Glioblastoma (GBM) is a primary brain cancer with an extremely poor prognosis. Recent studies in Drosophila and mammalian models (e.g., xenografts of human cancer cells into small animals) are summarized to elucidate the intercellular interactions between apoptosis-prone cancer cells and hyperproliferative cancer cells. These evolving investigations will yield insights on molecular signaling interactions in the context of post-therapeutic phenotypic changes in human cancers.
SUMMARY
Glioblastoma (GBM) is a primary brain cancer with an extremely poor prognosis. GBM tumors contain heterogeneous cellular components, including a small subpopulation of tumor cells termed glioma stem cells (GSCs). GSCs are characterized as chemotherapy- and radiotherapy-resistant cells with prominent tumorigenic ability. Studies in Drosophila cancer models demonstrated that interclonal cooperation and signaling from apoptotic clones provokes aggressive growth of neighboring tumorigenic clones, via compensatory proliferation or apoptosis induced proliferation. Mechanistically, these aggressive tumors depend on activation of Jun-N-terminal kinase (upstream of c-JUN), and Drosophila Wnt (Wg) in the apoptotic clones. Consistent with these nonmammalian studies, data from several mammalian studies have shown that c-JUN and Wnt are hyperactivated in aggressive tumors (including GBM). However, it remains elusive whether compensatory proliferation is an evolutionarily conserved mechanism in cancers. In the present report, we summarize recent studies in Drosophila models and mammalian models (e.g., xenografts of human cancer cells into small animals) to elucidate the intercellular interactions between the apoptosis-prone cancer cells (e.g., non-GSCs) and the hyperproliferative cancer cells (e.g., GSCs). These evolving investigations will yield insights about molecular signaling interactions in the context of post-therapeutic phenotypic changes in human cancers. Furthermore, these studies are likely to revise our understanding of the genetic changes and post-therapeutic cell-cell interactions, which is a vital area of cancer biology with wide applications to many cancer types in humans.
Introduction
Glioblastoma (GBM) is the most frequent and lethal form of primary brain cancer, with current therapy, such as surgery and radio- and/or chemotherapy, providing only palliation. Among heterogeneous GBM cells, glioma stem cells (GSCs) are operationally defined as a subpopulation that is relatively resistant to chemo- and radiotherapy with prominent tumorigenic ability. Recent studies, however, have raised questions whether therapy should be aimed only toward GSCs among the various tumor cells.
Recently, a series of elegant studies in Drosophila mosaic cancer models in epithelial imaginal discs has demonstrated interclonal cooperation via cell-to-cell signals between apoptotic clones and their neighboring tumorigenic clones [1–4]. In these Drosophila cancers, mosaic clones with activation of apoptosis signals and tumorigenic signals upregulated Drosophila Jun-N-terminal kinase (dJNK) (and an upstream regulator of c-JUN) [5, 6], Drosophila Casp 9 (I. Waghmare, S. Verghese, A. Roebke et al., manuscript in preparation), and Wg (Drosophila Wnt1 homolog) (I. Waghmare, S. Verghese, A. Roebke et al., manuscript in preparation). To study these molecular signals in a system more relevant to human GBM, the Drosophila glioma model was recently established in several laboratories, including ours, using the genetic combinations known to induce competitive and/or compensatory interactions [7–9]. This model has enabled us to determine interclonal signaling events between dying cells and surviving cells in brain cancers in vivo. Furthermore, our recent published data of the patient-derived GBM models suggest that irradiation-induced apoptosis of human non-GSCs upregulates at least some of the complementary genes (c-JUN, JNK, and Wnt), in addition to the oncogenic binding partner of c-JUN, maternal embryonic leucine-zipper kinase [10]. Collectively, these mammalian and nonmammalian data indicate that intercellular cooperation between the clonal populations of the tumor cells could be an evolutionarily conserved mechanism. These studies also suggest the presence of an interclonal molecular mechanism for “tumor tissue repair” in human cancers.
Current therapies, including irradiation and chemotherapy, will subsequently fail in virtually all patients with GBM. The concept that dying cancer cells activate the proliferation of remaining cancer cells would shed light on a novel mechanism for post-treatment tumor evolution into a more therapy resistant phenotype. If this hypothesis is true and most of the cancer cells dying of therapeutic insult are non-GSCs, a paradigm shift in the field could occur, highlighted by the refined understanding of the postirradiation molecular events in non-GSCs as a potentially vital therapeutic target in GBM. In addition, this novel concept has potential effects on broad areas of cancer biology and patient care.
Clinical Hurdle of GBM Therapies—Generation of Refractory Tumors
GBM is highly aggressive and therapy resistant [11–13]. Consequently, in developed countries, including the United States, virtually all patients die of recurrent tumors and not of newly diagnosed de novo tumors. Some patients will respond relatively well to first-line chemo- and radiotherapy regimens but then do not survive subsequent recurrence owing to the lack of a therapeutic response of the recurrent tumors to the standard of care therapies. How recurrent tumors gain, or are selected for, therapy resistance remains largely unknown. Although emerging evidence indicates that therapy resistance is, at least in part, mediated by GSCs and development of effective GBM therapies appears to require effective targeting of GSCs, recent studies have raised questions whether GSCs are the only therapeutic target in GBM. For example, at least in an experimental setting, non-GSCs acquire stem cell characteristics when challenged in glucose-deprived condition [14]. This finding has led to the possibility that the elimination of existing GSCs might not be sufficient to control GBM tumors.
In the clinical setting, we often see patients with GBM returning to the clinics during, or shortly after, irradiation because of rapidly regrowing therapy-refractory tumors. The preferential upregulation of the DNA damage repair genes in GSCs has been reported as a causal mechanism for their radioresistance [10]. To extend these findings, we recently demonstrated that GSCs that have overcome a radiation insult undergo phenotypic and genetic changes into mesenchymal-like cells that are even more radioresistant [15]. We found that recurrent GBM samples tend to contain more radioresistant mesenchymal-type GSCs than naïve tumors, which might be associated with the difficulty in treating recurrent tumors. It has been convincingly proven that GSCs are one, if not the exclusive, critical therapeutic target. Nonetheless, the investigation of isolated GSCs from heterogeneous tumor cells might not be clinically and physiologically relevant, because these oncogenic stem cells are tightly supported by the tumor microenvironment composed of various cell types, including non-GSCs. However, the intercellular signals between heterogeneous tumor cells caused by therapeutic insult are largely unknown.
Interclonal Cooperation in Cancer: Lessons From Drosophila Cancer Models
Human tumors display a large degree of genetic and phenotypic heterogeneity, partially owing to chromosomal instability in cancer cells [16, 17]. Complex signaling interactions between cancer cells and their microenvironment and the cooperation or competition between heterogeneous cancer clones contribute to tumorigenesis and malignant transformation. Given these complexities, Drosophila has proved to be an excellent, if not the perfect, model for cancer studies, not only because of its rich history as a genetic model and the conservation of genetic and cell biological processes from flies to humans, but also because of the arsenal of genetic tools and techniques available for study in flies [2, 18–20]. Similar to human cancers, Drosophila cancers can invade and breach the extracellular matrix, recruit stromal cells, and metastasize to other organs [2, 21–23]. Although the Drosophila model lacks an adaptive immune response, fibroblasts, and the other vascular cells required to study angiogenesis, the Drosophila models enable studies of very early oncogenic events that pertain to cell-cell signaling and cell-matrix interactions to track the clonal origin of cancers in vivo in a whole animal model [24]. Tracking these early molecular changes has proved challenging in vertebrate experimental models such as mice and in human clinical patients.
Improved experimental designs have allowed fly biologists to recapitulate oncogenic cooperation in flies to study the cell-to-cell signaling events in tumors of “clonal” origin caused by multiple genetic alterations using the epithelial imaginal discs as a model system [2, 4, 6, 25, 26]. Studies in Drosophila revealed that pathways regulating cell proliferation and apoptosis are central to the cell-cell interactions. Surgical ablation or irradiation was used in the initial studies to test the tissue response; in both cases, the cells responded by inducing cell proliferation to restore tissue homeostasis after cytotoxic insults [27–30]. Additional analysis revealed that cells undergoing apoptosis become metabolically active and release signals (mitogenic or toxic) to their microenvironment [31] that drive different intercellular behaviors. Several cell-cell interactions were identified by studying the interclonal interactions in flies (e.g., cell competition and compensatory proliferation). Cell competition is a homeostatic mechanism in which cells sense a damaged cell and eliminate it by activating cell death [32–34]. This is followed by compensatory proliferation in which the neighboring normal cells reactivate proliferation and thereby restore the tissue size [35–38] in response to apoptosis of the damaged cell. Recently, the concept of “supercompetition” was recognized as another cell behavior in which mutant cells grow even more aggressively when their apoptosis has been blocked and actively eliminate normal cells [39–42]. Apoptosis appears to drive the cell proliferation of surviving cells in all these interactions, and tissue homeostasis is disrupted if these phenomena are blocked. However, the molecular nature of the intercellular signal and how cell interactions contribute to oncogenic cooperation remain elusive and are an area of intensive study in flies. It is likely that most, if not all, of these cellular events are evolutionarily conserved. Assuming a few surviving human cancer cells remain after radiation therapy, it would be interesting to known how they behave adjacent to a large pool of cancer cells undergoing therapy-induced apoptosis.
Role of Paracrine Signaling From Apoptotic Cells in Tumor Growth and Progression
Some of the earliest models of interclonal cooperation were generated by overexpression of oncogenic Ras (RasV12) in cells mutant for the apical-basal polarity gene scribble (scrib) in eye imaginal discs of flies [25] using a powerful technique termed mosaic analysis with a repressible cell marker (MARCM) [43]. The initial studies revealed several important characteristics of oncogenic cooperation in clonal cancers. Cells with loss of scrib were outcompeted by their neighboring cells, suggesting that the scrib mutant cells were less fit than normal clones. Overexpression of oncogenic Ras alone resulted in mild hyperplasia without creating malignant cancers. In contrast, in the phenotype of flies carrying both, the overexpression of oncogenic Ras and scrib mutation (twin clones of RasV12 plus scrib−/−) was remarkable [25]. This genetic combination caused a dramatic growth of cells that showed increased proliferation and decreased apoptosis, mimicking human cancer lesions in terms of depicting increased mitotic rate, reduced differentiation, and increased metastatic potential. In addition, this oncogenic cooperation resulted in decreased adhesion (as seen by the downregulation of cyclin E) and degradation of basement membrane (as seen by the loss of collagenase IV), leading to formation of large eye cancers that were capable of metastasizing to other organs. Later models showed that overexpression of Ras and the loss of scrib in neighboring cells can also lead to oncogenic cooperation [1]. This combination mimics a condition in human cancers, which often show multiple genetic lesions that promote oncogenic cooperation between activated oncogenes and mutated tumor suppressor genes. These two cell populations actively cooperate via signaling interactions that promote changes in cell behaviors, leading to tumors that can metastasize. Another interesting aspect was that later during tumor growth, the interclonal tumors were comprised almost exclusively of RasV12 overexpressing cells, and the scrib−/− cells had been progressively eliminated from the eye cancer. Wu et al. established that scrib−/− cells were required for initial tumor formation and that the apoptotic signal induced to eliminate the scrib−/− cells was critical in the interclonal interactions between the RasV12 and scrib−/− populations to form aggressively growing metastatic tumors.
As a downstream target of the RasV12 and scrib−/− signaling axis, the JNK pathway has emerged as a key molecular player for the paracrine signal that promotes both intraclonal [6, 25] and interclonal [1] interactions [44]. JNK-mediated apoptosis is responsible for eliminating scrib−/− cells. In turn, expression of oncogenic Ras prevents cells from JNK-mediated apoptosis and promotes paradoxical tumor growth by activating the Fos-mediated transcriptional activation of matrix metalloprotease 1 [5, 25, 45, 46]. Therefore, JNK might play context-dependent roles as both a tumor suppressor and an oncogene [47]. In its role as an oncogene, JNK activates the cytokines of the JAK-STAT pathway, and the JNK-mediated JAK-STAT signaling axis might promote oncogenic Ras-mediated tumor growth. In other scenarios (e.g., by activation of oncogenic Ras after tissue damage [1]), JNK might activate components of the Drosophila innate immune response via activation of the tumor necrosis factor (TNF) family protein Eiger in circulating hemocytes (cells of the Drosophila immune response) [47]. These interactions resemble the effects of chronic inflammation in human cancers, in which TNF is secreted by both the tumor cells and the associated immune cells [48]. In addition, JNK interacts with the Hippo pathway through a variety of cell-cell interactions, including cell competition, compensatory proliferation, and supercompetition, which form another key signaling axis promoting tumor growth [49–51]. JNK cooperates with oncogenic Ras to inactivate the Hippo pathway, leading to upregulation of its targets Unpaired (an interleukin-6 homolog) and Wingless (a Wnt homolog) [52] (I. Waghmare, S. Verghese, A. Roebke et al., manuscript in preparation). The apoptosis caused by radiation also involves activation of JNK-mediated Hippo inactivation, leading to compensatory responses. Thus, tumor cells appear to co-opt mechanisms previously described for tissue remodeling, regeneration, and wound healing—other contexts in which cell proliferation is reactivated in response to paracrine signaling from cells undergoing apoptosis. Overall, the Drosophila cancer models recapitulate important cell-cell signaling interactions in the clonal tumors and have proved very informative for studying pathways involved in cancer growth and progression. These initial findings obtained from the Drosophila cancer models must be carefully validated in mammalian cancer models and clinical tumor samples.
Other Pathways in Drosophila Tumorigenesis
In addition to activated Ras and loss of apical-basal polarity genes such as scrib, many other pathways, including lethal giant larva and discs large, have been studied for their role in tumorigenesis and metastasis in Drosophila. Prominent among these is the study of invasive growth caused by the Notch pathway. Similar to oncogenic Ras, Notch signaling can synergize with JNK to promote tumorigenesis and invasive behavior in Drosophila eye and wing cancer models [6, 53]. Recent studies have suggested that epigenetic silencing (Polycomb group silencers) mechanisms could play a role in activated Notch mediated tumorigenesis [54]. Similarly, several components of the endosomal or endocytic sorting machinery are known to cause metastatic eye cancers in flies in a context-dependent manner through upregulation of Notch, JNK, JAK-STAT, and Yorkie activity [55–57]. The proliferative effects of oncogenic cooperation could be limited to the mutant cells (cell autonomous) or affect the normal cells adjacent to the mutant cells (non–cell autonomous) [58]. Mitochondrial dysfunction was recently shown to cooperate with oncogenic Ras to cause nonautonomous cancers. In this phenomenon, mitochondrial dysfunction induces apoptosis in cells that then signal to the neighboring cells, which form cancers that overproliferate and show invasive properties [58]. Drosophila cancer models have also been developed to study specific pathways (e.g., Notch, Wnt/Wg pathway) involved in the maintenance of adult stem cells [59–61]. The stem cell niches and their interactions with neighboring mitotic cells is well studied in flies for adult stem cells (e.g., follicle stem cells in the ovaries, neural stem cells in the brain) [59–61]. Other cancer models studied in Drosophila larvae include Drosophila cancers generated in the larval central nervous system that cause glioma by the expansion of glial cells from overexpression of endothelial growth factor receptor (EGFR)-Ras, PI3K, and other tyrosine kinase receptors (e.g., EGFR, platelet-derived growth factor receptor, fibroblast growth factor receptor) [9, 62] or neoplastic brain tumors due to an increase in the population of neuroblasts resulting from the defects in asymmetric cell division by the loss of tumor suppressor genes such as lethal (3) malignant brain tumor (l(3)mbt), brain tumor (brat), scrib, prospero (pros) [63].
Compensatory Proliferation of Surviving Cancer Cells Induced by Dying Cancer Cells—Causal for Repopulation of Cancer Cells at Recurrence?
The molecular mechanisms that induce intercellular signals leading to compensatory proliferation remain elusive. Nonetheless, taking advantage of several genetic mosaic models in Drosophila, the nature of some signals underlying these interactions is beginning to emerge [4, 19, 20]. In general, apoptotic clones are unable to induce effector caspases (termed “undead cells”) and continue to secrete proliferation signals (e.g., morphogens such as Wingless, transforming growth factor-β, or Hedgehog) to the neighboring oncogenic clones. Recent studies, including our own, have suggested that the JNK pathway might promote aggressive proliferation of cells within tumors via compensatory mechanisms [1, 5, 36, 45, 51–53, 64, 65].
An emerging area of research is to investigate whether human cancers show similar compensatory type signaling interactions. Recently, a correlative study to these Drosophila studies was published indicating the presence of intercellular cooperation in human cancer cells. In that study, Huang et al. presented several interesting pieces of evidence that surviving human breast cancer cells xenografted in the leg of nude mice receive proliferation signals from irradiated breast cancer cells and thereby undergo repopulation of the tumor cells [66]. Collectively, from the results of these Drosophila and human studies, it is tempting to speculate that intercellular signaling between heterogeneous tumor cells (or with neighboring nontumor cells) in brain tumors might co-opt the tissue “repair” mechanisms in response to therapeutic insult [67, 68]. Furthermore, taking the cancer stem cell theory into account, we speculate that dying non–cancer stem cells drive compensatory proliferation of cancer stem cells, which is a reason, at least in part, for the aggressiveness of recurrent brain tumors. It is important to elucidate the evolutionarily conserved molecular mechanisms for intercellular interaction to facilitate tumor cell survival, proliferation, and therapy resistance.
Limitation in the Current Understanding—Drosophila and Human
For innovative genome-wide genetic screens, Drosophila has proved a useful model organism for the identification of cancer-related genes in vivo, evidenced by the first identification of the pathways highly implicated in human cancers, such as the Hippo pathway, Notch pathway, Hedgehog, and JAK-STAT in flies [21, 69–71]. Another strength of the Drosophila models is the mosaic models with which we can investigate the interactions of multiple clones side-by-side with distinct mutations of oncogenes and tumor suppressor genes in situ that induce tumorigenesis [4, 26, 72]. However, similar to other model systems, the Drosophila models also have limitations. Obvious differences exist between the physiologic and immune systems of flies and humans. For example, the metabolic changes in cancer cells (e.g., glycolysis, glutaminolysis, and lipid metabolism) that affect the behavior of cancer cells cannot be directly compared between the two models. Drosophila lacks an adaptive immune response; however, the cellular and humoral aspects of the innate immune response are conserved in flies [18]. Flies have an open circulatory system; therefore, angiogenesis cannot be modeled in flies. Also, histopathology, is a guiding force for staging and grading tumors, cannot be compared between Drosophila and humans. Another limitation includes investigation of the metastatic behavior of secondary tumors in Drosophila tumor models.
Conclusion
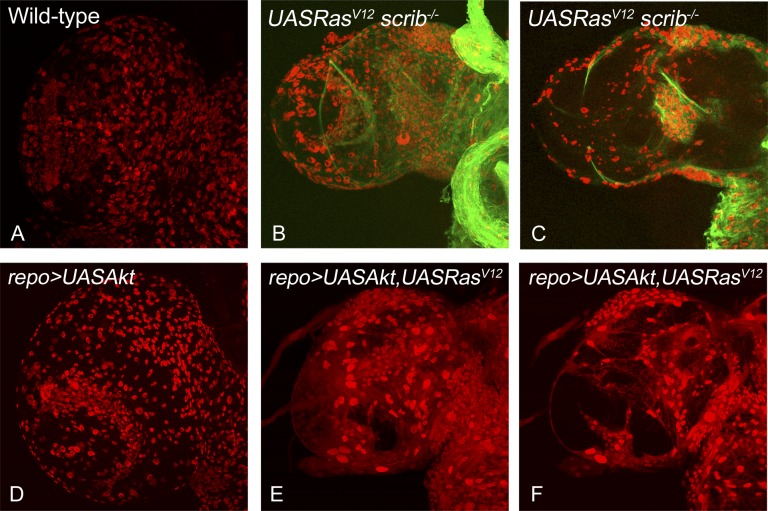
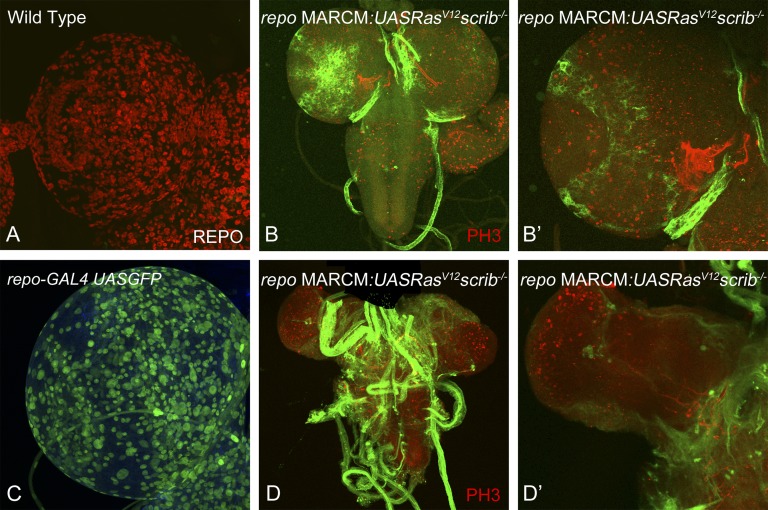
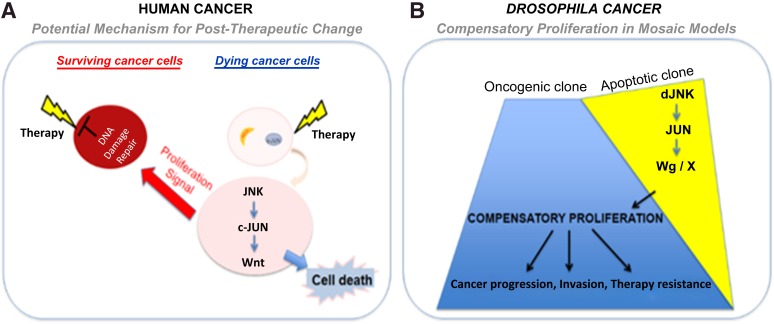
Recent advances in genetic tools available in Drosophila have just started to allow us to generate complex Drosophila tumor models (e.g., colorectal cancer model to study tumor progression [73], an intestinal stem cell tumor model to study paradoxical signaling between stem cells and neighboring cells [74], and human brain tumor models, including glioma [7, 62]). Overexpression of PI3K and oncogenic Ras (or scrib mutant cells overexpressing oncogenic Ras) specifically in glial cells using the repo-GAL4 in larval brains is sufficient to cause overproliferation of glial cells, leading to overgrowth of the brain lobes (Fig. 1). Furthermore, by combining the repo-GAL4 system with mosaic techniques, it is now possible to generate a glioma model to study interclonal cooperation between dying cells and their neighboring oncogenic cells (Fig. 2). These models will lead us to investigate the paradoxical in vivo signals from apoptotic cells that likely influence the behavior of neighboring surviving cancer cells and the interactions between heterogeneous populations of tumors (Fig. 3). Live imaging of Drosophila brain cells, including cancer cells by green fluorescent protein labeling, will further deepen our understanding of cell-to-cell signaling and cell-matrix interactions. In addition, this system is amenable to a variety of genetic screens and drug testing to identify novel anticancer chemotherapeutic agents or pharmacological compounds that either affect the cell-cell signals or inhibit the growth of human cancers. We can then translate this knowledge into the development of novel and effective cancer therapeutic agents. Successful achievement of the ongoing research will likely elucidate cancer stem/non-stem cell-specific signaling crosstalk. Eventually, these studies will lead to the identification of novel mechanisms for therapy resistance in cancers, one of the major hurdles for patients whose disease has failed to respond to current therapies and subsequently die of this devastating disease.
Figure 1.
Drosophila glioma models to study cell-cell signaling interactions. Comparisons of overgrowth and glial cell numbers in the dorsal lobe of Drosophila larval brain from mature third instar larvae are shown for the following genotypes: wild-type (A), w; repo-Gal4[4.3] UAS-mCD8:GFP repo-flp5/yw; +/+; FRT82B Tub-Gal80/ UAS RasV12 FRT82B scrib2 (B, C), UASAkt (D), and yw/UAS Akt; +/UAS RasV12; repo-Gal4 UASGFP/+ (E, F). All samples were stained for antibody against the glial-specific marker Repo (red). The samples in (A, B, D, E) show the surface view, and (C, F) show the medial view through the dorsal lobe of the brain. The glioma in (B, C) were generated using the MARCM approach, resulting in positively marked clones (green fluorescent protein expressing) of glial cells that are mutant for the tumor suppressor gene scribble and simultaneously overexpress oncogenic Ras. The glioma in (E, F) were induced by misexpression of oncogenic Ras and Akt in the glial cells using repo-Gal4. Note that both approaches cause overrepresentation of the glial cells specifically and cause overgrowth in the dorsal brain lobes compared with the normal wild-type controls. All images were scanned at identical magnification. Magnification, ×40. Abbreviation: MARCM, mosaic analysis with a repressible cell marker.
Figure 2.
The MARCM approach allows tracking of glioma of clonal origin. We established a system to positively mark clones induced specifically in the glial cells in the Drosophila brain using the MARCM approach. This system drives the expression of transgenes (e.g., UAS GFP, UAS RasV12) specifically in the glial cells and also causes recombination specifically in the glial cells because the expression of the flippase enzyme is under the control of the repo promoter (repo-flp). (A): Dorsal lobe of wild-type mature third instar larva stained for antibodies to Repo can be compared with (C): dorsal lobe of repo-Gal4 UAS GFP, which shows the expression of the repo-Gal4 transgene using UAS GFP reporter expression. Images in (A, C) show that the repo-Gal4 driver is capable of driving transgenes in all glial cells (red in [A]) in the brain. (B, D): Examples of positively marked clones are shown. Higher magnification images in (B′) and (D′) show the effects of clones early (day 5) and later (day 7) in development. Magnification, ×40 (A, C, B′, D′), ×20 (B, D). Abbreviation: MARCM, mosaic analysis with a repressible cell marker.
Figure 3.
Human and Drosophila models of intercellular interactions and their effect on cancer. The cartoon depicts the similarities in cell-cell signaling interactions that can occur in human cancer cells (A) or in Drosophila cancer models (B). (A): The cartoon illustrates how radiation could produce signals that affect the heterogeneous cancer cells such that the cells that die of radiotherapy (radiation sensitive) produce a signal that induces proliferation of the surviving (radiation-resistant) cancer cells. (B): A similar interaction occurs in Drosophila mosaic cancer models, in which dying cells (apoptotic clones induced by the loss of tumor suppressor genes, e.g., scrib−/−) produce signals that can synergize with the neighboring oncogenic clones (induced by activation of oncogene, e.g., RasV12), to promote growth, progression, and therapy resistance of cancer cells. Abbreviations: dJNK, Drosophila Jun-N-terminal kinase; Wg, Drosophila Wnt.
Acknowledgments
We thank the Developmental Studies Hybridoma Bank for the mouse α Repo antibody, Drs. K.D. Irvine and C. Klämbt for the w, repo-Gal4 [4.3] UAS-mCD8:GFP repo-flp5, and FRT82B Tub Gal80 flies. M.K.-S. is supported by funds from the University of Dayton, and I.N. is supported by American Cancer Society Grant MRSG-08-108-01, NIH/National Cancer Institute Grants P01-CA163205 and R21-CA175875, and NIH/National Institute of Neurological Disorders and Stroke Grants R01-NS083767 and R01-NS087913.
Author Contributions
I.W., A.R., and M.M.: manuscript writing; M.K.-S. and I.N.: manuscript writing, final approval of the manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Wu M, Pastor-Pareja JC, Xu T. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature. 2010;463:545–548. doi: 10.1038/nature08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudrapatna VA, Cagan RL, Das TK. Drosophila cancer models. Dev Dyn. 2012;241:107–118. doi: 10.1002/dvdy.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryoo HD, Bergmann A. The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harb Perspect Biol. 2012;4:a008797. doi: 10.1101/cshperspect.a008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel PH, Edgar BA. Tissue design: How Drosophila tumors remodel their neighborhood. Semin Cell Dev Biol. 2014;28:86–95. doi: 10.1016/j.semcdb.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Igaki T, Pagliarini RA, Xu T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr Biol. 2006;16:1139–1146. doi: 10.1016/j.cub.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 6.Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22:5769–5779. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Read RD. Drosophila melanogaster as a model system for human brain cancers. Glia. 2011;59:1364–1376. doi: 10.1002/glia.21148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miles WO, Dyson NJ, Walker JA. Modeling tumor invasion and metastasis in Drosophila. Dis Model Mech. 2011;4:753–761. doi: 10.1242/dmm.006908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witte HT, Jeibmann A, Klämbt C, et al. Modeling glioma growth and invasion in Drosophila melanogaster. Neoplasia. 2009;11:882–888. doi: 10.1593/neo.09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu C, Banasavadi-Siddegowda YK, Joshi K, et al. Tumor-specific activation of the C-JUN/MELK pathway regulates glioma stem cell growth in a p53-dependent manner. Stem Cells. 2013;31:870–881. doi: 10.1002/stem.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 12.Sathornsumetee S, Rich JN. New approaches to primary brain tumor treatment. Anticancer Drugs. 2006;17:1003–1016. doi: 10.1097/01.cad.0000231473.00030.1f. [DOI] [PubMed] [Google Scholar]
- 13.Nakano I, Saigusa K, Kornblum HI. BMPing off glioma stem cells. Cancer Cell. 2008;13:3–4. doi: 10.1016/j.ccr.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Ye F, Zhang Y, Liu Y, et al. Protective properties of radio-chemoresistant glioblastoma stem cell clones are associated with metabolic adaptation to reduced glucose dependence. PLoS One. 2013;8:e80397. doi: 10.1371/journal.pone.0080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao P, Joshi K, Li J, et al. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci USA. 2013;110:8644–8649. doi: 10.1073/pnas.1221478110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen SY, Huang YC, Liu SP, et al. An overview of concepts for cancer stem cells. Cell Transplant. 2011;20:113–120. doi: 10.3727/096368910X532837. [DOI] [PubMed] [Google Scholar]
- 17.Cheng L, Wu Q, Guryanova OA, et al. Elevated invasive potential of glioblastoma stem cells. Biochem Biophys Res Commun. 2011;406:643–648. doi: 10.1016/j.bbrc.2011.02.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bangi E. Drosophila at the intersection of infection, inflammation, and cancer. Frontiers in cellular and infection microbiology. Front Cell Infect Microbiol. 2013;3:103. doi: 10.3389/fcimb.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christofi T, Apidianakis Y. Drosophila and the hallmarks of cancer. Adv Biochem Eng Biotechnol. 2013;135:79–110. doi: 10.1007/10_2013_190. [DOI] [PubMed] [Google Scholar]
- 20.Deng WM. Molecular genetics of cancer and tumorigenesis: Drosophila models. J Genet Genomics. 2011;38:429–430. doi: 10.1016/j.jgg.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Vidal M, Cagan RL. Drosophila models for cancer research. Curr Opin Genet Dev. 2006;16:10–16. doi: 10.1016/j.gde.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Kango-Singh M, Halder G. Drosophila as an emerging model to study metastasis. Genome Biol. 2004;5:216. doi: 10.1186/gb-2004-5-4-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang AC, Starz-Gaiano M, Montell DJ. Modeling migration and metastasis in Drosophila. J Mammary Gland Biol Neoplasia. 2007;12:103–114. doi: 10.1007/s10911-007-9042-8. [DOI] [PubMed] [Google Scholar]
- 24.St John MA, Xu T. Understanding human cancer in a fly? Am J Hum Genet. 1997;61:1006–1010. doi: 10.1086/301619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagliarini RA, Xu T. A genetic screen in Drosophila for metastatic behavior. Science. 003;302:1227–1231. doi: 10.1126/science.1088474. [DOI] [PubMed] [Google Scholar]
- 26.Brumby AM, Richardson HE. Using Drosophila melanogaster to map human cancer pathways. Nat Rev Cancer. 2005;5:626–639. doi: 10.1038/nrc1671. [DOI] [PubMed] [Google Scholar]
- 27.Bryant PJ. Cell proliferation control in Drosophila: Flies are not worms. BioEssays. 1996;18:781–784. doi: 10.1002/bies.950181003. [DOI] [PubMed] [Google Scholar]
- 28.Bryant PJ. Growth factors controlling imaginal disc growth in Drosophila. Novartis Found Symp. 2001;237:182–194. discussion 194–202. [PubMed] [Google Scholar]
- 29.Bryant PJ, Fraser SE. Wound healing, cell communication, and DNA synthesis during imaginal disc regeneration in Drosophila. Dev Biol. 1988;127:197–208. doi: 10.1016/0012-1606(88)90201-1. [DOI] [PubMed] [Google Scholar]
- 30.Haynie JL, Bryant PJ. The effects of X-rays on the proliferation dynamics of cells in the imaginal wing disc of Drosophila melanogaster. Roux Arch Dev Biol. 1977;183:85–100. doi: 10.1007/BF00848779. [DOI] [PubMed] [Google Scholar]
- 31.Bergmann A, Steller H. Apoptosis, stem cells, and tissue regeneration. Sci Signal. 2010;3:re8. doi: 10.1126/scisignal.3145re8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morata G, Ripoll P. Minutes: Mutants of Drosophila autonomously affecting cell division rate. Dev Biol. 1975;42:211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- 33.Baker NE, Li W. Cell competition and its possible relation to cancer. Cancer Res. 2008;68:5505–5507. doi: 10.1158/0008-5472.CAN-07-6348. [DOI] [PubMed] [Google Scholar]
- 34.Tamori Y, Deng WM. Cell competition and its implications for development and cancer. J Genet Genomics. 2011;38:483–495. doi: 10.1016/j.jgg.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pérez-Garijo A, Martín FA, Morata G. Caspase inhibition during apoptosis causes abnormal signalling and developmental aberrations in Drosophila. Development. 2004;131:5591–5598. doi: 10.1242/dev.01432. [DOI] [PubMed] [Google Scholar]
- 36.Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell! Trends Cell Biol. 2008;18:467–473. doi: 10.1016/j.tcb.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan Y, Bergmann A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev Cell. 2008;14:399–410. doi: 10.1016/j.devcel.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levayer R, Moreno E. Mechanisms of cell competition: Themes and variations. J Cell Biol. 2013;200:689–698. doi: 10.1083/jcb.201301051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreno E, Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117:117–129. doi: 10.1016/s0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- 41.de la Cova C, Abril M, Bellosta P, et al. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117:107–116. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- 42.Rhiner C, Moreno E. Super competition as a possible mechanism to pioneer precancerous fields. Carcinogenesis. 2009;30:723–728. doi: 10.1093/carcin/bgp003. [DOI] [PubMed] [Google Scholar]
- 43.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 44.Chen F. JNK-induced apoptosis, compensatory growth, and cancer stem cells. Cancer Res. 2012;72:379–386. doi: 10.1158/0008-5472.CAN-11-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uhlirova M, Jasper H, Bohmann D. Non-cell-autonomous induction of tissue overgrowth by JNK/Ras cooperation in a Drosophila tumor model. Proc Natl Acad Sci USA. 2005;102:13123–13128. doi: 10.1073/pnas.0504170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uhlirova M, Bohmann D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 2006;25:5294–5304. doi: 10.1038/sj.emboj.7601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cordero JB, Macagno JP, Stefanatos RK, et al. Oncogenic Ras diverts a host TNF tumor suppressor activity into tumor promoter. Dev Cell. 2010;18:999–1011. doi: 10.1016/j.devcel.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 49.Menéndez J, Pérez-Garijo A, Calleja M, et al. A tumor-suppressing mechanism in Drosophila involving cell competition and the Hippo pathway. Proc Natl Acad Sci USA. 2010;107:14651–14656. doi: 10.1073/pnas.1009376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun G, Irvine KD. Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Dev Biol. 2011;350:139–151. doi: 10.1016/j.ydbio.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen CL, Schroeder MC, Kango-Singh M, et al. Tumor suppression by cell competition through regulation of the Hippo pathway. Proc Natl Acad Sci USA. 2012;109:484–489. doi: 10.1073/pnas.1113882109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohsawa S, Sugimura K, Takino K, et al. Elimination of oncogenic neighbors by JNK-mediated engulfment in Drosophila. Dev Cell. 2011;20:315–328. doi: 10.1016/j.devcel.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Leong GR, Goulding KR, Amin N, et al. Scribble mutants promote aPKC and JNK-dependent epithelial neoplasia independently of Crumbs. BMC Biol. 2009;7:62. doi: 10.1186/1741-7007-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferres-Marco D, Gutierrez-Garcia I, Vallejo DM, et al. Epigenetic silencers and Notch collaborate to promote malignant tumours by Rb silencing. Nature. 2006;439:430–436. doi: 10.1038/nature04376. [DOI] [PubMed] [Google Scholar]
- 55.Chi C, Zhu H, Han M, et al. Disruption of lysosome function promotes tumor growth and metastasis in Drosophila. J Biol Chem. 2010;285:21817–21823. doi: 10.1074/jbc.M110.131714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herz HM, Bergmann A. Genetic analysis of ESCRT function in Drosophila: A tumour model for human Tsg101. Biochem Soc Trans. 2009;37:204–207. doi: 10.1042/BST0370204. [DOI] [PubMed] [Google Scholar]
- 57.Menut L, Vaccari T, Dionne H, et al. A mosaic genetic screen for Drosophila neoplastic tumor suppressor genes based on defective pupation. Genetics. 2007;177:1667–1677. doi: 10.1534/genetics.107.078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohsawa S, Sato Y, Enomoto M, et al. Mitochondrial defect drives non-autonomous tumour progression through Hippo signalling in Drosophila. Nature. 2012;490:547–551. doi: 10.1038/nature11452. [DOI] [PubMed] [Google Scholar]
- 59.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 60.Jiang H, Edgar BA. Intestinal stem cells in the adult Drosophila midgut. Exp Cell Res. 2011;317:2780–2788. doi: 10.1016/j.yexcr.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh SR, Hou SX. Multipotent stem cells in the Malpighian tubules of adult Drosophila melanogaster. J Exp Biol. 2009;212:413–423. doi: 10.1242/jeb.024216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Read RD, Cavenee WK, Furnari FB, et al. A Drosophila model for EGFR-Ras and PI3K-dependent human glioma. PLoS Genet. 2009;5:e1000374. doi: 10.1371/journal.pgen.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caussinus E, Gonzalez C. Induction of tumor growth by altered stem-cell asymmetric division in Drosophila melanogaster. Nat Genet. 2005;37:1125–1129. doi: 10.1038/ng1632. [DOI] [PubMed] [Google Scholar]
- 64.Bogoyevitch MA, Ngoei KR, Zhao TT, Yeap YY, Ng DC. c-Jun N-terminal kinase (JNK) signaling: Recent advances and challenges. Biochim Biophys Acta. 2010;1804:463–475. doi: 10.1016/j.bbapap.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Martín FA, Peréz-Garijo A, Morata G. Apoptosis in Drosophila: Compensatory proliferation and undead cells. Int J Dev Biol. 2009;53:1341–1347. doi: 10.1387/ijdb.072447fm. [DOI] [PubMed] [Google Scholar]
- 66.Huang Q, Li F, Liu X, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17:860–866. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Byun JS, Gardner K. Wounds that will not heal: Pervasive cellular reprogramming in cancer. Am J Pathol. 2013;182:1055–1064. doi: 10.1016/j.ajpath.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 69.Hariharan IK, Haber DA. Yeast, flies, worms, and fish in the study of human disease. N Engl J Med. 2003;348:2457–2463. doi: 10.1056/NEJMon023158. [DOI] [PubMed] [Google Scholar]
- 70.Hariharan IK, Bilder D. Regulation of imaginal disc growth by tumor-suppressor genes in Drosophila. Annu Rev Genet. 2006;40:335–361. doi: 10.1146/annurev.genet.39.073003.100738. [DOI] [PubMed] [Google Scholar]
- 71.Bernards A, Hariharan IK. Of flies and men—Studying human disease in Drosophila. Curr Opin Genet Dev. 2001;11:274–278. doi: 10.1016/s0959-437x(00)00190-8. [DOI] [PubMed] [Google Scholar]
- 72.Tipping M, Perrimon N. Drosophila as a model for context-dependent tumorigenesis. J Cell Physiol. 2014;229:27–33. doi: 10.1002/jcp.24427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bell GP, Thompson BJ. Colorectal cancer progression: Lessons from Drosophila? Semin Cell Dev Biol. 2014;28:70–77. doi: 10.1016/j.semcdb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 74.Markstein M, Dettorre S, Cho J, et al. Systematic screen of chemotherapeutics in Drosophila stem cell tumors. Proc Natl Acad Sci USA. 2014;111:4530–4535. doi: 10.1073/pnas.1401160111. [DOI] [PMC free article] [PubMed] [Google Scholar]