of protein per lane) were separated by SDS-PAGE using a 10% polyacrylamide gel, and electroblotted on to a polyvinylidene difluoride (PVDF) membrane. After blocking in Tris-buffered saline with Tween 20 (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.05% Tween 20) containing 5% nonfat milk for 1 h at room temperature, the PVDF membrane was incubated with anti-Cnnm3 rabbit serum (1:10,000), anti-FLAG monoclonal antibody M2 (3.5 μg/ml; Sigma-Aldrich), or preimmune rabbit serum (1:10,000) for 16 h at 4°C. Bound antibodies were detected using peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) and chemiluminescent substrate solution (Immobilon Western Chemiluminescent HRP Substrate; EMD Millipore, Billerica, MA), and the signals were captured using an LAS 4000 Mini biomolecular imager (GE Healthcare, Piscataway, NJ).
Immunohistochemistry.
SW mefugu kidney specimens were fixed with paraformaldehyde (PFA) solution, and frozen sections (6 μm) were prepared as previously described (21, 22). The sections were blocked with 10% FBS in PBS for 1 h at room temperature and then reacted with anti-Cnnm3 or preimmune sera (1:2,000) for 16 h at room temperature. Sections were then washed with PBS and incubated with a mixture of Alexa Fluor 488-labeled anti-rabbit secondary antibody (1:2,000), tetramethylrhodamine isothiocyanate (TRITC)-labeled phalloidin (0.15 μM; Sigma-Aldrich), and Hoechst 33342 (100 ng/ml) in PBS containing 10% FBS at 20°C for 1 h. After washing with PBS, the sections were mounted with a cover slip, and fluorescence images were obtained using a laser confocal microscope as described earlier.
Functional analyses of expression of cyclin M3 in X. laevis oocytes.
The entire coding region of mefugu Cnnm3 cDNA was inserted into the pGEMHE X. laevis expression vector. The plasmid was linearized with NotI, and cRNAs transcribed in vitro using the T7 mMessage mMachine Kit (Ambion, Austin, TX). X. laevis oocytes were dissociated using collagenase, as previously described (34) and injected with 50 nl of water or a solution containing cRNA at 0.5 μg/μl (25 ng/oocyte) using a Nanoject II Injector (Drummond Scientific, Broomall, PA). Oocytes were incubated at 16°C in OR3 medium and were studied 3–4 days after injection.
Intracellular localization of Cnnm3 was analyzed by immunohistochemistry. Oocytes were washed in ND96 medium, fixed in 2% PFA in 100 mM phosphate buffer (pH 7.4) at 4°C for 1 h, and washed in ND96. The ND96 medium contained 96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.5). Frozen sections (6 μm) were prepared and stained as previously described (28). Anti-Cnnm3 rabbit serum (1:1,000) was used as the primary antibody. After washing with PBS, the sections were incubated with anti-rabbit Alexa Fluor 488-labeled secondary antibody (1:2,000), and fluorescence images were obtained using a laser confocal microscope as described above.
The extracellular [Mg2+]-dependent membrane current of oocytes was analyzed by the two-electrode voltage clamp method, as previously described (23). The current-voltage (I/V) relationships of cRNA or water-injected oocytes in the presence of different Mg2+ concentrations were analyzed. Each oocyte was perfused with ND96 solution and clamped at a holding potential of −60 mV in ND96. Subsequently, each oocyte was perfused with ND96 containing different Mg2+ concentrations. At each change of solution, oocytes were perfused in solution until the current stabilized, following which the I-V relationship was recorded. We prepared 65 Mg-ND96 by replacing 96 mM NaCl with 64 mM MgCl2. We prepared 0 Mg-ND96 (Mg2+-free ND96) by replacing 1 mM MgCl2 with 1.5 mM NaCl. Test solutions with differing Mg2+ concentrations were prepared by mixing 65 Mg-ND96 and 0 Mg-ND96. Mg2+-elicited currents were measured using ND96 containing 0, 1, and 20 mM Mg2+ and were calculated by subtracting I(no Mg2+) from I(Mg2+).
Total Mg2+ content in X. laevis oocytes was analyzed using inductively coupled plasma atomic emission spectrometry (ICP-MS; model ICPS-8100; Shimadzu, Kyoto, Japan). Oocytes injected with water or cRNA for mefugu Cnnm3 were washed in ND96 solution and incubated in ND96 solution for 1 h at 23°C. Subsequently, the oocytes were washed briefly with water and dried at 65°C for 1 h. The oocytes (five oocytes/tube) were digested with 1 ml of concentrated nitric acid in polytetrafluoroethylene tubes at 90°C for 30 min and 120°C for 3 h, in that order, and then left to dry completely at 90°C. The residues were dissolved in 0.08 M nitric acid containing 5 μg/ml Be, and Mg2+ concentration was measured using ICP-MS with Be as an internal standard. The average volume of oocytes was estimated on the basis of their diameters measured using a microscope under the assumption that the oocytes were spherical.
The intracellular free ion activity of oocytes was analyzed as previously described (35). Mg2+ activity (aMgi) of oocytes was analyzed with Mg2+-selective microelectrodes, which were filled with magnesium ionophore II-cocktail A (Fluka) at the tip and backfilled with 1 mM MgCl2 solution. aMgi was measured as the difference between the Mg2+ electrode and a KCl electrode impaling the oocyte. The Mg2+ electrodes were calibrated with 1 and 0.1 mM MgCl2 solutions containing 100 mM KCl, and were ∼1,000-fold selective for Mg2+ vs. Na+ and K+. The Mg2+ electrodes detected Ca2+ approximately eight-fold more sensitively than Mg2+, and we used Ca2+-free ND96 solution for a point calibration of each experiment. In the cytosol, the Ca2+ level is generally ∼100 nM (i.e., ∼10,000 times lower than that of Mg2+); thus, the aMgi measured by the Mg2+ electrodes was unaffected by intracellular Ca2+.
The Mg2+ content and aMgi of each oocyte are presented as means ± SE. Values were compared between Cnnm3- and water-injected oocytes, and the statistical significance (P values) was calculated by unpaired two-sided Student's t-test using the GraphPad Prism software.
RESULTS
Identification of members of the takifugu cyclin M family and phylogenetic analysis.
Phylogenetic analyses of six Cnnm genes in the genome database of torafugu (T. rubripes; Fig. 1) demonstrated that torafugu Cnnm genes comprise two Cnnm1, one Cnnm2, one Cnnm3, and two Cnnm4 orthologs. The numbers of Cnnm paralogs differed among teleosts (Table 2).
Fig. 1.
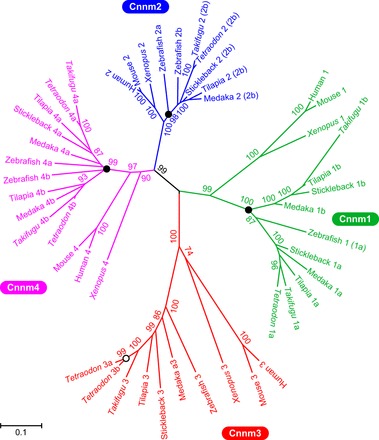
Phylogenetic analysis of the Cyclin M family. The tree was generated on the deduced amino acid sequences using the maximum likelihood method with CLUSTALW (42) and MEGA5 (40). Solid and open circles indicate branches corresponding to the teleost-specific third-round whole genome duplication (R3) and the linage-specific gene duplication, respectively. The numbers show bootstrap values (200 replications). The scale bar represents a genetic distance of 0.05 amino acid substitutions per site.
Table 2.
Cyclin M genes in the mammalian and teleost genomes
| Species | Cnnm gene (Ensembl gene ID or GenBank/EMBL/DDBJ accession number) | |||
|---|---|---|---|---|
| Human | Chromosome 10 | Chromosome 2 | ||
| CNNM1 (ENSG00000119946) | CNNM2 (ENSG00000148842) | CNNM3 (ENSG00000168763) | CNNM4 (ENSG00000158158) | |
| Mouse | Chromosome 19 | Chromosome 1 | ||
| Cnnm1 (ENSMUSG00000025189) | Cnnm2 (ENSMUSG00000064105) | Cnnm3 (ENSMUSG00000001138) | Cnnm4 (ENSMUSG00000037408) | |
| Rat | Chromosome 1 | Chromosome 9 | ||
| Cnnm1 (ENSRNOG00000016302) | Cnnm2 (ENSRNOG00000020113) | Cnnm3 (ENSRNOG00000016032) | Cnnm4 (ENSRNOG00000015886) | |
| Cow | Chromosome 26 | Chromosome 11 | ||
| Cnnm1 (ENSBTAG00000011956) | Cnnm2 (ENSBTAG00000012857) | Cnnm3 (ENSBTAG00000010846) | Cnnm4 (ENSBTAG00000019213) | |
| Zebrafish | Chromosome 13 | Chromosome 5 | ||
| Cnnm1 (1a) (ENSDARG00000078087) | Cnnm2a (ENSDARG00000061195) | Cnnm4a (ENSDARG00000074309) | ||
| Chromosome 1 | Chromosome 11 | |||
| Cnnm2b (ENSDARG00000078733) | Cnnm3 (ENSDARG00000074853) | Cnnm4b (ENSDARG00000077508) | ||
| Medaka | Chromosome 15 | Ultracontig 107 | ||
| Cnnm1a (ENSORLG00000007759) | Cnnm4a (XM_004086453) | |||
| Chromosome 1 | Chromosome 12 | |||
| Cnnm1b (ENSORLG00000000036) | Cnnm2 (2b) (ENSORLG00000012918) | Cnnm3 (ENSORLG00000000826) | Cnnm4b (ENSORLG00000000803) | |
| Stickleback | Group VI | Group XIII | ||
| Cnnm1a (ENSGACG00000007529) | Cnnm4 (4a) (ENSGACG00000014606) | |||
| Scaffold 369 | Group IX | Group XIV | ||
| Cnnm1b (ENSGACG00000000245) | Cnnm2 (2b) (ENSGACG00000018261) | Cnnm3 (BR001184) | ||
| Tetraodon | Chromosome 17 | Chromosome 12 | ||
| Cnnm1 (1a) (ENSTNIG00000002757) | Cnnm4a (ENSTNIG00000015632) | |||
| Chromosome 18 | Chromosome 4 | |||
| Cnnm2 (2b) (ENSTNI G00000012089) | Cnnm3-1 (ENSTNIG00000013561) Cnnm3-2 (ENSTNIG00000013554) | Cnnm4b (ENSTNIG00000013551) | ||
| Takifugu | Scaffold 24 | Scaffold 4 | ||
| Cnnm1a (ENSTRUG00000007603) | Cnnm4a (ENSTRUG00000015973) | |||
| Scaffold 7891/102 | Scaffold 62 | Scaffold 27 | ||
| Cnnm1b (AB871955) | Cnnm2 (2b) (ENSTRUG00000014689) | Cnnm3 (ENSTRUG00000018580) | Cnnm4b (ENSTRUG00000018576) | |
Paralogous genes in the genomes of teleosts are generally of two types: paralogs generated by the teleost-specific third-round whole-genome duplication [known as “ohnologs” after R3 (9, 41)]; and paralogs generated by lineage-specific gene duplications.
To understand the evolutionary history of the Cnnm paralogs, synteny analyses were performed using the human, mouse, rat, cow, zebrafish, medaka, stickleback, and pufferfish genome databases (Tetraodon and Takifugu; data not shown). As in the human CNNM family (44), Cnnm1 and Cnnm2 are located on the same chromosome, whereas Cnnm3 and Cnnm4 are tandemly arranged on another chromosome in mammals (Table 2). Two paralogs of Cnnm1 (Cnnm1a and Cnnm1b) and Cnnm4 (Cnnm4a and Cnnm4b) were identified in the genome databases of teleosts, indicating that these are ohnologs after R3. Cnnm1b was not present in the genome databases of zebrafish and Tetraodon, and Cnnm4b was absent in that of the stickleback, suggesting that these ohnologs have been deleted in each lineage. Two ohnologs of Cnnm2 (Cnnm2a and Cnnm2b) were present in the genome database of zebrafish; however, only one Cnnm2 was found in those of medaka, stickleback, Tetraodon, and Takifugu. Synteny and phylogenetic analyses demonstrated that the single Cnnm2 gene in each teleost is the ortholog of zebrafish Cnnm2b. Lineage-specific tandem gene duplication of Cnnm3 was found in the Tetraodon genome database.
Tissue distribution and salinity-dependent expression of members of the takifugu cyclin M family.
The tissue distribution of torafugu (SW) and mefugu (SW and FW) Cnnms was analyzed by semiquantitative RT-PCR (Fig. 2). In torafugu and mefugu, Cnnm3 and Cnnm4a were highly expressed in various tissues, including the kidneys (Fig. 2, A and B, left, 27 cycles). Cnnm1a, Cnnm1b, Cnnm2, and Cnnm4b were primarily expressed in the brain. A high level of Cnnm2 expression was also observed in the kidneys of FW mefugu, but not in SW mefugu or torafugu.
Fig. 2.
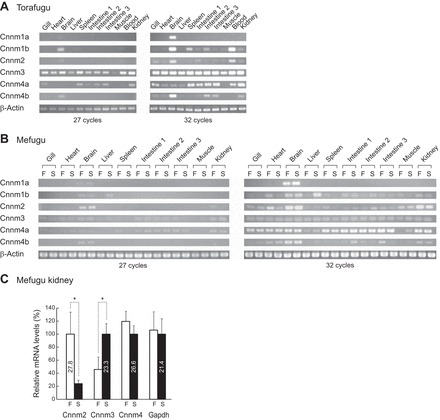
Expression of the members of the cyclin M family in seawater and euryhaline pufferfishes. A and B: expression of cyclin M (Cnnm) family members in the seawater (SW) pufferfish (torafugu, Takifugu rubripes) and the euryhaline pufferfish (mefugu, Takifugu obscurus) using semiquantitative reverse transcription-PCR. β-actin was used as an internal control. C: real-time PCR quantification of Cnnm2, Cnnm3, and Cnnm4a expressions in the kidney of freshwater- and SW-acclimated mefugu. Values are expressed as means ± SE of the relative expression levels (*P < 0.05; n = 3 or 4 for each group). F, freshwater; S, seawater.
Quantitative real-time PCR analysis demonstrated that the renal expression of Cnnm3 in SW mefugu was 2.2 times higher than that in FW mefugu (P < 0.05, n = 4). In contrast, renal expression of Cnnm2 in FW mefugu was 4.2 times higher than that in SW mefugu (P < 0.05, n = 3 or 4). No significant difference was observed in the expression of Cnnm4a between cDNAs isolated from the kidneys of SW and FW mefugu.
Expression of cyclin M3 in the proximal tubule.
A DIG-labeled antisense cRNA probe for Cnnm3 produced strong signals in some renal tubules and weak signals in the remaining renal tubules and in a number of cells that do not constitute nephrons (Fig. 3, A and G). No hybridization was observed with sense probes (Fig. 3B). To identify nephron segments, serial sections were stained with anti-Na+/K+-ATPase antibody (Fig. 3, C and F), nonimmune rabbit serum as a negative control (Fig. 3D), or hematoxylin (Fig. 3E). Strong positivity was evident in cells in the proximal tubule, which were characterized by the presence of a brush border and shallow basolateral infoldings labeled by the anti-Na+/K+-ATPase antibody (Fig. 3, E–G) (25).
Fig. 3.
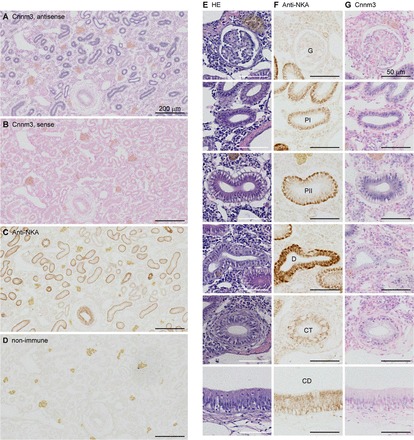
In situ hybridization analysis of cyclin M3 in the seawater mefugu kidney. Paraffin-embedded kidney sections were stained with digoxigenin-labeled antisense (A and G) and sense (B) cRNA probes for mefugu cyclin M3, anti-Na+/K+-ATPase antibody (C and F), nonimmune rabbit serum (D), and hematoxylin and eosin (E). Low- (A–D) and high- (E–G) magnification images are shown. PI, proximal tubule segment I; PII, proximal tubule segment II; D, distal tubule; CT, collecting tubule; CD, collecting duct; G, glomerulus. Scale bars: 50 μm.
Lateral membrane localization of cyclin M3 in the proximal tubule and in Madin-Darby canine kidney cells.
The polarized mammalian epithelial cell line MDCK was transfected with the Cnnm3 expression vector, and the localization of Cnnm3 was determined by indirect immunofluorescence using anti-Cnnm3 antiserum and optical sectioning microscopy. Cnnm3 was localized to the lateral membrane in MDCK cells (Fig. 4A). No signal was obtained from untransfected MDCK cells.
Fig. 4.
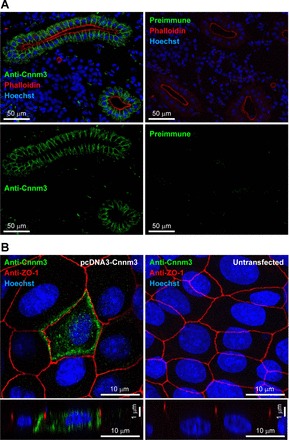
Localization of cyclin M3 in the proximal tubules of the seawater mefugu. A: renal immunolocalization of cyclin M3 (Cnnm3) in the seawater mefugu. Sections of mefugu kidney were stained with anti-Cnnm3 antiserum (green), phalloidin-tetramethylrhodamine isothiocyanate (red), and Hoechst 33342 (blue). B: Polarized distribution of Cnnm3 in Madin-Darby canine kidney (MDCK) cells. Anti-Cnnm3 antiserum (green), anti-zona occludens-1 antibody (red), and Hoechst 33342 (blue) were used to stain MDCK cells transiently transfected with or without an expression vector pcDNA3-Cnnm3. Confocal XY maximum projection image and XZ (vertical) sections are shown.
In frozen sections of the kidneys of SW-acclimated mefugu, strong immunoreactivity for Cnnm3 was detected at the lateral membrane of the proximal tubule (Fig. 4B). The proximal tubules were identified by staining the apical brush borders with the F-actin marker phalloidin-TRITC (36). No signals were observed in negative controls stained with preimmune serum.
Western blotting analysis of cyclin M3 expressed in HEK293 cells.
The specificity of anti-Cnnm3 antiserum was also confirmed by Western blot analyses of cultured mammalian cells exogenously expressing Cnnm3 (Fig. 5). Specific signals for the Cnnm3 monomer (∼85 kDa) and potential dimer (∼180 kDa) were detected by anti-Cnnm3 or anti-FLAG antibodies. A similar pattern was observed in the Western blot analysis of mouse Cnnm2 (12). The calculated molecular masses of Cnnm3 and FLAG-tagged Cnnm3 are 83 and 87 kDa, respectively. No signal was detected when preimmune serum was used for the analysis.
Fig. 5.
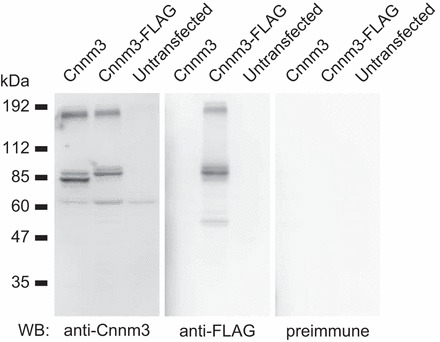
Western blot analysis of Cnnm3 in HEK293 cells. Proteins from HEK293 cells transfected with or without vectors expressing Cnnm3 or FLAG-tagged Cnnm3 were analyzed with anti-Cnnm3 antiserum, anti-FLAG monoclonal antibody, or preimmune rabbit serum.
Multiple sequence alignment of cyclin M3.
Figure 6 shows multiple sequence alignment of Cnnm3. Amino acid sequences of Cnnm3 in T. rubripes and T. obscurus share 100% identity; thus, they are termed Takifugu Cnnm3 here. Takifugu Cnnm3 has five hydrophobic regions homologous to the four transmembrane domains and one intermembrane domain of mouse Cnnm2 (12). Takifugu Cnnm3 also has a domain of unknown function (DUF21), two cystathionine β-synthase (CBS) domains, and a cNMP-binding domain, similarly to other members of the mammalian Cnnm family (12, 44). Amino acid sequences of the NH2 terminus (1–175) and DUF21 domain (176–331) in Takifugu Cnnm3 were only 31% and 34% identical to those in human CNNM3, respectively. Conversely, the amino acid sequences of the CBS domain pairs (365–499) and COOH terminus (500–747) were 81% and 64% identical to those of human CNNM3, respectively.
Fig. 6.

Multiple sequence alignment of cyclin M3. Conserved residues are indicated by black boxes. Four predicted hydrophobic segments of human and Takifugu cyclin M3 (Cnnm3) are indicated by gray overlines and underlines, respectively. A hydrophobic segment homologous to the intermembrane domain of human Cnnm2 (12) is indicated by a gray dotted line. The domain of unknown function (DUF21), cystathionine β-synthase (CBS) domain, cyclin box motif, and cyclic nucleotide (CNMP)-binding domain of human Cnnm3 defined by Wang et al. (44) are indicated by black overlines. Cnnm3 of T. rubripes and that of T. obscurus share 100% identity in amino acid sequences.
Activity of cyclin M3 expressed in Xenopus laevis oocytes.
Immunohistochemical analysis of X. laevis oocytes injected with cRNA for mefugu Cnnm3 showed that the majority of exogenously expressed Cnnm3 localizes to the plasma membrane (Fig. 7A). Two-electrode voltage-clamp analyses observed no significant changes in membrane current between Cnnm3- and water-injected oocytes incubated in media containing 0, 1, and 20 mM Mg2+ (Fig. 7D). Whole Mg2+ content of Cnnm3-injected oocytes was 12.7 ± 0.14 nmol (n = 10), which was 9% lower than that of water-injected oocytes (13.9 ± 0.1 nmol, n = 10; P < 0.001). Using an estimated average oocyte volume of 1.05 μl, the calculated concentrations of whole Mg2+ were 12.1 and 13.2 mM in Cnnm3-injected and control oocytes, respectively (Fig. 7B). Intracellular free Mg2+ (aMgi) of Cnnm3-injected oocytes measured using a Mg2+ electrode was ∼40% lower than that of control oocytes (P < 0.05, n = 5–7; Fig. 7C).
Fig. 7.
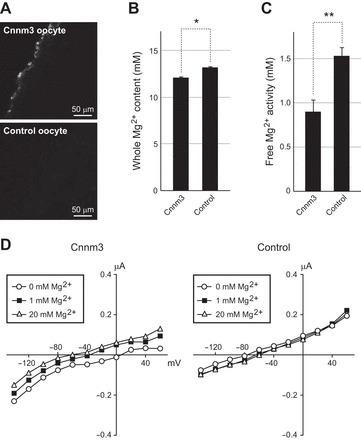
Reduced cellular Mg2+ content in Xenopus laevis oocytes expressing cyclin M3. A: surface expression of cyclin M3 (Cnnm3) in oocytes. Immunohistochemical analysis was performed on oocytes injected with cRNAs for Cnnm3 or water (control) using anti-Cnnm3 antibody. B: reduced basal Mg2+ content in Cnnm3 oocytes. Cnnm3 oocytes and control oocytes were incubated in ND96 saline for 1 h and cellular Mg2+ content was measured by inductively coupled plasma atomic emission spectrometry. *P < 0.001; n = 10 for each group. C: reduced intracellular free Mg2+ activity (aMgi) in Cnnm3 oocytes. aMgi of Cnnm3 and control oocytes were analyzed with Mg2+-selective microelectrodes in ND96 saline. **P < 0.05; n = 5–7 for each group. D: current voltage (I-V) relationships of Cnnm3 and control oocytes in solution containing various Mg2+ concentrations. Extracellular Mg2+-elicited current was not observed in either Cnnm3 or control oocytes.
DISCUSSION
In the present study, Cnnm3 was identified as a candidate Mg2+ transporter that mediates proximal tubular Mg2+ secretion in marine teleosts. Cnnm3 is highly expressed in the kidneys of pufferfishes, and renal expression of Cnnm3 significantly increases in euryhaline mefugu acclimated to SW, suggesting that Cnnm3 is involved in SW acclimation in pufferfishes. In the kidney, Cnnm3 is expressed in the proximal tubule, which is known to be the site of active Mg2+ secretion in marine teleosts (3, 4). In the proximal tubule, mefugu Cnnm3 is expressed in the lateral membrane, similarly to CNNM2 in the human kidney (38) and Cnnm4 in the mouse intestine (46). We were unable to detect Mg2+-elicited current, i.e., Mg2+-channel activity, in Cnnm3 oocytes; however, whole Mg2+ content and free intracellular Mg2+ activity are significantly reduced in Cnnm3-injected oocytes, suggesting that pufferfish Cnnm3 mediates or regulates electroneutral Mg2+ efflux. Single nucleotide polymorphisms in human CNNM3 loci are associated with serum Mg2+ levels (30), and the kidney is a tissue that highly expresses Cnnm3 in the mouse (45). These studies and our present data on pufferfishes strongly suggest that Cnnm3 is involved in renal Mg2+ handling in vertebrates. However, a recent study (19) showed that no significant Mg2+-efflux activity is observed in HEK293 cells expressing human CNNM3, whereas expression of mouse Cnnm2 and human CNNM4 in HEK293 cells causes significant Mg2+ efflux. Further analyses are required to clarify the role of Cnnm3 in Mg2+ extrusion by the cell.
In the genome databases of teleosts, at least one orthologous gene existed for each member of the mammalian Cnnm family. Tissue expression patterns of each member of the Cnnm family are similar between the mouse (45) and Takifugu species (torafugu and mefugu). Mouse Cnnm1 and Takifugu ohnologs are expressed mainly in the brain. Cnnm3 and Cnnm4 are widely expressed in both the mouse and Takifugu tissues. In Takifugu species, Cnnm4b is mainly expressed in the brain, whereas Cnnm4a is widely expressed except in specific tissues, including the brain, suggesting that the expression of the two Cnnm4 ohnologs diversified in teleosts after R3.
In the mouse, Cnnm2 is expressed in the brain and kidney (45). Conversely, in SW pufferfishes, Cnnm2 is primarily expressed in the brain, and only weak expression of Cnnm2 is evident in the kidney. In mefugu acclimated to FW, relatively high-level expression of Cnnm2 was evident in the kidneys. In the mouse, renal expression of Cnnm2 increases when they are fed a low-Mg2+ diet, and the expression of Cnnm2 in distal convoluted tubule cells increases when cultured in low-Mg2+ medium (16). In humans, CNNM2 is mutated in dominant familial hypomagnesemia (38) and is associated with serum Mg2+ levels (30), coronary artery disease (13), and hypertension (39). Arjona et al. (2) reported that renal and intestinal expression of Cnnm2 increases in zebrafish fed a Mg2+-deficient diet. These results suggest that Cnnm2 is commonly involved in Mg2+ retention in Mg2+-poor environments in both mammals and teleosts (8), and that the expression of teleost Cnnm2 is downregulated in Mg2+-rich environments, such as SW.
Perspectives and Significance
If Takifugu Cnnm3 mediates or regulates Mg2+ efflux across the lateral membrane of the proximal tubule, this is quite advantageous for SW fish for the following reasons: 1) the proximal tubules of SW fish have a weak lumen-negative transepithelial potential that drives paracellular cation secretion (5, 6); and 2) Cnnm3-mediated or -stimulated Mg2+ secretion may concentrate Mg2+ into the narrow intercellular space, increasing Mg2+ concentration there and enhancing paracellular Mg2+ secretion (Fig. 8).
Fig. 8.
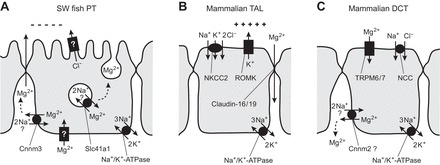
Comparison of hypothetical transport models for Mg2+ in the kidneys of seawater fish and mammals. A: Mg2+ secretion by the proximal tubule of seawater fish. Two candidate Mg2+-efflux transporters, solute carrier family 41 member 1 (Slc41a1; 21), and cyclin M3 (Cnnm3) are localized to the intracellular vesicles and the lateral membrane, respectively. Although 2 Na+/Mg2+ exchange activity of each transporter has not yet been demonstrated in fish, human SLC41A1 and CNNM4 are reportedly 2 Na+/Mg2+ exchangers (26, 46). An apical Cl− channel that produces lumen-negative transepithelial potential and a basolateral channel for Mg2+ loading have not yet been identified in the fish kidney. B: Mg2+ reabsorption by the thick ascending limb of Henle in mammals (20). Lumen-positive transepithelial membrane potential is maintained by the renal outer medullary K+ channel (ROMK; inwardly rectifying K+ channel Kir1.1) in the mammalian kidney and drives paracellular Mg2+ reabsorption. C: Mg2+ reabsorption by the distal convoluted tubule in mammals. Transient receptor potential (TRP) M6 and TRPM7 heteromers are considered to form an Mg2+ channel at the apical membrane (43). Cnnm2 is present in the basolateral membrane and is the strongest candidate for mediation or regulation of basolateral Mg2+ extrusion in the mammalian kidney (38).
It is believed that the transepithelial potential is insufficient for paracellular Mg2+ secretion against the ∼20-fold concentration gradient, but this conclusion was made under the assumption that Mg2+ concentration in the narrow intercellular space is equal to that in the peritubular fluid. When luminal Mg2+ is 27 mM and the transepithelial potential is 1.9 mV (lumen-negative; 5), Mg2+ is secreted paracellularly if the Mg2+ concentration in the narrow intercellular space exceeds 23 mM through Cnnm3-mediated or -stimulated Mg2+-efflux activity. The direction of paracellular movement of Mg2+ proposed here opposes that of the paracellular reabsorption of Mg2+ by the thick ascending limb of Henle in the mammalian kidney, where lumen-positive membrane potential is generated by an apical K+ channel (20) (Fig. 8B). Further detailed analyses of Cnnm3 function in the lateral membrane of the proximal tubule are required to confirm this hypothesis. Previously, Slc41a1 Mg2+ transporter was identified on vacuoles of the proximal tubule in mefugu (21), supporting the hypotheses that Mg2+ secretion is achieved using a vesicular exocytosis-mediated system in teleosts (10) and elasmobranch fish (17) (Fig. 8). It may be speculated that the proximal tubular cells of marine teleosts have multiple transport systems used to secrete Mg2+ into the lumen to deal with large system Mg2+ loads (from SW) and/or to more tightly control systemic Mg2+ homeostasis.
GRANTS
This work was supported by the Sumitomo Foundation (100535), Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI 22370029 and 26292113); the Global Center of Excellence Program of the Ministry of Education, Culture, Sports, Science and Technology of Japan; and the Program for Promoting the Enhancement of Research Universities at Tokyo Institute of Technology. Work in the Romero laboratory was supported by National Institutes of Health grants EY-017732 and DK-083007.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Z.I., N.H., and A.K. conception and design of research; Z.I., N.H., H.I., T.U., Y.K., H.D., and A.K. performed experiments; Z.I., N.H., H.I., and A.K. analyzed data; Z.I., N.H., M.F.R., and A.K. interpreted results of experiments; Z.I., N.H., and A.K. prepared figures; Z.I., N.H., and A.K. drafted manuscript; Z.I., N.H., H.I., T.U., Y.K., H.D., M.F.R., S.H., and A.K. approved final version of manuscript; M.F.R., S.H., and A.K. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. Min-Hwang Chang, Dr. An-Ping Chen, Dr. Taku Hirata, Dr. Michiko Tashiro, Dr. Masato Konishi, and Dr. Kimitoshi Denda for the discussion; Shinpei Nakamura for the ICP-MS experiments; Heather L. Holmes, Elyse M. Scileppi, Yoko Yamamoto, Ayako Takada, Nana Shinohara, and Noriko Isoyama for their technical assistance; Dr. Masayuki Komada for the use of laboratory facilities; and Yuriko Ishii and Megumi Ohmaki for secretarial assistance.
REFERENCES
- 1.Aparicio S, Chapman J, Stupka E, Putnam N, Chia JM, Dehal P, Christoffels A, Rash S, Hoon S, Smit A, Gelpke MD, Roach J, Oh T, Ho IY, Wong M, Detter C, Verhoef F, Predki P, Tay A, Lucas S, Richardson P, Smith SF, Clark MS, Edwards YJ, Doggett N, Zharkikh A, Tavtigian SV, Pruss D, Barnstead M, Evans C, Baden H, Powell J, Glusman G, Rowen L, Hood L, Tan YH, Elgar G, Hawkins T, Venkatesh B, Rokhsar D, Brenner S. Whole-genome shotgun assembly and analysis of the genome of Fugu rubripes. Science 297: 1301–1310, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Arjona FJ, Chen YX, Flik G, Bindels RJ, Hoenderop JG. Tissue-specific expression and in vivo regulation of zebrafish orthologues of mammalian genes related to symptomatic hypomagnesemia. Pflugers Arch 465: 1409–1421, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Beyenbach KW. Direct demonstration of fluid secretion by glomerular renal tubules in a marine teleost. Nature 299: 54–66, 1982 [DOI] [PubMed] [Google Scholar]
- 4.Beyenbach KW. Kidneys sans glomeruli. Am J Physiol Renal Physiol 286: F811–F827, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Beyenbach KW. Renal handling of magnesium in fish: from whole animal to brush border membrane vesicles. Front Biosci 5: D712–D719, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Beyenbach KW, Freire CA, Kinne RK, Kinne-Saffran E. Epithelial transport of magnesium in the kidney of fish. Miner Electrolyte Metab 19: 241–249, 1993 [PubMed] [Google Scholar]
- 7.Bijvelds M, Kolar Z, Bonga S, Flik G. Mg2+ transport in plasma membrane vesicles of renal epithelium of the Mozambique tilapia (Oreochromis mossambicus). J Exp Biol 200: 1931–1939, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Bijvelds MJ, Velden JA, Kolar ZI, Flik G. Magnesium transport in freshwater teleosts. J Exp Biol 201: 1981–1990, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Catchen JM, Conery JS, Postlethwait JH. Automated identification of conserved synteny after whole-genome duplication. Genome Res 19: 1497–1505, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandra S, Morrison GH, Beyenbach KW. Identification of Mg-transporting renal tubules and cells by ion microscopy imaging of stable isotopes. Am J Physiol Renal Physiol 273: F939–F948, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Cliff WH, Sawyer DB, Beyenbach KW. Renal proximal tubule of flounder II. Transepithelial Mg secretion. Am J Physiol Regul Integr Comp Physiol 250: R616–R624, 1986 [DOI] [PubMed] [Google Scholar]
- 12.de Baaij JH, Stuiver M, Meij IC, Lainez S, Kopplin K, Venselaar H, Muller D, Bindels RJ, Hoenderop JG. Membrane topology and intracellular processing of cyclin M2 (CNNM2). J Biol Chem 287: 13644–13655, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dichgans M, Malik R, Konig IR, Rosand J, Clarke R, Gretarsdottir S, Thorleifsson G, Mitchell BD, Assimes TL, Levi C, Odonnell CJ, Fornage M, Thorsteinsdottir U, Psaty BM, Hengstenberg C, Seshadri S, Erdmann J, Bis JC, Peters A, Boncoraglio GB, Marz W, Meschia JF, Kathiresan S, Ikram MA, McPherson R, Stefansson K, Sudlow C, Reilly MP, Thompson JR, Sharma P, Hopewell JC, Chambers JC, Watkins H, Rothwell PM, Roberts R, Markus HS, Samani NJ, Farrall M, Schunkert H. Shared genetic susceptibility to ischemic stroke and coronary artery disease: a genome-wide analysis of common variants. Stroke 45: 24–36, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freire CA, Kinne RK, Kinne-Saffran E, Beyenbach KW. Electrodiffusive transport of Mg across renal membrane vesicles of the rainbow trout Oncorhynchus mykiss. Am J Physiol Renal Fluid Electrolyte Physiol 270: F739–F748, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Gibson MM, Bagga DA, Miller CG, Maguire ME. Magnesium transport in Salmonella typhimurium: the influence of new mutations conferring Co2+ resistance on the CorA Mg2+ transport system. Mol Microbiol 5: 2753–2762, 1991 [DOI] [PubMed] [Google Scholar]
- 16.Goytain A, Quamme GA. Functional characterization of ACDP2 (ancient conserved domain protein), a divalent metal transporter. Physiol Genomics 22: 382–389, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Hentschel H, Zierold K. Morphology and element distribution of magnesium-secreting epithelium: the proximal tubule segment PII of dogfish, Scyliorhinus caniculus (L.). Eur J Cell Biol 63: 32–42, 1994 [PubMed] [Google Scholar]
- 18.Hickman CP, Jr, Trump BF. The Kidney. In: Fish Physiology, edited by Hoar WS, Randall DJ. New York: Academic, 1969, p. 91–239 [Google Scholar]
- 19.Hirata Y, Funato Y, Takano Y, Miki H. Mg2+-dependent interactions of ATP with the cystathionine-β-synthase (CBS) domains of a magnesium transporter. J Biol Chem In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou J, Goodenough DA. Claudin-16 and claudin-19 function in the thick ascending limb. Curr Opin Nephrol Hypertens 19: 483–488, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Islam Z, Hayashi N, Yamamoto Y, Doi H, Romero MF, Hirose S, Kato A. Identification and proximal tubular localization of the Mg2+ transporter, Slc41a1, in a seawater fish. Am J Physiol Regul Integr Comp Physiol 305: R385–R396, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Islam Z, Kato A, Romero MF, Hirose S. Identification and apical membrane localization of an electrogenic Na+/Ca2+ exchanger NCX2a likely to be involved in renal Ca2+ excretion by seawater fish. Am J Physiol Regul Integr Comp Physiol 301: R1427–R1439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato A, Chang MH, Kurita Y, Nakada T, Ogoshi M, Nakazato T, Doi H, Hirose S, Romero MF. Identification of renal transporters involved in sulfate excretion in marine teleost fish. Am J Physiol Regul Integr Comp Physiol 297: R1647–R1659, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato A, Doi H, Nakada T, Sakai H, Hirose S. Takifugu obscurus is a euryhaline fugu species very close to Takifugu rubripes and suitable for studying osmoregulation. BMC Physiol 5: 18, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato A, Muro T, Kimura Y, Li S, Islam Z, Ogoshi M, Doi H, Hirose S. Differential expression of Na+-Cl− cotransporter and Na+-K+-Cl− cotransporter 2 in the distal nephrons of euryhaline and seawater pufferfishes. Am J Physiol Regul Integr Comp Physiol 300: R284–R297, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Kolisek M, Nestler A, Vormann J, Schweigel-Rontgen M. The human gene SLC41A1 encodes for the Na+/Mg2+ exchanger. Am J Physiol Cell Physiol 302: C318–C326, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Kurita Y, Nakada T, Kato A, Doi H, Mistry AC, Chang MH, Romero MF, Hirose S. Identification of intestinal bicarbonate transporters involved in formation of carbonate precipitates to stimulate water absorption in marine teleost fish. Am J Physiol Regul Integr Comp Physiol 294: R1402–R1412, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Li S, Kato A, Takabe S, Chen AP, Romero MF, Umezawa T, Nakada T, Hyodo S, Hirose S. Expression of a novel isoform of Na+/H+ exchanger 3 (NHE3) in the kidney and intestine of banded houndshark Triakis scyllium. Am J Physiol Regul Integr Comp Physiol 304: R865–R876, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall WS, Grosell M. Ion transport, osmoregulation, and acid-base balance. In: The Physiology of Fishes, edited by Evans DH, Claiborne JB. New York: CRC, 2005, p. 177–224 [Google Scholar]
- 30.Meyer TE, Verwoert GC, Hwang SJ, Glazer NL, Smith AV, van Rooij FJ, Ehret GB, Boerwinkle E, Felix JF, Leak TS, Harris TB, Yang Q, Dehghan A, Aspelund T, Katz R, Homuth G, Kocher T, Rettig R, Ried JS, Gieger C, Prucha H, Pfeufer A, Meitinger T, Coresh J, Hofman A, Sarnak MJ, Chen YD, Uitterlinden AG, Chakravarti A, Psaty BM, van Duijn CM, Kao WH, Witteman JC, Gudnason V, Siscovick DS, Fox CS, Kottgen A. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six loci influencing serum magnesium levels. PLoS Genet 6: 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakada T, Westhoff CM, Kato A, Hirose S. Ammonia secretion from fish gill depends on a set of Rh glycoproteins. FASEB J 21: 1067–1074, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Natochin YV, Gusev GP. The coupling of magnesium secretion and sodium reabsorption in the kidney of teleost. Comp Biochem Physiol 37: 107–111, 1970 [DOI] [PubMed] [Google Scholar]
- 33.Renfro JL. Recent developments in teleost renal transport. J Exp Zool 283: 653–661, 1999 [Google Scholar]
- 34.Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol Renal Physiol 274: F425–F432, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Romero MF, Henry D, Nelson S, Harte PJ, Dillon AK, Sciortino CM. Cloning and characterization of a Na+-driven anion exchanger (NDAE1). A new bicarbonate transporter. J Biol Chem 275: 24552–24559, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Smith HW. The absorption and excretion of water and salts by marine teleosts. Am J Physiol 93: 480–505, 1930 [Google Scholar]
- 37.Sponder G, Svidova S, Schweigel M, Vormann J, Kolisek M. Splice-variant 1 of the ancient domain protein 2 (ACDP2) complements the magnesium-deficient growth phenotype of Salmonella enterica sv. typhimurium strain MM281. Magnes Res 23: 105–114, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Stuiver M, Lainez S, Will C, Terryn S, Gunzel D, Debaix H, Sommer K, Kopplin K, Thumfart J, Kampik NB, Querfeld U, Willnow TE, Nemec V, Wagner CA, Hoenderop JG, Devuyst O, Knoers NV, Bindels RJ, Meij IC, Muller D. CNNM2, encoding a basolateral protein required for renal Mg2+ handling, is mutated in dominant hypomagnesemia. Am J Hum Genet 88: 333–343, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeuchi F, Isono M, Katsuya T, Yamamoto K, Yokota M, Sugiyama T, Nabika T, Fujioka A, Ohnaka K, Asano H, Yamori Y, Yamaguchi S, Kobayashi S, Takayanagi R, Ogihara T, Kato N. Blood pressure and hypertension are associated with 7 loci in the Japanese population. Circulation 121: 2302–2309, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor JS, Braasch I, Frickey T, Meyer A, Van de Peer Y. Genome duplication, a trait shared by 22000 species of ray-finned fish. Genome Res 13: 382–390, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem 279: 19–25, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Wang CY, Shi JD, Yang P, Kumar PG, Li QZ, Run QG, Su YC, Scott HS, Kao KJ, She JX. Molecular cloning and characterization of a novel gene family of four ancient conserved domain proteins (ACDP). Gene 306: 37–44, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Wang CY, Yang P, Shi JD, Purohit S, Guo D, An H, Gu JG, Ling J, Dong Z, She JX. Molecular cloning and characterization of the mouse Acdp gene family. BMC Genomics 5: 7, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamazaki D, Funato Y, Miura J, Sato S, Toyosawa S, Furutani K, Kurachi Y, Omori Y, Furukawa T, Tsuda T, Kuwabata S, Mizukami S, Kikuchi K, Miki H. Basolateral Mg2+ extrusion via CNNM4 mediates transcellular Mg2+ transport across epithelia: a mouse model. PLoS Genet 9: e1003983, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang M, Jensen LT, Gardner AJ, Culotta VC. Manganese toxicity and Saccharomyces cerevisiae Mam3p, a member of the ACDP (ancient conserved domain protein) family. Biochem J 386: 479–487, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]


