Abstract
The Developmental Origin of Health and Disease (DOHaD) hypothesis is a broad theoretical framework that emphasizes how early risk factors have a causal influence on psychopathology. Researchers have raised concerns about the causal interpretation of statistical associations between early risk factors and later psychopathology because most existing studies have been unable to rule out the possibility of environmental and genetic confounding. In this paper we illustrate how family-based quasi-experimental designs can test the DOHaD hypothesis by ruling out alternative hypotheses. We review the logic underlying sibling-comparison, co-twin control, offspring of siblings/twins, adoption, and in vitro fertilization designs. We then present results from studies using these designs focused on broad indices of fetal development (low birth weight and gestational age) and a particular teratogen, smoking during pregnancy. The results provide mixed support for the DOHaD hypothesis for psychopathology, illustrating the critical need to use design features that rule out unmeasured confounding.
The Developmental Origin of Health and Disease (DOHaD) hypothesis originated in research that linked indices of fetal development (e.g., low birth weight) to adult health, particularly cardiovascular disease (Barker, 1998). Ongoing research has identified key mediating mechanisms through which early risk factors influence subsequent medical problems, including epigenetic factors (Gluckman, Hanson, Cooper, & Thornburg, 2008). The DOHaD hypothesis now provides a broad framework for exploring associations between early risk factors and later psychopathology (Bale et al., 2010), which is the focus of the current review. The DOHaD hypothesis suggests that early life influences can causally impact later functioning because the insult is experienced during a sensitive developmental period (Barker, 1998). It is important to stress that the DOHaD hypothesis is a causal hypothesis, which emphasizes how early risk factors have a causal effect—independent of confounding factors—on later outcomes.
Despite the popularity and influence of the DOHaD hypothesis, researchers have expressed skepticism regarding the interpretation of statistical associations between early risk factors and later psychopathology because of the inability of most studies to rule out alternative hypotheses for the associations (e.g., Thapar & Rutter, 2009). Quantitative genetic studies have found that correlations between environmental and genetic factors are pervasive (Jaffee & Price, 2012), which brings about the possibility that genetic confounds may account for the statistical associations between putative environmental risk factors and developmental outcomes (Rutter, Pickles, Murray, & Eaves, 2001). As a consequence, research that tests causal theories must rule out the plausible alternative hypothesis that genetic confounding accounts for the statistical associations. Similarly, researchers must also rigorously examine the possibility that environmental confounds account for both the exposures and the outcomes.
Although drawing strong causal inferences requires identifying and systematically ruling out plausible competing alternative hypotheses (Shadish, Cook, & Campbell, 2002), relatively few of the existing studies of pregnancy-related and perinatal risk factors have used rigorous research designs to test competing hypotheses. Testing the DOHaD hypothesis is particularly difficult because researchers cannot randomly assign individuals to these exposures, and there are concerns about the generalizability of the results from animal studies of pregnancy-related factors (e.g., Mitchell & Taggert, 2009). Unless research addresses the alternative hypotheses of genetic and environmental confounding, the findings related to early risk factors may not be useful for guiding subsequent translational research.
We propose that the use of several family-based quasi-experimental designs enables researchers to rigorously test competing, theory-driven hypotheses and help identify the mechanisms through which early risk factors are associated with psychopathology. Quasi-experimental approaches rely on design features to rule out confounding factors rather than relying solely on controlling measured covariates in analyses (Shadish et al., 2002). Leading researchers in numerous fields (review in D’Onofrio, Lahey, Turkheimer, & Lichtenstein, 2013), have all stressed the critical need to use such design to test competing hypotheses. In the current manuscript we briefly review the logic underlying several family-based quasi-experimental designs. We then provide the results from some existing studies of broad indices of pregnancy risk factors and a specific teratogen.
Review of Family-Based Quasi-Experimental Designs
Family-based quasi-experimental designs rely on variability among family members in exposure to risk factors and later psychopathology (i.e., the designs do not compare unrelated individuals, which is the standard approach for studying the DOHaD hypothesis in humans). Extensive reviews of the strengths and limitations of each of the designs are available elsewhere (D’Onofrio et al., 2013; Knopik, 2009; Rutter et al., 2001).
Sibling-Comparison Design
This design compares siblings who are differentially exposed to a risk factor, which accounts for all genetic and environmental factors that make siblings similar. If an association between an early risk factor and later psychopathology is observed, the results would be consistent with the DOHaD hypothesis. In contrast, if there is no within-family association (i.e., differentially exposed siblings have the same levels of psychopathology), the results would suggest that family-level confounds account for the association. Sibling comparisons rely on a number of important assumptions and limitations, however. The approach does not prove causation because genetic and environmental confounders that influence only one sibling (i.e., individual-specific factors) could account for the statistical association. There also are assumptions about the generalizability of the findings from differentially exposed siblings (Frisell, Oberg, Kuja-Halkola, & Sjolander, 2012; Lahey & D’Onofrio, 2010). In addition, the design assumes no carry-over effects, which occur when the exposure of one sibling influences the outcome of another (Donovan & Susser, 2011; Frisell et al., 2012; Lahey & D’Onofrio, 2010). Finally, the results of sibling-comparison studies may be ambiguous if there is large measurement error (McGue, Osler, & Christensen, 2010).
However, researchers can use different approaches to test many of these assumptions. For instance, researchers can include measured covariates (e.g., birth order and within-family covariates) in sibling-comparison analyses to help account for individual-specific confounds. Sibling-comparison studies can also be combined with longitudinal assessments; using temporal ordering can help rule out individual-specific genetic confounds when the risk factor (e.g., parental age at childbearing) cannot be influenced by the offspring (Lahey & D’Onofrio, 2010). And, researchers can test for carry-over effects by conducting bidirectional case-crossover studies, which explore differentially exposed siblings across birth order (i.e., the first-versus the second-born child is exposed: D’Onofrio et al., 2013). Other family-based quasi-experimental designs can test additional assumptions of siblings-comparisons.
Co-twin Control Design
The comparison of differentially exposed identical twins provides an approach that accounts for all genetic confounding (identical twins share 100% of their DNA) and environmental factors shared by siblings (McGue et al., 2010). As such, the approach allows researchers to make stronger causal inferences by ruling out all genetic confounds. The design cannot account for environmental confounds that influence only one twin and are correlated with the exposure, however. There also are particular concerns about the generalizability of findings from such studies. For example, the results from co-twin control studies of early risk factors may not apply to singletons because of the pregnancy-related differences in the births of multiples. Furthermore, the design cannot examine important pregnancy-related risk factors that are shared by twins (e.g., gestational age), although we illustrate below how researchers have used the design to examine low birth weight.
Offspring of Siblings/Twins Design
The comparison of differentially exposed offspring of siblings and twins accounts for environmental factors shared by cousins. The degree to which the design accounts for genetic factors depends on the type of cousins being compared; the offspring of half-siblings (6.25%), offspring of full-siblings and fraternal twins (12.5%), and offspring of identical twins (25%) account for different amounts of genetic confounding. The offspring of identical twins represents a unique case; the offspring are genetically half-siblings, but socially they are cousins. The offspring of siblings/twins design, therefore, provide tests of the DOHaD hypothesis that are independent of the genetic and environmental factors that make cousins similar. Cousin-comparisons account for fewer confounds than sibling-comparisons and the co-twin control design. Cousin-comparisons, however, can be used to examine early risk factors that are shared by twins (e.g., gestational age). Furthermore, the offspring of siblings/twins can be used to test some of the assumptions in other designs. For example, the comparison of first-born cousins can explore risk factors independent of confounding by birth order, and the concern about carry-over effects is mitigated in cousin-comparisons as compared to sibling-comparisons. As such, the offspring of siblings/twins design provides additional designs that can shed light on early risk factors.
Adoption-at-birth design
The adoption-at-birth design can be used to explore whether the statistical associations between prenatal factors and later outcomes are confounded by postnatal environmental factors. This is because the biological mothers provide genetic and prenatal environmental factors, but not the postnatal environment to the children. Thus, the design cannot remove effects of genetic confounding from prenatal environmental influences on children, such as smoking during pregnancy (SDP) (Gaysina, Fergusson, Leve, & et al., 2013). By using a sample of children adopted-at-birth and their genetically unrelated rearing parents the design is, on the other hand, able to control for genetic confounding when examining the association between postnatal environmental factors (e.g., parenting) and child outcomes (Harold et al., 2013). This is because adoptive mothers provide postnatal environmental factors, but not genetic or prenatal environmental factors to children.
In Vitro Fertilization Design
The in vitro fertilization (IVF) design, sometimes referred to as an adoption-at-conception design, examines families that use reproductive technologies to conceive a child to rule out genetic confounding when studying pregnancy-related risk factors. The largest IVF study to date includes offspring who are genetically related to mothers (i.e., the mother’s own egg was fertilized) and offspring who are not genetically related to their birth mothers (i.e., the birth mother became pregnant through an embryo donation) (Thapar et al., 2007). The strongest test of the DOHaD hypothesis in an IVF study is whether an early risk factor is associated with offspring psychopathology in the subsample of offspring and mothers who are not genetically related because the design rules out genetic factors passed down from the mother to the offspring. Although the design provides a strong test of causal influences, obtaining large samples of such families is difficult, and the findings from IVF studies may not generalize to other populations because such families are a highly selected subgroup.
Quasi-Experimental Studies of Pregnancy-Related Risk factors
Broad Indices of Early Risk Factors
Previous human studies have found that low birth weight, an index of fetal development and the physiological adaptations to the intrauterine environment (Barker, 1998), is correlated with later psychological and cognitive problems. Differences in brain development correlated with birth weight (Walhovd et al., 2012) may be driving these associations. Yet, low birth weight is a complex trait that is influenced by environmental and genetic factors that could account for the association with later outcomes. To address the possibility that the association between low birth weight and later psychopathology may be due to confounding environmental and genetic factors, researchers have used several family-based quasi-experimental designs. For instance, co-twin control studies have found that low birth weight is associated with Attention Deficit Hyperactivity Disorder (ADHD) (Hultman, Torrång, Cnattingius, Larsson, & Lichtenstein, 2007) and autism (Losh, Esserman, Anckarsäter, Sullivan, & Lichtenstein, 2012) independent of all genetic and environmental factors shared by twins. Furthermore, sibling- and cousin-comparisons have similarly found that low birth weight is independently associated with ADHD and autism (Class, Rickert, Larsson, Lichtenstein, & D’Onofrio, submitted), which provides additional support for the DOHaD hypothesis that low birth weight and/or the environmental processes that influence it cause these two neurodevelopmental disorders. Although more quasi-experimental research is needed, the existing findings suggest that future research should explore the biological mechanisms that specifically account for the associations between low birth weight and psychopathology.
Researchers have likewise found that short gestational age (i.e., preterm birth) is correlated with later psychopathology, and neuroimaging studies suggest that alterations in brain development mediate the association with later psychopathology (Whitaker et al., 2011). A recent family-based quasi-experimental study has provided a more nuanced view of the consequences of preterm birth, however (D’Onofrio et al., 2013). Figure 1 presents some of the findings from a sibling-comparison study based on a large population-based sample. The panels present the magnitude of associations (using a Hazard Ratio) between gestational age and (a) autism, (b) psychotic or bipolar disorders, and (c) suicide attempts using three analytical models. The Baseline Model presents the magnitude of the association in the entire population. The Adjusted Model presents the association while controlling for measured covariates (e.g., maternal and paternal age at childbearing, education, and psychiatric problems). The Fixed-Effects model represents the association when comparing with differing gestational ages (i.e., one sibling had a shorter gestational age than the other) and controlling for the measured covariates.
Figure 1. Associations between Gestational Age and Three Measures of Psychopathology Using Different Research Designs.
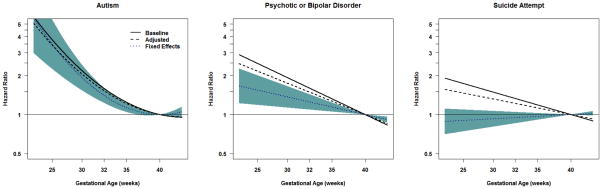
Note. The panels present the magnitude of the associations (in Hazard Ratios) between gestational age (measured in weeks and centered at 40 weeks of gestation) and Autism (Panel A), Psychotic or Bipolar Disorder (Panel B), and Suicide Attempt (Panel C). The lines represent the best fitting quadratic models. The Baseline Model (the solid black line) compares unrelated individuals in the entire population. The Adjusted Model (the dotted black line) compares unrelated individuals in the population while also controlling for measured covariates, such as maternal and paternal age at childbearing, highest level of education, and psychiatric problems. The Fixed Effects Model (the dotted blue line, with the 95% confidence intervals represented in the shaded blue areas) presents the associations when comparing differentially exposed siblings and controlling for the measured covariates. The dotted blue line, therefore, presents the magnitude of the association that is independent of genetic and environmental factors shared by siblings, as well as the measured covariates. For more details about the results, see D’Onofrio et al., (2013).
Panel A shows that early gestational age was correlated with greater risk for autism, and the association remained large when controlling for measured covariates and comparing differentially exposed siblings. These results illustrate that the association is independent of environmental and genetic factors shared by siblings and all of the measured covariates, which is consistent with the DOHaD hypothesis. The results for psychotic and bipolar disorders (Panel B) present a slightly different pattern. Early gestational age was correlated with increased risk for these disorders in the entire population. The magnitude was somewhat attenuated when the analyses controlled for the measured covariates. The association was further attenuated, albeit still robust, when comparing differentially exposed siblings. These results suggest that unmeasured confounds explain part, but not all, of the association between gestational age and psychotic and bipolar disorders, providing partial support for the DOHaD hypothesis. Finally, the results for suicide attempts (Panel C) support a different conclusion. Although gestational age was associated with suicide attempts in the population and when controlling measured covariates, the association was completely attenuated when comparing differentially-exposed siblings. These findings, which were replicated in cousin-comparisons and bidirectional case-crossover analyses, strongly suggest that early gestational age does not cause an increased risk for suicide attempts, thus failing to support the DOHaD hypothesis for this outcome.
Quasi-Experimental Studies of Maternal Smoking during Pregnancy
The accepted view among researchers in the field was that maternal SDP causes later psychopathology, including disruptive behaviors, ADHD, substance use problems, and academic problems (e.g., Wakschlag, Pickett, Cook, Benowitz, & Leventhal, 2002). However, Knopik (2009) first proposed that the consequences of SDP would depend on the outcomes being explored, with the associations between SDP and later psychopathology being due to familial confounding. As we recently reviewed elsewhere (D’Onofrio et al., 2013), numerous sibling-comparison studies published in the past several years have all found that familial confounding accounts for most, if not all, of the associations with offspring conduct problems during childhood, disruptive behavior and criminality during adolescence and young adulthood, ADHD, substance use problems, academic difficulties, and lower academic achievement. Although the initial sibling-comparison studies generated controversy because they mostly predicted extreme forms of psychopathology (Talati & Weissman, 2010), more recent sibling-comparison studies, including those focused on more normative outcomes [e.g., substance use during adolescence (D’Onofrio et al., 2012) and measures of early temperament (Ellingson, Goodnight, Van Hulle, Waldman, & D’Onofrio, 2014)], have consistently found that familial factors confound the associations with SDP.
Research with other quasi-experimental designs has provided converging evidence. IVF (Rice et al., 2009; Thapar et al., 2009) and offspring of siblings/twins studies (D’Onofrio et al., 2010; Kuja-Halkola et al., 2010) suggest that SDP does not cause psychopathology; rather, genetic factors that vary between families account for the association between SDP and offspring psychopathology. These findings are consistent with a recent adoption study that suggests that postnatal genetic factors (e.g., the children’s genetic factors influencing the adoptive parents behaviors) do not account for the association between SDP and offspring conduct problems (Gaysina et al., 2013). Family-based quasi-experimental studies, therefore, strongly suggest that most of the existing research on SDP and offspring psychopathology using traditional comparisons of unrelated individuals have overstated the causal influence (D’Onofrio et al., 2013; Jaffee, Strait, & Odgers, 2012; Knopik, 2009), although there remains considerable debate in the field (Slotkin, 2013).
It is important to note, however, that recent quasi-experimental studies have replicated and expanded the findings that SDP is associated with pregnancy-related problems (e.g., low birth weight) and infant mortality (Johansson, Dickman, Kramer, & Cnattingius, 2009), which is consistent with a strong causal hypothesis (Knopik, 2009). Reducing SDP, therefore, remains an important public health concern, consistent with the DOHaD hypothesis.
Summary and Future Directions
We briefly reviewed several family-based quasi-experimental designs and how research using these approaches has shed light on the DOHaD hypothesis as it relates to psychopathology. There are certainly other quasi-experimental approaches (Rutter et al., 2001), such as Mendelian randomization (Wiebe, Fang, Johnson, James, & Espy, 2014) and natural experiments (e.g., across pregnancy; Class et al., 2014), as well as advances in the use of statistical covariates (e.g., propensity scores; West et al., 2014), that can help provide insight into causal mechanisms through which early risk factors influence later psychopathology because the approaches have different assumptions and limitations than those discussed here. We stress that the existing research using quasi-experimental designs as a whole clearly illustrates that such approaches are absolutely critical for studying early risk factors (Academy of Medical Sciences Working Group, 2007).
Most of the existing quasi-experimental studies on early risk factors have not used detailed assessment of exposures that are available in smaller, intensive studies of pregnancy. As such, future research will need collaborative efforts to combine advanced research designs with detailed assessment of risk factors (D’Onofrio & Lahey, 2010). Because of the relative difficulty in identifying twins and offspring of IVF, conducing sibling- and cousin-comparison studies may best facilitate collaborations using family-based quasi-experimental designs (Lahey & D’Onofrio, 2010). Furthermore, most quasi-experimental studies reviewed here solely tested main effects. Yet, new statistical developments allow tests of more complex developmental questions, such as mediation and moderation, within quasi-experimental designs (Lahey, D’Onofrio, Van Hulle, & Rathouz, in press). In addition, we stress the need for quasi-experimental research that simultaneously examines multiple early risk factors. For instance, although SDP is associated with low birth weight in quasi-experimental studies, the mechanisms through SDP and low birth weight influence later developmental may be independent, as suggested by recent studies (e.g., Wiebe et al., 2014), but more research is needed. And, quasi-experimental research should also explore outcomes at multiple levels of analysis, including biomarkers (e.g., epigenetic factors), that are thought to mediate the consequences of early risk factors (D’Onofrio & Lahey, 2010).
Although the DOHaD hypothesis has shed great light on the importance of early risk factors, the results of the existing quasi-experimental research on pregnancy and perinatal risk factors for later psychopathology is mixed. This illustrates the need for researchers to rule out plausible alternative hypotheses (e.g., genetic and environmental confounding) before drawing strong causal inferences. Using multiple quasi-experimental designs and other advanced approaches to rule out confounding factors, therefore, is not merely an incremental advance over traditional human research that only includes one child per family; rather, these approaches are necessary for drawing conclusions about the causal and confounding processes that account for the associations with many early risk factors (Academy of Medical Sciences Working Group, 2007).
Acknowledgments
The manuscript was supported by grants from the National Institute of Child Health and Human Development (HD061817 and HD061384), Swedish Research Council (Medicine), and Swedish Prison and Probation Services.
References
- Academy of Medical Sciences Working Group. Identifying the environmental causes of disease: How should we decide what to believe and when to take action? London: Academy of Medical Sciences; 2007. [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nestler EJ. Early Life Programming and Neurodevelopmental Disorders. Biological Psychiatry. 2010;68(4):314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP. Mothers, babies and health in later life. 2. Edinburgh: Churchill Livingstone; 1998. [Google Scholar]
- Class QA, Abel KM, Khashan AS, Rickert ME, Dalman C, Larsson H, D’Onofrio BM. Offspring psychopathology following preconception, prenatal, and postnatal maternal bereavement stress. Psychological Medicine. 2014;44:71–84. doi: 10.1017/S0033291713000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Class QA, Rickert ME, Larsson H, Lichtenstein P, D’Onofrio BM. A population-based sibling-comparison study of the associations between fetal growth and psychiatric and socioeconomic outcomes; submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Class QA, Rickert ME, Larsson H, Langstrom N, Lichtenstein P. Preterm birth and mortality and morbidity: A population-based quasi-experimental study. JAMA Psychiatry. 2013;70:1231–1240. doi: 10.1001/jamapsychiatry.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Lahey BB. Biosocial influences on the family: A decade review. Journal of Marriage and Family. 2010;72:762–782. doi: 10.1111/j.1741-3737.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Singh AL, Iliadou A, Lambe M, Hultman C, Neiderhiser JM, Lichtenstein P. A quasi-experimental study of maternal smoking during pregnancy and offspring academic achievement. Child Development. 2010;81:80–100. doi: 10.1111/j.1467-8624.2009.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Lahey BB, Turkheimer E, Lichtenstein P. The critical need for family-based, quasi-experimental research in integrating genetic and social science research. American Journal of Public Health. 2013;103:S46–S55. doi: 10.2105/AJPH.2013.301252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Rickert ME, Långström N, Donahue KL, Coyne CA, Larsson H, Lichtenstein P. Familial confounding of the associations between maternal smoking during pregnancy and offspring substance use and problems. Archives of General Psychiatry. 2012;69:1140–1150. doi: 10.1001/archgenpsychiatry.2011.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan SJ, Susser E. Commentary: Advent of sibling designs. International Journal of Epidemiology. 2011;40:345–349. doi: 10.1093/ije/dyr057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson JM, Goodnight JA, Van Hulle CA, Waldman ID, D’Onofrio BM. A sibling-comparison study of smoking during pregnancy and childhood psychological traits. Behavior Genetics. 2014;44:25–35. doi: 10.1007/s10519-013-9618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisell T, Oberg S, Kuja-Halkola R, Sjolander A. Sibling comparison designs: Bias from non-shared confounders and measurement error. Epidemiology. 2012;23:713–720. doi: 10.1097/EDE.0b013e31825fa230. [DOI] [PubMed] [Google Scholar]
- Gaysina D, Fergusson DM, Leve LD, et al. Maternal smoking during pregnancy and offspring conduct problems: Evidence from 3 independent genetically sensitive research designs. JAMA Psychiatry. 2013;70(9):956–963. doi: 10.1001/jamapsychiatry.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KI. Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold GT, Leve LD, Barrett D, Elam K, Neiderhiser JM, Natsuaki MN, Thapar A. Biological and rearing mother influences on child ADHD symptoms: revisiting the developmental interface between nature and nurture. Journal of Child Psychology and Psychiatry. 2013;54(10):1038–1046. doi: 10.1111/jcpp.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman C, Torrång A, Cnattingius S, Larsson JO, Lichtenstein P. Birth weight and Attention-Deficit/Hyperactivity symptoms in childhood and early adolescence: A prospective Swedish twin study. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(3):370–377. doi: 10.1097/01.chi.0000246059.62706.22. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. The implications of genotype–environment correlation for establishing causal processes in psychopathology. Development and Psychopathology. 2012;24(Special Issue 04):1253–1264. doi: 10.1017/S0954579412000685. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Strait LB, Odgers CL. From correlates to causes: Can quasi-experimental studies and statistical innovations bring us closer to identifying the causes of antisocial behavior? Psychological Bulletin. 2012;138(2):272–295. doi: 10.1037/a0026020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ALV, Dickman PW, Kramer MS, Cnattingius S. Maternal smoking and infant mortality: Does quitting smoking reduce the risk of infant death? Epidemiology. 2009;20:1–8. doi: 10.1097/EDE.0b013e31819dcc6a. [DOI] [PubMed] [Google Scholar]
- Knopik VS. Maternal smoking during pregnancy and child outcomes: Real or spurious effect? Developmental Neuropsychology. 2009;34:1–36. doi: 10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuja-Halkola R, D’Onofrio BM, Illiadou A, Pawitan Y, Langstrom N, Lichtenstein P. Prenatal smoking exposure and stress coping in late adolescence: No causal link. International Journal of Epidemiology. 2010;39:1531–1540. doi: 10.1093/ije/dyq133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, D’Onofrio BM, Van Hulle CA, Rathouz PJ. Prospective association of childhood receptive vocabulary and conduct problems with self-reported adolescent delinquency: Tests of mediation and moderation in sibling-comparison analyses. Journal of Abnormal Child Psychology. doi: 10.1007/s10802-014-9873-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, D’Onofrio BM. All in the family: Comparing siblings to test causal hypotheses regarding environmental influences on behavior. Current Directions in Psychological Science. 2010;19:319–323. doi: 10.1177/0963721410383977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losh M, Esserman D, Anckarsäter H, Sullivan PF, Lichtenstein P. Lower birth weight indicates higher risk of autistic traits in discordant twin pairs. Psychological Medicine. 2012;42(05):1091–1102. doi: 10.1017/S0033291711002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Osler M, Christensen K. Causal inference and observational research: The utility of twins. Perspectives on Psychological Science. 2010;5:546–556. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell BF, Taggert MJ. Are animal models relevant to key aspects of human parturition? American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2009;297:R525–R545. doi: 10.1152/ajpregu.00153.2009. [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Boivin J, Hay DF, Van den Bree M, Thapar A. Disentangling prenatal and inherited influences in humans with an experimental design. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2464–2467. doi: 10.1073/pnas.0808798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Pickles A, Murray R, Eaves LJ. Testing hypotheses on specific environmental causal effects on behavior. Psychological Bulletin. 2001;127:291–324. doi: 10.1037/0033-2909.127.3.291. [DOI] [PubMed] [Google Scholar]
- Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. New York: Houghton Mifflin; 2002. [Google Scholar]
- Slotkin TA. Maternal smoking and conduct disorder in the offspring. JAMA Psychiatry. 2013;70(9):901–902. doi: 10.1001/jamapsychiatry.2013.1951. [DOI] [PubMed] [Google Scholar]
- Talati A, Weissman MM. In utero smoking exposure warrants further investigation. Archives of General Psychiatry. 2010;67:1094. doi: 10.1001/archgenpsychiatry.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Harold G, Rice F, Ge X, Boivin J, Hay D, Lewis A. Do intrauterine or genetic influences explain the foetal origins of chronic disease? A novel experimental method for disentangling effects. BMC Medical Research Methodology. 2007;7:25. doi: 10.1186/1471-2288-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Rice F, Hay D, Boivin J, Langley K, Van den Bree M, Harold G. Prenatal smoking might not cause Attention-Deficit/Hyperactivity Disorder. Evidence from a novel design. Biological Psychiatry. 2009;66:722–727. doi: 10.1016/j.biopsych.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Rutter M. Do prenatal risk factors cause psychiatric disorder? Be wary of causal claims. British Journal of Psychiatry. 2009;195:100–101. doi: 10.1192/bjp.bp.109.062828. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Pickett KE, Cook E, Benowitz NL, Leventhal BL. Maternal smoking during pregnancy and severe antisocial behavior in offspring: A review. American Journal of Public Health. 2002;92:966–974. doi: 10.2105/ajph.92.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Brown TT, Kuperman JM, Chung Y, Hagler DJ, Study G. Long-term influence of normal variation in neonatal characteristics on human brain development. Proceedings of the National Academy of Sciences. 2012 doi: 10.1073/pnas.1208180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West SG, Cham H, Thoemmes F, Renneberg B, Schulze J, Weiler M. Propensity score as a basis for equating groups: Basic principles and applicaiton in clinical treatment outcome research. Journal of Consulting and Clinical Psychology. 2014 doi: 10.1037/a0036387. epub April 7, 2014. [DOI] [PubMed] [Google Scholar]
- Whitaker AH, Feldman JF, Lorenz JM, McNicholas F, Fisher PW, Shen S, Paneth N. Neonatal head ultrasond abnormalities in preterm infants and adolescent psychiatric disorders. Archivies of General Psychiatry. 2011;68:742–752. doi: 10.1001/archgenpsychiatry.2011.62. [DOI] [PubMed] [Google Scholar]
- Wiebe SA, Fang H, Johnson C, James KE, Espy KA. Determining the impact of prenatal tobacco exposure on self-regulation at 6 months. Developmental Psychology. 2014 doi: 10.1037/a0035904. epub February 10, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


