The goal of this study was to investigate the immunological properties of adipogenic differentiated adipose-derived stem cells (ASCs) to evaluate their suitability for allogenic applications, including in vitro, in vivo, and clinical applications, through comprehensive studies. The allogenic immune response of adipogenic differentiated ASCs was investigated by flow cytometry, mixed lymphocyte culture, and allogenic implantation. The results suggest that adipogenic differentiated ASCs can be used as a universal donor for soft-tissue engineering in major histocompatibility complex-mismatched recipients.
Keywords: Adipose-derived stem cells, Adipogenic differentiated ASCs, Immunogenicity, Autologous transplantation, Allogenic transplantation
Abstract
We recently reported that autologous adipogenic differentiated adipose-derived stem cells (ASCs) can potentially be used as an effective and safe therapy for soft-tissue regeneration. In the present study, we investigated whether adipogenic differentiated ASCs can be used for allogenic applications to enlarge their therapeutic use. The allogenic immune response of adipogenic differentiated ASCs was investigated by flow cytometry and mixed lymphocyte culture. To determine whether adipogenic differentiated ASCs can form new adipose tissue without immune rejection, these cells were implanted subcutaneously into allo- or xenogenic recipient mice. In addition, the safety of the allogenic implantation of adipogenic differentiated ASCs was explored in a phase I clinical study. Adipogenic differentiated ASCs do not express major histocompatibility complex (MHC) class II molecules and costimulatory molecules, and the expression levels of MHC class I decreased after differentiation. In addition, these cells do not elicit an immune response against MHC-mismatched allogenic lymphocytes and formed new adipose tissue without immune rejection in the subcutaneous region of MHC-mismatched mice. Moreover, these cells did not induce clinically significant local and systemic immune responses or adverse events in the subcutaneous region of donor-independent healthy subjects. These results suggest that adipogenic differentiated ASCs can be used as a “universal donor” for soft-tissue engineering in MHC-mismatched recipients.
Introduction
Millions of plastic and reconstructive surgical procedures are performed each year to repair soft-tissue defects resulting from tumor resections, complex traumas, and congenital defects. Various implants made from biological, artificial, or autologous materials are developed and used in the clinic, but most biological materials are largely absorbed within a few months of surgery, and artificial implants can cause complications. The implantation of autologous adipose tissue was established for the augmentation of soft tissue over the past quarter century.
Unfortunately, implanted adipose tissue is largely absorbed and replaced by fibrous tissue and oil cysts because of the insufficient supply of nutrients and oxygen [1]. In contrast to mature adipose tissue, adipose-derived mesenchymal stem cells (ASCs) are an ideal cell source for soft-tissue regeneration because these cells (a) are easy to isolate from an abundant tissue source, (b) exhibit a high proliferation rate, and (c) differentiate into adipose tissue after reimplantation. Therefore, ASCs have been extensively studied over the last decade with respect to the repair of soft-tissue defects [2, 3].
Many attempts have been made to use undifferentiated ASCs for the regeneration of adipose tissue. The use of a combination of an appropriate scaffold and adipogenic inducing agents or growth factors is required to engineer adipose tissue from ASCs in vivo because ASCs alone are much less effective in inducing the formation of adipose tissue [4–8]. This observation suggests that the host environment may not be sufficient to induce adipogenic differentiation after ASC transplantation. Therefore, the use of stem cells that are already differentiated into a specific cell type ex vivo may be an alternative strategy for the enhancement of therapeutic efficacy in the absence of an appropriate microenvironment for in vivo differentiation after transplantation. Our previous preclinical study showed that adipogenic differentiated ASCs can generate adipose tissue more effectively than undifferentiated ASCs [9]. In addition, a phase II/III clinical study also demonstrated that autologous adipogenic differentiated ASCs can potentially be used as an effective method for volume recovery to treat various disorders that involve soft-tissue defects [10]. In addition, Jeong et al. [11] confirmed that autologous adipogenic differentiated ASC implantation is technically feasible and effective for correcting wrinkles and for soft-tissue augmentation. These results indicate that adipogenic differentiated ASCs are an ideal material for soft-tissue regeneration.
However, the use of autologous adipogenic differentiated ASCs still exhibits some potential limitations, including inadequate cell number and donor site morbidity. These limitations can be resolved through allogenic application if these cells are not immunogenic.
It is well known that undifferentiated ASCs and bone marrow mesenchymal stem cells (BM-MSCs) have no/low immunogenicity and immune modulatory function [12–14]. Undifferentiated ASCs express a low level of major histocompatibility complex (MHC) class I molecules and do not express MHC class II and costimulatory molecules, such as CD40, CD80 (B7-1), and CD86 (B7-2), which allows the undifferentiated ASCs to escape immune surveillance [12, 15]. ASCs also have an immunosuppressive effect exerted through the release soluble factors, such as prostaglandin E2 and indoleamine 2,3-dioxygenase [15, 16]. Several clinical studies using the immunomodulatory properties of ASCs have been performed in our [17] and other [18, 19] laboratories, and some clinical trials on the allogenic transplant application of BM-MSCs [20, 21] and ASCs [22] are being conducted. However, it has not been reported whether ASCs can maintain their nonimmunologic properties after adipogenic differentiation. Although Le Blanc et al. [13] reported that not only undifferentiated but also adipogenic differentiated BM-MSCs are inherently nonimmunogenic in an in vitro study, it is still necessary to confirm nonimmunogenic property of the differentiated ASCs in vivo.
Thus, the goal of this study was to investigate the immunological properties of adipogenic differentiated ASCs to evaluate their suitability for allogenic applications, including in vitro, in vivo, and clinical applications, through comprehensive studies. We assessed adipogenic differentiated human and mouse ASCs (hASCs and mASCs, respectively) for immunologically relevant cell surface molecules. Moreover, we examined the allogenic immune responses and allosensitization of T cells, which were used as the stimulatory cells in a mixed lymphocyte culture (MLC) assay. We also evaluated whether adipogenic differentiated ASCs can form new adipose tissue without immune rejection even in immunocompetent allo- or xenogenic recipients. Finally, we explored the safety of allogenically implanting these cells in a phase I clinical study. Our results demonstrated that adipogenic differentiated ASCs have no immunogenicity and are clinically safe; therefore, these cells can be used for soft-tissue regeneration in allogenic recipients.
Materials and Methods
Isolation and Expansion of Adipose-Derived Stem Cells
Human
hASCs were isolated from lipoaspirates of human subcutaneous fat tissue obtained from healthy donors who provided informed consent as described previously [9]. The lipoaspirates were washed at least three times with phosphate-buffered saline (PBS) and digested in an equal volume of PBS containing 1% bovine serum albumin and 0.025% collagenase type I (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) for 80 minutes at 37°C with intermittent shaking. The mixture was centrifuged for 5 minutes at 300g, and floating adipocytes and collagenase solution were removed from the cell pellet, stromal vascular fractions (SVFs). The SVFs were resuspended in Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 (F12; Invitrogen) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, http://www.hyclone.com) and 1× antibiotic-antimycotic (Invitrogen) and then plated at 10,000 cells per cm2 and cultured at 37°C, a humidified 5% CO2 atmosphere for 24–72 hours. Cultures were washed with PBS to remove nonadherent cells and fed with DMEM supplemented with 10% FBS, 0.25 ng/ml human transforming growth factor β1, 5 ng/ml human epidermal growth factor, and 1 ng/ml human basic fibroblast growth factor. Cells were harvested at 80% confluence and subcultured to passage 2–3.
Mice
mASCs were isolated from the inguinal fat fad of BALB/c mice (8 weeks old, males). The fat pads were minced into small pieces using sterile scissors and processed using the same protocol as described for the preparation of the human cells.
Adipogenic Differentiation
The expanded cells at passages 2–3 were plated at a density of 5,000 cells per cm2 to induce the adipogenic differentiation of ASCs. After the cells reached 100% confluence, they were treated with the following differentiation medium: DMEM/F12 supplemented with 3% FBS (10% FBS in mice), 33 μM biotin, 17 μM pantothenate, 1 μM insulin, 1 μM dexamethasone, 0.1875 mM isobutylmethylxanthine (IBMX), and 0.2 mM indomethacin. After 3 days of adipogenic induction, the cells were fed the same medium without IBMX and indomethacin for an additional 2–5 days. The adipogenic differentiated ASCs were harvested by incubation with 0.1% Trypsin-EDTA for 5–10 minutes at 37°C. Trypsin-EDTA was inactivated by the addition of DMEM. Then the cell suspension was washed three times at room temperature with phenol red-free DMEM. The adipogenic differentiation was assessed in the presence of intracellular lipid droplets by observing the cellular morphology through Oil Red O staining [9] and by reverse transcription-polymerase chain reaction (RT-PCR).
RT-PCR
The total RNA was extracted from ASCs using the TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. The purity and concentration of the extracted RNA were assessed spectrophotometrically at 260 and 280 nm. The total RNA (10 mg) was reverse-transcribed using SuperScriptTM II Reverse Transcriptase (Invitrogen). The synthesized first-strand cDNA (1 or 2 ml) was used as a template for PCR. The primer sequences were as follows: human peroxisome proliferator-activated receptor γ (PPARγ) (sense, 5′-TGGGTGAAACTCTGGGAGATTC-3′; antisense, 5′-CATGAGGCTTATTGTAGAGCTG-3′), mice PPARγ (sense, 5′-GGTTGACACAGAGATGCCATTCTGGCC-3′; antisense, 5′-GGTGGAGATGCAGGTTCTACTTTGATC-3′), human adipocyte protein 2 (aP2) (sense, 5′-GGCCAGGAATTTGACGAAGTC-3′; antisense, 5′-ACAGAATGTTGTAGAGTTCAATGCGA-3′), mice aP2 (sense, 5′-CTGGACTTCAGAGGCTCAATGCA-3′; antisense, 5′-TACTCTCTGACCGGATGGTGACCAA-3′), human hypoxanthine guanine phosphoribosyl transferase (HPRT; sense, 5′-CAGCCCTGGCGTGATTA-3′; antisense, 5′-AGCAAGACGTTCAGTCCTGTC-3′), and mice HPRT (sense,5′-GGTGGCAGAGGCCTTTG-3′; antisense, 5′-TGCCCATTTAGCATCTCCTT-3′). The reactions were performed in a total volume of 20 ml, and the amplification of all of the investigated genes was conducted over 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s. The PCR products were separated on 1.5% agarose gels containing ethidium bromide. The PCR product intensities were visualized using a UV transilluminator.
Flow Cytometric Analysis
The undifferentiated and adipogenic differentiated hASCs and human peripheral blood mononuclear cells (hPBMCs) were subjected to immunophenotypic analysis. The cells were washed with PBS containing 1% FBS, incubated with fluorescein isothiocyanate-conjugated or phycoerythrin-conjugated monoclonal antibodies for 30 minutes at 4°C, washed, and analyzed. The antibodies used were the following: MHC class I (human leukocyte antigen [HLA]-ABC), MHC class II (HLA-DR), CD40, CD80, and CD86 (BD Pharmingen, San Diego, CA, http://www.bdbiosciences.com). Isotype-matched negative controls were used to define the background staining. The data were analyzed by collecting 10,000 events on a BD FACSCanto II flow cytometer system using the FACS Diva software (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com).
Human Leukocyte Antigen Typing
HLA typing was performed using a PCR-specific oligonucleotide (PCR-SSO) probe assay. The genomic DNA was extracted from human ASCs. The PCR-SSO amplification of the HLA-A, -B, and -DRB1 loci was performed using INNO-LiPA HLA-A Update, INNO-LiPA HLA-B Update, and INNO-LiPA HLA-DRB1 kits, respectively, according to the manufacturer’s instructions (Innogenetics, Ghent, Belgium, http://www.fujirebio-europe.com). The detection and genotyping of all PCR products were performed using the reverse dot blot hybridization assay.
Mixed Lymphocyte Culture
Responder Preparation
hPBMCs were collected from the peripheral blood of healthy donors who provided informed consent through Ficoll-Paque (1.077 g/ml; Amersham Biosciences, Uppsala, Sweden, http://www.amersham.com) density gradient centrifugation; these cells were then frozen until their use as responder cells in a MLC. Splenocytes were isolated from BALB/c, C3H, and C57BL/6 mice; were treated with 160 mM NH4Cl (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) to lyse the erythrocytes; and subsequently washed three times with RPMI 1640.
Stimulator Preparation
Prior to MLC, adipogenic differentiated ASCs and undifferentiated ASCs were pretreated with 50 μg/ml mitomycin C (MMC) at 37°C for 3 hours. PBMCs and splenocytes were pretreated for 30 minutes with 50 μg/ml MMC at 37°C and then washed three times with RPMI 1640.
MLC
MMC-pretreated adipogenic differentiated or undifferentiated mMSCs (stimulators) were plated in 96-well plates (1 × 104 cells per well) and allowed to adhere to the plate for 2 hours. As a positive control, MMC-pretreated splenocytes (5 × 105 cells per well) were also added to the 96-well plates. Splenocytes (5 × 105 cells per well, responders) were then added to the wells containing the stimulators. After 2 days of incubation, the supernatants were collected and analyzed to determine the levels of the proinflammatory cytokines interferon γ (IFN-γ) and tumor necrosis factor α (TNF-α) through enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, http://www.rndsystems.com). The lymphocyte proliferation was analyzed using the remaining cells by 5-bromo-2′-deoxyuridine (BrdU) incorporation assay (BD Pharmingen). Briefly, the remaining cells were treated with BrdU for 20 hours, harvested, pooled, stained with anti-BrdU antibody, and analyzed by flow cytometry. MMC-pretreated undifferentiated hASCs, adipogenic differentiated hASCs, and hPBMCs (stimulators) were plated into 96-well plates (2 × 104 cells per well). hPBMCs (2 × 105 cells per well, responders) were then added to the wells containing the stimulators. The supernatants were collected and analyzed to determine the levels of IFN-γ after 3 days of incubation.
In Vivo Experiment
Implantation
Adipogenic differentiated ASCs (2 ×106 cells per head) from BALB/c mice were implanted into the subcutaneous region of the dorsum of 8-week-old BALB/c (group I, syngraft, n = 5) or C3H mice (group II, allograft, n = 5). Adipogenic differentiated ASCs from humans were also implanted into BALB/c mice (group III, xenograft, n = 5).
Biopsy
The mice were sacrificed 2 weeks after implantation, and the implants, including skin, were retrieved. The implants were imbedded in Tissue-Tek OCT compound, frozen, and cut into 5-μm-thick sections.
Histology and Immunofluorescence Analysis
The sections were fixed for 30 minutes in 3.7% formaldehyde. The sections were washed with distilled water and stained with Oil Red O reagent or hematoxylin and eosinto observe the newly formed adipose tissue at the implanted site. The fixed sections were washed three times with PBS and incubated with PBS containing 5% normal goat serum and 0.1% Triton X-100, and the sections were then incubated with rabbit polyclonal antibody to CD45 (1:50; Abcam, Cambridge, U.K., http://www.abcam.com) and human DNA-protein kinase catalytic subunit (DNA-PKcs) (1:100; Abcam) for 1 hour at 37°C. After washing three times, the sections were incubated with Alexa Fluor 488-conjugated goat anti-rabbit antibody (1:200; Molecular Probes, Eugene, OR, http://probes.invitrogen.com) for 30 minutes at room temperature. The sections were then mounted with mounting medium containing 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com) and were observed using a fluorescence microscope (Axiovert 200; Carl Zeiss, Oberkochen, Germany, http://www.zeiss.com).
Phase I Clinical Study to Evaluate the Safety of Adipogenic Differentiated ASCs
This study was an open-labeled phase I study to evaluate the safety of adipogenic differentiated ASCs (ClinicalTrials.gov identifier NCT01739530). The protocol was approved by the institutional review board of Samsung Medical Center (Seoul, Korea; 2009-01-054-002) and the Korea Food and Drug Administration. Written informed consent was obtained from all of the subjects before enrollment.
Preparation of Adipogenic Differentiated ASCs
The adipogenic differentiated ASCs for the clinical study were prepared using adipose tissue obtained from donors meeting strict selection criteria, including formal medical assessment, age younger than 40 years, body mass index more than 20, and negative testing for mandatory infectious disease markers. ASC isolation, expansion, and adipogenic differentiation were carried out using the same protocol that the one described for in preclinical study, but all manufacturing process were performed under good manufacturing practices authorized by the Korea Food and Drug Administration. The final product was suspended at a concentration of 3.8 × 107 cells per ml in DMEM and packaged in single-use vials based on the efficacy results of the previous clinical study using the autologous adipogenic differentiated ASCs [10]. For lot release testing, the cells were tested for quality control parameters, such as viability (≥ 80.0%), identification (observation of lipid droplets), purity (adipogenic differentiation rate ≥ 80.0%), potency (amount of Oil Red O eluted ≥200 μg/ml), and negative microbial contamination testing.
Enrollment and Cell Implantation
Five healthy men (mean age, 27.0 ± 3.4 years) were enrolled. The eligibility was determined by completing a health history form and physical examination. Each subject was subcutaneously administered 1 ml of adipogenic differentiated ASCs 2– cm from the center of the left axillary region using an 18–21-gauge blunt-end needle.
Follow-up Schedule
Postoperative follow-up examinations were performed on days 1 (after implantation), 7, 14, and 28 and at week 8 after implantation. At each visit, a photograph was taken, and any adverse events (AEs) were evaluated. Clinical laboratory testing was performed on days 1 and 7 and at week 8. A lymphocyte analysis and a comparison of the CD4/CD8 ratios were performed to evaluate the immunological response on day 7 and at week 8. On the final follow-up visit, the implant site was biopsied by incisional biopsy and analyzed for the infiltration of immune cells through histology based on five basic parameters: 0, no evidence of immune cell infiltration in all tissue areas; 1, slight immune cell infiltration visible in some of the tissue area (barely visible); 2, obvious immune cell infiltration visible in some of the tissue areas; 3, slight immune cell infiltration visible in all of the tissue areas; and 4 intense immune cell infiltration visible in all of the tissue areas.
Statistical Analysis
The data are presented as the means ± SD. The statistical analysis was performed using analysis of variance followed by Tukey’s test; a p value of less than 0.05 was considered to indicate statistical significance.
Results
Characterization of the Adipogenic Differentiation of ASCs
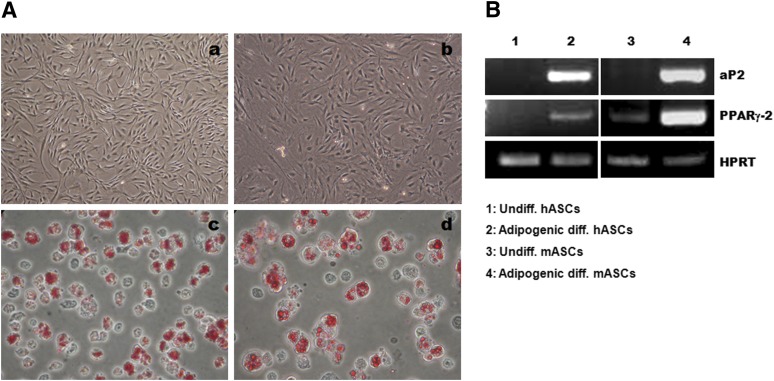
ASCs isolated from human and mouse adipose tissues were expanded up to passages 2–3 and differentiated into adipocytes. The undifferentiated ASCs exhibited an elongated fibroblast-like morphology. The adipogenic differentiated ASCs contained intracellular lipid droplets stained by Oil Red O and expressed the adipocyte-specific markers aP2 and PPARγ-2 (Fig. 1). The viability and differentiation rates of adipogenic differentiated ASCs were >90% and 80%, respectively.
Figure 1.
Identification of adipogenic differentiated ASCs. (A): Undifferentiated hASCs (Aa) and mASCs (Ab) showed a fibroblast-like morphology. Adipogenic differentiated hASCs (Ac) and mASCs (Ad) contained lipid droplets that stained positively for Oil Red O. (Aa, Ab): Magnification, ×40; (Ac, Ad): Magnification, ×400. (B): Adipogenic differentiated hASCs expressed the adipocyte-specific genes aP2 and PPARγ-2. Abbreviations: aP2, adipocyte protein 2; diff., differentiated; hASCs, human adipose-derived stem cells; HPRT, hypoxanthine guanine phosphoribosyl transferase; mASCs, mouse adipose-derived stem cells; PPARγ2, peroxisome proliferator-activated receptor γ2; Undiff., undifferentiated.
Immunophenotype of Adipogenic Differentiated hASCs
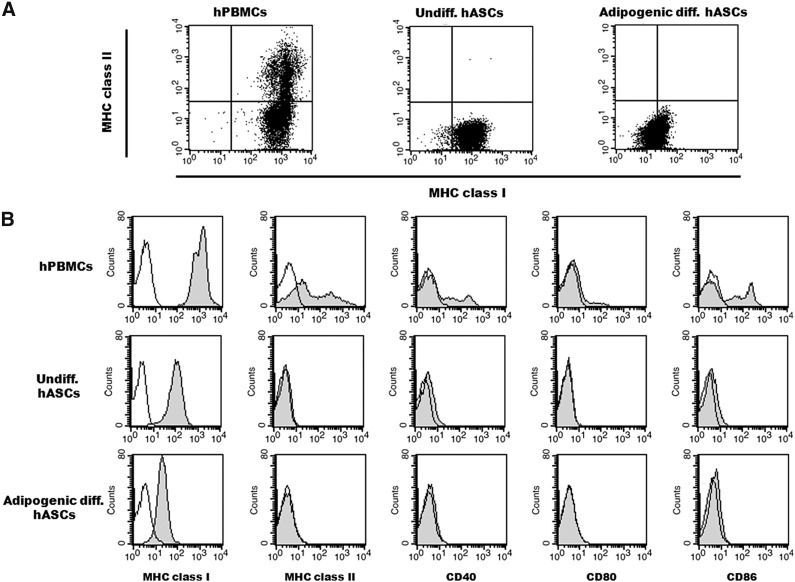
The adipogenic differentiated hASCs were evaluated for the expression of immunologically relevant cell surface molecules, such as MHC class I and II and costimulatory molecules, by flow cytometry. The expression pattern of MHC class II molecules in the adipogenic differentiated hASCs was similar to that of the undifferentiated hASCs that did not express MHC class II. The expression level of MHC class I was markedly reduced after the adipogenic differentiation of hASCs (Fig. 2A), which is consistent with the results of a previous study [13]. The adipogenic differentiated hASCs also did not express any of the costimulatory molecules, namely CD40, CD80, and CD86, which are required for the full activation of immune cells (Fig. 2B). The positive control hPBMCs highly expressed MHC class I and II molecules and costimulatory molecules. The immunophenotypes of the adipogenic differentiated mASCs were similar to those of the hASCs (data not shown). These results indicate that the nonimmunogenic features of ASCs may persist even after adipogenic differentiation.
Figure 2.
Flow cytometry of adipogenic differentiated hASCs. (A, B): Representative dot plot (A) and histogram (B) of hPBMCs and undifferentiated and adipogenic differentiated hASCs. Adipogenic differentiated and undifferentiated hASCs and hPBMCs were stained with antibodies against immunologically relevant cell surface proteins. Representative histograms are shown (gray), and the isotype controls are indicated (black). Abbreviations: diff., differentiated; hASCs, human adipose-derived stem cells; hPBMCs, human peripheral blood mononuclear cells; MHC, major histocompatibility complex; Undiff., undifferentiated.
Nonimmunogenicity of Adipogenic Differentiated ASCs
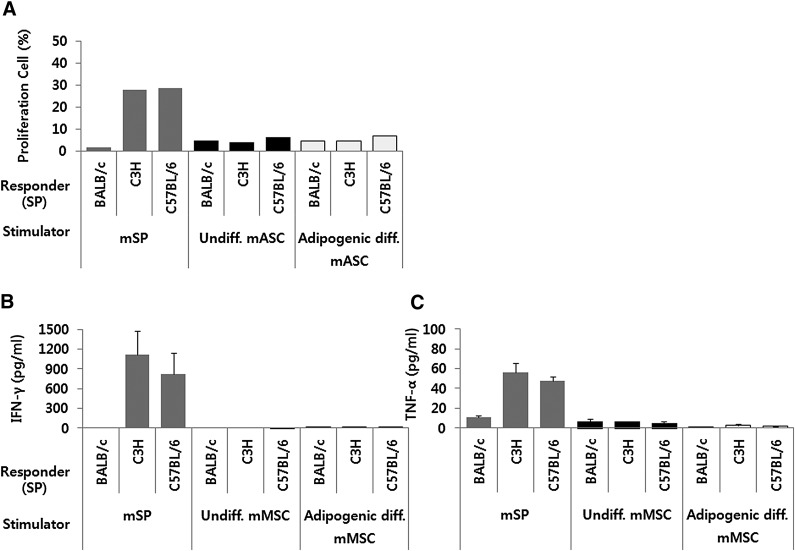
MLC was performed using mouse cells to determine whether adipogenic differentiated mASCs continue to exhibit no/low immunogenicity. MMC-pretreated adipogenic differentiated mASCs, undifferentiated mASCs, and splenocytes from BALB/c mice were cocultured with splenocytes from BALB/c (syngenic), C3H (allogenic), and C57BL/6 (allogenic) for 3 days. The immune response was evaluated based on the proliferation of splenocytes and the secretion of IFN-γ and TNF-α. As shown in Figure 3A, the proliferation of the responder splenocytes increased significantly when they were cocultured with allogenic splenocytes compared with syngenic splenocytes. In contrast, no significant proliferation of the responder splenocytes was observed when these cells were cocultured with either adipogenic differentiated mASCs or undifferentiated mASCs. No significant secretion of IFN-γ and TNF-α was observed in the coculture with allogenic undifferentiated or adipogenic differentiated mASCs (Fig. 3B, 3C).
Figure 3.
Adipogenic differentiated mASCs do not elicit a significant proliferative response and a significant secretion of IFN-γ and TNF-α when cocultured with allogenic splenocytes. Mitomycin C-pretreated adipogenic differentiated mASCs, undifferentiated mASCs, and mSPs were cocultured with splenocytes from BALB/c, C3H, and C57BL/6 for 2 days. (A): The proliferation was assessed with the remaining splenocytes by BrdU incorporation. (B, C): The IFN-γ (B) and TNF-α (C) production was determined through the analysis of the culture supernatants by enzyme-linked immunosorbent assay. The results are presented as the means ± SD of one proliferation assay and a selected set of four IFN-γ and TNF-α production assays. Each experiment was performed in duplicate. Abbreviations: diff., differentiated; IFN-γ, interferon γ; mASC, mouse adipose-derived stem cell; mMSC, mouse mesenchymal stem cell; mSP, splenocytes from BALB/c; SP, splenocyte; TNF-α, tumor necrosis factor α; Undiff., undifferentiated.
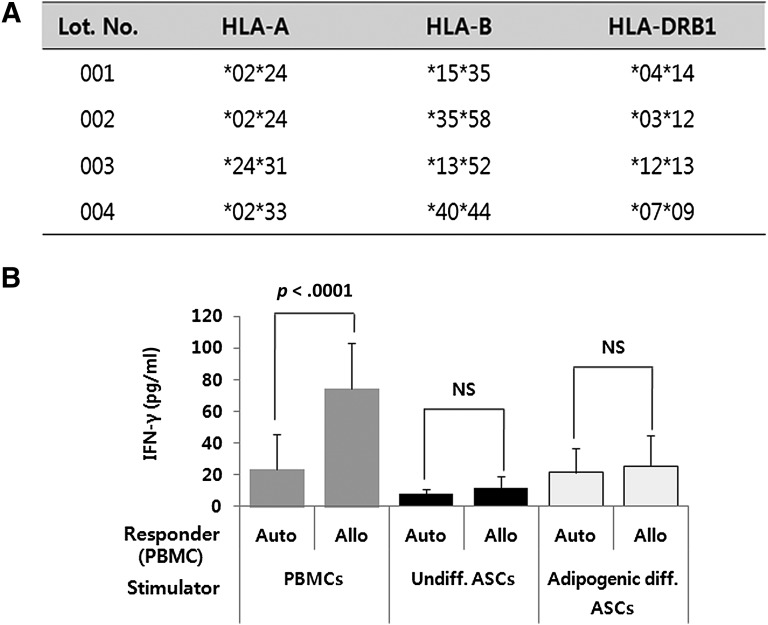
We also determined whether adipogenic differentiated hASCs can induce an immune response. HLA typing was performed using the PCR-based line-strip SSO hybridization method prior to MLC to confirm the allogenicity of each sample. The HLA-A, -B, and -DRB1 alleles are shown in Figure 4A. Two of the four samples had the same type of HLA-A. The other alleles were mismatched with one another. Consistent with the results obtained with the mASCs, adipogenic differentiated hASCs did not elicit the secretion of IFN-γ when they were cocultured with MHC-mismatched allogenic hPBMCs. In contrast, PBMCs induced a vigorous secretion of IFN-γ when cocultured with allogenic PBMCs, as expected (p < .0001) (Fig. 4B). These results demonstrate that adipogenic differentiated hASCs are immunogenically similar to undifferentiated hASCs.
Figure 4.
Adipogenic differentiated human ASCs (hASCs) do not elicit a significant secretion of IFN-γ when cocultured with allogenic human PBMCs (hPBMCs). (A): HLA typing was performed using the polymerase chain reaction-based line-strip specific oligonucleotide hybridization method. (B): Mitomycin C-pretreated adipogenic differentiated hASCs, undifferentiated hASCs, and hPBMCs from four different donors were cocultured with autologous or major histocompatibility complex-mismatched allogenic hPBMCs. The IFN-γ secretion was measured by enzyme-linked immunosorbent assay after 3 days. The experiment was conducted in duplicate, and the results are expressed as the means ± SD of 4 autologous reaction experiments and 12 allogenic reaction experiments. Abbreviations: Allo, allogenic; ASCs, adipose-derived stem cells; Auto, autologous; diff., differentiated; HLA, human leukocyte antigen; IFN-γ, interferon γ; NS, not significant; PBMCs, peripheral blood mononuclear cells; Undiff., undifferentiated.
New Adipose Tissue Formation From Adipogenic Differentiated ASCs In Vivo
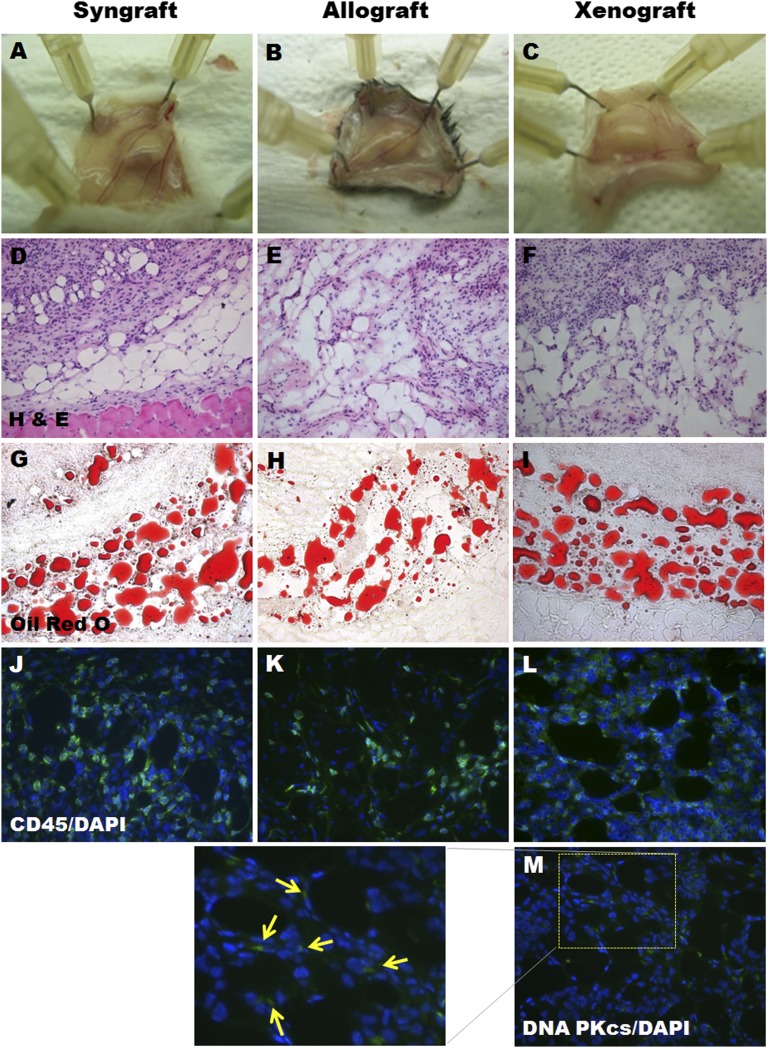
Adipogenic differentiated mASCs from BALB/c mice (2 × 106 cells per head) were implanted into the subcutaneous regions of BALB/c (syngraft) or C3H (allograft) mice to investigate whether adipogenic differentiated mASCs can generate new adipose tissue without immune rejection in allogenic recipients. Adipogenic differentiated hASCs (2 × 106 cells per head) were also implanted into BALB/c mice for the xenograft model. The mice were sacrificed 2 weeks after implantation, and the implantation sites were retrieved. The implants remained until they were harvested from all of the groups, and there were no significant differences in the dimensions between the experimental groups (Fig. 5A–5C). The histological analysis revealed typical adipose tissue in the syngenic (4 of 5 cases), allogenic (5 of 5 cases), and xenogenic (4 of 5 cases) recipients (Fig. 5D–5I). In the xenograft group, the newly formed tissue was also stained positively for anti-human DNA PKcs antibody, which reacts only with the human cell nucleus (Fig. 5M). This finding indicates that this tissue originated from the implanted adipogenic differentiated cells from humans. We then determined whether the newly formed adipose tissue was infiltrated with immune cells using a CD45 antibody. As shown in Figure 5J–5L, all of the sections were positively stained with CD45. However, no significant difference was observed between the syngraft, allograft, and xenograft models.
Figure 5.
Adipogenic differentiated adipose-derived stem cells (ASCs) formed new adipose tissue without immune rejection in the subcutaneous region of syngenic, allogenic, and xenogenic recipients. Adipogenic differentiated mouse ASCs from BALB/c mice were implanted subcutaneously into BALB/c (n = 5) or C3H mice (n = 5). Adipogenic differentiated human ASCs were implanted into the subcutaneous region of BALB/c mice (n = 5). The implants were harvested and analyzed 14 days after implantation. (A–C): Macroscopic appearance of the implants after harvesting. (D–M): The implants were sectioned and stained with H&E (D–F), Oil Red O (G–I), anti-CD45 (J–L), and anti-DNA PKcs (arrows in [M]). (A–I): Magnification, ×200. (J–M): Magnification, ×400. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; DNA-PKcs, DNA-protein kinase catalytic subunit; H&E, hematoxylin and eosin.
Adipogenic Differentiated ASCs Did Not Induce Immune Rejection in Allogenic Recipients
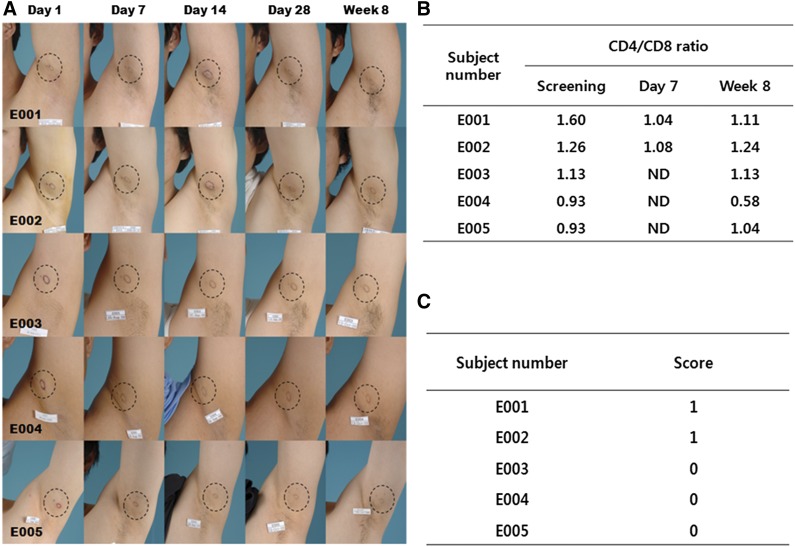
The phase I clinical study was conducted to evaluate the safety of the allogenic application of adipogenic differentiated ASCs. The five healthy men (mean age, 27.0 ± 3.4 years) enrolled in this study were implanted with 1 ml of 3.8 × 107 cells per ml of adipogenic differentiated hASCs obtained from an unrelated healthy donor. The cell viability of adipogenic differentiated ASCs was greater than 90%. The differentiation rate and the quantity of Oil Red O eluted were 96.3 ± 0.8% and 297.7 ± 79.8 μg/ml, respectively. No contamination with bacteria, fungi, mycoplasma, or adventitious viruses was detected in the final product. All of the subjects completed the clinical study (Fig. 6A). No serious AEs were reported during the study. Throughout the follow-up period, a total of 10 AEs of severity 1 were reported by four of the subjects; three of these AEs resulted from biopsy incision and were thus judged to be unrelated to the investigational drug, whereas the observed redness (one case), hardness (two cases), and itching (three cases) were judged to be possibly related to the implanted cells. However, these AEs were resolved within the study period without additional treatment and were thus not considered evidence of immune rejection. The immunological tests performed on blood samples to evaluate the CD4/CD8 ratio showed no clinically significant changes (Fig. 6B). The histological examination of the implantation site revealed a slight infiltration of score 1 in two of the subjects (Fig. 6C). The routine laboratory results, physical examinations, and vital signs showed no clinically significant abnormalities.
Figure 6.
Adipogenic differentiated human adipose-derived stem cells (hASCs) do not elicit an alloreactive immune response in the subcutaneous regions of different recipients. Adipogenic differentiated hASCs (3 × 107 cells per ml) from healthy donors were implanted subcutaneously into the axillary region of five different healthy subjects. (A): On days 1 (after implantation), 7, 14, and 28 and at week 8, the implantation sites were photographed and assessed to determine the local immune response. (B): On screening, on day 7, and at week 8, CD4/CD8 ratios were assessed for immunological response. (C): At week 8, the implantation site was biopsied and analyzed for infiltration of immune cells by histology based on five basic parameters.
Discussion
Based on our previous study [10], which demonstrated the efficacy and safety of autologous adipogenic differentiated ASCs implanted subcutaneously into soft-tissue defects, the present study was designed to evaluate the safety and feasibility of “allogenic” differentiated adipocytes from ASCs for soft-tissue regeneration. The results showed that (a) adipogenic differentiated ASCs did not express MHC class II and costimulatory molecules and that the expression levels of MHC class I decreased after differentiation, (b) these cells did not elicit an immune response against MHC-mismatched lymphocytes, (c) these cells formed new adipose tissue without immune rejection in the subcutaneous region of MHC-mismatched mice, and (d) these cells did not induce clinically significant local and systemic immune responses or AEs in the subcutaneous region of donor-independent healthy subjects. We used >80% differentiated cells as assessed by Oil Red O staining in all of the experiments to exclude the influence of undifferentiated ASCs on the results.
The immunogenicity of allogenic implanted cells is determined primarily by the presence of antigen presenting cells (APCs) within the implanted cell population. APCs express MHC class I and II molecules in addition to costimulatory molecules, such as CD80 and CD86 [23]. We observed that the adipogenic differentiation of ASCs did not result in the expression of MHC class II molecules and costimulatory molecules, similar to that observed in undifferentiated ASCs. Interestingly, the expression level of MHC class I decreased markedly in the differentiated cells compared with undifferentiated ASCs. These results are consistent with those from a previous study on BM-MSCs, which showed that adipogenic differentiation did not increase the levels of immunologically relevant cell surface molecules [13]. These results suggest that adipogenic differentiated ASCs do not act as APCs; thus, these cells may not be recognized by immune cells, even in MHC-mismatched allogenic recipients, and can therefore escape immune rejection. Adipogenic differentiated ASCs, similarly to undifferentiated ASCs, did not lead to any significant immune response against allogenic lymphocytes (Figs. 3 and 4), which confirms this hypothesis.
It should be recognized that even intrinsically nonimmunogenic BM-MSCs may induce a memory T-cell response under certain conditions [24]. The immune response can usually be accelerated after immunological memory [25]. This sensitization is important for clinical applications because it may lead to active immune responses when cells with a low threshold of immunogenicity are repeatedly implanted in the desired site. Thus, we conducted a two-step MLC to determine whether adipogenic differentiated ASCs can cause immune sensitization but did not detect any sensitization in vitro or in vivo in the secondary MLC using adipogenic differentiated ASCs, which supports the hypothesis that these cells may be completely nonimmunogenic (data not shown).
Although the lack of an active immune response to allogenic differentiated ASCs is certainly a positive sign for the survival of these implanted cells, all of these observations are based on in vitro experimentation, which cannot completely guarantee the survival of these cells in the host tissue because of the complexity of the immune system in vivo. Osteogenic differentiated BM-MSCs and ASCs are not immunogenic in vitro [26] but fail to engraft in vivo [27]. Our results showed that adipogenic differentiated ASCs successfully survived and developed into adipose tissue without immune rejection in the subcutaneous region of both allogenic and syngenic recipients. Additionally, these cells also formed new adipose tissue even in xenogenic recipients (human cells to mice) without any remarkable events. We confirmed that these newly formed adipose tissues originated from the implanted cells using an antibody specific for the human nucleus.
To the best of our knowledge, this is the first report of a clinical trial using adipogenic differentiated ASCs for allogenic cell therapy. Although the present study has some limitations, including its small scale and the lack of a comparison group, our findings indicate that adipogenic differentiated ASCs are safe in donor-independent allogenic recipients. A finding of this study was the mild degree of some events related to symptoms of immune responses, including redness, hardness, and itching at the implant site. However, these events can also result from most dermal fillers, including autologous fat, during the implantation process and can be prevented through the use of proper implantation techniques [28]. Based on our preclinical study (Fig. 5J–5O), which showed immune cell infiltration in syngrafts, allografts, and xenografts, the results also showed that these slight infiltrations in the implant sites were not alloreactive responses, and the degrees of infiltration were similar. Nevertheless, the possibility that such events are an alloreactive immune response was not definitively excluded. Further comparative clinical studies will be required to clarify the reasons for this observation.
Autologous adipose tissue is prepared for implantation using aspiration and centrifugation and contains a large number of ruptured and nonviable cells, together with a large proportion of other cells, such as erythrocytes and fibrotic tissue. In addition, mature adipocytes are susceptible to nutrient supply deficiencies and mechanical irritation [29]. Therefore, implanted autologous adipose tissue is absorbed within 3 months of implantation, and only 25%–50% of the adipose tissue takes. On the other hand, adipogenic differentiated ASCs are healthy (more than 90% are alive), pure, and immature adipocytes with diameters of 20–40 μm. After implantation of the cells, the diameter gradually increases to 100 μm or greater as they mature. As a result, they are able to fill the depressed volume. Moreover, adipogenic differentiated ASCs are more durable and tolerant to ischemia than mature adipocyte [30]. These properties of adipogenic differentiated ASCs are valuable because they survive during the time when the grafts are not sufficiently vascularized. According to our previous study, when autologous adipogenic differentiated ASCs were implanted into depressed scars, the scars gradually decreased in volume until 4 weeks after implantation and then stabilized. They maintained well during the long-term follow-up of more than 1 year. We thus presume that the allogenic implantation of adipogenic differentiated ASCs will also show this effect, along with autologous implantation. A future phase II clinical study to determine the efficacy and safety of the allogenic implantation of adipogenic differentiated ASCs in patients with depressed scars will be performed.
Conclusion
We demonstrated that adipogenic differentiated ASCs maintain nonimmunogenicity and are clinically safe and feasible in HLA-mismatched allogenic recipients. Further large-scale clinical trials are required to demonstrate the efficacy and clinical benefit of the allogenic administration of adipogenic differentiated ASCs.
Acknowledgments
This work was supported by a research grant from the Korea Health 21 R&D Project, the Ministry of Health Welfare and Family Affairs of the Republic of Korea (A050569), and a National Research Foundation of Korea grant funded by the Korean government (2012R1A2A1A0300692).
Author Contributions
I.K. and S.I.B.: conception and design, experiments, collection and assembly of data, data analysis and interpretation, manuscript writing; S.K.L.: conception and design, administrative support; S.Y.P.: experiments, collection and assembly of data; M.K. and H.H.: conception and design, manuscript writing, final approval of the manuscript.
Disclosure of Potential Conflicts of Interest
I.K., M.K., and S.K.L. have uncompensated employment. S.Y.P. has compensated employment from Anterogen Co., Ltd.
References
- 1.Peer LA. The neglected free fat graft, its behavior and clinical use. Am J Surg. 1956;92:40–47. doi: 10.1016/s0002-9610(56)80009-3. [DOI] [PubMed] [Google Scholar]
- 2.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for facial lipoatrophy: Efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008;34:1178–1185. doi: 10.1111/j.1524-4725.2008.34256.x. [DOI] [PubMed] [Google Scholar]
- 3.Brayfield CA, Marra KG, Rubin JP. Adipose tissue regeneration. Curr Stem Cell Res Ther. 2010;5:116–121. doi: 10.2174/157488810791268582. [DOI] [PubMed] [Google Scholar]
- 4.Cho SW, Kim SS, Rhie JW, et al. Engineering of volume-stable adipose tissues. Biomaterials. 2005;26:3577–3585. doi: 10.1016/j.biomaterials.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Hemmrich K, von Heimburg D, Rendchen R, et al. Implantation of preadipocyte-loaded hyaluronic acid-based scaffolds into nude mice to evaluate potential for soft tissue engineering. Biomaterials. 2005;26:7025–7037. doi: 10.1016/j.biomaterials.2005.04.065. [DOI] [PubMed] [Google Scholar]
- 6.Kawaguchi N, Toriyama K, Nicodemou-Lena E, et al. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc Natl Acad Sci USA. 1998;95:1062–1066. doi: 10.1073/pnas.95.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torio-Padron N, Baerlecken N, Momeni A, et al. Engineering of adipose tissue by injection of human preadipocytes in fibrin. Aesthetic Plast Surg. 2007;31:285–293. doi: 10.1007/s00266-006-0221-6. [DOI] [PubMed] [Google Scholar]
- 8.Rophael JA, Craft RO, Palmer JA, et al. Angiogenic growth factor synergism in a murine tissue engineering model of angiogenesis and adipogenesis. Am J Pathol. 2007;171:2048–2057. doi: 10.2353/ajpath.2007.070066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho SW, Kim I, Kim SH, et al. Enhancement of adipose tissue formation by implantation of adipogenic-differentiated preadipocytes. Biochem Biophys Res Commun. 2006;345:588–594. doi: 10.1016/j.bbrc.2006.04.089. [DOI] [PubMed] [Google Scholar]
- 10.Kim M, Kim I, Lee SK, et al. Clinical trial of autologous differentiated adipocytes from stem cells derived from human adipose tissue. Dermatol Surg. 2011;37:750–759. doi: 10.1111/j.1524-4725.2011.01765.x. [DOI] [PubMed] [Google Scholar]
- 11.Jeong SH, Han SK, Kim WK. Soft tissue augmentation using in vitro differentiated adipocytes: A clinical pilot study. Dermatol Surg. 2011;37:760–767. doi: 10.1111/j.1524-4725.2011.01950..x. [DOI] [PubMed] [Google Scholar]
- 12.Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 13.Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 14.McIntosh K, Zvonic S, Garrett S, et al. The immunogenicity of human adipose-derived cells: Temporal changes in vitro. Stem Cells. 2006;24:1246–1253. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- 15.Cui L, Yin S, Liu W, et al. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng. 2007;13:1185–1195. doi: 10.1089/ten.2006.0315. [DOI] [PubMed] [Google Scholar]
- 16.DelaRosa O, Lombardo E, Beraza A, et al. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng Part A. 2009;15:2795–2806. doi: 10.1089/ten.TEA.2008.0630. [DOI] [PubMed] [Google Scholar]
- 17.Cho YB, Lee WY, Park KJ, et al. Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: A phase I clinical study. Cell Transplant. 2013;22:279–285. doi: 10.3727/096368912X656045. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: A phase II clinical trial. Dis Colon Rectum. 2009;52:79–86. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- 19.García-Olmo D, García-Arranz M, Herreros D, et al. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–1423. doi: 10.1007/s10350-005-0052-6. [DOI] [PubMed] [Google Scholar]
- 20.Prasad VK, Lucas KG, Kleiner GI, et al. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal™) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant. 2011;17:534–541. doi: 10.1016/j.bbmt.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de la Portilla F, Alba F, García-Olmo D, et al. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: Results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28:313–323. doi: 10.1007/s00384-012-1581-9. [DOI] [PubMed] [Google Scholar]
- 23.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 24.Nauta AJ, Westerhuis G, Kruisselbrink AB, et al. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valujskikh A, Li XC. Frontiers in nephrology: T cell memory as a barrier to transplant tolerance. J Am Soc Nephrol. 2007;18:2252–2261. doi: 10.1681/ASN.2007020151. [DOI] [PubMed] [Google Scholar]
- 26.Niemeyer P, Kornacker M, Mehlhorn A, et al. Comparison of immunological properties of bone marrow stromal cells and adipose tissue-derived stem cells before and after osteogenic differentiation in vitro. Tissue Eng. 2007;13:111–121. doi: 10.1089/ten.2006.0114. [DOI] [PubMed] [Google Scholar]
- 27.Niemeyer P, Vohrer J, Schmal H, et al. Survival of human mesenchymal stromal cells from bone marrow and adipose tissue after xenogenic transplantation in immunocompetent mice. Cytotherapy. 2008;10:784–795. doi: 10.1080/14653240802419302. [DOI] [PubMed] [Google Scholar]
- 28.Lemperle G, Rullan PP, Gauthier-Hazan N. Avoiding and treating dermal filler complications. Plast Reconstr Surg. 2006;118(suppl):92S–107S. doi: 10.1097/01.prs.0000234672.69287.77. [DOI] [PubMed] [Google Scholar]
- 29.Katz AJ, Llull R, Hedrick MH, et al. Emerging approaches to the tissue engineering of fat. Clin Plast Surg. 1999;26:587–603, viii. [PubMed] [Google Scholar]
- 30.von Heimburg D, Hemmrich K, Zachariah S, et al. Oxygen consumption in undifferentiated versus differentiated adipogenic mesenchymal precursor cells. Respir Physiol Neurobiol. 2005;146:107–116. doi: 10.1016/j.resp.2004.12.013. [DOI] [PubMed] [Google Scholar]