Abstract
Background
Brain imaging studies suggest that volume reductions and compromised white matter integrity occur in schizophrenia and bipolar disorder (BD). However, the cellular correlates have not yet been identified. To address this issue we assessed oligodendrocyte, astrocyte and microglial populations in postmortem white matter from schizophrenia, BD and nonpsychiatric control samples.
Methods
The density, areal fraction and spatial distribution of glial fibrillary acidic protein (GFAP)-expressing astrocytes and ionized calcium-binding adaptor molecule-1 (IBA-1)-expressing microglia as well as the density, nuclear size and spatial distribution of Nissl-stained oligodendrocytes were quantified in postmortem white matter adjacent to the dorsolateral prefrontal cortex (Brodmann area 9) in schizophrenia, BD and control samples (n = 20). In addition, the oligodendrocyte-associated proteins myelin basic protein and 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase) were quantified in the same samples by enzyme-linked immunosorbent assay and immunoblotting.
Results
Oligodendrocyte density (p = 0.012) and CNPase protein levels (p = 0.038) differed between groups, being increased in BD compared with control samples. The GFAP area fraction (p = 0.05) and astrocyte spatial distribution (p = 0.040) also differed between groups, reflecting decreased area fraction and increased cell clustering in both schizophrenia and BD samples.
Limitations
Oligodendrocytes were identified using morphological criteria.
Conclusion
This study provides evidence for glial pathology in prefrontal white matter in schizophrenia and BD. Changes in oligodendrocyte and astrocyte populations in white matter in the major psychiatric disorders may reflect disruptions in structural or metabolic support of axons.
Introduction
Schizophrenia and bipolar disorder (BD) are 2 disabling mental illnesses, each with a prevalence of approximately 25–30 million people worldwide.1 While the causes are unknown, brain imaging studies have implicated white matter dysfunction in individuals with these disorders.2,3 Specifically, disruption of frontal white matter tracts has been identified by diffusion tensor imaging in both schizophrenia4 and BD populations,5 while meta-analyses have revealed reduced cross-sectional area of the corpus callosum.6,7 In addition, frontal white matter hyperintensities are more common in BD than in control populations.7
The molecular and cellular correlates of these imaging findings remain to be elucidated, although glial cells have been implicated in both disorders. While we previously reported lower glial density in temporal white matter in a schizophrenia sample,8 it is unknown if this deficit is specific to any individual glial population (i.e., oligodendrocytes, astrocytes, microglia). Hof and colleagues9 have reported decreased oligodendrocyte density in frontal white matter in a schizophrenia sample, while molecular alterations related to oligodendrocytes and the myelin sheath, including reduced expression of 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase), have been noted in this disorder.10,11 However, others have found no significant change in oligodendrocyte density in white matter in schizophrenia or BD samples12–15 and no change in the oligodendrocyte-associated proteins myelin basic protein (MBP) or CNPase in schizophrenia samples.16,17 Astrocyte and microglial populations have rarely been studied in white matter in individuals with these disorders, although decreased astrocyte density in cingulate white matter in schizophrenia, but not BD samples,15 and increased density of activated microglia in frontal white matter in schizophrenia samples18 have been reported.
Considering that white matter plays an essential role in communication between brain regions, abnormalities of white matter glia could lead to alterations in structural or functional support of axons, ultimately contributing to disturbed neural connectivity in individuals with schizophrenia and BD. The purpose of the present study was to assess the 3 major glial populations in well-characterized postmortem tissues from schizophrenia, BD and nonpsychiatric control samples. The density, area fraction and spatial distribution of glial fibrillary acidic protein (GFAP)–expressing astrocytes and ionized calcium binding adaptor molecule-1 (IBA-1)-expressing microglia as well as the density, nuclear size and spatial distribution of Nissl-stained oligodendrocytes were quantified in white matter underlying the dorsolateral prefrontal cortex (DLPFC). In addition, given that we did not use a cell-specific marker for oligodendrocytes, we further examined alterations in this glial population by quantifying levels of the oligodendrocyte-associated proteins MBP and CNPase in the same sample using enzyme-linked immunosorbent assay (ELISA) and immunoblotting.
Methods
Population
We obtained postmortem brain tissue from the Stanley Medical Research Institute’s brain collection (array collection). The population comprised samples from 60 people: 20 who had schizophrenia, 20 who had BD and 20 who were nonpsychiatric controls. Brain tissue was collected as previously described.19 Diagnoses were made according to DSM-IV criteria. Routine neuropathological examinations found no evidence of neurodegenerative changes or pathological lesions. Demographic and clinical details are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of the study sample
| Group; mean ± SD* | |||
|---|---|---|---|
|
|
|||
| Characteristic | Control, n = 20 | Schizophrenia, n = 20 | BD, n = 20 |
| Age, yr | 45.3 ± 6.5 | 44.7 ± 6.9 | 47.4 ± 0.7 |
| PMI, h | 29.9 ± 12.7 | 30.7 ± 11.2 | 37.4 ± 19.6 |
| Brain pH | 6.7 ± 0.2 | 6.5 ± 0.2 | 6.4 ± 0.3 |
| Fixed hemisphere, right:left | 11:9 | 10:10 | 11:9 |
| Sex, male:female | 14:6 | 13:7 | 8:12 |
| Cause of death, no. | |||
| Cardiac | 18 | 8 | 5 |
| Cancer | 1 | — | — |
| Asthma | 1 | — | — |
| Suicide | — | 4 | 6 |
| Pneumonia | — | 3 | 1 |
| Overdose | — | 2 | 4 |
| Cirrhosis | — | 1 | — |
| Drowning | — | — | 2 |
| Acute pancreatitis | — | 1 | — |
| Sleep apnea | — | — | 1 |
| Thromboembolism | — | 1 | — |
| Ketacidosis | — | — | 1 |
| Age at onset, yr | NA | 20.9 ± 6.7 | 24.0 ± 8.5 |
| Illness duration, yr | NA | 23.8 ± 10.9 | 23.4 ± 9.9 |
| Lifetime antipsychotic dose, mg† | NA | 90 383 (108,083) | 13 960 (28,708) |
| Illicit drug use, low:high‡ | 19:1 | 11:7 | 10:10 |
| Alcohol use, low:high‡ | 17:3 | 11:9 | 6:14 |
BD = bipolar disorder; NA = not applicable; PMI = postmortem interval; SD = standard deviation.
Unless otherwise indicated.
Fluphenazine equivalents.
Data on smoking and substance use is not available for all individuals.
Immunohistochemistry
Tissue blocks were dissected from the middle frontal gyrus, rostral to the genu of the corpus callosum, from either the left or right hemisphere. Tissue was embedded in paraffin, serially sectioned in the coronal plane at 6 μm and mounted onto glass slides. A series of 15 paraffin-embedded sections containing the DLPFC (Brodmann Area [BA] 9) and underlying white matter was made available to us. For each antibody, 3 sections were selected per individual, with an interval of 30 μm between sections. To optimize antigen retrieval, sections were heated at 95°C in 0.01 M citrate buffer (pH 6) for 10 minutes (IBA-1) or 15 minutes (GFAP). We performed GFAP immunostaining using an ABC kit (Vector Laboratories), with overnight incubation at 4°C in a mouse monoclonal antibody (Sternberger Monoclonal Inc; 1:8000) and visualization with diaminobenzadine (DAB; Vector Laboratories). For IBA-1 immunostaining, sections were incubated overnight at 4°C in a rabbit polyclonal antibody (Wako; 1:8000). Sections were then incubated in biotinylated goat anti-rabbit (Jackson Immunoresearch; 1:1000) followed by peroxidase-conjugated streptavidin (Jackson Immunoresearch; 1:12000). We visualized IBA-1 using an enhanced ImmPACT DAB substrate (Vector Laboratories). All sections were counterstained with cresyl violet before being cover-slipped. Immunostaining was performed using the Dako Autostainer Plus (Dako, Canada Inc.). Omission of the primary antibody resulted in the absence of immunostaining.
Microscopic image analysis
Images were obtained using an Olympus BX61 light microscope with ×40 objective (NA 0.95) Olympus DP71 camera and Prior ProScan II motorized stage system, coupled with the software Image-Pro Plus (version 6.3.1.542; Media Cybernetics). For each stained section, 3 counting frames (each 700 μm × 700 μm) were randomly placed directly below the grey matter–white matter border of a straight section of DLPFC. Each counting frame comprised a tiled series of contiguous images, with a total of 9 counting frames per individual (total area analyzed per individual = 4.41 mm2) for each antibody. A single investigator (C.H.) performed all cell assessments blind to diagnosis.
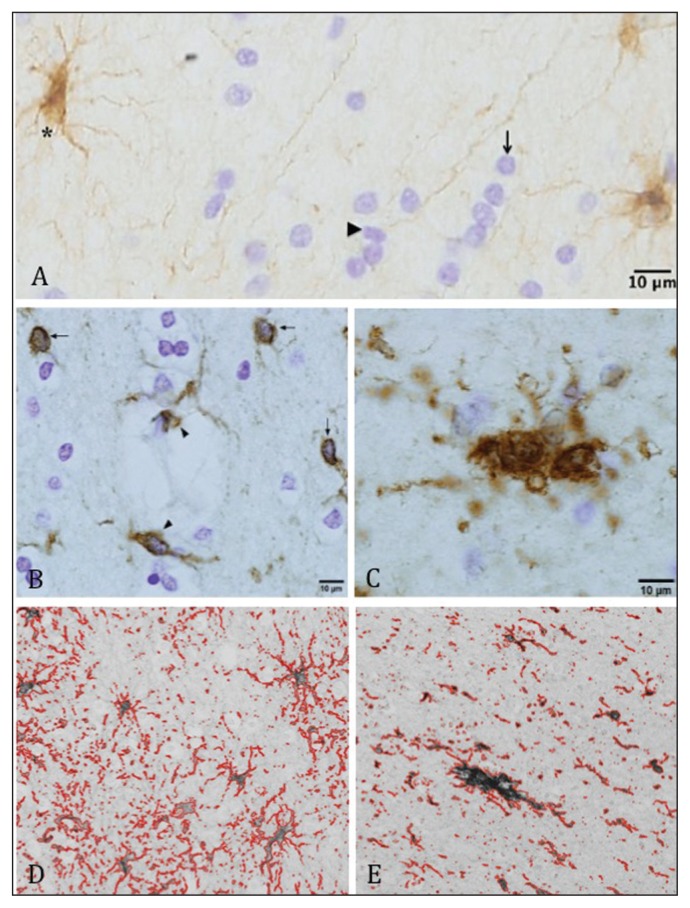
Astrocytes and oligodendrocytes were counted on composite images from GFAP-stained sections. Astrocytes were identified as cells with GFAP-positive cell bodies and processes that displayed classic astrocytic morphology (Fig. 1A). Nissl-stained oligodendrocytes were differentiated from other cell types by the presence of a small round or oval nucleus, more heterogeneous chromatin within the nucleus and a narrow rim of cytoplasm (Fig. 1A). Only oligodendrocytes with nuclei 3–7 μm in diameter were counted.20 Oligodendrocyte nuclear size was assessed in 1 counting frame per individual (approximately 460 cells/ individual) using a 2-step editing process.8,21 The Abercrombie correction factor22 was applied to oligodendrocyte density data to correct for potential bias due to section thickness and cell size. Microglial cells were counted on composite images from IBA-1-stained sections. Perivascular microglial cells located within blood vessels (Fig. 1B) were excluded from the microglial counts. Considering IBA-1 labels both activated and resting microglia, a qualitative neuropathological assessment of microglia morphology was also conducted. Microglia were classified as activated if cell bodies were enlarged and poorly defined, with thickening of processes near the soma and overall fewer and shorter processes (Fig. 1C). Cases containing areas of unambiguous microglial activation were noted.
Fig. 1.
Photomicrographs showing glial fibrillary acidic protein (GFAP)–labelled astrocytes, Nissl-stained oligodendrocytes and ionized calcium binding adaptor molecule-1 (IBA-1)–labelled microglia. (A) GFAP-immunoreactive astrocytes (asterisk), Nissl-stained oligodendrocytes (arrow) and microglia (arrowhead). (B) Filled arrows indicate resting microglia and arrowheads indicate perivascular microglial cells. (C) Activated microglia showing enlarged, poorly defined cell bodies and overall fewer and shorter processes. (D) GFAP area fraction. Percentage area fraction was calculated by dividing the area covered by GFAP-immunoreactive cells and processes by the area of the counting frame. (E) IBA-1 area fraction. Percentage area fraction was calculated by dividing the area covered by IBA-1-immunoreactive cells and processes by the area of the counting frame.
A total of 302 040 cells were counted, comprising 236 731 oligodendrocytes, 33 593 astrocytes and 31 716 microglia. Coefficients of error23 were 0.016 for oligodendrocytes and 0.043 for astrocytes and microglia. The reliability of astrocyte, oligodendrocyte and microglial cell counts was assessed by requantifying 5 cases chosen at random. Intrarater correlation coefficients were 0.981, 0.985 and 0.990, respectively.
Area fraction
The area occupied by GFAP-positive astrocytes and IBA-1-positive microglia was examined in each counting frame. Briefly, colour composite images were converted into greyscale and immunoreactive structures segmented by first defining an average background level in an area without staining, then subtracting a fixed level of 30 grey values from the background intensity level. Grey levels ranged from 0 (darkest) to 255 (lightest). Thresholded areas were outlined and summed, yielding the percentage area of GFAP (Fig. 1D) or IBA-1 (Fig. 1E) staining.
Spatial pattern analysis
To estimate the spatial distribution of oligodendrocytes, astrocytes and microglia, we used a 2-dimensional Voronoi tessellation approach.24,25 Maps of the distribution of oligodendrocytes, astrocytes and microglia were constructed separately with Voron software (courtesy of Dr. Charles Duyckaerts) used to compute Voronoi tessellation. All counting frames were analyzed, and mean coefficients of clustering (CC) were obtained. The CC yields a measure of the distribution of cells; a CC tending toward 1 represents a random distribution, whereas a higher ratio represents a more clustered distribution.
Quantification of oligodendrocyte-associated proteins
Frozen white matter from the opposite hemisphere of the same individuals was homogenized in 10 volumes of ice-cold tris buffered saline and stored at −80°C until required. Total protein concentrations were determined using the DC Protein Assay (BioRad).
We quantified MBP using ELISA, as previously described16,26 with minor modifications. Briefly, duplicate samples were serially diluted over a 128-fold range with a starting concentration of 15 μg/mL. Plates were incubated overnight at 4°C with a mouse monoclonal antibody (Sternberger Monoclonal Inc.; 1:1000). Plates were subsequently incubated with peroxidase-conjugated goat antimouse (Jackson Immunoresearch; 1:1000), then with 2,2′-azino-di-3-ethylbenzthiazoline (KPL Inc.). The optical density of each well was determined at 405 nm using a microplate reader (Molecular Devices). The optical density was determined at a protein concentration of 5 μg, within the linear range for each sample, and compared between samples. Each sample was run twice on different days, resulting in a between-run correlation of r = 0.80, with mean values used for analysis.
We quantified CNPase by immunoblotting. Briefly, 3 μg protein/lane was run on 12% polyacrylamide gels, then transblotted to polyvinylidene fluoride membranes. Pilot studies indicated that this concentration fell within the linear range of detection of our assay. Blots were stained for total protein using MemCode reversible protein stain (ThermoScientific) and imaged using a LAS 3000 Luminescence Image Analyzer (FujiFilm). Blots were then incubated with a mouse monoclonal antibody CNPase (Sternberger Monoclonal Inc.; 1:3000). Following incubation with peroxidase-conjugated goat antimouse (Jackson Immunoresearch; 1:1000), chemiluminescence substrate (ECL, GE Healthcare) was applied and blots reimaged. Four pooled standard samples were included on each gel to act as a between-gel reference. Blot images were quantified using Science Laboratory Image Gauge (FujiFilm). The intensity of each band of interest was normalized both to the average band intensity of the adjacent pooled standards (to account for blot-to-blot variability) and to the total protein stain for that lane (to account for variations in sample loading and transfer), as previously described.27 Samples were run twice on different days, resulting in a between-run correlation of r = 0.84, with mean values used for analysis.
Statistical analysis
The main objective of this study was to compare oligodendrocyte, astrocyte and microglial measures between each of the patient groups and the control group. Normality was verified using Shapiro–Wilks tests and, when necessary, data were transformed to conform to a normal distribution. Between-group comparisons were made by univariate analysis of variance (ANOVA) using diagnoses as contrasts. Type-I error was set at 0.05. We performed Pearson correlations to examine the association between age, postmortem interval (PMI) and pH and cellular or protein measures. When data were not normally distributed we used Spearman rank correlations. We examined the influence of the variables sex and hemisphere separately using 2-way ANOVA. If a significant association was found between any of these continuous or categorical variables and cellular or protein measures, we included them as covariates or fixed factors in an additional analysis of covariance (ANCOVA) model.
Secondary analyses were performed to assess the potential impact of suicide, prescribed medication and substance use on cellular or protein measures. The effect of suicide was assessed by ANOVA, with post hoc least significant difference (LSD) tests. In the schizophrenia group, the association between estimated lifetime antipsychotic dose (fluphenazine equivalents) and cellular and protein measures was determined using Pearson or Spearman rank correlations. We used ANOVA or ANCOVA analyses to examine the effect of prescribed mood stabilizers and antidepressants within the BD group and the effect of illicit drug or alcohol use within both patient groups. We divided the samples into those with none/social drug or alcohol use and those with moderate/heavy drug or alcohol use according to Brain Bank criteria. Analyses were carried out in SPSS software version 15.
Results
Influence of demographic and clinical characteristics
Mean brain pH differed among the groups (F2,57 = 5.45, p = 0.007), with both the schizophrenia (p = 0.039) and BD (p = 0.002) groups having lower values than the control group. Age, PMI, sex and hemisphere did not differ significantly among the groups.
Correlations were observed between GFAP area fraction and pH (r = 0.49, p < 0.001). The IBA-1 area fraction (r = 0.28, p = 0.037), MBP (r = 0.32, p = 0.012), oligodendrocyte nuclear area (r = −0.33, p = 0.011) and oligodendrocyte nuclear diameter (r = −0.32, p = 0.012) all correlated with PMI.
Increasing evidence suggests that men and women exhibit distinct pathophysiology in schizophrenia and BD.28,29 We therefore assessed the influence of sex on our cellular and protein measures using 2-way ANOVAs. We observed a main effect of sex on oligodendrocyte nuclear area (F1,54 = 5.20, p = 0.027) and oligodendrocyte diameter (F1,54 = 5.28, p = 0.025), with men having larger cell sizes than women. We also noted an effect of sex on MBP levels (F1,54 = 6.60, p = 0.013), with protein expression being higher in men. There was no main effect of hemisphere on any cytoarchitectural measure, although we observed an effect of hemisphere on CNPase levels (F1,54 = 4.85, p = 0.032), with protein expression higher in the left hemisphere in all groups.
Cell density
Cell density results are listed in Table 2. We observed a significant effect of diagnosis on oligodendrocyte density (F2,57 = 4.78, p = 0.012), reflecting increased oligodendrocyte density in the BD group compared with the control group (17%, p = 0.011). There was no difference between the schizophrenia and control groups. Given the controversy surrounding the application of the Abercrombie correction for cell size,30 we repeated the analysis without the use of the Abercrombie correction. In this case the nature of the results was not altered (F2,57 = 5.10, p = 0.009). The oligodendrocyte nuclear area and diameter did not differ among the groups. While the density of GFAP-immunoreactive (GFAP-IR) astrocytes did not differ significantly among the groups, we observed a trend toward decreased astrocyte density in the schizophrenia group (p = 0.06, 10%). We excluded samples from 4 individuals from the analysis of IBA-IR microglia owing to lack of staining. Following logarithmic transformation, microglial density data attained a normal distribution. The density of IBA-1-stained microglia did not differ among the groups; however, a qualitative assessment of microglial morphology found numerous activated microglial cells in 3 schizophrenia samples, but not in control or BD samples.
Table 2.
Summary of glial cell measures
| Group; mean ± SD | ||||
|---|---|---|---|---|
|
|
||||
| Glial cell measure | Control | Schizophrenia | BD | p value |
| Oligodendrocyte density, cells/mm2 | 345.29 ± 54.65 | 343.21 ± 68.86 | 402.50 ± 80.53 |
*0.012 †0.92 ‡0.011 |
| Astrocyte density, cells/mm2 | 133.62 ± 17.73 | 119.64 ± 18.39 | 127.62 ± 30.48 | *0.16 |
| Microglia density, cells/mm2 | 126.59 ±26.62 | 132.61 ± 33.11 | 124.32 ± 27.86 | *0.74 |
| GFAP area fraction, % | 35.17 ± 12.30 | 26.70 ± 13.45 | 25.83 ± 13.16 |
*0.05 †0.044 ‡0.027 |
| IBA-1 area fraction, % | 9.55 ± 1.79 | 9.13 ± 1.82 | 9.96 ± 2.40 | *0.46 |
| Oligodendrocyte nuclear area, μm2 | 26.25 ± 4.87 | 26.33 ± 4.64 | 25.29 ± 3.91 | *0.72 |
| Oligodendrocyte nuclear diameter, μm | 5.58 ± 0.62 | 5.55 ± 0.53 | 5.43 ± 0.45 | *0.65 |
| Oligodendrocyte CC | 1.49 ± 0.09 | 1.54 ± 0.14 | 1.53 ± 0.10 | *0.33 |
| Astrocyte CC | 1.24 ± 0.03 | 1.28 ± 0.04 | 1.28 ± 0.07 |
*0.040 †0.031 ‡0.025 |
| Microglia CC | 1.21 ± 0.03 | 1.23 ± 0.03 | 1.22 ± 0.05 | *0.14 |
BD = bipolar disorder; CC = coefficient of clustering; GFAP = glial fibrillary acidic protein; IBA-1 = ionized calcium binding adaptor molecule 1; SD = standard deviation.
Between-subjects effect.
Schizophrenia compared with controls.
Bipolar disorder compared with controls.
Area fraction
The ANOVA model indicated that GFAP area fraction differed among the groups (F2,57 = 3.16, p = 0.05), reflecting decreased GFAP area fraction in both the schizophrenia (24%, p = 0.044) and BD (27%, p = 0.027) groups compared with the control group. However, following addition of brain pH as a covariate in the model, this finding did not attain statistical significance. The IBA-1 area fraction did not differ among the groups.
Spatial distribution
Oligodendrocyte CC and microglial CC data were transformed using logarithmic and reciprocal transformations, respectively. We observed a significant effect of group on astrocyte CC (F2,57 = 3.41, p = 0.040), reflecting significantly higher astrocyte CC in the schizophrenia (p = 0.031) and BD (p = 0.025) groups than in the control group, which is indicative of a more clustered distribution of GFAP-positive astrocytes in the psychiatric groups. Oligodendrocyte and microglial CC did not differ among the groups.
Oligodendrocyte-associated proteins
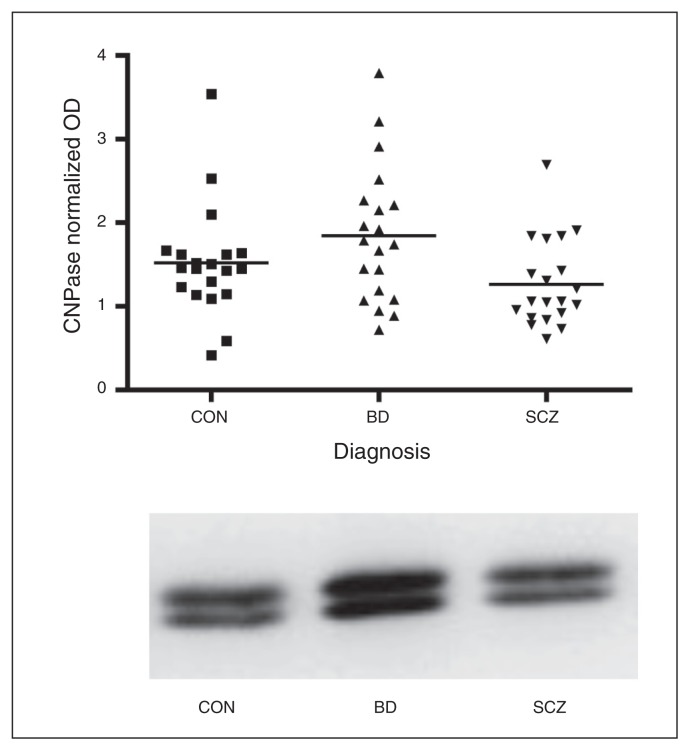
We observed a significant effect of diagnosis on CNPase (F2,57 = 3.46, p = 0.038). Addition of hemisphere in an ANCOVA model did not alter the nature of the results (F2,54 = 3.39, p = 0.041). Protein levels were increased by 21% in the BD group and decreased by 17% in the schizophrenia group (Fig. 2); however, this did not reach statistical significance for either group individually. There was no difference in MBP among the groups.
Fig. 2.
Levels of the oligodendrocyte-associated protein 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase) in prefrontal white matter in the control (CON), schizophrenia (SCZ) and bipolar disorder (BD) groups. CNPase optical density (OD) differed among the groups (ANOVA F2,57 = 3.46, p = 0.038), being increased by 21% in the BD group and decreased by 17% in the schizophrenia group. Examples of CNPase bands from Western blotting experiments are shown.
Influence of suicide, medication and substance use
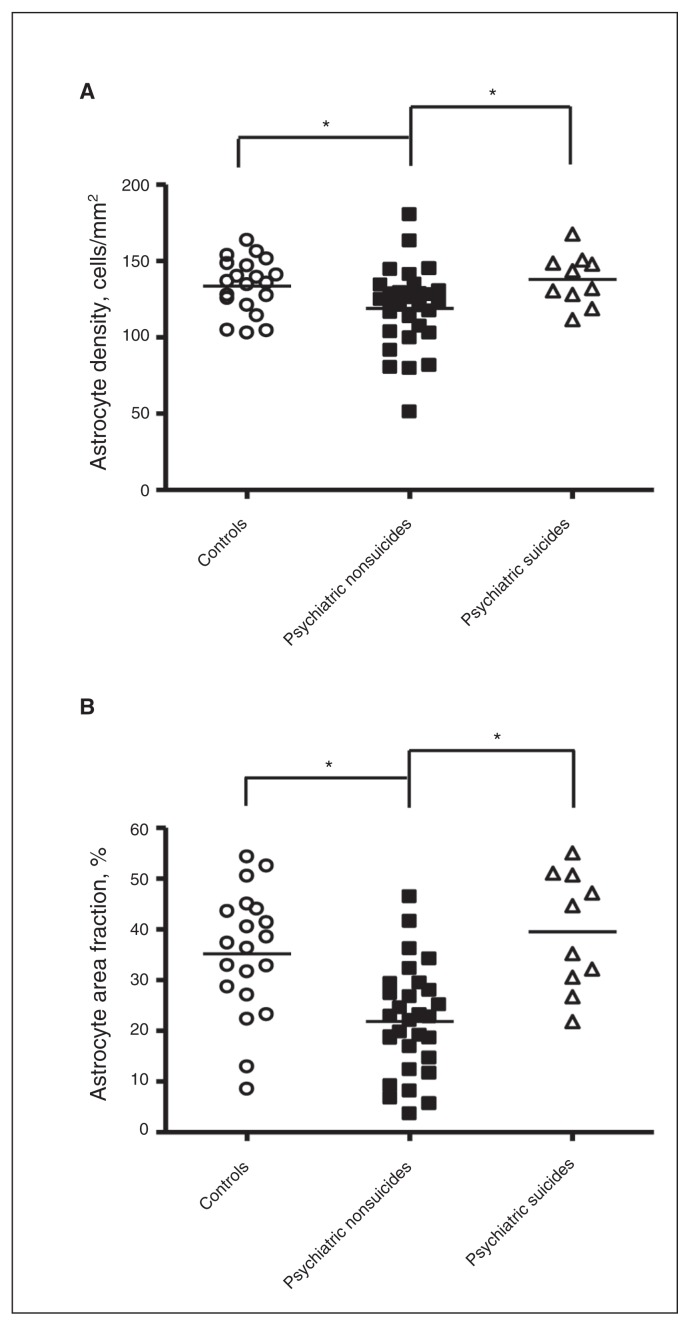
Suicidal behaviours may share common pathologies not associated with any specific psychiatric diagnosis.31,32 Measures were compared in all psychiatric patients who died by suicide (n = 10), all psychiatric patients who died by other means (n = 30) and controls (n = 20). Astrocyte densities differed significantly among the groups (F2,57 = 4.12, p = 0.021), with post hoc LSD tests indicating that the psychiatric nonsuicide group had significantly lower astrocyte densities than the control group (p = 0.025) and the suicide group (p = 0.022; Fig. 3A). Similar differences were also seen for GFAP area fraction (F2,57 = 13.34, p < 0.001), with post hoc LSD tests indicating that the psychiatric nonsuicide group had significantly lower GFAP area fraction than the control group (p < 0.001) and the suicide group (p < 0.001; Fig. 3B). Following addition of brain pH as a covariate in the model, a significant group effect remained (F2,56 = 9.51, p < 0.001), with post hoc tests indicating that the psychiatric nonsuicide group had significantly lower GFAP area fraction than the control group (p = 0.008) and the suicide group (p < 0.001). In addition, the spatial distribution of GFAP-IR cells differed among the groups (F2,57 = 3.44, p = 0.039), with the psychiatric nonsuicide group having significantly higher astrocyte CC than the control group (p = 0.013), indicating increased astrocyte clustering. Oligodendrocyte CC also differed among the groups (F2,57 = 4.17, p = 0.020), with the suicide group showing higher oligodendrocyte CCs than the control group (p = 0.007) and the psychiatric nonsuicide group (p = 0.018).
Fig. 3.
Astrocyte density and glial fibrillary acidic protein (GFAP) area fraction in controls (n = 20), psychiatric individuals who died by suicide (n = 10) and psychiatric individuals who died by other means (n = 30). (A) Astrocyte densities were significantly different among the groups (ANOVA F2,57 = 4.12, p = 0.021), being lower in the psychiatric nonsuicide group than the control group (p = 0.025) and the suicide group (p = 0.022). (B) The GFAP area fraction differed among the groups (ANOVA F2,57 = 13.34, p < 0.001), being significantly lower in the psychiatric nonsuicide group than the control group (p < 0.001) and the suicide group (p < 0.001).
In the schizophrenia group lifetime estimated antipsychotic dose correlated with oligodendrocyte CC (r = −0.58, p = 0.007), but no other measure. The patients who had BD and who had been prescribed mood stabilizers (n = 14) showed a trend toward increased oligodendrocyte density relative to those who had not been prescribed mood stabilizers (n = 6; F1,18 = 4.27, p = 0.05). The patients who had BD and who had been prescribed antidepressants (n = 14) had increased MBP immunoreactivity relative to those who had not been prescribed antidepressants (n = 6; F1,18 = 7.44, p = 0.014). However, this finding failed to reach statistical significance following addition of PMI and sex in an ANCOVA model.
To assess the potential influence of substance use on our findings, we examined the effect of alcohol use and illicit drug use on cellular and protein measures in the psychiatric groups. Oligodendrocyte densities were higher in the patients with BD who had documented moderate/heavy alcohol use (n = 14; F1,18 = 6.11, p = 0.024) and moderate/heavy drug use (n = 10; F1,18 = 4.78, p = 0.042) than in those with no or low alcohol (n = 6) or drug (n = 10) use. CNPase levels were increased in patients with schizophrenia who had documented moderate/heavy alcohol use (n = 9; F1,18 = 5.00, p = 0.038) and moderate/heavy drug use (n = 7; F1,16 = 9.47, p = 0.007) than in those with no or low alcohol (n = 11) or drug (n = 11) use. Addition of hemisphere in an ANCOVA model did not change the nature of the results.
Discussion
While imaging studies have reported white matter abnormalities in individuals with schizophrenia and BD, the cellular correlates of these changes remain to be elucidated. To address this we undertook a comprehensive assessment of individual glial subpopulations in white matter underlying the DLPFC in postmortem samples of schizophrenia, BD and nonpsychiatric controls.
Our main finding was a significant increase in oligodendrocyte density in the BD group. This outcome is contrary to 3 previous studies that found no significant change in oligodendrocyte density, also determined using Nissl staining, in the white matter of patients with this disorder.13–15 This inconsistency may reflect variations in methodology, for example use of 2-dimensional versus 3-dimenstional counting methods or brain region examined (prefrontal versus cingulate or corpus callosum). In addition, these studies used separate brain collections with different sample characteristics. Of note, we also found a significant effect of diagnosis on CNPase levels; CNPase was increased by 21% in the BD group, and although this finding did not attain statistical significance, we believe it to be consistent with increased oligodendrocytes in this disorder. CNPase is present in oligodendrocyte cell bodies and is frequently used as a marker for this cell population.33 One explanation for the observed increase in oligodendrocyte density in BD is abnormal regulation of the oligodendrocyte cell cycle, as previously implicated in schizophrenia.34 An alternative theory is that increased cell density could reflect a compensatory mechanism to help replace damaged myelin. Increased lipid peroxidation has been noted in the myelin fraction in individuals with BD.35 Oligodendrocyte precursor cells (OPCs) present during development typically differentiate into mature oligodendrocytes during myelination; however, a population of OPCs persists in the adult brain.36 In response to demyelination signals, adult OPCs proliferate and differentiate into mature oligodendrocytes capable of remyelination.37 We found no significant change in MBP, an oligodendrocyte-associated protein primarily located in compact myelin, in the BD group, which implies that the increase in oligodendrocytes may not be accompanied by higher white matter myelin concentration. To our knowledge, MBP and CNPase levels have not previously been quantified in white matter in individuals with BD. We failed to find any significant change in oligodendrocyte density in white matter in individuals with schizophrenia, consistent with several previous studies of this disorder.12–15
Our data suggest that mood stabilizers, alcohol use and illicit drug use may influence oligodendrocyte density in individuals with BD. While our sample sizes are small, increased oligodendrocyte density was only present in the patients with BD who had been prescribed mood stabilizers. The effect of mood stabilizers on white matter is not fully understood. Lithium increases axial diffusivity in white matter in patients with BD38 and reduces demyelination in an animal model of experimental autoimmune encephalomyelitis.39 However, an inhibitory effect of lithium on proliferating OPCs has also been noted,40 along with decreased expression of myelin-associated genes in mice exposed to lithium carbonate.41 In addition, we found that patients with BD who had documented moderate/high alcohol or illicit drug use had significantly greater oligodendrocyte densities than those with no/low alcohol or drug use. This appears contrary to previous reports of white matter loss and decreased expression of myelin-related genes in chronic alcohol and cocaine abusers.42–44 However, increased expression of the oligodendrocyte-associated gene myelin-associated basic protein (MOBP) was noted in the DLPFC white matter in patients with schizophrenia who had a history of substance abuse.17 In addition, we observed a 16% increase in MBP protein expression in patients with BD who had been prescribed antidepressants compared to those who had not been prescribed antidepressants. This finding is consistent with that of Mosebach and colleagues,13 who reported a trend toward increased MBP immunoreactivity with mean antidepressant dosage in patients with MDD. Further examination of the effects of psychotropic medications, alcohol and illicit drugs on white matter is warranted.
Few studies have examined astrocytes in white matter in the major psychiatric disorders. Williams and colleagues15 reported decreased density of GFAP-labelled astrocytes in cingulate white matter in patients with schizophrenia, but not in those with BD, whereas Falkai and colleagues45 found no significant change in astrocyte density in premotor white matter in individuals with schizophrenia. Astrocyte density did not differ among the groups in our study, although contrast analyses revealed a trend toward decreased astrocyte density in the schizophrenia group, and secondary analyses indicated that astrocyte density was significantly lower in psychiatric patients who died by means other than suicide than in controls. The GFAP area fraction was reduced in both psychiatric groups relative to controls, although this finding was no longer significant following inclusion of brain pH as a covariate in the model. The GFAP area fraction was also lower in psychiatric patients who died by means other than suicide relative to controls. To our knowledge, GFAP area fraction has not previously been quantified in white matter in individuals with the major psychiatric disorders, although decreased GFAP area fraction has been reported in prefrontal grey matter in individuals with schizophrenia.46 Reduced GFAP area fraction could be the result of fewer, less complex or atrophied astrocyte processes, decreased astrocyte density, downregulation of GFAP expression, or a combination of these factors. In addition, GFAP-labelled astrocytes exhibited significantly altered spatial distribution in the schizophrenia and BD groups, such that astrocytes were more clustered in the psychiatric groups than the control group. Unlike the protoplasmic astrocytes present in grey matter, fibrous astrocytes in white matter are not organized into nonoverlapping spatial domains. However, their cell bodies are typically equally spaced, with their processes being radially oriented in the direction of the axon bundles;47 this regular spacing arrangement is thought to reflect the structural support that fibrous astrocytes provide for axonal tracts. Increased astrocyte clustering could therefore result in disruption of structural support of axons in individuals with schizophrenia and BD.
There is a paucity of information on white matter microglia in patients with schizophrenia and BD. We found no significant alteration in the density or distribution of IBA-1-labelled microglia among the groups. Our data appear contrary to those of Fillman and colleagues,18 who reported a 9% increase in microglial density in frontal white matter. However, it should be noted that our marker IBA-1 labels both resting and activated microglial populations, whereas the human leukocyte antigen antibody used previously18 is considered specific to activated microglia. Qualitative examination of our samples revealed areas of microglial activation in 3 schizophrenia samples, but not in controls. We were not able to identify any obvious confounding reason for this finding (e.g., cause of death). Inflammation may therefore be present in a subset of patients with schizophrenia, consistent with findings in grey matter in individuals with this disorder.48,49
Limitations
When interpreting our results some methodological limitations should be kept in mind. First, we identified oligodendrocytes using a nonspecific histological stain, an approach used in several previous studies of this cell population.9,12–15 This could lead to the misclassification of a small number of other cells as oligodendrocytes. However, we employed stringent morphological criteria and counted only round or oval cells with a diameter of 3–7 μm to exclude most OPCs, neurons, microglia and any unstained astrocytes. In addition, counting oligodendrocytes on GFAP-stained sections reduced the possibility of misclassifying astrocytes as oligodendrocytes. While GFAP is a poor marker of protoplasmic astrocytes in grey matter, it stains mature fibrous astrocytes in white matter much more robustly.50 Other groups have quantified oligodendrocytes expressing CNPase,9 olig-1,12 prohibitin51 and ADAM-1252 in individuals with schizophrenia. CNPase immunohistochemistry was not consistent in our formalin-fixed paraffin-embedded tissue, a problem discussed previously.15 Furthermore, prohibitin is not specific to oligodendrocytes,51 while only a minority of white matter oligodendrocytes express ADAM-12.53 Second, technical limitations required 2-dimensional measures of cell density and size to be acquired. The implications of 2-dimensional and 3-dimensional counting methods have been discussed in detail previously.54 One advantage of our method is that we were able to assess greater numbers of glial cells using larger sampling frames, thus allowing us to undertake spatial pattern analyses.
A further potential confounding influence is the use of medication in the psychiatric samples. While information was available regarding prescription of medications, we did not determine compliance. In addition to mood stabilizers and antidepressants, which have been discussed, several patients had been prescribed antipsychotics. The effects of these medications on white matter are poorly understood and may differ between typical and atypical classes. Animal and cell culture studies suggest that antipsychotics may have protective and proliferative effects on immature and progenitor oligodendrocytes,55–58 although decreased astrocyte number has been reported in monkeys exposed to antipsychotics.59 We found no significant association between antipsychotic dose and any measure.
Conclusion
To our knowledge, this is the first study to concurrently quantify oligodendrocyte, astrocyte and microglial densities in white matter in schizophrenia and BD samples. These data provide further evidence for a morphological basis for white matter abnormalities, which may contribute to disrupted neural connectivity, in individuals with these disorders. Our findings do not support a coordinated alteration in multiple glial cell populations and are not suggestive of a gliotic response. Increased oligodendrocyte density was specific to the BD group and could reflect a compensatory mechanism to restore damaged myelin. However, the influence of factors such as use of medications, alcohol and illicit drugs should be considered when interpreting our data. Astrocyte spatial arrangement was compromised in individuals with schizophrenia and BD. While astrocytes play multiple roles in white matter, we propose that these alterations may be associated with disturbances in myelin preservation or axonal support.
Acknowledgements
Postmortem brain tissue was donated by the Stanley Medical Research Institute’s brain collection. We would like to thank Dr. Athena Ypsilanti for performing the ELISA studies and Dr. Charles Duyckaerts for generously donating the Voron software and providing technical assistance. This study was supported by the Mind Foundation of British Columbia, the Stanley Medical Research Institute, the Michael Smith Foundation for Health Research and the Canadian Institutes of Health Research (#81112, #102525).
Footnotes
Competing interests: C.L. Beasley declares grants from the Canadian Institutes of Health Research, Stanley Medical Research Institute and Michael Smith Foundation for Health Research. No other competing interests declared.
Contributors: C.L. Beasley designed the study. C. Hercher and V. Chopra acquired the data, which C. Hercher and C.L. Beasley analyzed. C. Hercher and C.L. Beasley wrote the article, which V. Chopra reviewed. All authors approved the final version for publication.
References
- 1.World Health Organization (WHO) Global burden of disease. [accessed 2013 Jan. 23]. Available: http:///www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf.
- 2.Mahon K, Burdick KE, Szeszko PR. A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neurosci Biobehav Rev. 2010;34:533–54. doi: 10.1016/j.neubiorev.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walterfang M, Wood SJ, Velakoulis D, et al. Neuropathological, neurogenetic and neuroimaging evidence for white matter pathology in schizophrenia. Neurosci Biobehav Rev. 2006;30:918–48. doi: 10.1016/j.neubiorev.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Nortje G, Stein DJ, Radua J, et al. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J Affect Disord. 2013;150:192–200. doi: 10.1016/j.jad.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 6.Arnone D, McIntosh AM, Tan GM, et al. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in schizophrenia. Schizophr Res. 2008;101:124–32. doi: 10.1016/j.schres.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Kempton MJ, Geddes JR, Ettinger U, et al. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65:1017–32. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- 8.Beasley CL, Honavar M, Everall IP, et al. Two-dimensional assessment of cytoarchitecture in the superior temporal white matter in schizophrenia, major depressive disorder and bipolar disorder. Schizophr Res. 2009;115:156–62. doi: 10.1016/j.schres.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 9.Hof PR, Haroutunian V, Friedrich VL, Jr, et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53:1075–85. doi: 10.1016/s0006-3223(03)00237-3. [DOI] [PubMed] [Google Scholar]
- 10.Barley K, Dracheva S, Byne W. Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophr Res. 2009;112:54–64. doi: 10.1016/j.schres.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 11.McCullumsmith RE, Gupta D, Beneyto M, et al. Expression of transcripts for myelination-related genes in the anterior cingulate cortex in schizophrenia. Schizophr Res. 2007;90:15–27. doi: 10.1016/j.schres.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segal D, Schmitz C, Hof PR. Spatial distribution and density of oligodendrocytes in the cingulum bundle are unaltered in schizophrenia. Acta Neuropathol. 2009;117:385–94. doi: 10.1007/s00401-008-0379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosebach J, Keilhoff G, Gos T, et al. Increased nuclear Olig1-expression in the pregenual anterior cingulate white matter of patients with major depression: A regenerative attempt to compensate oligodendrocyte loss? J Psychiatr Res. 2013;47:1069–79. doi: 10.1016/j.jpsychires.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Uranova NA, Vostrikov VM, Orlovskaya DD, et al. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–75. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 15.Williams MR, Hampton T, Pearce RK, et al. Astrocyte decrease in the subgenual cingulate and callosal genu in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2013;263:41–52. doi: 10.1007/s00406-012-0328-5. [DOI] [PubMed] [Google Scholar]
- 16.Beasley CL, Dwork AJ, Rosoklija G, et al. Metabolic abnormalities in fronto-striatal-thalamic white matter tracts in schizophrenia. Schizophr Res. 2009;109:159–66. doi: 10.1016/j.schres.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitkus SN, Hyde TM, Vakkalanka R, et al. Expression of oligodendrocyte-associated genes in dorsolateral prefrontal cortex of patients with schizophrenia. Schizophr Res. 2008;98:129–38. doi: 10.1016/j.schres.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18:206–14. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- 19.Torrey EF, Webster M, Knable M, et al. The stanley foundation brain collection and neuropathology consortium. Schizophr Res. 2000;44:151–5. doi: 10.1016/S0920-9964(99)00192-9. [DOI] [PubMed] [Google Scholar]
- 20.Wolswijk G. Oligodendrocyte survival, loss and birth in lesions of chronic-stage multiple sclerosis. Brain. 2000;123:105–15. doi: 10.1093/brain/123.1.105. [DOI] [PubMed] [Google Scholar]
- 21.Chana G, Landau S, Beasley C, et al. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for decreased neuronal somal size and increased neuronal density. Biol Psychiatry. 2003;53:1086–98. doi: 10.1016/s0006-3223(03)00114-8. [DOI] [PubMed] [Google Scholar]
- 22.Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–47. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz C. Variation of fractionator estimates and its prediction. Anat Embryol (Berl) 1998;198:371–97. doi: 10.1007/s004290050191. [DOI] [PubMed] [Google Scholar]
- 24.Duyckaerts C, Godefroy G. Voronoi tessellation to study the numerical density and the spatial distribution of neurones. J Chem Neuroanat. 2000;20:83–92. doi: 10.1016/s0891-0618(00)00064-8. [DOI] [PubMed] [Google Scholar]
- 25.Duyckaerts C, Godefroy G, Hauw JJ. Evaluation of neuronal numerical density by Dirichlet tessellation. J Neurosci Methods. 1994;51:47–69. doi: 10.1016/0165-0270(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 26.Beasley CL, Honer WG, Bergmann K, et al. Reductions in cholesterol and synaptic markers in association cortex in mood disorders. Bipolar Disord. 2005;7:449–55. doi: 10.1111/j.1399-5618.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 27.Feresten AH, Barakauskas V, Ypsilanti A, et al. Increased expression of glial fibrillary acidic protein in prefrontal cortex in psychotic illness. Schizophr Res. 2013;150:252–7. doi: 10.1016/j.schres.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 28.Byne W, Dracheva S, Chin B, et al. Schizophrenia and sex associated differences in the expression of neuronal and oligodendrocyte-specific genes in individual thalamic nuclei. Schizophr Res. 2008;98:118–28. doi: 10.1016/j.schres.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackay CE, Roddick E, Barrick TR, et al. Sex dependence of brain size and shape in bipolar disorder: an exploratory study. Bipolar Disord. 2010;12:306–11. doi: 10.1111/j.1399-5618.2010.00804.x. [DOI] [PubMed] [Google Scholar]
- 30.Hedreen JC. What was wrong with the Abercrombie and empirical cell counting methods? A review. Anat Rec. 1998;250:373–80. doi: 10.1002/(SICI)1097-0185(199803)250:3<373::AID-AR12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 31.Mann JJ, Currier DM. Stress, genetics and epigenetic effects on the neurobiology of suicidal behavior and depression. Eur Psychiatry. 2010;25:268–71. doi: 10.1016/j.eurpsy.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turecki G, Ernst C, Jollant F, et al. The neurodevelopmental origins of suicidal behavior. Trends Neurosci. 2012;35:14–23. doi: 10.1016/j.tins.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Quarles RH, Macklin WB, Morell P. Myelin formation, structure and biochemistry. In: Brady S, Siegel G, Albers RW, et al., editors. Basic neurochemistry: molecular, cellular and medical aspects. 7th ed. Waltham (MA): Academic Press; 2005. pp. 51–73. [Google Scholar]
- 34.Katsel P, Davis KL, Li C, et al. Abnormal indices of cell cycle activity in schizophrenia and their potential association with oligodendrocytes. Neuropsychopharmacology. 2008;33:2993–3009. doi: 10.1038/npp.2008.19. [DOI] [PubMed] [Google Scholar]
- 35.Andreazza AC, Wang JF, Salmasi F, et al. Specific subcellular changes in oxidative stress in prefrontal cortex from patients with bipolar disorder. J Neurochem. 2013;127:552–61. doi: 10.1111/jnc.12316. [DOI] [PubMed] [Google Scholar]
- 36.McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- 37.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–55. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 38.Benedetti F, Bollettini I, Barberi I, et al. Lithium and GSK3-β promoter gene variants influence white matter microstructure in bipolar disorder. Neuropsychopharmacology. 2013;38:313–27. doi: 10.1038/npp.2012.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Sarno P, Axtell RC, Raman C, et al. Lithium prevents and ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2008;181:338–45. doi: 10.4049/jimmunol.181.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orre K, Wennström M, Tingström A. Chronic lithium treatment decreases NG2 cell proliferation in rat dentate hilus, amygdala and corpus callosum. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:503–10. doi: 10.1016/j.pnpbp.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 41.McQuillin A, Rizig M, Gurling HM. A microarray gene expression study of the molecular pharmacology of lithium carbonate on mouse brain mRNA to understand the neurobiology of mood stabilization and treatment of bipolar affective disorder. Pharmacogenet Genomics. 2007;17:605–17. doi: 10.1097/FPC.0b013e328011b5b2. [DOI] [PubMed] [Google Scholar]
- 42.Harper C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J Neuropathol Exp Neurol. 1998;57:101–10. doi: 10.1097/00005072-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Lewohl JM, Harris RA, et al. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology. 2006;31:1574–82. doi: 10.1038/sj.npp.1300947. [DOI] [PubMed] [Google Scholar]
- 44.Albertson DN, Pruetz B, Schmidt CJ, et al. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–9. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Falkai P, Honer WG, David S, et al. No evidence for astrogliosis in brains of schizophrenic patients. A post-mortem study. Neuropathol Appl Neurobiol. 1999;25:48–53. doi: 10.1046/j.1365-2990.1999.00162.x. [DOI] [PubMed] [Google Scholar]
- 46.Rajkowska G, Miguel-Hidalgo JJ, Makkos Z, et al. Layer-specific reductions in GFAP-reactive astroglia in the dorsolateral prefrontal cortex in schizophrenia. Schizophr Res. 2002;57:127–38. doi: 10.1016/s0920-9964(02)00339-0. [DOI] [PubMed] [Google Scholar]
- 47.Oberheim NA, Takano T, Han X, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–87. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foster R, Kandanearatchi A, Beasley C, et al. Calprotectin in microglia from frontal cortex is up-regulated in schizophrenia: Evidence for an inflammatory process? Eur J Neurosci. 2006;24:3561–6. doi: 10.1111/j.1460-9568.2006.05219.x. [DOI] [PubMed] [Google Scholar]
- 49.Steiner J, Mawrin C, Ziegeler A, et al. Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol. 2006;112:305–16. doi: 10.1007/s00401-006-0090-8. [DOI] [PubMed] [Google Scholar]
- 50.Molofksy AV, Krencik R, Ullian EM, et al. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernstein HG, Smalla KH, Dürrschmidt D, et al. Increased density of prohibitin-immunoreactive oligodendrocytes in the dorsolateral prefrontal white matter of subjects with schizophrenia suggests extraneuronal roles for the protein in the disease. Neuromolecular Med. 2012;14:270–80. doi: 10.1007/s12017-012-8185-y. [DOI] [PubMed] [Google Scholar]
- 52.Farkas N, Lendeckel U, Dobrowolny H, et al. Reduced density of ADAM 12-immunoreactive oligodendrocytes in the anterior cingulate white matter of patients with schizophrenia. World J Biol Psychiatry. 2010;11:556–66. doi: 10.3109/15622970903497936. [DOI] [PubMed] [Google Scholar]
- 53.Bernstein HG, Keilhoff G, Bukowska A, et al. ADAM (a disintegrin and metalloprotease) 12 is expressed in rat and human brain and localized to oligodendrocytes. J Neurosci Res. 2004;75:353–60. doi: 10.1002/jnr.10858. [DOI] [PubMed] [Google Scholar]
- 54.Benes FM, Lange N. Two-dimensional versus three-dimensional cell counting: a practical perspective. Trends Neurosci. 2001;24:11–7. doi: 10.1016/s0166-2236(00)01660-x. [DOI] [PubMed] [Google Scholar]
- 55.Niu J, Mei F, Li N, et al. Haloperidol promotes proliferation but inhibits differentiation in rat oligodendrocyte progenitor cell cultures. Biochem Cell Biol. 2010;88:611–20. doi: 10.1139/O09-178. [DOI] [PubMed] [Google Scholar]
- 56.Selemon LD, Lidow MS, Goldman-Rakic PS. Increased volume and glial density in primate prefrontal cortex associated with chronic antipsychotic drug exposure. Biol Psychiatry. 1999;46:161–72. doi: 10.1016/s0006-3223(99)00113-4. [DOI] [PubMed] [Google Scholar]
- 57.Steiner J, Sarnyai Z, Westphal S, et al. Protective effects of haloperidol and clozapine on energy-deprived OLN-93 oligodendrocytes. Eur Arch Psychiatry Clin Neurosci. 2011;261:477–82. doi: 10.1007/s00406-011-0197-3. [DOI] [PubMed] [Google Scholar]
- 58.Wang H, Xu H, Niu J, et al. Haloperidol activates quiescent oligodendroglia precursor cells in the adult mouse brain. Schizophr Res. 2010;119:164–74. doi: 10.1016/j.schres.2010.02.1068. [DOI] [PubMed] [Google Scholar]
- 59.Konopaske GT, Dorph-Petersen KA, Sweet RA, et al. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol Psychiatry. 2008;63:759–65. doi: 10.1016/j.biopsych.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]