In the first study of human subjects, the safety and feasibility of treatment with CD34+ cells delivered intra-arterially in patients with acute ischemic stroke were determined. CD34+ cells were collected from the bone marrow of the subjects before being delivered by catheter angiography into the ipsilesional middle cerebral artery. Autologous CD34+ selected stem/progenitor cell therapy delivered intra-arterially into the infarct territory can be achieved safely in patients with acute ischemic stroke.
Keywords: Ischemic stroke, Stem cells, CD34+, Intra-arterial
Abstract
Treatment with CD34+ hematopoietic stem/progenitor cells has been shown to improve functional recovery in nonhuman models of ischemic stroke via promotion of angiogenesis and neurogenesis. We aimed to determine the safety and feasibility of treatment with CD34+ cells delivered intra-arterially in patients with acute ischemic stroke. This was the first study in human subjects. We performed a prospective, nonrandomized, open-label, phase I study of autologous, immunoselected CD34+ stem/progenitor cell therapy in patients presenting within 7 days of onset with severe anterior circulation ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score ≥8). CD34+ cells were collected from the bone marrow of the subjects before being delivered by catheter angiography into the ipsilesional middle cerebral artery. Eighty-two patients with severe anterior circulation ischemic stroke were screened, of whom five proceeded to treatment. The common reasons for exclusion were age >80 years (n = 19); medical instability (n = 17), and significant carotid stenosis (n = 13). The procedure was well tolerated in all patients, and no significant treatment-related adverse effects occurred. All patients showed improvements in clinical functional scores (Modified Rankin Score and NIHSS score) and reductions in lesion volume during a 6-month follow-up period. Autologous CD34+ selected stem/progenitor cell therapy delivered intra-arterially into the infarct territory can be achieved safely in patients with acute ischemic stroke. Future studies that address eligibility criteria, dosage, delivery site, and timing and that use surrogate imaging markers of outcome are desirable before larger scale clinical trials.
Introduction
A major unmet need exists for treatments that can reduce tissue injury progression and enhance functional recovery in patients with stroke. Stem cells offer the promise of a novel reparative strategy for acute brain injury. Recent nonhuman studies of stroke using stem cell therapy have demonstrated significant behavioral recovery and, in some cases, reductions in lesion volume [1–13]. A variety of stem cell types have been investigated in this context, including CD34+ stem/progenitor cells. CD34+ cells are a subset of bone marrow mononuclear cells (BMMNCs) and act as the main endogenous source of hematopoietic and endothelial cell precursors. Several lines of evidence have suggested that they might act as a potentially useful therapy for acute stroke. First, after a stroke, mobilization of CD34+ cells from the bone marrow into the periphery is increased [14], which shows a strong positive correlation with clinical recovery [15]. Second, two trials of CD34+ cells in rodent models of stroke have demonstrated improved functional recovery and a reduced infarct size [12, 13]. At the cellular level, CD34 cell transplantation resulted in increased perilesional angiogenesis and subsequent neurogenesis [13]. Finally, a recently published clinical trial of intra-arterial BMMNC treatment of acute stroke showed a trend toward a positive correlation between the number of CD34+ cells injected and functional outcome at 1 month [16].
Potentially, three main routes of central nervous system delivery are available: intravenous, intra-arterial, and direct surgical cranial implantation. Stereotactic surgical approaches—although likely to be the most efficient method of delivery—are also likely to be particularly challenging in the context of acute stroke, given the risks of general anesthesia (especially in medically unstable patients) and the potential for worsening cerebral inflammation and edema. Conversely, with intravenous delivery, the overwhelming majority of transplanted cells will be absorbed by nontarget organs—especially the lungs, spleen, and liver—before they can reach the brain [17–19]. Direct arterial delivery, therefore, represents an intermediate strategy that offers directed therapy with fewer of the risks of major surgery.
To date, five phase I trials using unselected BMMNCs in stroke have been published [16, 20–23]. These have examined the effect of BMMNCs in acute and chronic stroke, using intravenous, intra-arterial, and intracerebral approaches. They have all shown safety and feasibility in the patients studied. BMMNCs contain a number of subpopulations of cells (including ∼1% CD34+ cells), and it is not known which fraction contributes to the recovery seen in preclinical studies. It might be that the CD34+ fraction, which contains populations of both hematopoietic stem cells and endothelial progenitor cells, is the active component. There is some evidence to support this. One animal study compared CD34+ cells and CD34-negative cells in a rodent model of cortical stroke [13]. Benefits were seen with the CD34+ treatment arm, which were not reproduced in the CD34-negative group. Additional data in favor of using CD34+ cells comes from a recently reported clinical trial of intra-arterially delivered BMMNCs in patients with acute severe ischemic stroke. They found no correlation between recovery and the numbers of BMMNCs injected but did see a trend toward better outcomes with higher numbers of CD34+ cells injected (those present within the BMMNC infusate) [16].
Granulocyte-colony stimulating factor (G-CSF), used to mobilize CD34+ cells into the peripheral circulation, has been shown to improve outcomes in rodent models of stroke [24, 25]. The recently reported Stem cell Trial of recovery EnhanceMent after Stroke 2 (phase IIb) clinical trial showed that G-CSF given to patients 3–30 days after stroke onset was safe, with a trend toward a reduction in the magnetic resonance imaging (MRI)-determined ischemic volume in the treated group [26]. However, to date, no investigators have studied CD34+ selected cells via the intra-arterial delivery route within an early period after stroke. The aim of our study, therefore, was to establish the safety and feasibility of autologous CD34+ selected stem/progenitor cell therapy—delivered directly into the ipsilesional middle cerebral artery—in patients with acute (<7 days) ischemic stroke. Because this was an early-phase development study, for ethical reasons, we restricted our target population to those with clinically severe anterior circulation ischemic stroke.
Materials and Methods
Design
We performed a prospective, nonrandomized, open-label, proof of concept study of autologous immunoselected CD34+ stem/progenitor cell therapy in patients with acute severe ischemic stroke. The study was approved by the ethical committee of Hammersmith Hospital, Imperial College Healthcare National Health Services Trust, London, United Kingdom, and the Medicines and Healthcare Products Regulatory Agency. It was conducted according to the Declaration of Helsinki and was registered at ClinicalTrials.gov (unique identifier: NCT00535197; available at: http://www.clinicaltrials.gov).
Subjects
Patients were eligible for inclusion within 7 days of stroke onset if they had had a clinically definite severe ischemic stroke, conforming to the total anterior circulation stroke (TACS; Oxford Community Stroke Project classification) phenotype. Detailed inclusion and exclusion criteria are listed in Table 1.
Table 1.
Inclusion and exclusion criteria

Recruitment started December 2007. Owing to the low recruitment/screen ratio (as described in the Discussion), the criteria were expanded from August 2010 onward to include the partial anterior circulation stroke (PACS) subtype of ischemic stroke, including (a) imaging confirmation of an infarct in the territory of the middle cerebral artery, and (b) a significant neurologic deficit with an National Institutes of Health Stroke Scale (NIHSS) score of ≥8 at screening.
Intervention
Potential subjects were clinically evaluated by one of three of us (S.B., P.B., or J.C.) at Imperial College Healthcare National Health Services Trust (London, United Kingdom). The patients provided written informed consent (day 0, within 7 days of stroke onset). If the patient was dysphasic or obtunded, assent could be obtained from the next of kin. A bone marrow aspirate was collected on day 0, and the CD34+ cells were separated by immunoselection using standard procedures (see below). On day 1 or 2, depending on completion of the CD34+ stem/progenitor cell immunoselection process, the cells were reinfused into the patients directly via the intra-arterial route.
Production of a CD34+ stem/progenitor cell concentrate occurred in two stages. First, under the institutional Human Tissue Authority procurement license, the patients were screened serologically for the following infections before bone marrow acquisition (in line with standard stem cell transplantation protocols): HIV 1 and 2, hepatitis B and C, human T lymphotropic virus 1 and 2, and syphilis. Bone marrow aspiration was performed by an experienced hematologist under aseptic conditions, using lidocaine anesthesia only. A single-pull 10-ml aspirate (on guidance from our ethical review board) was collected directly into a syringe containing 1.4 ml acid citrate dextrose as anticoagulant.
The final cell product, defined as an “Investigational Medicinal Product” (IMP), required the second stage of production to be performed to Good Manufacturing Practice (GMP) standards within a facility holding IMP manufacturing authorization. Under GMP manufacturing, this stage of the process was conducted under aseptic conditions. The aspirated bone marrow was first washed in phosphate-buffered saline, EDTA, and human albumin solution (HAS) to remove any cellular debris, followed by the addition of a set volume of immunoselection labeling type reagent composed of CD34+ antibody-coated magnetic beads (CliniMACS; Miltenyi Biotec Inc., San Diego, CA, https://www.miltenyibiotec.com). After mixing, the bag was aseptically connected to the CliniMACS machine (Miltenyi Biotec Inc.). An automated process applied the cells to the separation column, performed a series of washes, and, finally, the purified CD34+ cells were obtained. These cells were resuspended in HAS and loaded into a syringe for administration to the patient. Quality control of the final cell product was performed to determine the total number of CD34+ cells, sterility, and viability. The criterion set for the CD34+ dosage was a maximum of 1 × 108 cells to be infused.
The immunoselected CD34+ cells were infused via ipsilateral middle cerebral arterial cannulation (via femoral artery puncture), performed by an experienced interventional radiologist, with the patient under local anesthesia and with appropriate monitoring facilities. The infusion was performed slowly over 10 minutes.
The clinical outcome measures and neuroimaging studies in the form of MRI was performed at specified points: days 0, 1–2 (after infusion), 7, 14, 30, and 180. The use of computed tomography was acceptable if the patient was unable to tolerate MRI. Data were collected on symptoms and adverse events. All patients received concurrent best medical treatment.
Outcomes
Primary Endpoints
The primary endpoint was to assess the safety and tolerability of the intervention. Safety was evaluated in terms of adverse events graded according to the Common Toxicity Criteria and laboratory test results. All adverse events (classified using the International Conference on Harmonisation/Good Clinical Practice criteria) were graded for their relationship to treatment and as expected and unexpected. They were reported to the stem cell group, the appropriate ethics committee, and competent authority, as required, and to an independent safety monitoring committee.
Secondary Endpoints
Clinical function was assessed using the Modified Rankin Score (mRS) and NIHSS. Data were analyzed using the paired t test, and statistical significance was taken at p < .05.
Neuroimaging outcomes comprised qualitative assessments of T2, fluid attenuated inversion recovery (FLAIR), and gradient-echo or susceptibility-weighted MRI at each evaluation point by a consultant neuroradiologist and estimation of the lesion volume determined from T2-weighted MRI scans. The latter was performed by manual delineation of the original images in MRIcron (available at: http://www.mccauslandcenter.sc.edu/mricro/mricron/main.html) by an experienced stroke-neurologist (P.B.), who was unaware of the temporal order of the scans.
Results
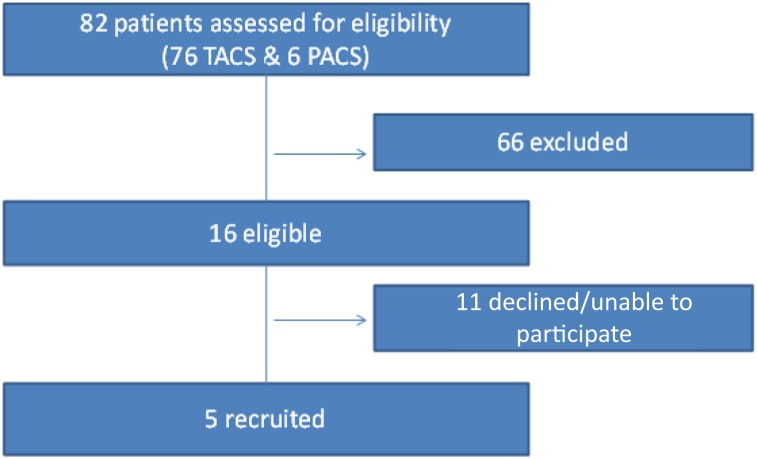
A total of 76 patients with the TACS and 6 with the PACS subtype were considered for the trial. Five patients ultimately were suitable and received the intervention (Fig. 1). The major reasons for ineligibility were age >80 years (n = 19), significant comorbidity or medical instability (n = 17), and ipsilateral internal carotid artery occlusion or significant stenosis (n = 13).
Figure 1.
Patient flow. Abbreviations: PACS, partial anterior circulation stroke; TACS, total anterior circulation stroke.
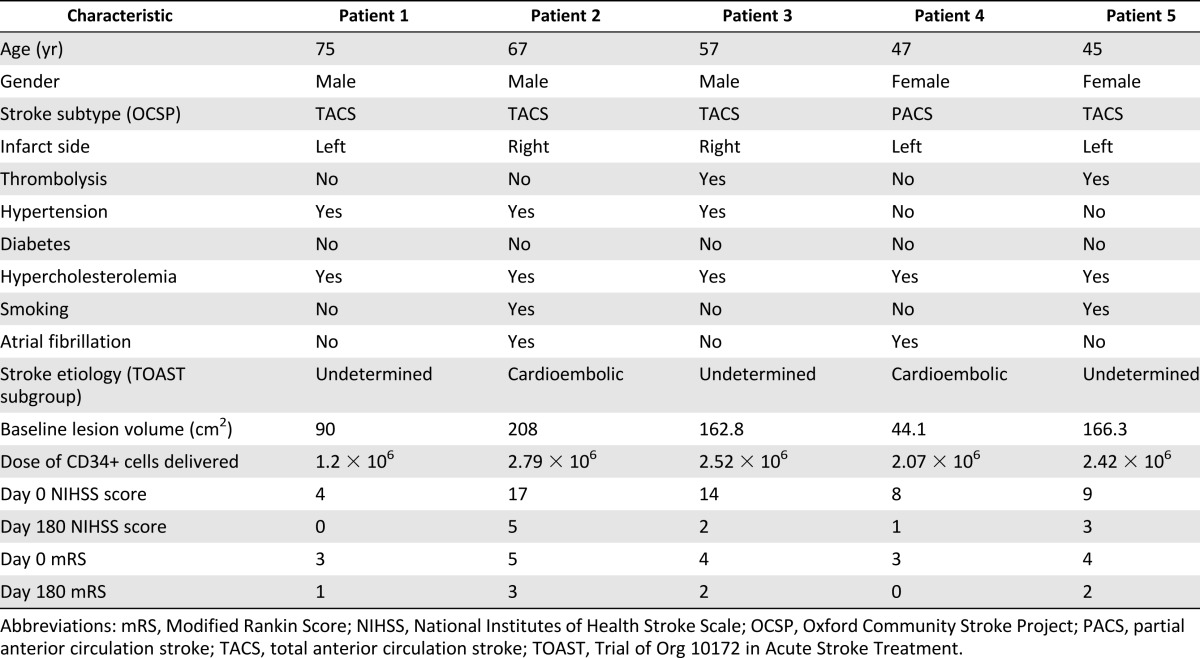
The clinical and imaging characteristics and CD34+ cell dosage delivered are documented in Table 2. The percentage of CD34+ cells in the infusate was in the range of 45.3%–67.2%. Cell viability was between 81.6% and 100%. The CD34+ cell dosage data were corrected for viability. All cells obtained by immunoselection were delivered.
Table 2.
Cohort characteristics
Safety and Trial Conduct
All five patients tolerated the procedure well, with no complications. No recurrent strokes developed during the follow-up period, and no postprocedure neurologic deterioration was observed. No patient died. One patient developed renal dysfunction 2 weeks after the infusion and subsequently experienced an episode of pneumonia. These resolved and were deemed to be serious adverse events because they led to a prolongation of the hospital stay but were not related to the intervention. No protocol deviations occurred.
Clinical Outcomes
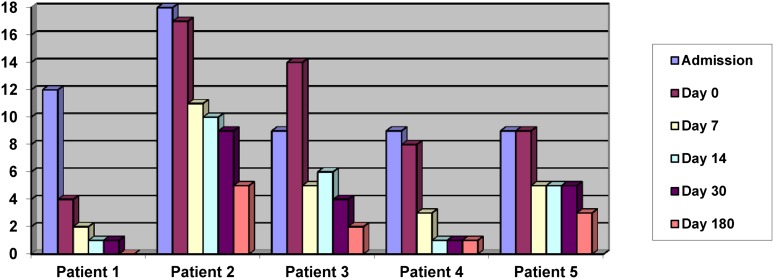
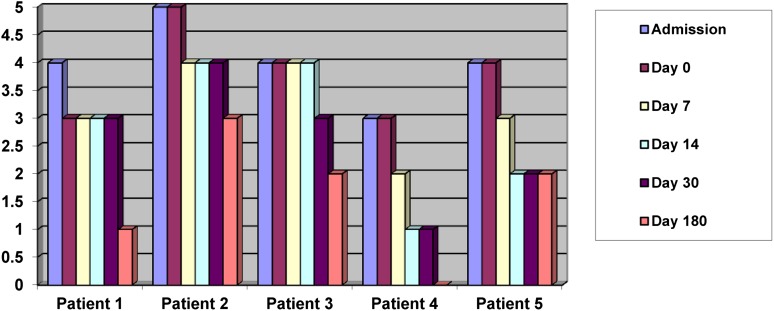
The serial NIHSS scores and mRSs are shown in Figures 2 and 3. All patients had improved by the day 180 review. The median NIHSS score was 9 (interquartile range [IQR] 8–14) at day 0 and 2 (IQR 1–3) at day 180. The median mRS was 4 (IQR 3–4) at day 0 and 2 (IQR 1–2) at day 180.
Figure 2.
National Institutes of Health Stroke Scale scores on admission and at days 0–180.
Figure 3.
Modified Rankin Scores on admission and at days 0–180.
Significant improvement was seen in the mean NIHSS score, from 10.40 to 2.20 (95% confidence interval [CI] 3.69–12.71; p = .007), and mean mRS, from 3.80 to 1.60 (95% CI 1.64–2.76; p = .0004), from day 0 to day 180.
Radiological Outcomes
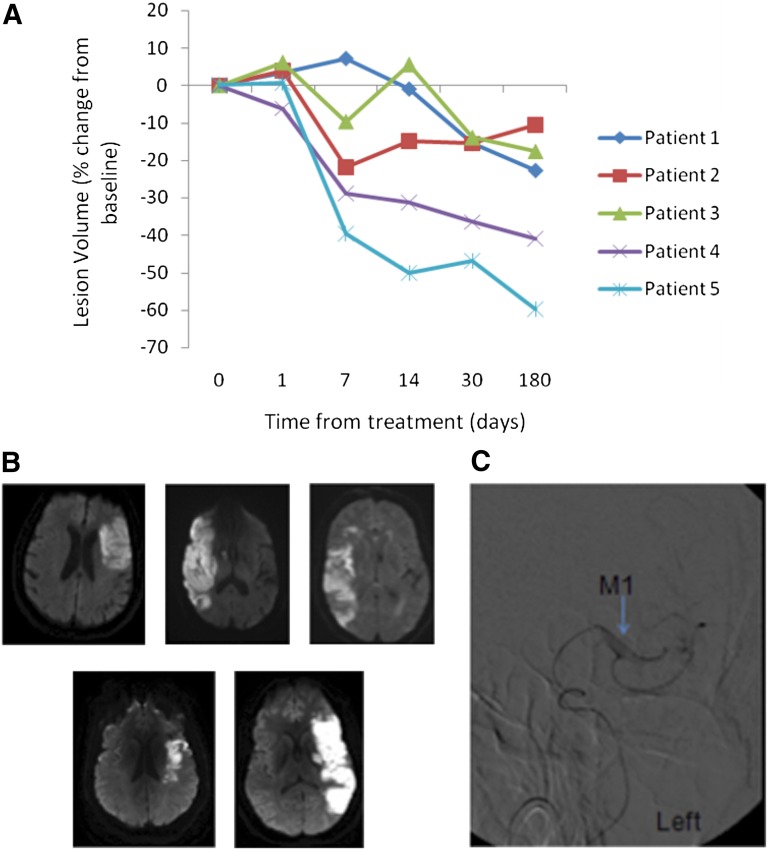
The lesion volumes estimated from T2-weighted MRI at baseline are listed in Table 2, and the relative changes in lesion volume during the 6-month follow-up period from CD34+ cell treatment are shown in Figure 4A. A blinded determination of lesion volume showed a reduction in the lesion volume relative to baseline in all patients at the final follow-up examination. A nonsignificant reduction was seen in the mean lesion volume from 134.24 to 96.49 cm2 (95% CI −5.46 to 80.97; p = .07) from day 0 to day 180. The mean percentage of change from baseline was 28% at 6 months of follow-up.
Figure 4.
Findings from imaging studies. (A): Percentage of change in infarct volume from day 0 to 180 for patients 1–5. (B): Baseline diffusion weighted magnetic resonance images for patients 1–5 (shown left to right). (C): Angiographic image of intra-arterial catheter delivery of cells to M1 segment (blue arrow) of left middle cerebral artery.
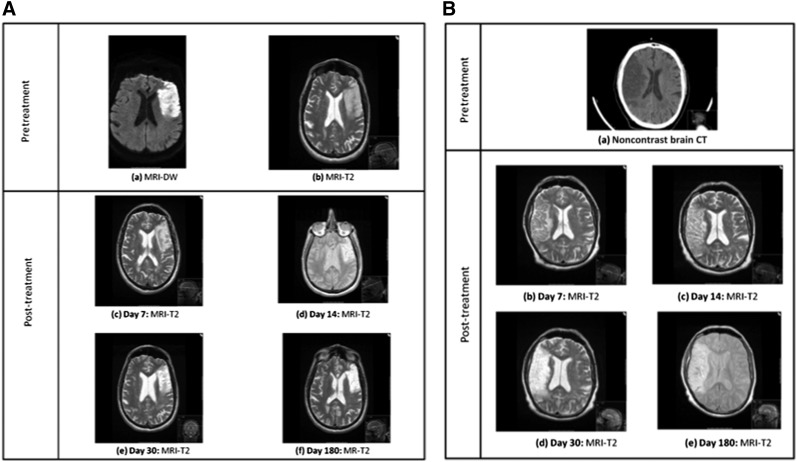
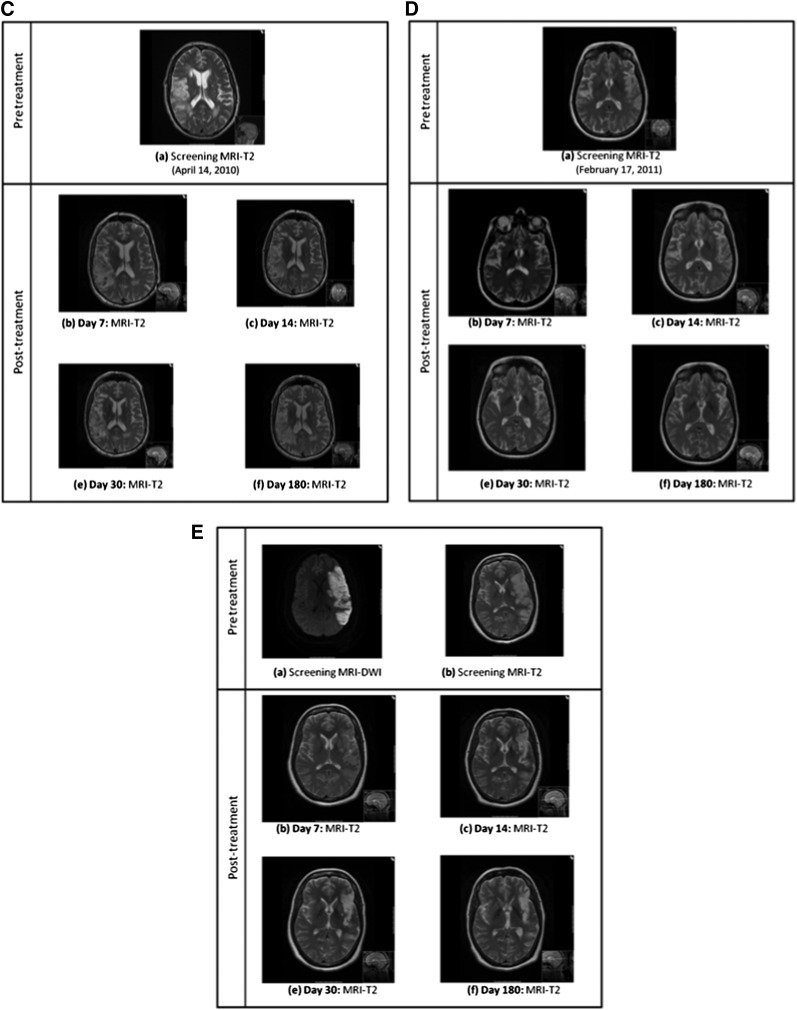
Qualitative assessment of the MRI studies (T2, FLAIR, and gradient-echo or susceptibility-weighted images) at each follow-up point showed the typical evolution of cerebral infarction with no new lesions (including edema, hemorrhage, arteriovenous malformation, or tumor). Baseline diffusion weighted MRI scans are shown in Figure 4B. An angiographic image of intra-arterial cell delivery is shown in Figure 4C. Serial imaging of all 5 patients up to day 180 is shown in Figure 5.
Figure 5.
Serial brain imaging from baseline to day 180 in patients 1–5. (A): Serial MRI scans of patient 1 showing progressive maturation of a left middle cerebral artery (MCA) infarct. (B): Baseline CT and subsequent MRI scans of patient 2 showing progressive maturation of a right MCA infarct. (C): Serial MRI scans patient 3 showing progressive maturation of a right MCA infarct. (D): Serial MRI scans of patient 4 showing progressive maturation of a left MCA infarct. (E): Serial MRI scans of patient 5 showing progressive maturation of a left MCA infarct. Abbreviations: CT, computed tomography; DW, diffusion weighted; MRI, magnetic resonance imaging.
Discussion
Subsequent to evidence of preclinical therapeutic benefit of CD34+ stem/progenitor cells in acute stroke, we have demonstrated in a phase I clinical trial that autologous CD34+ stem/progenitor cells, delivered directly into the middle cerebral artery within the first week of stroke symptoms, is both possible and safe. All patients tolerated the treatment well, with no identifiable treatment-related serious adverse events during a 6-month follow-up period. We attempted to minimize the risks of treatment by performing both bone marrow aspiration (for cell acquisition) and subsequent cerebral angiography (for cell delivery), with the patient under local anesthesia, rather than general anesthesia. Furthermore, all patients showed improvements in clinical scores and reductions in lesion volume within 6 months. Although such patterns of recovery are well-recognized in the usual natural history of strokes, these findings are nevertheless reassuring for future trials of CD34+ cell therapy. In particular, we found no evidence of postintervention stroke (ischemic or hemorrhagic), vascular malformation, or tumor.
Preclinical studies of CD34+ cell transplantation have shown evidence of functional recovery in rodent models of ischemic stroke. Although our proof of concept study was not designed with a control group or powered to be able to detect efficacy, the assessment of outcome did show a trend toward functional improvement, with all 5 patients in our study showing improvements up to 6 months of follow-up in their NIHSS and mRSs. Previously, patients with TACS have been shown to have a poor prognosis, with 96% of such patients having died or become dependent (mRS >2) at 6 months of follow-up [27]. Of the 5 patients recruited, 4 had the TACS subtype, and of these, 3 were independent at the final follow-up visit (defined as an mRS of ≤2). Our results showing that 75% of the TACS patients (or 80% of the total cohort) were independent at the final follow-up visit are therefore encouraging.
We deliberately chose an early timescale to maximize the potential neuroreparative effects of CD34+ cells. Two preclinical studies, in particular, have demonstrated increased angiogenesis in perilesional tissue after CD34+ cell transplantation, with evidence of possible angiogenesis-mediated neurogenesis [12, 13]. The timing of transplantation in these 2 studies was at 48 hours after stroke and 7 days after stroke, respectively. Both studies showed evidence of functional improvement and reduced infarct volume. Most preclinical studies have shown very little evidence of engraftment of significant numbers of transplanted cells; therefore, it has been thought that the mechanism of recovery occurs via a trophic effect on the brain (e.g., secretion of growth factors), including recruitment of endogenous repair processes. We hypothesized that this reparative effect would be maximal early after the ischemic insult. On the basis of the cited preclinical studies, we chose an early timescale within 7 days of the event.
Because this was a phase I trial of a potentially hazardous intervention, for ethical reasons we limited our treatment population to those most severely affected (TACS subtype). Furthermore, we were deliberately cautious in our specification of the inclusion criteria. Of the 76 TACS patients considered, only 4 were eligible. The slow recruitment to this trial was a surprise and seemed to be mainly related to the nature of the patients with the TACS subtype, in particular, medical instability and arterial anatomy. To increase the external validity and recruitment rate for this technique, we widened the inclusion criteria to include the PACS subtype (and an NIHSS score of ≥8), resulting in 1 patient recruited of 6 screened. Age older than 80 years was another significant reason for exclusion from this trial. Future studies will need to consider extending this upper age limit, which has been done in other acute stroke trials, such as the third international stroke trial [28].
To date, a number of clinical trials examining human stem cells in ischemic stroke have been completed across the world. These have used a variety of different types of stem cells (mesenchymal, bone marrow mononuclear cells, neuronal immortalized cells), at different time points, and via different methods of delivery [16, 20–23, 29–32]. The ideal timing of transplantation remains unclear. Only three of the currently published clinical trials have considered stem cell delivery in the acute phase. All 3 studies investigated the effects of autologous BMMNCs: 1 at 24–72 hours after the event, given intravenously; another at 3–7 days after the event, given intra-arterially; and the third at 5–9 days after the event, given intra-arterially [16, 22, 23]. All three trials demonstrated safety and feasibility in their subjects.
The ideal dose of stem cells to deliver also remains unclear. In our study, the dosage was limited by the number of cells obtained via bone marrow aspiration with the patient under local anesthesia. Performing the procedure with the patient under general anesthesia would have allowed acquisition of greater volumes of marrow and hence immunoselection of more cells. However, the use of a general anesthetic would have been potentially problematic in such a severe subgroup of patients, so early after the stroke. Future trials will need to clarify issues regarding dosage and, ideally, would incorporate a dose-escalation design. Comparisons of different methods of delivery will also be important. Tracking of transplanted cells and elucidation of the mechanism of action are other critical issues that still need additional investigation [19].
Conclusion
This is the first study in humans demonstrating that direct intra-arterial infusion of autologous CD34+ selected cells in patients with acute (within 7 days of stroke onset) severe ischemic stroke is safe and feasible. All patients improved (NIHSS score and mRS) up to 6 months of follow-up, although this study was not powered to examine efficacy. Our study was hindered by slow recruitment owing to the severity of the subtype of patients included.
Acknowledgments
This study was funded by Omnicyte Ltd. P.B. is funded by a Higher Education Funding Council for England Department of Health award. J.C. received a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme. The funding sources had no role in the study design, data collection, interpretation, or compilation of the report. The corresponding author had full access to all the data in the study, and final responsibility for the decision to submit for publication.
Author Contributions
S.B. and P.B.: manuscript writing, collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; M.H., S.L.J., and M.G.: final approval of manuscript; S.M. and J.D.: manuscript writing, final approval of manuscript; A.S., D.A.W., and J.C.: conception and design, final approval of manuscript; J.N.: administrative support; N.H.: conception and design, financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
S.B. has a compensated consultancy with Clearview Health Partners for stem cell trial design. M.H. has a compensated consultancy role with Hansen Medical/Gore Medical. J.N. and N.H. have compensated ownership interest.
References
- 1.Chu K, Kim M, Jeong SW, et al. Human neural stem cells can migrate, differentiate, and integrate after intravenous transplantation in adult rats with transient forebrain ischemia. Neurosci Lett. 2003;343:129–133. doi: 10.1016/s0304-3940(03)00174-5. [DOI] [PubMed] [Google Scholar]
- 2.Chu K, Kim M, Park KI, et al. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004;1016:145–153. doi: 10.1016/j.brainres.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 3.Kelly S, Bliss TM, Shah AK, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borlongan CV, Tajima Y, Trojanowski JQ, et al. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp Neurol. 1998;149:310–321. doi: 10.1006/exnr.1997.6730. [DOI] [PubMed] [Google Scholar]
- 5.Stroemer P, Patel S, Hope A, et al. The neural stem cell line CTX0E03 promotes behavioral recovery and endogenous neurogenesis after experimental stroke in a dose-dependent fashion. Neurorehabil Neural Repair. 2009;23:895–909. doi: 10.1177/1545968309335978. [DOI] [PubMed] [Google Scholar]
- 6.Smith EJ, Stroemer RP, Gorenkova N, et al. Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem Cells. 2012;30:785–796. doi: 10.1002/stem.1024. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Chen J, Wang L, et al. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56:1666–1672. doi: 10.1212/wnl.56.12.1666. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Chopp M, Chen J, et al. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Shen LH, Li Y, Chen J, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Chen J, Chen XG, et al. Human marrow stromal cell therapy for stroke in rat: Neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 12.Shyu WC, Lin SZ, Chiang MF, et al. Intracerebral peripheral blood stem cell (CD34+) implantation induces neuroplasticity by enhancing beta1 integrin-mediated angiogenesis in chronic stroke rats. J Neurosci. 2006;26:3444–3453. doi: 10.1523/JNEUROSCI.5165-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paczkowska E, Larysz B, Rzeuski R, et al. Human hematopoietic stem/progenitor-enriched CD34(+) cells are mobilized into peripheral blood during stress related to ischemic stroke or acute myocardial infarction. Eur J Haematol. 2005;75:461–467. doi: 10.1111/j.1600-0609.2005.00536.x. [DOI] [PubMed] [Google Scholar]
- 15.Dunac A, Frelin C, Popolo-Blondeau M, et al. Neurological and functional recovery in human stroke are associated with peripheral blood CD34+ cell mobilization. J Neurol. 2007;254:327–332. doi: 10.1007/s00415-006-0362-1. [DOI] [PubMed] [Google Scholar]
- 16.Moniche F, Gonzalez A, Gonzalez-Marcos JR, et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: A pilot clinical trial. Stroke. 2012;43:2242–2244. doi: 10.1161/STROKEAHA.112.659409. [DOI] [PubMed] [Google Scholar]
- 17.Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lappalainen RS, Narkilahti S, Huhtala T, et al. The SPECT imaging shows the accumulation of neural progenitor cells into internal organs after systemic administration in middle cerebral artery occlusion rats. Neurosci Lett. 2008;440:246–250. doi: 10.1016/j.neulet.2008.05.090. [DOI] [PubMed] [Google Scholar]
- 19.McColgan P, Sharma P, Bentley P. Stem cell tracking in human trials: A meta-regression. Stem Cell Rev. 2011;7:1031–1040. doi: 10.1007/s12015-011-9260-8. [DOI] [PubMed] [Google Scholar]
- 20.Battistella V, de Freitas GR, da Fonseca LM, et al. Safety of autologous bone marrow mononuclear cell transplantation in patients with nonacute ischemic stroke. Regen Med. 2011;6:45–52. doi: 10.2217/rme.10.97. [DOI] [PubMed] [Google Scholar]
- 21.Suárez-Monteagudo C, Hernández-Ramírez P, Alvarez-González L, et al. Autologous bone marrow stem cell neurotransplantation in stroke patients: An open study. Restor Neurol Neurosci. 2009;27:151–161. doi: 10.3233/RNN-2009-0483. [DOI] [PubMed] [Google Scholar]
- 22.Savitz SI, Misra V, Kasam M, et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70:59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- 23.Friedrich MA, Martins MP, Araújo MD, et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant. 2012;21(suppl 1):S13–S21. doi: 10.3727/096368912x612512. [DOI] [PubMed] [Google Scholar]
- 24.Shyu WC, Lin SZ, Yang HI, et al. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004;110:1847–1854. doi: 10.1161/01.CIR.0000142616.07367.66. [DOI] [PubMed] [Google Scholar]
- 25.Schäbitz WR, Kollmar R, Schwaninger M, et al. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke. 2003;34:745–751. doi: 10.1161/01.STR.0000057814.70180.17. [DOI] [PubMed] [Google Scholar]
- 26.England TJ, Abaei M, Auer DP, et al. Granulocyte-colony stimulating factor for mobilizing bone marrow stem cells in subacute stroke: The stem cell trial of recovery enhancement after stroke 2 randomized controlled trial. Stroke. 2012;43:405–411. doi: 10.1161/STROKEAHA.111.636449. [DOI] [PubMed] [Google Scholar]
- 27.Bamford J, Sandercock P, Dennis M, et al. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–1526. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 28.IST-3 Collaborative Group. Sandercock P, Wardlaw JM, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): A randomised controlled trial. Lancet. 2012;379:2352–2363. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondziolka D, Wechsler L, Goldstein S, et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55:565–569. doi: 10.1212/wnl.55.4.565. [DOI] [PubMed] [Google Scholar]
- 30.Kondziolka D, Steinberg GK, Wechsler L, et al. Neurotransplantation for patients with subcortical motor stroke: A phase 2 randomized trial. J Neurosurg. 2005;103:38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- 31.Bang OY, Lee JS, Lee PH, et al. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 32.Honmou O, Houkin K, Matsunaga T, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790–1807. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]