This review examines clinical applications of animal substance-free human embryonic stem cells. Following studies on factors regulating pluripotency and differentiation, there are now techniques to establish and effectively expand these cells in animal substance-free conditions, even from single cells biopsied from eight-cell stage embryos in chemically defined feeder-free cultures. Early clinical trials are ongoing.
Keywords: Clinical translation, Embryonic stem cells, Pluripotent stem cells, Serum-free, Stem cell culture
Abstract
Human embryonic stem cells have been considered the gold standard as a cell source for regenerative medicine since they were first cultured in 1998. They are pluripotent and can form principally all the cells types in the body. They are obtained from supernumerary human in vitro fertilization embryos that cannot be used for infertility treatment. Following studies on factors regulating pluripotency and differentiation, we now have techniques to establish and effectively expand these cells in animal substance-free conditions, even from single cells biopsied from eight-cell stage embryos in chemically defined feeder-free cultures. The genetic stability and absence of tumorigenic mutations can be determined. There are satisfactory animal tests for functionality and safety. The first clinical trials are ongoing for two indications: age-related macular degeneration and spinal cord injury.
Derivation of Human Embryonic Stem Cell Lines
The first human embryonic stem cell (hESC) lines were established similarly to mouse embryonic stem cells, that is, on murine embryonic fibroblasts as feeder cells using a fetal bovine serum-containing culture medium [1–4]. In our laboratory, we initiated the use of human neonatal fibroblasts as feeder cells in derivation of new hESC lines [5] and then serum replacement (SR) instead of bovine serum to develop a better defined culture medium [6]. Subsequently, several hESC lines were established using SR media [7]. Although human feeder cells do not provide a chemically defined culture system, their use has been adopted worldwide. We also gave up the originally used immunosurgery methods using a mouse antibody against human trophectoderm and guinea pig complement in the isolation of the inner cell mass (ICM) and started to use mechanical isolation of the ICM instead [8].
Conditioned medium from feeder cells was then used to avoid two different cell populations in the cultures [9, 10]. The most commonly used culture substrate was previously Matrigel, a protein extract from whole mouse EHS sarcoma tumor tissue [11]. It is a very complex mixture of basement membrane components such as type IV collagen, laminin-111, and perlecan, as well as multiple other matrix and cellular proteins and growth factors. Although Matrigel has extensive batch-to-batch variation and is far from being a defined matrix, it has been widely used, and together with conditioned medium from MEFs it became a kind of standard matrix in hESC research for a while. The International Stem Cell Initiative performed studies aiming at more standardized cultures using chemically defined media [12].
Feeder-free derivation of hESC lines proved challenging, and all the clinical-grade hESC lines until this year had been first established on feeder cells and then transferred to feeder-free culture [13]. Animal substrate-free human feeder cells were used by us and other groups [14], but such cultures were not chemically defined. An earlier chemically defined derivation [15] did not result in genetically stable hESCs. Clinical-grade hESC lines have earlier been established, in both animal substance-containing conditions [16] and xeno-free conditions using clinical-grade human feeder cells [17].
However, a real step forward came with a specific cell culture coating of human recombinant laminin (LN-511) [18] that had been originally identified in the early embryo [19] and synthetized by Karl Tryggvason’s team [20]. The cultures were now chemically defined, because we used a xeno-free chemically defined medium, partially manufactured in our laboratories. Important from a practical standpoint was that the cells could be cultured as monolayers and plated from single-cell suspensions (Fig. 1). At the same time, two other feeder-free defined culture systems were published in the same journal, but we are not aware of any wider use of those systems [21, 22].
Figure 1.
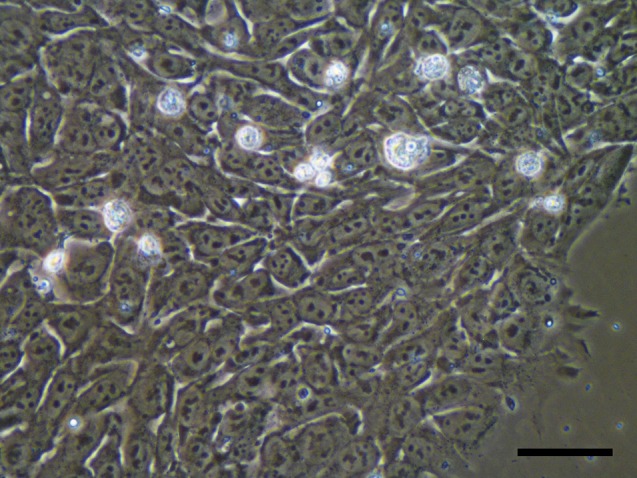
A colony of the human embryonic stem cell line HS983 growing on human LN-521 surface as single cell layer. This cell line was derived from a single biopsied cell of an 8-cell embryo 3 days after in vitro fertilization. It was donated for stem cell derivation after informed consent and approval of the regional ethics board in Stockholm. Scale bar = 50 mm.
Following successful synthesis of another human laminin, LN-521, an adhesion protein also present in the ICM and other in vivo stem cell niches such as the hair follicle and small intestine crypts [23], we developed yet another robust chemically defined, xeno-free cell culture matrix that allows culturing of highly stable hESC cultures [4]. Importantly, we can derive new hESC lines from a single blastomere biopsied from an eight-cell in vitro fertilization (IVF) embryo by culturing it on a mixture of LN-521 and E-cadherin, which provides a cell-cell contact inducing signal. This is important from an ethical standpoint because the procedure does require destruction of the IVF embryo. The blastomere biopsy is similar to that normally carried out to obtain a single cell for preimplantation genetic diagnostics (PGD). In our IVF unit, we regularly get pregnancies and infants from such embryos, from which one cell has been removed. The pregnancy rate per transfer of a single blastocyst after removal of a single cell for PGD, has been in our clinic 39% in 2013 (41 embryo transfers) and 43% in January–February 2014 (29 transfers). In our regular IVF program, the pregnancy rate per transferred blastocyst was 35% in 2013 and 38% (1,066 embryo transfers) and in January–February 2014 it was 38% (142 embryo transfers). The slightly higher pregnancy rates after the PGD-biopsied blastocysts can probably be explained by the fact that these women are not infertile. We can cryopreserve the biopsied embryos or transfer them to the woman, if she wishes it, to get a family-specific or child-specific hESC line. Using this new hESC derivation procedure, we can derive new hESC lines with an as high efficacy as 60% per ICM of donated blastocysts [4]. The splitting ratio of the hESC in these cultures is 1:30 instead of 1:3 in the conventional cell clump cultures. This means that we can obtain large numbers of hESCs for regenerative medicine with fewer passages, which is faster and safer. hESC lines have been established in coculture of existing hESC, but our system is the first totally defined one [24].
Because we need not destroy the original embryo, hESC lines established using this method should be acceptable for those who see ethical problems in destroying supernumerary preimplantation embryos for hESC derivation from the ICM. If the line is family-specific, the need for immunosuppression in regenerative medicine is much smaller than when using completely allogenic hESC lines. Another important advantage with this new cell derivation method is that it enables the establishment of a hESC bank of ~150 haplotypes (or even more), which is the calculated number enabling lower doses of immunosuppression [25]. Such a hESC bank would make it possible to generate various differentiated cell type lines for cell therapy purposes. One option to establish patient-specific hESC lines is to make them from parthenotes. This option is feasible for women who still produce oocytes [26].
There are currently research-grade hESC lines in registries and banks, such as the U.K. stem cell bank and the NIH stem cell registry. They are available for researchers from the stem cell banks and laboratories [27]. Our hESC lines are also available for research.
Advantages of Using hESCs in Regenerative Medicine
Pluripotent stem cells, either hESCs or induced pluripotent stem cells (iPSCs) have the advantage that they can be differentiated to almost any cell type. In contrast, adult stem cells are limited to only some tissue types. Mesenchymal stem cells from bone marrow or adipose tissue are useful in treating graft versus host reaction after blood stem cell transplantation, and they also form bone, connective tissue, and adipose tissue cells. However, their expansion potential is significantly more limited than that of pluripotent stem cells. Blood stem cells can form all different cells of the blood, and they have been used for over 40 years in renewing the blood cell formation. For other regenerative purposes, the cells and tissues can be differentiated from pluripotent stem cells. hESCs are genetically more stable than iPSCs, and so far the most well-known source for therapies.
An advantage with embryonic stem cells is that they normally exist in the human embryo for 3–6 days after fertilization. Using the specific culture condition described above, they can maintain their nondifferentiated state for long periods of time and then differentiate to the opted cell and tissue type using particular differentiation protocols. This makes them excellent for various regenerative medicine purposes for replacing injured and dead cells in tissues. They are always allogenic cells for the recipient, because each hESC line has its own genome. There is an exception if the hESC line is derived from a biopsied cell of an embryo that was then transferred to a woman’s uterus to give origin to a new individual. Then the genome is identical to that of that particular individual, and no immunological reaction will develop. If the hESC line is made of a sibling embryo, the immunogenicity will be lower than when using unrelated cells. If family-specific lines are not available, the immunologically best-matching hESC line could be obtained from a hESC bank with cells representing most human leukocyte antigen histocompatibility classes [28].
The pluripotent stem cells differ from cord blood cells in being capable of dividing without limits. Large amounts of cells for several recipients and treatments can be obtained, and all human cell types can be produced. Cord blood stem cells and mesenchymal stem cells have more limited differentiation and expansion potentials. For the time being, it has not been possible to expand fully functional cord blood stem cells.
iPSCs generated by incorporation of certain transcription factor genes into the genome of somatic cells are likely to be less immunogenic to the original host than other foreign cells, but they can also be immunogenic to the previous host [29]. More problematic is that the transcription factor genes that have been transferred to their genome have caused genomic changes that may make the cells tumorigenic. Because of genetic instability, human iPSCs are not considered as safe as hESCs [30].
Characterization of hESCs
Cultured hESCs should be characterized to determine their real potential for being used in clinical applications. It is important that hESCs destined for differentiation protocols and subsequent transplantation into patients have been rigorously characterized for normal phenotype prior to differentiation and transplantation procedures. Although numerous features of the hESCs have been identified, we focus here on a minimal set of properties that concern their differentiation potential and safety. Thus, hESCs expressing pluripotency markers Oct-4, Nanog, Sox-2, and SSEA-4 need to be genetically normal, and they should differentiate into all three germ layers of the human embryo in in vitro and in vivo assays.
Expression of pluripotency markers can be determined using three complementary methods: (a) Immunostaining of fixed hESCs reveals expression of the markers and allows estimation of their homogeneity in monolayer culture (Fig. 2). The method is quick and easy, but it is qualitative and requires a pluripotency marker negative culture for subtracting the background signal. Because partially differentiated cultures and even embryoid bodies continue expressing certain amounts of pluripotency markers for long periods of time [31], it is important to use quantitative methods to confirm stable and high expression of the markers. (b) Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis allows reliable quantitation of expression levels. Analysis of cells taken at different time points of the experiment can determine similar expression levels of the pluripotency markers. Also, expression of differentiation markers can be compared to prove their stable and low expression levels [18]. The markers should represent all three germline lineages of the human embryo and especially markers of neuro-ectoderm because it is the major differentiation path of hESCs [32]. Although qRT-PCR is powerful and informative, it reveals the phenotype of the whole hESC culture showing average expression levels and lacking information on homogeneity of the cellular population. (c) Fluorescence-activated cell sorting (FACS) complements qRT-PCR and provides information on different cellular populations in hESC cultures. Apart from membrane pluripotency marker SSEA-4, FACS analysis can be also done with Oct-4 or Nanog, because they are the most specific markers of pluripotent cells [33].
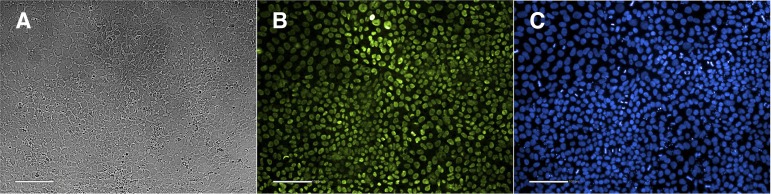
Figure 2.
Images of human embryonic stem (hES) cells growing on LN-521. (A): Brightfield image of hES cells cultured on LN-521. (B): Immunostaining of hES cells grown on LN-521 for Oct-4, a marker of pluripotency (green). (C): 4′,6-Diamidino-2-phenylindole staining shown in blue. Scale bars = 100 μm.
hESCs commonly undergo adaptive genetic changes during prolonged culturing in vitro [34]. Although this has been regarded as a feature of hESCs themselves [35], cells with genetic abnormalities could be dangerous and should not be used in patients. Karyotyping of cultured hESCs aimed for cell therapy procedures should be performed after every 10th passage (2 months) in vitro. Recently, it has been shown that karyotyping does not reveal high enough resolution to detect all potentially tumorigenic genetic aberrations. Indeed, culture adaptation may have occurred in such a small area that it cannot be detected by karyotyping leading to multiplication of locus BCL2L1, which is an antiapoptotic factor, has been reported in many different hESC lines [35]. Therefore, a method with a resolution of at least 50 kb or higher should be used to confirm safety of the cells before use for cell transplantation purposes into patients. Genotyping of cultured hESCs using genome-wide single nucleotide polymorphism (SNP) arrays with analysis of copy-number variations and copy-number neutral loss-of-heterozygosity regions provides sufficient resolution and is now widely used to confirm genetic integrity of cultured hESCs. The method cannot fully replace karyotyping, because it cannot detect balanced translocations and inversions and has some other limitations.
The experiments described above reliably define hESCs and their quality for therapeutic purposes, but some additional characterization techniques can be used if needed. Thus, expression of other pluripotency markers, such as TRA-1-60, TRA-1-81, etc. [36], can be assessed using the methods described above. Alkaline phosphatase staining can also be used to define the hESC status. Immunoblots can provide semiquantitative information on expression of markers of pluripotency on the protein level [36]. Transcriptomes and proteomes of cultured cells can be compared with control hESC cells using RNA arrays and mass spectrometry, respectively, to detect multiple markers of pluripotency and differentiation simultaneously. Genetic stability has been successfully studied using comparative genomic hybridization (CGH) arrays [37, 38]. Fluorescent in situ hybridization can be used to detect aneuploidies in the cells and provide further information on genetic integrity of hESCs. Because hESCs have peculiar chromatic organization and pattern of histone modifications in the vicinity of Oct-4 and Nanog promoters and some other genetic loci [39], chromatin immunoprecipitation followed by high-throughput DNA sequencing (ChIP-Seq) may be also used for hESC characterization.
Although successful instrumental characterization of cultured hESCs is important, alone it is not sufficient to prove pluripotency of the cells. Differentiation into cells of all three germ layers of the human embryo in in vitro and in vivo arrays must be demonstrated to prove that. Generation of embryoid bodies (EBs) is a standard in vitro assay for spontaneous differentiation of hESCs into all three germ layers [31]. To generate them, hESCs are cultured in solution on nonadherent dishes [18] or in hanging drops [40] in a medium that does not contain any pluripotency promoting factors, such as basic fibroblast growth factor. Two to three weeks later, the cells are analyzed by RT-PCR [31], by immunostaining [18], and by flow cytometry to detect expression of markers of all three germ layers. One week prior the analysis, the EBs are plated on gelatin-coated slides or cell culture plates. Additionally, direct differentiation of hESCs into various cellular lineages without EB stage is useful to further prove pluripotency. Direct differentiation of hESC is needed to establish populations that can be used in cell transplantation for various indications. At the same time, directed differentiation to components of the three germ layers can be used as a characterization method to demonstrate the capacity of each cell line to form various tissues. For ectoderm differentiation, there are simple neural differentiation protocols [41]. For endodermal differentiation, directed hepatocyte differentiation is often used [42], and for mesoderm differentiation, cartilage differentiation is often used [43]. For in vivo pluripotency testing, the cells can be injected to immunodeficient mice in a similar manner as for the exclusion of tumorigenic cells (described in Exclusion of Tumorigenic Cells).
Exclusion of Tumorigenic Cells
The safety of transplantation of cells differentiated from pluripotent hESCs is still a significant concern, and certain gene expression networks are conserved between cancers and pluripotent stem cells [34, 44]. Both cancer cells and pluripotent stem cells have extensive proliferation capacity. Pluripotent stem cells may grow as teratoma-like tumors, a property that we use in the characterization of their pluripotency in vivo. This means that a cell population aimed for transplantation in regenerative medicine has to be extremely well differentiated, and one has to remove all the possibly remaining pluripotent cells from the population to be used. Regarding iPSCs, the transduced additional genes cause a larger tumor risk than that belonging to ingeniously pluripotent hESC [44]. Also, the so-called culture adaptation [45] may during several passages increase the tumorigenic properties of stem cells (described in Characterization of hESCs).
FACS or magnetic cell sorting is applied for enrichment of the differentiated cell population at the first stage. At the end, antibodies against pluripotency genes can be used to remove possibly remaining pluripotent cells [46, 47]. Ben-David et al. [48] developed an elimination system for pluripotent cells using an oleate synthesis inhibitor. Inducible apoptosis is also an option [49].
Clinical-Grade hESCs
There are specific requirements for hESC lines intended for clinical use. Good manufacturing practice (GMP) [13] is a quality system that is required by the authorities. It is described in this chapter. In clinical work, all the possible risks have to be minimized. These risks include infection that can be avoided by carefully selecting clean culture constituents and having high grade cleanliness in the laboratory. GMP deals with all such factors. Tumor risks have to minimized. That the cells fulfill their function has to be confirmed in advance by careful in vitro and in vivo characterization. The cells have to be optimally differentiated for each purpose as described above. Testing of the quality according to the GMP is the most expensive part of establishing cell lines for clinical use [27]. Genetic stability has to be shown for the time being by SNP or CGH arrays and karyotyping.
Authorities regulate therapies using cell transplantation, including the Food and Drug Administration in the United States of America and the European Union Regulations for Advanced Therapies in Europe. The European laws are presented by the European Science Foundation [50]. In addition, stem cell banks must comply with guidelines established by the International Society for Stem Cell Research (Guidelines for the Conduct of Human Embryonic Stem Cell Research, 2005, http://www.ISSCR.org), in which the ethics principles are also discussed, and national guidelines such as in the United States (U.S. National Academy of Science, NAS 2005, http://www.nap.edu; NIH Guidelines for Human Embryonic Stem Cell Research, http://stemcells.NIH.gov).
The First Clinical Trials
Currently, there are two ongoing clinical trials using hESC-derived differentiated cells [51] for two indications: a safety trial using oligodendrocytes in spinal cord injury [52] and treatment of age-related macular degeneration [53]. Cavities in the spinal cord were first reported from the spinal cord injury study. Similar findings were also seen in a trial in which fetal cells were used [54]. The oligodendrocyte study has since been reported to proceed without complications [52]. Retinal pigment epithelial cells have been injected into two patients in a safety study, and so far, no side effects have been reported [53].
Conclusion
It is now feasible to establish clinical-grade hESC lines in animal substance-free chemically defined conditions. Genetically stable hESC lines can be derived even from single biopsied blastomeres without destroying the embryo. Efficient differentiation methods exist and are being further improved.
Author Contributions
O.H., S.R., L.A., and K.T.: manuscript writing.
Disclosure of Potential Conflicts of Interest
O.H. and S.R. are shareholders in Biolamina. K.T. has uncompensated intellectual property rights, is an uncompensated shareholder in Biolamina, and has uncompensated relationships with BioLamina and NephroGenex.
References
- 1.Fishel SB, Edwards RG, Evans CJ. Human chorionic gonadotropin secreted by preimplantation embryos cultured in vitro. Science. 1984;223:816–818. doi: 10.1126/science.6546453. [DOI] [PubMed] [Google Scholar]
- 2.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 3.Reubinoff BE, Pera MF, Fong CY, et al. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 4.Rodin S, Antonsson L, Niaudet C, et al. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat Commun. 2014;5:3195. doi: 10.1038/ncomms4195. [DOI] [PubMed] [Google Scholar]
- 5.Hovatta O, Mikkola M, Gertow K, et al. A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Hum Reprod. 2003;18:1404–1409. doi: 10.1093/humrep/deg290. [DOI] [PubMed] [Google Scholar]
- 6.Koivisto H, Hyvärinen M, Strömberg AM, et al. Cultures of human embryonic stem cells: Serum replacement medium or serum-containing media and the effect of basic fibroblast growth factor. Reprod Biomed Online. 2004;9:330–337. doi: 10.1016/s1472-6483(10)62150-5. [DOI] [PubMed] [Google Scholar]
- 7.Inzunza J, Gertow K, Strömberg MA, et al. Derivation of human embryonic stem cell lines in serum replacement medium using postnatal human fibroblasts as feeder cells. Stem Cells. 2005;23:544–549. doi: 10.1634/stemcells.2004-0201. [DOI] [PubMed] [Google Scholar]
- 8.Ström S, Rodriguez-Wallberg K, Holm F, et al. No relationship between embryo morphology and successful derivation of human embryonic stem cell lines. PLoS One. 2010;5:e15329. doi: 10.1371/journal.pone.0015329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter MK, Rosler E, Rao MS. Characterization and differentiation of human embryonic stem cells. Cloning Stem Cells. 2003;5:79–88. doi: 10.1089/153623003321512193. [DOI] [PubMed] [Google Scholar]
- 11.Kleinman HK, Martin GR. Matrigel: Basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Akopian V, Andrews PW, Beil S, et al. Comparison of defined culture systems for feeder cell free propagation of human embryonic stem cells. In Vitro Cell Dev Biol Anim. 2010;46:247–258. doi: 10.1007/s11626-010-9297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unger C, Skottman H, Blomberg P, et al. Good manufacturing practice and clinical-grade human embryonic stem cell lines. Hum Mol Genet. 2008;17:R48–R53. doi: 10.1093/hmg/ddn079. [DOI] [PubMed] [Google Scholar]
- 14.Ilic D, Giritharan G, Zdravkovic T, et al. Derivation of human embryonic stem cell lines from biopsied blastomeres on human feeders with minimal exposure to xenomaterials. Stem Cells Dev. 2009;18:1343–1350. doi: 10.1089/scd.2008.0416. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig TE, Levenstein ME, Jones JM, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 16.Crook JM, Peura TT, Kravets L, et al. The generation of six clinical-grade human embryonic stem cell lines. Cell Stem Cell. 2007;1:490–494. doi: 10.1016/j.stem.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Tannenbaum SE, Turetsky TT, Singer O, et al. Derivation of xeno-free and GMP-grade human embryonic stem cells—platforms for future clinical applications. PLoS One. 2012;7:e35325. doi: 10.1371/journal.pone.0035325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodin S, Domogatskaya A, Ström S, et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol. 2010;28:611–615. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- 19.Klaffky E, Williams R, Yao CC, et al. Trophoblast-specific expression and function of the integrin alpha 7 subunit in the peri-implantation mouse embryo. Dev Biol. 2001;239:161–175. doi: 10.1006/dbio.2001.0404. [DOI] [PubMed] [Google Scholar]
- 20.Doi M, Thyboll J, Kortesmaa J, et al. Recombinant human laminin-10 (alpha5beta1gamma1). Production, purification, and migration-promoting activity on vascular endothelial cells. J Biol Chem. 2002;277:12741–12748. doi: 10.1074/jbc.M111228200. [DOI] [PubMed] [Google Scholar]
- 21.Melkoumian Z, Weber JL, Weber DM, et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol. 2010;28:606–610. doi: 10.1038/nbt.1629. [DOI] [PubMed] [Google Scholar]
- 22.Villa-Diaz LG, Nandivada H, Ding J, et al. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat Biotechnol. 2010;28:581–583. doi: 10.1038/nbt.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domogatskaya A, Rodin S, Tryggvason K. Functional diversity of laminins. Annu Rev Cell Dev Biol. 2012;28:523–553. doi: 10.1146/annurev-cellbio-101011-155750. [DOI] [PubMed] [Google Scholar]
- 24.Klimanskaya I, Chung Y, Becker S, et al. Human embryonic stem cell lines derived from single blastomeres. Nature. 2006;444:481–485. doi: 10.1038/nature05142. [DOI] [PubMed] [Google Scholar]
- 25.Taylor CJ, Bolton EM, Pocock S, et al. Banking on human embryonic stem cells: Estimating the number of donor cell lines needed for HLA matching. Lancet. 2005;366:2019–2025. doi: 10.1016/S0140-6736(05)67813-0. [DOI] [PubMed] [Google Scholar]
- 26.Daughtry B, Mitalipov S. Concise review: Parthenote stem cells for regenerative medicine: Genetic, epigenetic, and developmental features. Stem Cells Translational Medicine. 2014;3:290–298. doi: 10.5966/sctm.2013-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murdoch A, Braude P, Courtney A, et al. The procurement of cells for the derivation of human embryonic stem cell lines for therapeutic use: Recommendations for good practice. Stem Cell Rev. 2012;8:91–99. doi: 10.1007/s12015-011-9288-9. [DOI] [PubMed] [Google Scholar]
- 28.Jacquet L, Stephenson E, Collins R, et al. Strategy for the creation of clinical grade hESC line banks that HLA-match a target population. EMBO Mol Med. 2013;5:10–17. doi: 10.1002/emmm.201201973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao T, Zhang ZN, Rong Z, et al. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 30.Ma H, Morey R, O’Neil RC, et al. Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature. 2014;511:177–183. doi: 10.1038/nature13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhattacharya B, Cai J, Luo Y, et al. Comparison of the gene expression profile of undifferentiated human embryonic stem cell lines and differentiating embryoid bodies. BMC Dev Biol. 2005;5:22. doi: 10.1186/1471-213X-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vallier L, Reynolds D, Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–421. doi: 10.1016/j.ydbio.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 33.Silva J, Smith A. Capturing pluripotency. Cell. 2008;132:532–536. doi: 10.1016/j.cell.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Närvä E, Autio R, Rahkonen N, et al. High-resolution DNA analysis of human embryonic stem cell lines reveals culture-induced copy number changes and loss of heterozygosity. Nat Biotechnol. 2010;28:371–377. doi: 10.1038/nbt.1615. [DOI] [PubMed] [Google Scholar]
- 35.Amps K, Andrews PW, Anyfantis G, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol. 2011;29:1132–1144. doi: 10.1038/nbt.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adewumi O, Aflatoonian B, Ahrlund-Richter L, et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25:803–816. doi: 10.1038/nbt1318. [DOI] [PubMed] [Google Scholar]
- 37.Hovatta O, Jaconi M, Töhönen V, et al. A teratocarcinoma-like human embryonic stem cell (hESC) line and four hESC lines reveal potentially oncogenic genomic changes. PLoS One. 2010;5:e10263. doi: 10.1371/journal.pone.0010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobs K, Mertzanidou A, Geens M, et al. Low-grade chromosomal mosaicism in human somatic and embryonic stem cell populations. Nat Commun. 2014;5:4227. doi: 10.1038/ncomms5227. [DOI] [PubMed] [Google Scholar]
- 39.Bibikova M, Laurent LC, Ren B, et al. Unraveling epigenetic regulation in embryonic stem cells. Cell Stem Cell. 2008;2:123–134. doi: 10.1016/j.stem.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Cerdan C, Hong SH, Bhatia M. Curr Protoc Stem Cell Biol. 2007. Formation and hematopoietic differentiation of human embryoid bodies by suspension and hanging drop cultures. Chapter 1Unit 1D2. [DOI] [PubMed] [Google Scholar]
- 41.Nat R, Nilbratt M, Narkilahti S, et al. Neurogenic neuroepithelial and radial glial cells generated from six human embryonic stem cell lines in serum-free suspension and adherent cultures. Glia. 2007;55:385–399. doi: 10.1002/glia.20463. [DOI] [PubMed] [Google Scholar]
- 42.Cai J, Zhao Y, Liu Y, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–1239. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- 43.Qu C, Puttonen KA, Lindeberg H, et al. Chondrogenic differentiation of human pluripotent stem cells in chondrocyte co-culture. Int J Biochem Cell Biol. 2013;45:1802–1812. doi: 10.1016/j.biocel.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 44.Lee AS, Tang C, Rao MS, et al. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19:998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Draper JS, Smith K, Gokhale P, et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004;22:53–54. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- 46.Choo AB, Tan HL, Ang SN, et al. Selection against undifferentiated human embryonic stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1. Stem Cells. 2008;26:1454–1463. doi: 10.1634/stemcells.2007-0576. [DOI] [PubMed] [Google Scholar]
- 47.Tang C, Lee AS, Volkmer JP, et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011;29:829–834. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ben-David U, Gan QF, Golan-Lev T, et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013;12:167–179. doi: 10.1016/j.stem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Di Stasi A, Tey SK, Dotti G, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hovatta O, Stojkovic M, Nogueira M, et al. European scientific, ethical, and legal issues on human stem cell research and regenerative medicine. Stem Cells. 2010;28:1005–1007. doi: 10.1002/stem.436. [DOI] [PubMed] [Google Scholar]
- 51.http://www.clinicaltrials.gov/. Clinical trials. Available at http://www.clinicaltrials.gov/
- 52.Piltti KM, Salazar DL, Uchida N, et al. Safety of human neural stem cell transplantation in chronic spinal cord injury. Stem Cells Translational Medicine. 2013;2:961–974. doi: 10.5966/sctm.2013-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 54.Seiger A. Collection and use of fetal central nervous system tissue. Fetal Ther. 1989;4(suppl 1):104–107. doi: 10.1159/000263474. [DOI] [PubMed] [Google Scholar]