SUMMARY
Wilson disease, Menkes disease, occipital horn syndrome, and X-linked distal hereditary motor neuropathy are genetic disorders of copper metabolism that span a broad spectrum of neurological dysfunction (Table 180.1). The occurrence of these disorders indicates the fundamental importance of ATP7A and ATP7B. Further research to clarify the mechanisms suggested by these clinical and biochemical phenotypes may yield insight about the roles of ATP7A and ATP7B in neuronal cells, and lead to improved treatments.
INTRODUCTION
Copper is an essential trace metal that requires exquisite homeostatic control; its regulation involves mechanisms which govern gastrointestinal uptake, transport to the developing brain, targeted intracellular delivery to copper enzymes, and hepatic excretion of copper into the biliary tract (Lutsenko et al., 2008). These functions are largely fulfilled by a pair of evolutionarily related copper-transporting ATPases, ATP7A and ATP7B. Defects in ATP7B cause a single known phenotype, Wilson disease (Kaler, 2008), whereas mutations in ATP7A are associated with three distinct conditions: (1) Menkes disease, a severe infantile-onset neurodegenerative disorder (Kaler, 1994); (2) occipital horn syndrome, similar to Menkes disease in many clinical and biochemical aspects, with a less severe neurologic phenotype (Kaler et al., 1994); and (3) an isolated distal motor neuropathy, often with adult onset and without overt signs of copper metabolic derangements (Kennerson et al., 2010; Yi et al., 2012). This chapter reviews the neurological and other clinical signs, the biochemical manifestations, and the molecular underpinnings associated with these four entities, as well as treatment considerations for each.
WILSON DISEASE
Wilson disease is an autosomal recessive disorder of copper metabolism with an incidence of approximately 1 in 30 000 live births. Affected individuals accumulate abnormal levels of copper in the liver and (later) in the brain due to mutations in both alleles of the Wilson disease gene (ATP7B). This gene was identified in 1993 and encodes a copper-transporting ATPase, ATP7B, expressed primarily in the liver, where its major function is excretion of hepatic copper into the biliary tract (Bull et al., 1993). The clinical condition was first described in 1912 by S.A.K. Wilson, an American-born neurologist working in England. Thirty-six years later, the pathologist J.N. Cummings proposed an etiological connection with copper overload and, in 1956, therapy with copper chelation by penicillamine was introduced by J.M. Walshe, a British physician working in Boston (Walshe, 2003).
Clinical manifestations
Presenting clinical features of Wilson disease include nonspecific liver disease, neurological abnormalities, psychiatric illness, hemolytic anemia, renal tubular Fanconi syndrome, and various skeletal abnormalities (Kaler, 2008). Age influences the specific presentation in Wilson disease. Nearly all individuals who present with liver disease are less than 30 years of age, whereas those presenting with neurological or psychiatric signs may range in age from the first to the fifth decade. This reflects the sequence of events in the pathogenesis of this disease. However, regardless of clinical presentation, some degree of liver disease is invariably present.
In one series of 400 adult patients with Wilson disease, approximately 50% presented with neurological and psychiatric symptoms, 20% with neurological and hepatic symptoms, and 20% with purely hepatic symptoms (Dening and Berrios, 1989). In patients with neurological presentations, abnormalities include speech difficulty (dysarthria), dystonia, rigidity, tremor, or choreiform movements, abnormal gait, and uncoordinated handwriting. Wilson disease may properly be classified as a movement disorder. The neurological signs and symptoms reflect the predilection for basal ganglia (e.g., caudate, putamen) involvement in these individuals’ brains. Parkinson’s disease or other movement disorders may be mistakenly diagnosed. In psychiatric presentations, changes in personality (irritability, anger, poor self-control), depression, and anxiety are common symptoms. Typically, patients presenting in this fashion are in their late teens or early twenties, a period during which substance abuse is also a diagnostic consideration. Wilson disease should be formally excluded in all teenagers and young adults with new-onset psychiatric signs.
With hepatic presentations, signs and symptoms include jaundice, hepatomegly, edema, or ascites. Secondary endocrine effects of liver disease may include delayed puberty or amenorrhea. Viral hepatitis and cirrhosis are often initial diagnostic considerations in individuals who, in fact, have Wilson disease.
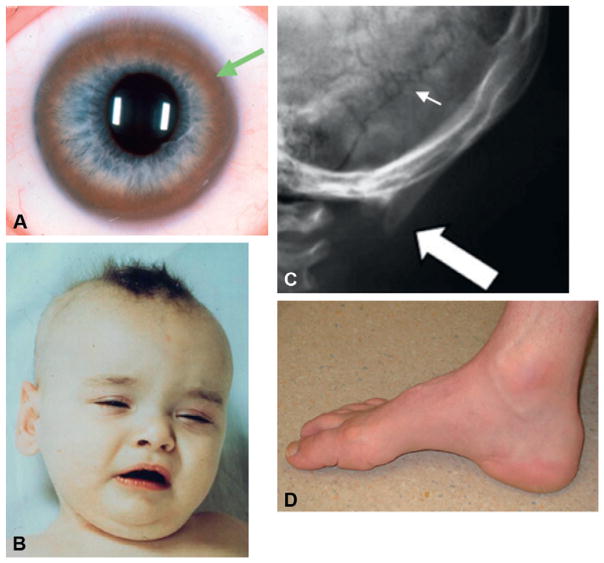
In addition to the brain and liver, the eye is a primary site of copper deposition in Wilson disease, producing a pathognomonic sign, the Kayser–Fleischer ring (Fig. 180.1A). The Kayser–Fleischer ring is a golden to greenish-brown annular deposition of copper in the periphery of the cornea. This important diagnostic sign first appears as a superior crescent, then develops inferiorly and ultimately becomes circumferential. Slit-lamp examinations are required to detect rings in their early stage of formation. Copper can also accumulate in the lens and produce “sunflower” cataracts. Approximately 95% of patients with neurological signs manifest the Kayser–Fleischer ring compared to approximately 65% of those with hepatic presentations. Copper chelation therapy causes fading and eventual disappearance of corneal copper.
Fig. 180.1.
Diagnostic signs in inherited disorders of copper transport. (A) Kayser–Fleischer ring (arrow) in the cornea of a newly diagnosed adult patient with Wilson disease. (Reproduced from Kaler (2008). Wilson disease. In: L Goldman, D Ausiello (Eds.), Cecil’s Textbook of Medicine, 23rd edn. Saunders, Philadelphia, ch. 230, pp. 1593–1595.) (B) Hair and facial appearance in classical Menkes disease at 8 months of age. (Reproduced from Kaler (1994). Adv Pediatr 41:263–304.) (C) Occipital exostoses (thick arrow) and wormian bones (small arrow) in a 4-year-old patient with occipital horn syndrome. (Modified from Tang et al. (2006). Genet Med 8: 711–718.) (D) Pes cavus foot deformity in a 43-year-old patient with ATP7A-related hereditary distal motor neuropathy. (Reproduced from Kennerson et al. (2010). Am J Hum Genet 86: 343–352.)
Renal tubular dysfunction in Wilson disease leads to abnormal losses of amino acids, electrolytes, calcium, phosphorus, and glucose. Presumably this effect is related to copper toxicity. High copper levels have been noted previously in the kidneys of patients with Wilson disease. Treatment with copper chelation often improves the renal disturbances. There can also be skeletal effects of Wilson disease, including osteoporosis and rickets, and these may be attributable to renal losses of calcium and phosphorus. Osteoarthritis primarily affecting the knees and wrists also occurs in Wilson disease patients and may involve excess copper deposition in the bone and cartilage.
Hemolytic anemia due to the direct toxic effects of copper on red blood cell membranes has been observed in Wilson disease, and is usually associated with release of massive quantities of hepatic copper into the circulation, a phenomenon that can be sudden and catastrophic.
Biochemical findings
Laboratory findings that support the diagnosis of Wilson disease include low levels of serum copper and serum ceruloplasmin, elevated hepatic transaminase levels, aminoaciduria, and hemolytic anemia. Incorporation of radiolabeled 64copper into serum ceruloplasmin, measured as the appearance of copper in the serum after an oral load is a highly specific diagnostic test; patients with Wilson disease incorporate very little 64copper into ceruloplasmin.
Aceruloplasminemia, a different autosomal-recessive disease caused by mutations in the ceruloplasmin gene (CP), may be confused with Wilson disease due to very low or absent serum ceruloplasmin level (Harris et al., 1995). This rare disorder of iron metabolism involves the triad of diabetes, retinal degeneration, and progressive basal ganglia degeneration with clinical symptoms including dysarthria, dystonia, and dementia. Since ceruloplasmin is required for oxidation of ferrous iron (Fe2+) to ferric iron (Fe3+), ferrous iron accumulates in pancreas, retina, and brain of aceruloplasmic patients. Magnetic resonance imaging reveals distinctive iron deposition in the basal ganglia – not seen in Wilson disease. Of note, given the pediatric focus of the Handbook, aceruloplasminemia has an average age of onset of 50 years and only one non-adult case (age 16 years) has been reported (McNeill et al., 2008).
Increased urinary excretion of copper (greater than 100 micrograms per 24 hours) is an easily performed and important diagnostic test for Wilson disease. Acid-washed (copper-free) collection containers should be used. A variation involving serial urine copper measurements is the penicillamine “challenge” in which 500 mg of penicillamine is administered orally after collecting a baseline 24 hour urine. The penicillamine dose is repeated after 12 hours, the midpoint of the second 24 hour urine collection. A several-fold increase in copper excretion in the second collection is suggestive of the diagnosis. Percutaneous needle liver biopsy for measurement of hepatic copper remains a gold standard, though invasive technique for Wilson disease diagnosis. Hepatic copper values greater than 200 micrograms per gram of dry weight (normal 20–50) are characteristic of Wilson disease. Atomic absorption spectrometry is the preferred method; histochemical staining for copper in a liver biopsy specimen is unreliable.
A Wilson disease mutation database (http://www.wilsondisease.med.ualberta.ca/database.asp) has been assembled and contains over 300 different mutations reported at the ATP7B locus (as of July 10, 2009). For families in which the mutant alleles have been determined, molecular diagnosis is highly reliable.
Prevention and treatment
The era of successful treatment of Wilson disease began in 1956 with Walshe’s use of penicillamine, a free thiol that binds (chelates) copper (Walshe, 2003). This drug does not formally correct the basic defect of impaired copper excretion in the bile. However, it greatly enhances urinary excretion of copper and thereby corrects and prevents copper overload and its effects. Pyridoxine (vitamin B6) is usually prescribed concomitantly, to counter the tendency for deficiency of this vitamin to develop during chronic penicillamine administration.
Certain individuals are intolerant of penicillamine, however, encountering significant side-effects that include nephrotoxicity, hematological abnormalities, and a distinctive rash, elastosis perforans serpiginosa (usually involving the neck and axillae). Furthermore, in some Wilson disease patients with neurological presentations, penicillamine treatment induces paradoxical worsening of the clinical picture. Triethylenetetramine dihydrochloride (trien) is a suitable alternative chelating agent with a somewhat lower side-effect profile.
Oral zinc acetate also has proven highly effective in Wilson disease. The mechanism involves induction of metallothionein synthesis in intestinal epithelial cells; increased metallothionein synthesis results in greater binding of dietary copper, and thus decreased absorption. Zinc therapy has particular value in (1) young, pre-symptomatic patients, (2) patients who are pregnant given the possible fetal teratogenic effects of other compounds, and (3) as maintenance therapy for patients after their initial “de-coppering” is accomplished. Zinc acetate has minimal side-effects. The only drawback to its use is the relatively long time (4–6 months) needed for restoration of proper copper balance when used as monotherapy in the initial stages of treatment.
Tetrathiomolybdate forms stable tripartite complexes between protein, copper and itself. This drug functions both to decrease copper absorption and to reduce circulating free copper. It is very fast acting and can restore normal copper balance within several weeks compared to the several months required with other copper chelators or with zinc.
Liver transplantation is a rare consideration in Wilson disease since the condition is typically responsive to medical therapy. This is generally necessary only in cases where delayed diagnosis or poor compliance results in irreversible hepatic damage.
Prognosis
The prognosis in Wilson disease is generally favorable; current therapeutic approaches can prevent or reverse most of the significant clinical signs and symptoms, including the Kayser–Fleisher rings. However, if treatment is stopped, irreversible and potentially fatal liver damage will inevitably occur.
Future directions
Gene therapy for Wilson disease is a theoretical possibility. Since the Wilson copper transporter is expressed most prominently and functions most critically in the liver, this organ could be specifically targeted using adenoviral (Ad), adeno-associated viral (AAV), or replication-deficient retroviral vectors. Hepatocyte transfer is an alternative to Ad, AAV or retroviral mediated gene transfer which is gaining credibility for treatment of liver-specific metabolic disorders through a process termed therapeutic liver repopulation.
ATP7A DISORDERS–Mutations in ATP7A, a close homolog of ATP7B discussed above, are associated with three distinct clinical phenotypes, based on the molecular pathology (Fig. 180.2):
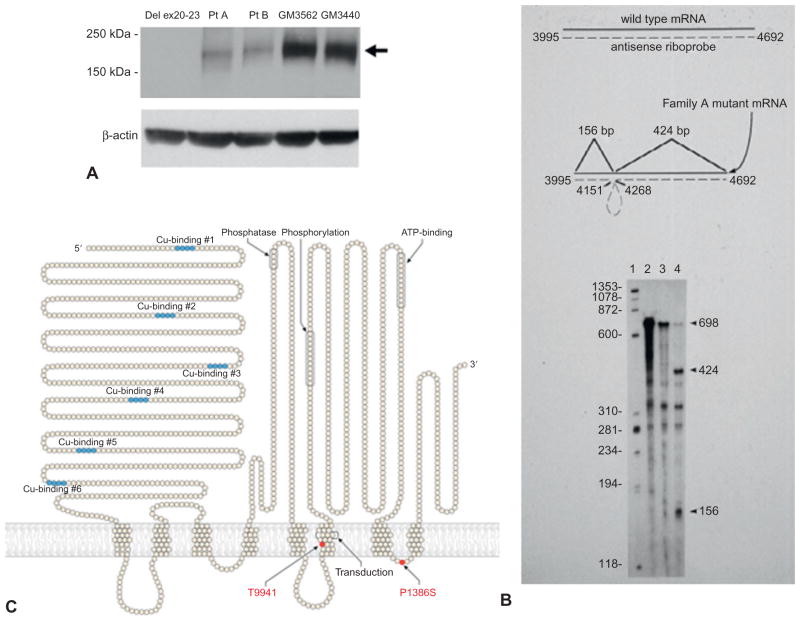
Fig. 180.2.
Molecular spectrum of ATP7A-related copper transport disorders. (A) Western analysis of fibroblast protein from patients with classical Menkes disease (Del ex20-23, Pt A, Pt B) show absent or reduced quantities of the 178 kDa ATP7A protein compared to two normal fibroblast cell lines (GM3562, GM3440). An antibody raised against the carboxyl-terminus of ATP7A was used. The membrane was stripped and re-probed with an anti-β-actin antibody to demonstrate equivalent loading. (Modified from Tang et al. (2008). Clinical outcomes in Menkes disease patients with a copper-responsive ATP7A mutation, G727R. PMID: 18752978. Molec Genet Metab 95:174–181.) (B) RNAse protection assay indicates a “leaky” splicing defect in an occipital horn syndrome patient (lane 4) whose cultured lymphoblasts contained some properly spliced ATP7A transcript (698 bp protected fragment) in addition to two mutant protected fragments (424 bp and 156 bp), in comparison to a normal control lymphoblast cell line GM3201 (lane 3). (Modified from Kaler et al. (1994). Nat Genet 8:195–202.) (C) ATP7A model indicating two missense mutations in unrelated families with X-linked distal hereditary motor neuropathy. These specific mutations have not been reported in Menkes disease or occipital horn syndrome. (Modified from Kennerson et al. (2010). Am J Hum Genet 86: 343–352.)
Menkes disease
Menkes disease is an X-linked recessive disorder of copper transport caused by diverse mutations in a copper transporting ATPase, ATP7A (Kaler, 1998a). As early as 1937, Australian veterinary scientists recognized the critical role of copper in mammalian neurodevelopment through the association of copper deficiency with demyelinating disease in ataxic lambs. In 1962, Menkes et al. described five male infants in a family of English-Irish heritage who were affected with a distinctive syndrome of neurological degeneration, peculiar hair, and failure to thrive. These boys appeared normal at birth and throughout the first several months of life, but then experienced seizures and developmental regression and ultimately passed away between the ages of 7 months and 3{1/2} years. The pedigree of the family strongly suggested that the condition was an X-linked genetic disease. In 1972, Danks et al. recognized that the unusual hair of infants with Menkes disease appeared similar in texture to the brittle wool of sheep raised on copper-deficient soil in Australia and found very low serum copper in seven Menkes disease patients.
Clinical manifestations
Menkes disease typically presents in males at 2–3 months of age with loss of previously obtained developmental milestones and the onset of hypotonia, seizures, and failure to thrive. Characteristic physical changes of the hair and facies (Fig. 180.1B), in conjunction with typical neurological findings, often suggest the diagnosis. The presenting signs and symptoms of 127 patients reported in the medical literature up to 1985 were compiled (Baerlocher and Nadal, 1988). The less distinctive appearance of very young affected infants before the onset of symptoms, is discussed separately below.
The scalp hair of classically affected infants is short, sparse, coarse, and twisted. Light microscopy of patient hair will illustrate pathognomonic pili torti (180° twisting of the hair shaft) and often other abnormalities including trichoclasis (transverse fracture of the hair shaft) and trichoptilosis (longitudinal splitting of the shaft). The hair tends to be lightly pigmented and may show unusual colors such as white, silver, or gray, but in some cases is normally pigmented. The face is jowly with sagging cheeks and ears that often appear large. The palate tends to be high-arched and tooth eruption delayed. Pectus excavatum is a common thoracic finding. Umbilical and/or inguinal herniae may be present. The skin often appears loose and redundant, particularly at the nape of the neck, the axillae, and on the trunk. Neurologically, profound truncal hypotonia with poor head control is invariably present. Appendicular tone may be increased with thumbs held in an adducted, cortical posture. Deep tendon reflexes are often hyperactive.
Certain clinical diagnostic tests are characteristic. White matter abnormalities reflecting impaired myelination, diffuse atrophy, ventriculomegaly, and tortuosity of cerebral blood vessels are typical findings on brain MRI. Subdural hematomas are common in infants, and cerebrovascular accidents can occur in patients who survive longer. The “corkscrew” appearance of cerebral vessels is well visualized by magnetic resonance angiography, a noninvasive method for study of the vasculature. Dysplastic coronary vessels may be detectable by echocardiography.
Electroencephalograms are usually moderately to severely abnormal including high rates of status epilepticus and infantile spasms (Friedman et al., 1978; Bahi-Buisson et al., 2006). Normal tracings may be recorded in some classically affected individuals, however (White et al., 1993). These three prior surveys indicated clinical seizures and electroencephalographic (EEG) abnormalities in a combined 27 of 29 (93%) symptomatic Menkes disease patients diagnosed at 2 months of age or older.
Pelvic ultrasonography reveals diverticula of the urinary bladder in nearly all patients. Radiographs often disclose abnormalities of bone formation in the skull (wormian bones), long bones (metaphyseal spurring), and ribs (anterior flaring, multiple fractures). Connective tissue problems in Menkes disease that have been identified more recently include neck masses due to dilation of internal jugular veins (Price et al., 2007), aneurysms of the brachial arteries (Godwin et al., 2006), and gastrointestinal polyps (Kaler et al., 1993).
Menkes disease in the neonatal period
Classical Menkes disease often escapes attention in the newborn period due to its very subtle manifestations in neonates (Gunn et al., 1984; Kaler et al., 2008) and the fact that healthy newborns have low serum copper levels which overlap those in affected infants. However, several nonspecific physical and metabolic findings are commonly cited when birth histories of these infants are reviewed. These include premature labor and delivery, large cephalohematomas, hypothermia, hypoglycemia, and jaundice. Occasionally, unusual hair pigmentation may suggest the diagnosis in newborns. Often, however, the appearance of the hair is unremarkable and the pili torti found on microscopic examination of hair from older Menkes patients is not evident in the hair of affected newborns. Neurologically, newborns with Menkes disease generally appear normal. Since the success of treatment with small copper complexes in this disorder depends heavily on early diagnosis and treatment (Kaler et al., 2008), newborn screening for Menkes disease based on neurochemical levels (see Biochemical findings) from dried blood spots, or via high throughput molecular assays, is desirable.
Biochemical findings
The biochemical phenotype in Menkes disease involves: (1) low levels of copper in plasma, liver, and brain due to impaired intestinal absorption, (2) reduced activities of copper-dependent enzymes, and (3) paradoxical accumulation of copper in certain tissues (duodenum, kidney, spleen, pancreas, skeletal muscle, placenta). The copper retention phenotype is also evident in cultured fibroblasts and lymphoblasts, in which reduced egress of radio-labeled copper is demonstrable in pulse-chase experiments (Kaler et al., 1994).
Certain clinical features of Menkes disease are related to deficient activity of specific copper-requiring enzymes (Kaler, 2011). Partial deficiency of dopamine-β-hydroxylase (DBH), a critical enzyme in the catecholamine biosynthetic pathway is responsible for a distinctively abnormal plasma and cerebrospinal fluid (CSF) neurochemical pattern in Menkes patients (Kaler, 1994; Kaler et al., 2008). Peptidylglycine α-amidating monooxygenase (PAM), is required for removal of the carboxy-terminal glycine residue characteristic of numerous neuroendocrine peptide precursors (e.g., gastrin, cholecstokinin, vasoactive intestinal peptide, corticotropin releasing hormone, thyrotropin releasing hormone, calcitonin, vasopressin), and failure to amidate these precursors results in 100- to 1000-fold diminution of bioactivity compared to the mature, amidated forms. Deficiency of tyrosinase, a copper enzyme needed for melanin biosynthesis, is considered responsible for reduced hair and skin pigmentation in Menkes disease patients. Deficient cytochrome c oxidase (CCO) activity is likely a major factor in the neuropathology of Menkes disease; the brain findings (marked neuronal cell loss in the cerebral cortex and cerebellum, severe demyelination, dystrophic Purkinje cells, mitochondrial proliferation) are partly similar to those in individuals with Leigh disease (subacute necrotizing encephalomyelopathy) in whom CCO deficiency is caused by complex IV respiratory chain defects. Deficiency of copper/zinc superoxide dismutase (Cu/Zn SOD) may lower protection against oxygen free radicals and theoretically have cytotoxic effects.
Treatment of Menkes disease
Three issues remain central in configuring therapeutic strategies for Menkes disease: (1) affected infants should be identified and treatment commenced within 10 days of birth, (2) the blood–CSF and/or blood–brain barriers for copper entry to the developing central nervous system must be bridged, and (3) copper should be available to the enzymes within all cells that require it as a cofactor.
A variety of small molecule copper complexes have been employed for treatment, including copper chloride, copper gluconate, copper histidine, and copper sulfate (Kaler, 2010), with variable clinical outcomes (Kaler, 1994). Delineation of individual ATP7A mutations in a cohort of early diagnosed and treated patients helped clarify the disparate outcomes issue; subjects with mutations shown to possess partial copper transport capacity were responsive to early copper replacement, showing dramatic improvements in survival, epilepsy, and overall neurodevelopment compared to late diagnosed historical controls (Kaler et al., 2008; Kaler et al., 2010). However, in patients with severe loss of function ATP7A mutations, outcomes were less successful – even in the context of very early diagnosis and treatment. For this latter category of patient (early diagnosed but with a severe mutation), gene therapy is a theoretical possibility, although a number of potential problems would be faced. Because gene therapy requires targeting of specific organs, a disease such as Menkes that affects nearly every cell in the body would not be amenable to full correction. Assuming the brain is the target organ, concern regarding the potential toxicity of gene therapy vectors would be heightened. The gene would need to be delivered to many cells, and expression would need to be sustained. Coproduction of native mutant forms of the copper ATPase could inhibit proper function of the normal molecule expressed by the introduced gene. Despite these significant caveats, functional characterization of ATP7A, progress in gene delivery to the brain, and the severe nature of the untreated condition may render Menkes disease a candidate for gene therapy in the future (see Future directions).
For some older symptomatic Menkes disease patients, copper injection treatment has been associated with modest improvements that parents often report as small joys in the face of a difficult disease (Kaler, 1998b). Decisions concerning copper treatment in symptomatic patients are perhaps best made by the parents, following discussion of the limited benefits to be expected (Sheela et al., 2005).
Prognosis
In the natural history of classical Menkes disease, death usually occurs by 3 years of age. Survival and outcomes are clearly improved through early diagnosis and treatment (Kaler et al., 2008).
Future directions
We recently rescued a mouse model of severe Menkes disease using combination brain-directed therapies: recombinant adeno-associated virus serotype 5 (AAV5) vector expressing a reduced size human ATP7A, plus copper chloride (Donsante et al., 2011). Neither treatment alone was effective, but combination therapy significantly enhanced survival. Identification of the mechanisms underlying this pronounced synergistic effect will help illuminate the normal processes of copper transport in brain, and the function(s) of ATP7A in various neuronal cell types. In addition, this advance in gene transfer may have future clinical implications for Menkes disease patients with severe ATP7A mutations, as well as for patients with ATP7A-related motor neuron disease.
Occipital horn syndrome
A milder allelic variant of Menkes disease is known as occipital horn syndrome, in reference to the pathognomonic wedge-shaped calcifications that form within the trapezius and sternocleidomastoid muscles at their attachment to the occipital bone in affected individuals (Fig. 180.1C). This protuberance can be palpated in some patients and is demonstrable radiographically on lateral and Towne’s view skull X-rays, or appropriate sagittal CT or MRI images.
Clinical manifestations
Occipital horn syndrome shares the hair and connective tissue abnormalities of classical Menkes disease and also features the gradual development of occipital exostoses, as described above. Because the neurological phenotype in this variant is mild (slight generalized muscle weakness, and dysautonomia including syncope, orthostatic hypotension, and chronic diarrhea), affected individuals often escape detection until mid-childhood or later.
Biochemical findings
Occipital horn syndrome patients have low-normal levels of serum copper and ceruloplasmin and abnormal plasma and CSF catecholamines. The neurochemical abnormalities are distinctive although of lower magnitude than in Menkes disease. The molecular basis for typical occipital horn syndrome most often involves exon skipping with reduction of correct mRNA processing compared to normal (Kaler et al., 1994). Six of eight typical occipital horn syndrome mutations reported in the literature, as well as the molecular defect in a mouse model of occipital horn syndrome, mottled-blotchy, involve such aberrant splicing. We reported on two brothers with typical occipital horn syndrome in whom we identified a novel missense mutation within the ATP-binding domain of ATP7A (Tang et al., 2006). Characterization of this defect contributed to understanding the relationship between neurological phenotypes, function of the ATP-binding domain, and residual copper transport in this syndrome.
Treatment
Copper replacement treatment in occipital horn syndrome has been limited to date. Based on studies in murine models, copper treatment is not predicted to improve the connective tissue problems. Although patients with occipital horn syndrome have borderline low or low-normal serum copper levels, they could benefit from copper treatment, especially if provided prior to developing neurological symptoms. Oral treatment with levo-dihydroxyphenylserine (L-DOPS), a neuro-chemical that bypasses the defect in dopamine-β-hydroxylase and bolsters norepinephrine levels, could provide a direct approach to management of dysautonomic symptoms in selected patients.
Prognosis
The natural history of patients with occipital horn syndrome is not well known due to the scarcity of patients for whom long-term follow-up has been reported. Potential vascular complications could be anticipated for these patients in terms of their connective tissue problems, although there are no reports of catastrophic vascular rupture, stroke, or cardiac events in patients with this phenotype.
Future directions
A pilot study of L-DOPS for occipital horn syndrome patients with dysautonomia is planned.
Distal hereditary motor neuropathy
A third clinical phenotype, distal motor neuropathy without overt copper metabolic abnormalities, was recently found in association with mutations in the ATP7A copper transporter (Kennerson et al., 2010). Distal hereditary motor neuropathies (distal HMNs) comprise a clinically and genetically heterogeneous group of disorders predominantly affecting motor neurons in the peripheral nervous system. Distal HMNs have been classified into seven subgroups based on mode of inheritance, age of onset, distribution of muscle weakness, and clinical progression. Fifteen genetic loci for distal HMN have been mapped with eight genes identified to date. These encode a functionally diverse array of gene products including a transfer RNA synthetase, two heat shock proteins, and a microtubule motor protein involved in axonal transport.
Clinical findings
This newly recognized ATP7A allelic variant involves progressive distal motor neuropathy with minimal or no sensory symptoms. Signs include distal muscle weakness with curled fingers, pes cavus foot deformities (Fig. 180.1D), and diminished deep tendon reflexes. Neurophysiological studies indicate reduced compound motor amplitudes with normal conduction velocities. Affected patients manifest neither the severe infantile central neurological deficits observed in Menkes disease, nor the signs of autonomic dysfunction seen in occipital horn syndrome, nor the hair and connective tissue abnormalities found in both conditions, nor any of the typical biochemical features of those well characterized phenotypes. These facts highlight the distinction between this isolated distal motor neuropathy and the syndromes previously associated with ATP7A mutations. The phenomenon of late, often adult-onset, distal muscular atrophy implies that the ATP7A missense mutations causing this phenotype are unique and have attenuated effects that require years to provoke pathological consequences (Yi et al., 2012).
Biochemical findings
No biochemical abnormalities have been identified to date in these patients.
Treatment
Although the patients with ATP7A-related distal motor neuropathy evaluated to date have serum copper levels in the range of 80–100 micrograms/dL, such individuals might benefit from copper replacement, based on the known relationship between acquired copper deficiency and peripheral neuropathy (Kaler, 2010). This treatment consideration seems especially relevant for pediatric patients in distal HMN families who possess a mutant allele but have not developed neurological symptoms. Ideally, response to treatment would be tracked with objective measures of distal motor neuron structure and function (e.g., diffusion tensor imaging and nerve conduction studies) obtained at regular intervals.
Prognosis
The natural history and estimation of long-term prognosis for ATP7A-related distal motor neuropathy require additional evaluation. To date, only two affected families are known, although it is anticipated that approximately 5–10% of distal motor neuropathy in males may have this molecular basis.
Future directions
Motor neuron-directed gene transfer is under investigation for spinal muscular atrophy (Werdnig–Hoffman disease) and could be relevant to ATP7A-related distal HMN. Knockin mouse models harboring specific human ATP7A missense mutations will be useful in evaluating this consideration. In the past 2 years (2012–2013), three new autosomal recessive copper metabolism conditions have been recognized: 1) Huppke—Brendel syndrome caused by mutations in an acetyl CoA transporter needed for acetylation of one or more copper proteins (Huppke et al., 2012), 2) CCS deficiency caused by mutations in the copper chaperone to SOD1 (Huppke et al., 2012a), and 3) MEDNIK syndrome, which revealed that mutations in the σ1A subunit of adaptor protein complex 1 (AP-1) have detrimental effects on trafficking of ATP7A and ATP7B (Martinelli et al., 2013). These conditions are also summarized in Table 180.1.
Table 180.1.
Neurological, biochemical, and molecular features in copper transport disorders
| Condition | Age of onset (years) |
Neurological signs | Other clinical manifestations | Biochemical findings | Molecular defects | Treatment options | Prognosis | Future directions |
|---|---|---|---|---|---|---|---|---|
| Wilson disease | 10–40 | Dysarthria, dystonia, rigidity, abnormal gait, poor handwriting, tremor | Kayser–Fleischer ring (corneal deposition of copper) | Low serum copper and ceruloplasmin; increased urinary copper excretion; high liver copper | Diverse mutations in ATP7B | Copper chelation with penicillamine, trien,*** tetrathiomolybdate, zinc acetate | Favorable, if patient compliant with medical treatment | Liver-directed gene therapy; hepatocyte transfer |
| Menkes disease | 0–1 | Hypotonia, seizures, developmental delay, brain atrophy | Coarse hair, jowly facies, lax skin and joints, decreased bone density, bladder diverticula, gastric polyps, vascular tortuousity | Low serum copper and ceruloplasmin; abnormal plasma and CSF neurochemicals; increased urine β2-microglobulin | Diverse mutations in ATP7A (Fig. 180.2A) | Early copper replacement | Difficult, unless very early diagnosis/ treatment (within 2 weeks of birth) | Newborn screening for early detection; brain-directed combination therapy: copper + ATP7A viral gene therapy |
| Occipital horn syndrome (OHS) | 3–10 | Dysautonomia,* muscle weakness | Coarse hair, occipital exostoses, hammer-shaped clavicular heads, lax skin and joints, bladder diverticula, vascular tortuousity | Low-normal serum copper and ceruloplasmin; abnormal plasma and CSF neurochemicals | “Leaky” splice junction and hypofunctional missense mutations in ATP7A (Fig. 180.2B) | L-dihydroxyphenylserine (L-DOPS) for dysautonomia | Fair (long-term natural history not known) | Newborn screening for early detection and early copper replacement |
| X-linked distal hereditary motor neuropathy (dHMN) | 5–50 | Atrophy and weakness of distal muscles, foot drop, abnormal nerve conduction studies** | No other specific clinical abnormalities | No specific laboratory abnormalities | Missense mutations in carboxyl half of ATP7A (Fig. 180.2C) | Copper replacement in selected patients | Unknown (long-term natural history uncertain) | Motor neuron-directed viral gene therapy |
| Huppke–Brendel syndrome | 0–1 | Global developmental delay, hypotonia, sensorineural deafness, brain atrophy | Cataracts, nystagmus | Low serum copper and ceruloplasmin | SLC33A1 (acetyl-CoA transporter) | None available at present | Poor | Possibly viral gene therapy to replete SLC33A1 |
| CCS deficiency | 0–1 | Neonatal hypotonia, global developmental delay, abnormal brain MRI, epilepsy | Pericardial effusion | Low SOD1 activity (superoxide dismutase) | CCS (copper chaperone to SOD) | None available at present | Poor | Possibly viral gene therapy to replete CCS |
| MEDNIK | 0–1 | Mental retardation, deafness, neuropathy | Enteropathy, ichthyosis, keratodermia | Low copper and ceruloplasmin, high liver copper, high plasma very long-chain fatty acids | AP1S1 (sigma1A subunit of adaptor protein complex 1) | Zinc acetate | Poor neurological prognosis, liver disease treatable | Possibly viral gene therapy to replete AP1S1 |
Syncope, dizziness, orthostatic hypotension, abnormal sinoatrial conduction, nocturnal bradycardia, and bowel or bladder dysfunction.
Decreased peroneal and median muscle amplitudes with normal conduction velocities.
Triethylene tetramine dihydrochloride.
Acknowledgments
I gratefully acknowledge the members of my laboratory for their intelligence and dedicated work, as well as the human subjects who have participated in our clinical research program, and their families.
References
- Baerlocher K, Nadal D. Das Menkes-syndrom. Ergeb Inn Med Kinderheilkd. 1988;57:77–144. [PubMed] [Google Scholar]
- Bahi-Buisson N, Kaminska A, Nabbout R, et al. Epilepsy in Menkes disease: analysis of clinical stages. Epilepsia. 2006;47:380–386. doi: 10.1111/j.1528-1167.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- Bull PC, Thomas GR, Rommens JM, et al. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327–337. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- Danks DM, Campbell PE, Walker-Smith JS, et al. Menkes’ kinky-hair syndrome. Lancet. 1972;1:1100–1102. doi: 10.1016/s0140-6736(72)91433-x. [DOI] [PubMed] [Google Scholar]
- Dening TR, Berrios GE. Wilson’s disease: clinical subgroups in 400 cases. Acta Neurol Scand. 1989;80:527–534. doi: 10.1111/j.1600-0404.1989.tb03922.x. [DOI] [PubMed] [Google Scholar]
- Donsante A, Yi L, Zerfas P, et al. ATP7A gene addition to the choroid plexus results in long-term rescue of the lethal copper transport defect in a Menkes disease mouse model. Mol Ther. 2011;19:2114–2123. doi: 10.1038/mt.2011.143. http://dx.doi.org/10.1038/mt.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E, Harden A, Koivikko M, et al. Menkes’ disease: neurophysiological aspects. J Neurol Neurosurg Psychiatry. 1978;41:505–510. doi: 10.1136/jnnp.41.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin SC, Shawker T, Chang M, et al. Brachial artery aneurysms in Menkes disease. J Pediatr. 2006;149:412–415. doi: 10.1016/j.jpeds.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Gunn TR, McFarlane S, Phillips LI. Difficulties in the neonatal diagnosis of Menkes’ kinky hair syndrome-trichopoliodystrophy. Clin Pediatr. 1984;23:514–516. doi: 10.1177/000992288402300915. [DOI] [PubMed] [Google Scholar]
- Harris ZL, Takahashi Y, Miyajima H, et al. Aceruloplasminemia: molecular characterization of this disorder of iron metabolism. Proc Natl Acad Sci U S A. 1995;92:2539–2543. doi: 10.1073/pnas.92.7.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppke P, Brendel C, Kalscheuer V, et al. Mutations in SLC33A1 cause a lethal autosomal-recessive disorder with congenital cataracts, hearing loss, and low serum copper and ceruloplasmin. Am J Hum Genet. 2012;90:61–68. doi: 10.1016/j.ajhg.2011.11.030. http://dx.doi.org/10.1016/j-ajhg.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppke P, Brendel C, Korenke GC, et al. Molecular and biochemical characterization of a unique mutation in CCS, the human copper chaperone to superoxide dismutase. Hum Mutation. 2012a doi: 10.1002/humu.22099. http://dx.doi.org/10.1002/humu.22099. [DOI] [PMC free article] [PubMed]
- Kaler SG. Menkes disease. In: Barness LA, editor. Advances in Pediatrics. Vol. 41. C.V. Mosby; St. Louis: 1994. pp. 263–304. [PubMed] [Google Scholar]
- Kaler SG. Molecular and metabolic bases of Menkes disease and occipital horn syndrome. Pediatr Dev Pathol. 1998a;1:85–98. doi: 10.1007/s100249900011. [DOI] [PubMed] [Google Scholar]
- Kaler SG. Diagnosis and therapy of Menkes disease, a genetic form of copper deficiency. Am J Clin Nutr. 1998b;67:S1029–S1034. doi: 10.1093/ajcn/67.5.1029S. [DOI] [PubMed] [Google Scholar]
- Kaler SG. Wilson disease. In: Goldman L, Ausiello D, editors. Cecil’s Textbook of Medicine. 23. ch 230. Saunders; Philadelphia: 2008. pp. 1593–1595. [Google Scholar]
- Kaler SG. Small copper complexes for treatment of ATP7A-related disorders. In: Thoene JG, editor. Small Molecule Therapy of Genetic Diseases. Cambridge University Press; Cambridge: 2010. pp. 202–212. [Google Scholar]
- Kaler SG. ATP7A-related copper transport diseases –emerging concepts and future trends. Nat Rev Neurol. 2011;7:15–29. doi: 10.1038/nrneurol.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler SG, Westman JA, Bernes SM, et al. Gastrointestinal hemorrhage associated with gastric polyps in Menkes disease. J Pediatr. 1993;122:93–95. doi: 10.1016/s0022-3476(05)83496-1. [DOI] [PubMed] [Google Scholar]
- Kaler SG, Gallo LK, Proud VK, et al. Occipital horn syndrome and a mild Menkes phenotype associated with splice site mutations at the MNK locus. Nat Genet. 1994;8:195–202. doi: 10.1038/ng1094-195. [DOI] [PubMed] [Google Scholar]
- Kaler SG, Holmes CS, Goldstein DS, et al. Neonatal diagnosis and treatment of Menkes disease. N Engl J Med. 2008;358:605–614. doi: 10.1056/NEJMoa070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler SG, Liew CJ, Donsante A, et al. Molecular correlates of epilepsy in early diagnosed and treated Menkes disease. J Inherit Metab Dis. 2010;33:583–589. doi: 10.1007/s10545-010-9118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerson ML, Nicholson GA, Kaler SG, et al. Missense mutations in the copper transporter gene ATP7A cause X-linked distal hereditary motor neuropathy. Am J Hum Genet. 2010;86:343–352. doi: 10.1016/j.ajhg.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsenko S, Gupta A, Burkhead JL, et al. Cellular multitasking: the dual role of human Cu-ATPases in cofactor delivery and intracellular copper balance. Arch Biochem Biophys. 2008;476:22–32. doi: 10.1016/j.abb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli D, Travaglini L, Drouin CA, et al. MEDNIK syndrome: a novel defect of copper metabolism treatable by zinc acetate therapy. Brain. 2013 doi: 10.1093/brain/awt012. http://dx.doi.org/10.1093/brain/awt012. [DOI] [PubMed]
- McNeill A, Pandolfo M, Kuhn J, et al. The neurological presentation of ceruloplasmin gene mutations. Eur Neurol. 2008;60:200–205. doi: 10.1159/000148691. [DOI] [PubMed] [Google Scholar]
- Menkes JH, Alter M, Steigleder GK, et al. A sex-linked recessive disorder with retardation of growth, peculiar hair, and focal cerebral and cerebellar degeneration. Pediatrics. 1962;29:764–779. [PubMed] [Google Scholar]
- Price D, Ravindranath T, Kaler SG. Internal jugular phlebectasia in Menkes disease. Int J Pediatr Otorhinolaryngol. 2007;71:1145–1148. doi: 10.1016/j.ijporl.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheela SR, Manoj L, Liu P-C, et al. Copper replacement treatment for symptomatic Menkes disease: ethical considerations. Clin Genet. 2005;68:278–283. doi: 10.1111/j.1399-0004.2005.00496.x. [DOI] [PubMed] [Google Scholar]
- Tang J, Robertson SP, Lem KE, et al. Functional copper transport explains neurologic sparing in occipital horn syndrome. Genet Med. 2006;8:711–718. doi: 10.1097/01.gim.0000245578.94312.1e. [DOI] [PubMed] [Google Scholar]
- Tang J, Donsante A, Desai V, et al. Clinical outcomes in Menkes disease patients with a copper-responsive ATP7A mutation, G727R. Mol Genet Metab. 2008;95:174–181. doi: 10.1016/j.ymgme.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walshe JM. The story of penicillamine: a difficult birth. Mov Disord. 2003;18:853–859. doi: 10.1002/mds.10458. [DOI] [PubMed] [Google Scholar]
- White SR, Reese K, Sato S, et al. Spectrum of EEG findings in Menkes disease. Electroencephalogr Clin Neurophysiol. 1993;87:57–61. doi: 10.1016/0013-4694(93)90175-u. [DOI] [PubMed] [Google Scholar]
- Yi L, Donsante A, Kennerson ML, et al. Altered intra-cellular localization and valosin-containing protein (p97 VCP) interaction underlie ATP7A-related distal motor neuropathy. Hum Mol Genet. 2012;21:1794–1807. doi: 10.1093/hmg/ddr612. [DOI] [PMC free article] [PubMed] [Google Scholar]