Abstract
Background
Schizophrenia is a complex neuropsychiatric disorder of unclear etiology. The strongest known genetic risk factor is the 22q11.2 microdeletion. Research has yet to confirm which genes within the deletion region are implicated in schizophrenia. The minimal 1.5 megabase deletion contains MIR185, which encodes microRNA 185.
Methods
We determined miR-185 expression in embryonic and adult mouse brains. Common and rare variants at this locus were then investigated using a human genetics approach. First, we performed gene-based analyses for MIR185 common variants and target genes using Psychiatric Genomics Consortium genome-wide association data. Second, MIR185 was resequenced in German patients (n = 1000) and controls (n = 500). We followed up promising variants by genotyping an additional European sample (patients, n = 3598; controls, n = 4082).
Results
In situ hybridization in mice revealed miR-185 expression in brain regions implicated in schizophrenia. Gene-based tests revealed association between common variants in 3 MIR185 target genes (ATAT1, SH3PXD2A, NTRK3) and schizophrenia. Further analyses in mice revealed overlapping expression patterns for these target genes and miR-185. Resequencing identified 2 rare patient-specific novel variants flanking MIR185. However, follow-up genotyping provided no further evidence of their involvement in schizophrenia.
Limitations
Power to detect rare variant associations was limited.
Conclusion
Human genetic analyses generated no evidence of the involvement of MIR185 in schizophrenia. However, the expression patterns of miR-185 and its target genes in mice, and the genetic association results for the 3 target genes, suggest that further research into the involvement of miR-185 and its downstream pathways in schizophrenia is warranted.
Introduction
Schizophrenia is a complex neuropsychiatric disorder of unclear etiology.1 The strongest known genetic risk factor is a hemizygous microdeletion in chromosomal region 22q11.2, which causes the phenotypically heterogenous 22q11.2 deletion syndrome (22q11.2DS),2 also known as velocardiofacial/DiGeorge syndrome. The overall prevalence of 22q11.2DS is 1 in 2000–4000 births.3,4 Affected individuals have an estimated 30% risk for schizophrenia.4,5 Since the symptoms of 22q11.2DS-related schizophrenia are largely indistinguishable from those of the idiopathic disease,4,5 risk genes for 22q11.2DS-related schizophrenia may also be implicated in idiopathic cases.6
The size of the 22q11.2 deletion varies. Most are 1.5 mega-bases (Mb) or 3 Mb in size, and span approximately 35 and 60 known genes, respectively.7,8 Although the 22q11.2DS phenotype is highly variable, its severity is not correlated with deletion size. This suggests that the minimal 1.5 Mb deletion region is of crucial etiological importance.3 Several genetic association studies have attempted to identify which genes in this 1.5 Mb region are responsible — through their absence — for the increased risk for schizophrenia.3 To date, however, no single gene has received consistent independent support.
One known gene within the minimal 1.5 Mb deletion region is MIR185,3 which encodes microRNA 185. MicroRNAs are small noncoding RNAs that control the translation of target messenger RNAs (mRNAs). Accumulating evidence suggests that microRNAs contribute to the basic mechanisms underlying brain development and plasticity,9,10 thus suggesting their possible involvement in the pathogenesis of several psychiatric disorders,11 including schizophrenia.12
This hypothesis is supported by a large genome-wide association study (GWAS) of schizophrenia conducted by the Psychiatric Genomics Consortium (PGC).13 Here, a single nucleotide polymorphism (SNP) in an intron of MIR137 was the second strongest finding. Four other loci with genome-wide significance were predicted targets of MIR137, thus providing genetic support for the hypothesis that microRNA-mediated dysregulation is an etiological mechanism in schizophrenia.13
Research in mouse-models has also implicated microRNAs in 22q11.2DS. Investigation of Df(16)A+/− mice — an engineered mouse strain carrying a chromosomal deficiency spanning a segment syntenic to the human 22q11.2 locus — demonstrated alterations in the biogenesis of brain microRNAs.11,14 Interestingly, miR-185 was the top-scoring downregulated microRNA in both the prefrontal cortex (PFC) and the hippocampus,14 brain areas that are key foci of schizophrenia research.15 A recent study confirmed a significant reduction in the expression of miR-185 in the hippocampus and PFC of Df(16)A+/− mice and showed that this reduction contributed to deficits in the dendritic and spine development of hippocampal neurons.16 Furthermore, Earls and colleagues6 identified miR-185 as a regulator of sarco(endo)plasmic reticulum Ca(2+) ATPase (SERCA2) in 22q11.2DS mouse models and showed that miR-185 depletion contributed to SERCA2 upregulation. This mechanism may be implicated in the elevation of SERCA2 protein observed in the postmortem brains of patients with schizophrenia.6
Further support for the involvement of MIR185 in schizophrenia is provided by evidence that 2 of its validated targets, RhoA and Cdc42,17 are associated with altered expression levels in patients with schizophrenia.18,19
We conducted the present study to investigate the role of MIR185 in schizophrenia using gene expression analyses in mice and human genetics approaches in patients with schizophrenia and controls.
Methods
Animal study of miR-185: in situ hybridization
These experiments involved the wild-type C57BL6 mouse strain. All procedures involving animals followed the guidelines of the German Animal Protection Legislation, and the experiments were approved by the Local Committee for Animal Health (Landesamt für Natur, Umwelt und Verbraucherschutz).
We determined miR-185 expression profiles to validate microarray-predicted expression in the PFC and hippocampus and to determine miR-185 expression in brain regions of relevance to 22q11.2DS psychiatric phenotypes. We applied in situ hybridization (ISH) in embryonic and adult mouse brains, as this was the best experimental alternative to microRNA expression profiling at the time of study.20
We performed ISH as described previously.21 Briefly, mouse embryos and isolated young and adult mouse brains for a total of 6 different stages were fixed overnight at 4°C with 4% paraformaldehyde (PFA) prepared in phosphate-buffered saline. Embryos and adult brains were then dehydrated using ethanol/saline solutions. Following Roti-Histol (Roth) treatment, the specimens were embedded in Paraplast sections. The specimens were dewaxed and rehydrated, re-fixed in 4% PFA, and treated with proteinase K (10 μg/mL). An miR-185 3′-DIG labelled detection probe (Exiqon, hsa-miR-185:TCAGGAACTGCCTTTCTCTCCA) was used. A sense probe was used as a negative control (NM_001163311, 263 to 1126 base pairs (bp)). After hybridization and immunological detection with antidigoxigenin Fab fragments (Roche), NBT/BCIP staining was performed. The slides were mounted with CC/Mount aqueous mounting medium (Sigma). Midday of the day on which the vaginal plug appeared was considered embryonic day 0.5 (E0.5).
Expression pattern of miR-185 target genes in the mouse brain
Two publicly available databases were used to determine the expression patterns of Atat1, Sh3pxd2a and Ntrk3: Eurexpress (www.eurexpress.org)22 for the developing mouse brain, and Mouse Allen Brain Atlas (mouse.brain-map.org)23 for the adult mouse brain. These genes have the following identifications: T31063 (Atat1), T37016 (Sh3pxd2a) and T36710 (Ntrk3) for Eurexpress, and 68522497 (Atat1), 69873720 (Sh3pxd2a), and 71234689 (Ntrk3) for Mouse Allen Brain Atlas.
Human genetics study of miR-185
The 3-step human genetics study was approved by the respective local ethics committees. Written informed consent was obtained from all participants.
The diagnoses of all German and Dutch patients were made according to DSM-IV criteria; the diagnoses of all Danish patients were made according to ICD-10 (1994–2005) criteria. German controls were screened for schizophrenia, but Dutch controls were not. None of the Danish controls had been assigned a diagnosis of schizophrenia at the time of inclusion according to Danish health registers.24
Step 1: gene-based tests for GWAS data
Sample
We used publicly available summary statistics of genotyping data from the PGC study.13 The PGC data were obtained from 9394 patients with schizophrenia or schizoaffective disorder (66.5% men) and 12 462 controls (48.5% men) of European ancestry. These included individuals from Mannheim/Bonn, Germany (474 patients and 1304 controls); Munich, Germany (434 patients and 351 controls); Utrecht, the Netherlands (704 patients and 631 controls); and Copenhagen, Denmark (482 patients and 457 controls).
Methods
We performed gene-based analyses to investigate whether common variants in the premature MIR185 sequence increased the risk for schizophrenia and whether common genetic variants associated with schizophrenia were over-represented in MIR185 target genes. Gene-based p values were computed for MIR185 and its predicted target genes using FORGE software.25 The MIR185 target gene set comprised 124 genes and was derived from the Molecular Signatures Database 3.1 (http://www.broadinstitute.org/gsea/msigdb/index.jsp).26 Using the Ensembl human gene annotation, SNPs located within the annotated gene coordinates ± 20 000 bp flanking regions were mapped to genes. Gene-wide p values were calculated for all 122 target genes in the autosomal data from the PGC using 2 test statistics in the FORGE software: the Sidak correction on minimum p value (SIDAK) and the fixed-effect z-score statistic (Z FIX). Both statistics are described in detail in the supplemental information of Pedroso and colleagues.25 While the SIDAK approach is most powerful in situations where only a single functionally relevant variant is observed within the gene, the Z FIX approach is most powerful in situations in which multiple variants are observed. We applied both test statistics, since the true pattern of functionally relevant variants for the presently investigated genes was unknown a priori. Although too conservative (since both tests are not independent), the calculated gene-based p values were corrected for multiple testing with twice the number of investigated target genes (n = 244).
Further analyses were performed to investigate whether any of the 17 SNPs in the gene-based analysis of MIR185 showed an individual association with schizophrenia.
Step 2: resequencing
Sample
The resequencing step involved 1000 patients with schizophrenia (57.3% men) of German ancestry and 500 controls (57.4% men) from a population-based sample collected in the Bonn area within the German National Genome Research Network (NGFN).27 In addition, we investigated 2 patients with schizophrenia with a 22q11.2 microdeletion. These 2 individuals were recruited through the psychiatric services in the German cities of Mannheim and Bonn, respectively. The patient from Bonn is described elsewhere.28
Methods
Resequencing was performed to investigate the role of rare variants in idiopathic schizophrenia. In addition, the MIR185 gene was resequenced in the 2 patients with 22q11.2 microdeletion to investigate the presence of recessive risk alleles on the nondeleted strand.
Prior to resequencing, the presence of a 22q11.2 microdeletion in the 1000 idiopathic patients was excluded using QuantiSNP v1.129 and SNP intensity data from Human-Hap550v3, HumanHap610v1 and HumanHap660W Bead Arrays (Illumina). These 1000 patients were also screened for small copy number variants (CNVs) pinpointing MIR185 ± 20 000 bp flanking regions. We retained the CNVs that had a log Bayes factor of 10 or greater and that spanned a minimum of 10 consecutive markers.
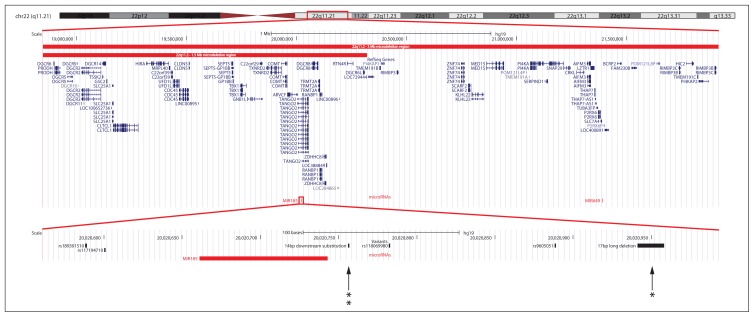
Genomic DNA sequences were obtained from the University of California, Santa Cruz (UCSC) Genome Browser (http://genome.ucsc.edu/cgi-bin/hgGateway, NCBI build 37.1, hg19; Fig. 1).30 Using the RefSeq gene definition31 of MIR185 (NR_029706.1), primers were designed to amplify a region of around 400 bp, including the target premature microRNA sequence of 82 bp (chr22:20,020,662–20,020,743) and flanking sequences. Primer sequences are available on request.
Fig. 1.
Overview of the 22q11.2 microdeletion region and the premature MIR185 sequence obtained from the University of California, Santa Cruz Genome Browser. The extent of the 3 megabase (Mb) and 1.5 Mb deletions are based on the supplementary information in the study by Karayiorgou and colleagues.3 The locations of the patient-specific novel 14 bp downstream substitution and 17 bp long deletion are indicated by arrows. The number of asterisks indicates the number of observations in patients detected through resequencing.
Resequencing was performed using the Sanger method. The sequencing information was generated and analyzed on the 3130xl Genetic Analyzer (Applied Biosystems). We investigated all obtained nucleotide sequences using SeqMan II (DNASTAR). All patient-specific rare variants were confirmed by sequencing a second, independent amplicon.
We classified variants as “rare” if their minor allele frequency (MAF) in the combined patients/controls was 1% or less. We used a Fisher exact test (2-tailed) to test for association with rare variants. A variant was designated as “novel” if it was not listed in either dbSNP build 135 (http://www.ncbi.nlm.nih.gov/projects/SNP/) or if it was not present in 379 individuals of European ancestry from the 1000 Genomes Project (http://browser.1000genomes.org/index.html).32 Novel variants were submitted to dbSNP, and identifiers (ss) are provided in the Results section.
Variant nomenclature was assigned according to den Dunnen and Antonarakis.33 Nucleotide positions correspond to genomic sequence positions (NCBI build 37.1) in human chromosome 22 (NC_000022.10). All variants were investigated for splice site changes, using Human Splicing Finder (http://www.umd.be/HSF/)34; for evolutionary conservation, using the Vertebrate Multiz Alignment and Conservation Track; and for localization within transcription factor binding sites, using the HMR Conserved Transcription Factor Binding Sites Track of the UCSC Genome Browser.30
Step 3: follow-up genotyping
Sample
This step involved an independent sample of 3598 patients with schizophrenia (61.4% men) and 4082 controls (51.4% men) from 5 centres in Germany (n = 3), the Netherlands (n = 1) and Denmark (n = 1). A subsample of the German control cohort (n = 1097) was drawn from the population-based Heinz Nixdorf Recall Study.35
Methods
Genotyping in the 3598 patients and 4082 controls was performed using the iPLEX Gold Sequenom Mass-ARRAY system (Sequenom). The iPLEX primer sequences and assay conditions are available on request. After the exclusion of 66 patients and 62 controls with missing genotypes, the genotyping sample consisted of 3532 patients and 4020 controls.
Results
MiR-185 expression pattern
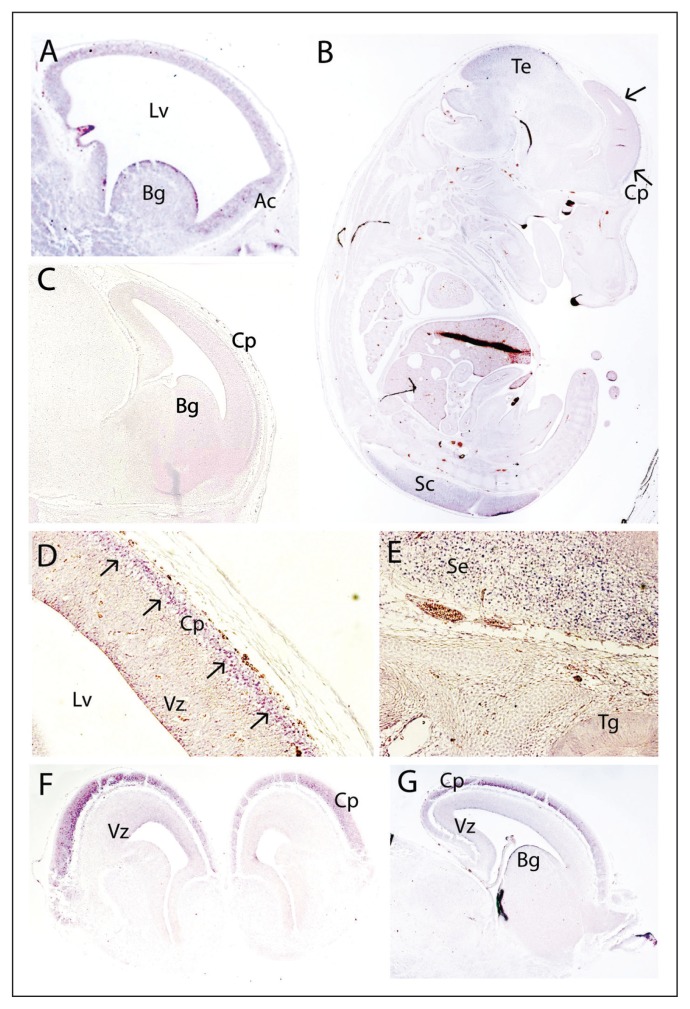
Table 1 summarizes miR-185 expression in the mouse brain. At E12.5 in the mouse embryos (Fig. 2A), miR-185 expression was detected in the cerebral cortex (most prominently in the anterior part) and in the basal ganglia. At E14.5, miR-185 expression appeared to be restricted to the developing central nervous system, in particular the tectum and the spinal cord (Fig. 2B). In the developing cerebral cortex, expression was clearly enriched in the mantle layer (Fig. 2B and D), with low expression being observed in the ventricular zone (Fig. 2D). MiR-185 expression was also prominent in the septum (Fig. 2E and 4A). At E15.5, miR-185 expression remained predominant in the cortical plate (Fig. 2F). In the ventricular zone, miR-185 expression was slightly higher than at E14.5, although it remained low (Fig. 2F). At E16.5, miR-185 expression was similar to that at E15.5, with prominent expression in the subcortical plate (Fig. 2G). We also observed miR-185 in the basal ganglia at this time point (Fig. 2G).
Table 1.
Summary of miR-185 expression in the mouse brain
| Ages | Regions | Details | Figure |
|---|---|---|---|
| E12.5 | Cerebral cortex | Most prominent rostrally | 2A |
| Basal ganglia | 2A | ||
| E14.5 | Cerebral cortex | Enriched in mantle layer, low in ventricular zone | 2B, 2D, 4A |
| Basal ganglia | Septum | 2E, 4A | |
| Tectum | 2B | ||
| Spinal cord | 2B | ||
| E15.5 | Cerebral cortex | Predominant in cortical plate, low in ventricular zone | 2F |
| Basal ganglia | 2F | ||
| E16.5 | Cerebral cortex | Prominent in cortical plate | 2G |
| Basal ganglia | 2G | ||
| P9 | Olfactory bulb | Mitral cell layer, granular layer | 3A |
| Frontal cortex | Strong expression | 3A | |
| Hippocampus | 3A | ||
| Cerebellum | External germinal and internal granular layers | 3A | |
| P46 | Olfactory bulb | Mitral cell layer, granular layer | 3C |
| Frontal cortex | 3C, 4M | ||
| Hippocampus | Strong in CA1, dentate gyrus; weak in CA3 | 3C, 4E | |
| Cerebellum | Granular layer | 3B, 3C, 4I |
CA = cornu ammonis.
Fig. 2.
Expression pattern of miR-185 in the developing mouse brain at E12.5 (A, sagittal), E14.5 (B, D, E, sagittal), E15.5 (F, coronal) and E16.5 (G, sagittal). A negative control with a sense probe is also included (C, E14.5, sagittal). At E12.5, miR-185 is present in the developing cerebral cortex, being most pronounced in the anterior cortex (A); miR-185 is also present in the basal ganglia (A). At E14.5, miR-185 expression is prominent in the cortical plate (B, D) as well as in the tectum and the spinal cord (B). The septum also shows enriched miR-185 expression (E), with no expression in the trigeminal ganglion (E). At E15.5 (F) and E16.5 (G), miR-185 is clearly observed in the cortical plate, with low expression in the ventricular zone. Arrows indicate miR-185 expression in the mantle layer. Ac = anterior cortex; Bg = basal ganglia; Cp = cortical plate; Lv = lateral ventricle; Sc = spinal cord; Se = septum; Te = tectum; Tg = trigeminal ganglion; Vz = ventricular zone.
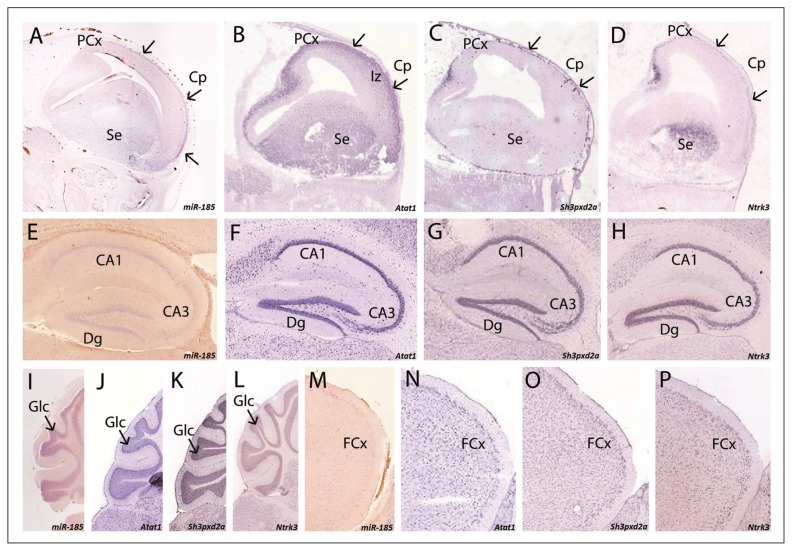
Fig. 4.
Comparison of the expression between miR-185 (A, E, I, M) and Atat1 (B, F, J, N), Sh3pxd2a (C, G, K, O) and Ntrk3 (D, H, L, P) in both embryonic (A–D, E14.5) and adult (E–P, P46 for miR-185, P56 for the 3 target genes) mouse brain. At E14.5, all 4 genes are detected in the developing cortex, although with differing intensities. While Atat1 (B) has a broad expression, including the intermediate zone, the other genes are restricted to the cortical plate. In addition, the expression of Atat1 (B), Sh3pxd2a (C) and Ntrk3 (D) is highlighted in the posterior developing cortex, where miR-185 (A) is not detected. All 4 genes are also detected in the septum (AD). In the adult mouse brain, all 4 genes are observed in the hippocampus (E–H), the cerebellum (I–L) and the frontal cortex (M–P). In the case of the hippocampus, miR-185 is predominant in the CA1 and the dentate gyrus (E), while the 3 target genes are also clearly seen in CA3 (F–H). In the cerebellum, all 4 genes are present in the granular layer (I–L). Regarding the frontal cortex, miR-185 (M) shows weaker expression than Atat1 (N), Sh3pxd2a (O), and Ntrk3 (P). Arrows indicate the expression of the 4 genes in the cortical plate. Panels B–D were derived from the Eurexpress database.22 Panels F–H, J–L and N–P were derived from the Allen Mouse Brain Atlas, Allen Institute for Brain Science.23 Abbreviations: CA = cornu ammonis; Cp = cortical plate; Dg = dentate gyrus; FCx = frontal cortex; Glc = granular layer of the cerebellum; Iz = intermediate zone; PCx = posterior developing cortex; Se = septum.
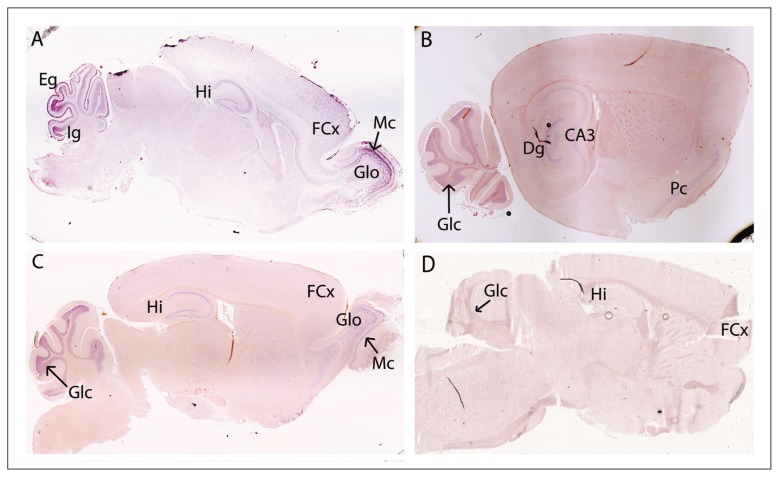
In the young (P9) and adult (P46) mouse brains, miR-185 was observed in the mitral cell and granular layers of the olfactory bulb (Fig. 3A and C). We also detected miR-185 in the cortex, being enriched at P9 (Fig. 3A) and still detectable at P46 (Fig. 3C and 4M). At P9, miR-185 was present in the hippocampus (Fig. 3A). At P46, strong expression was present in the cornu ammonis (CA)1 and the dentate gyrus, and lower expression was detected in the CA3 (Fig. 3B and C and Fig. 4E). At P9, miR-185 was visualized in both the external germinal and internal granular layers of the cerebellum (Fig. 3A). At P46, strong cerebellar expression was restricted to the granular layer (Fig. 3B and C and Fig. 4I).
Fig. 3.
Expression of miR-185 in the young (A, P9, sagittal) and adult (B, C, P46, sagittal) mouse brain. A negative control with a sense probe is also included (D, P46, sagittal). At P9, miR-185 is observed in both mitral cell and granular layers of the olfactory bulb, as well as in the hippocampus (A); miR-185 is also present in both the external germinal and internal granular layers of the cerebellum (A). At P9 (A) and P46 (C), miR-185 expression is detected in the frontal cortex. At P46, microRNA expression in the hippocampus remains (B, C); miR-185 expression also persists in the olfactory bulb (C) and the piriform cortex (B), and miR-185 is clearly enriched in the granular layer of the cerebellum (B, C). Dg = dentate gyrus; Eg = external germinal layer of the cerebellum; FCx = frontal cortex; Glc = granular layer of the cerebellum; Glo = granular layer of the olfactory bulb; Hi = hippocampus; Ig = internal germinal layer of the cerebellum; Mc = mitral cell layer; Pc = piriform cortex.
Gene-based association tests for MIR185 and its predicted target genes
Analysis of the PGC genotyping data13 revealed no association between MIR185 and schizophrenia at either the gene level (pSIDAK = 0.53 and pZ FIX = 0.11), or the level of individual SNPs (minimal p = 0.09).
With the SIDAK method, 20 of the 122 target genes showed nominally significant p values. With the Z FIX method, 22 target genes showed nominally significant p values. For both methods, the number of genes with a p < 0.05 was significantly higher than expected (n = 6), indicating that schizophrenia-associated genes were enriched within the targets of MIR185 (χ2 test, pSIDAK = 0.006, pZ FIX = 0.002). Thirty-one genes were nominally associated with schizophrenia according to at least 1 of the 2 methods (Table 2). After correction for multiple testing, 3 genes showed a significant association with schizophrenia: ATAT1 (pSIDAK < 0.001, corrected), SH3PXD2A (pSIDAK = 0.008, corrected) and NTRK3 (pZ FIX = 0.002, corrected).
Table 2.
Gene-based tests of miR-185 target genes
| Gene* | Chromosome | Minimal p value† | No. of SNPs | pSIDAK value‡ | pZ FIX value‡ |
|---|---|---|---|---|---|
| ADAMTS19 | 5 | 0.005 | 107 | > 0.99 | > 0.99 |
| ANXA2 | 15 | 0.008 | 50 | > 0.99 | > 0.99 |
| APBA1 | 9 | < 0.001 | 109 | > 0.99 | > 0.99 |
| APP | 21 | < 0.001 | 153 | 0.53 | > 0.99 |
| ARID1A | 1 | 0.013 | 13 | > 0.99 | > 0.99 |
| ATAT1 | 6 | < 0.001 | 16 | < 0.001 | > 0.99 |
| CDC42 | 1 | 0.011 | 22 | > 0.99 | > 0.99 |
| FAM134C | 17 | < 0.001 | 17 | > 0.99 | > 0.99 |
| FMO2 | 1 | < 0.001 | 50 | > 0.99 | > 0.99 |
| FOXN3 | 14 | < 0.001 | 281 | > 0.99 | > 0.99 |
| KCNN3 | 1 | 0.016 | 135 | > 0.99 | > 0.99 |
| LPHN1 | 19 | 0.005 | 12 | > 0.99 | > 0.99 |
| NFASC | 1 | < 0.001 | 151 | > 0.99 | > 0.99 |
| NTRK3 | 15 | < 0.001 | 187 | 0.22 | 0.002 |
| PAK6 | 15 | < 0.001 | 36 | > 0.99 | 0.13 |
| PBX1 | 1 | < 0.001 | 237 | > 0.99 | > 0.99 |
| PRX | 19 | 0.002 | 15 | > 0.99 | > 0.99 |
| PTPRE | 10 | < 0.001 | 132 | > 0.99 | > 0.99 |
| RHOA | 3 | 0.014 | 17 | > 0.99 | > 0.99 |
| RIC8B | 12 | 0.012 | 43 | > 0.99 | > 0.99 |
| SERF2 | 15 | < 0.001 | 16 | > 0.99 | 0.91 |
| SH3PXD2A | 10 | < 0.001 | 130 | 0.008 | 0.06 |
| SLC30A3 | 2 | 0.006 | 13 | > 0.99 | > 0.99 |
| SLC37A2 | 11 | < 0.001 | 41 | > 0.99 | > 0.99 |
| SNX19 | 11 | < 0.001 | 53 | 0.18 | > 0.99 |
| SYNGAP1 | 6 | 0.008 | 17 | > 0.99 | > 0.99 |
| SYNM | 15 | < 0.001 | 57 | > 0.99 | 0.25 |
| TCF12 | 15 | < 0.001 | 114 | > 0.99 | > 0.99 |
| TJP1 | 15 | < 0.001 | 113 | > 0.99 | > 0.99 |
| VEZF1 | 17 | 0.020 | 15 | > 0.99 | > 0.99 |
| ZNF385A | 12 | < 0.001 | 7 | 0.30 | > 0.99 |
SNP = single nucleotide polymorphism.
Thirty-one miR-185 target genes that showed nominal association with schizophrenia according to at least 1 of the 2 applied methods (SIDAK, Z FIX).
Single marker.
Corrected for multiple testing.
Mouse brain expression of Atat1, Sh3pxd2a and Ntrk3
The expression patterns of the 3 miR-185 target genes ATAT1, SH3PXD2A and NTRK3 were further investigated in the mouse brain to determine whether they were expressed in the same brain regions as miR-185 (Fig. 4). Identification of overlap in regions previously implicated in schizophrenia would provide further evidence for the pathogenic role of the miR-185 regulatory pathway.
At E14.5, all 4 genes were expressed in the cortical plate (Fig. 4A–D). Atat1, Sh3pxd2a and Ntrk3 were also detected in the posterior developing cerebral cortex (Fig. 4B–D), where miR-185 expression was not detected (Fig. 4A). This may indicate differing regulation of the 3 target genes in this brain region. Atat1 showed broader expression, as it was also observed in the intermediate zone (Fig. 4B).
In the adult mouse brain (P46 for miR-185; P56 for the target genes), the 4 genes were observed in the hippocampus (Fig. 4E–H), with lower miR-185 expression in the CA3 (Fig. 4E). In the cerebellum, all 4 genes were expressed in the granular cell layer (Fig. 4I–L). Overlapping expression of miR-185 and its 3 target genes was also observed in the frontal cortex (Fig. 4M–P).
Resequencing and array data
No small MIR185-spanning CNVs were observed during reinspection of the array intensity data.
Resequencing identified 2 common (MAF > 1%) and 4 rare variants (MAF ≤ 1%). All were located in regions flanking the premature microRNA sequence (Fig. 1). No variants were detected in the premature microRNA sequence per se. Both common variants were known SNPs (rs117194710 and rs9605051). Two of the 4 rare variants were known and 2 were novel, and all individuals with these alterations were heterozygous for the variant allele. The 2 known rare variants (rs189301510 and rs118069980) showed a similar distribution in patients and controls: MAF = 0.46% versus 0.31% (patients v. controls, p = 0.73) and MAF = 0.1% (patients and controls, p > 0.99), respectively. The 2 novel variants were observed only in patients with schizophrenia. A cytosine to thymine substitution 14 bp downstream of the premature microRNA sequence (g.20020757C > T, ss748772517) was observed in 2 patients. A 17 bp long deletion located 199 bp downstream was observed in 1 patient (g.20020942_20020958del17, ss748772518).
In silico analysis revealed no potential splice site changes for any of the 4 rare variants. None of the 4 variants was located within transcription factor binding sites. Interestingly, high across-species conservation was observed for the 14 bp downstream substitution (ss748772517). The site was conserved in humans, chimps, rhesus monkeys, mice and dogs.
In the 2 patients with 22q11.2 microdeletion, no rare variants were detected in the analyzed region.
Follow-up genotyping
The 2 patient-specific variants were genotyped in the follow-up sample. Given their similar frequencies in patients and controls, the 2 known rare variants were not followed up. Genotyping revealed that a control individual in the follow-up sample carried the 14 bp downstream substitution (ss748772517). In a combined analysis with the sequencing data, the overall patient and control frequencies for this variant were 0.02% and 0.01%, respectively (p > 0.99). None of the follow-up sample participants carried the 17 bp long deletion (p > 0.99).
Discussion
The ISH in embryonic and adult mice revealed miR-185 expression in brain regions implicated in schizophrenia. Of particular interest is the validation of miR-185 expression in the frontal cortex and hippocampus, with high expression being observed in the dentate gyrus and CA1, since independent studies have reported an association between manifestations of schizophrenia and dysfunction in these regions.15,36 Furthermore, the miR-185 expression patterns overlapped with those of validated miR-185 targets (RhoA and Cdc42), both of which have been previously associated with schizophrenia.18,19 In the adult rat brain, RhoA and Cdc42 are expressed in the hippocampus (predominantly in the dentate gyrus and CA1) as well as in the piriform cortex, the cerebral neocortex and the granular layer of the cerebellum.37 This is consistent with the localization of miR-185 in the present study.
The miR-185 expression pattern observed in the present study (Table 1) may provide insight into other 22q11.2DS brain abnormalities. For example, both cortical atrophy38 and abnormal cortical organization39 have been associated with 22q11.2DS, in particular polymicrogyria and pachygyria.40,41 Other structural abnormalities in 22q11.2DS affect the cerebellum. These include hypoplasia of the cerebellar vermis38 and cerebellar atrophy.42 Remarkably, miR-185 expression in the present study was particularly pronounced in these brain regions. As microRNAs play an important role in cortical and cerebellar development,9 prominent miR-185 expression in the developing cortical plate and the cerebellum may be related to 22q11.2DS brain abnormalities.38,39,42
Gene-based analysis of MIR185 and its predicted target genes revealed no individual or gene-wide association between schizophrenia and common variants at the MIR185 locus per se. Two possible explanations for this finding can be proposed. First, common functional variation at this locus, if it exists, may have no effect on schizophrenia susceptibility because MIR185 is not critically involved in disease-related processes. Second, the lack of association may simply reflect the absence of common functional variation at this locus. This latter explanation is supported by a recent study concerning the genetic regulation of microRNA expression.43 Here, no SNP had significant cis effects on miR-185 expression. Lack of common functional variation at the MIR185 locus would also explain why previous attempts to identify schizophrenia-related genes in the 22q11.2DS region through searches for association with common variants in the region have proven frustrating.
Genetic support for the involvement of MIR185 and its regulatory pathways is provided by the present association analysis of target genes, which showed that schizophrenia-associated genes were significantly enriched within MIR185 targets. The genes ATAT1, SH3PXD2A and NTRK3 showed individual association with schizophrenia after correction for multiple testing. The 2 validated targets RHOA and CDC42 received nominally significant support (Table 2).
The ATAT1 (MEC-17) gene encodes for an α-tubulin acetyl-transferase, which plays a conserved role in several microtubule-based processes.44 A study in rats showed that ATAT1 was highly expressed in the cerebral cortex during various developmental stages.45 In adult rats, relatively high ATAT1 expression was detected in the cerebral cortex, the hippocampus, and the cerebellum. In addition ATAT1 deficiency caused defects in the migration of cortical neurons, thus indicating that ATAT1 and α-tubulin acetylation are important for cortical development.45 The ATAT1 gene is located in the extended major histocompatibility complex (MHC) region on chromosome 6. One of the investigated SNPs at the locus (rs9262135) achieved genome-wide significance (p < 0.001) in the PGC GWAS.13 Previous studies have reported strong association between common SNPs in the MHC region and schizophrenia.13,46–48 However, owing to extensive linkage disequilibrium, the issues of whether the association signal is driven by 1 or several genes in the region and whether immune- or nonimmune-related genes are implicated remain unclear.48
The product of the SH3 and PX domains 2A gene (SH3PXD2A, TKS5) is a tyrosine kinase substrate with 5 Srchomology 3 domains. This is required for podosome and invadopodia formation and for cancer cell invasion.49 The presence of severe developmental defects (including craniofacial abnormalities and heart malformations) in zebrafish embryos with reduced SH3PXD2A expression suggests that this product is implicated in neural crest migration during embryonic development.50
The association findings for ATAT1 and SH3PXD2A suggest that alterations in neural and neural crest cell migration are plausible mechanisms in the pathophysiology of schizophrenia. This provides further support for the hypothesis that schizophrenia is a neurodevelopmental disorder.51
The NTRK3 (TRKC) gene encodes for a tyrosine protein kinase neurotrophin-3 receptor.52 NTRK3 is expressed in various structures within the developing mouse brain, including the caudoputamen, cerebellum and hippocampus.52 In the adult mouse brain, NTRK3 is expressed in the cerebral cortex, hippocampus, thalamus and hypothalamus.52 Research has identified reduced NTRK3 gene expression in the PFC of patients with schizophrenia53,54 as well as associations between SNPs in the NTRK3 gene and hippocampal function and various psychiatric disorders. These include bipolar disorder55 and schizophrenia.47,56 Interestingly, Cdc42 and RhoA — the 2 validated schizophrenia-associated targets of miR-18518,19 — are also involved in neurotrophin signalling. This may indicate that miR-185 influences neurodevelopment through regulation of different levels of the neurotrophin signalling pathway. In the case of NTRK3, only the Z FIX method generated a significant association, which might indicate that multiple common variants exert functional effects on this gene.
The expression patterns of the 3 schizophrenia-associated target genes were further analyzed to investigate whether they were expressed in the same brain regions as miR-185, and whether these overlapping regions have been previously implicated in schizophrenia. This revealed substantial overlap in the expression patterns of the 4 genes in brain regions that included the hippocampus and the frontal cortex. Since both regions have been implicated in schizophrenia,15,36 this finding provides further support for the involvement of the miR-185 regulatory pathway in the development of schizophrenia.
In the resequencing step, no genetic variants were identified in the 82 bp long premature MIR185 sequence. Although the existence of ultrarare variants cannot be excluded, the present observations suggest that this region is highly conserved. If this is the case, a plausible hypothesis is that alterations in the mature microRNA sequence could have more dramatic effects, which is also supported by the high conservation of microRNA sequences between species.57 This hypothesis is consistent with observations from previous studies of microRNA sequences.58 The present study identified 4 rare variants flanking the premature MIR185 sequence for which a possible regulatory role could not be excluded. Although the 2 patient-specific variants were followed up by genotyping large samples of patients and controls, their possible contribution to the risk for schizophrenia could be neither confirmed nor entirely excluded. Furthermore, re-sequencing data derived from the 2 patients with 22q11.2 microdeletion revealed no evidence for the presence of recessive risk alleles in MIR185 on the nondeleted DNA strand. The investigation of larger numbers of deletion carriers would be required to determine whether recessive alleles contribute to schizophrenia and 22q11.2DS.59
Limitations
Reduced miR-185 expression in 22q11.2DS is attributable to hemizygosity. Future studies of the role of miR-185 in schizophrenia must identify rare variants that functionally mimic this effect. Although the present samples included several thousand individuals, the power to detect associations with rare variants was limited,60 since such variants may be extremely rare.
Conclusion
The present human genetic analyses of common and rare variants generated no evidence for the involvement of MIR185 in the development of schizophrenia in idiopathic or 22q11.2DS patients. This may reflect the lack of common functional variation at this locus and the limited power to detect rare variant associations. However, the expression patterns of miR-185 and its target genes (ATAT1, SH3PXD2A, NTRK3) observed in the brains of embryonic and adult mice, as well as the significant association findings for the 3 target genes, suggest that further research into the possible involvement of miR-185 regulatory pathways in the risk for schizophrenia and (possibly) other neuropsychiatric phenotypes in 22q11.2DS, as well as in idiopathic schizophrenia, is warranted.
Acknowledgements
We are grateful to all of the patients and control individuals who contributed to this study. We also thank the probands from the Heinz Nixdorf Recall (HNR) study. We thank Marie Luise Dreisow and Peter Tessmann for their excellent technical assistance. We also thank Christine Schmäl for her careful reading of the manuscript. We are grateful to the Psychiatric Genomics Consortium for contributing to this study by sharing of their data. We confirm that these data will be used for noncommercial research purposes only. We have complied with all applicable state, local and federal laws or regulations and institutional policies regarding human subjects and genetic research. We acknowledge that secondary distribution of the data without registration by secondary parties is prohibited and confirm that we will cite the appropriate PGC publication in any communications or publications arising directly or indirectly from these data.
Footnotes
Funding: This study was supported by the German Federal Ministry of Education and Research (BMBF) through the Integrated Genome Research Network (IG) MooDS (Systematic Investigation of the Molecular Causes of Major Mood Disorders and Schizophrenia; grant 01GS08144 to M.M. Nöthen and S. Cichon; grant 01GS08147 to M. Rietschel), under the auspices of the National Genome Research Network plus (NGFNplus). M.M. Nöthen is a member of the DFG-funded Excellence-Cluster ImmunoSensation. M.M. Nöthen also received support from the Alfried Krupp von Bohlen und Halbach-Stiftung. A.J. Forstner received support from the BONFOR program of the Medical Faculty of the University of Bonn. This study was partially supported by a grant from the European Union (EUTwinsS network; RTN, FP6), which supported work in which F.B. Basmanav was a Marie Curie Fellow and M.M. Nöthen and S. Cichon were principal investigators. The HNR cohort was established with the support of the Heinz Nixdorf Foundation. These funding sources had no involvement in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the paper for publication.
Competing interests: A.D. Børglum is the co-founder and CEO of PsychoGenetics, which pursues strategies for new diagnostic and therapeutic approaches for mental disorders.
Contributors: A.J. Forstner, F.B. Basmanav, M.M. Mattheisen, M. Rietschel, A. Zimmer, M.M. Nöthen, X. Miró and S. Cichon designed the study. A.J. Forstner, F.B. Basmanav, A.C. Böhmer, M.V. Hollegaard, E. Janson, E. Strengman, W. Maier, R. Mössner, D. Rujescu, R.A. Ophoff, S. Moebus, P.B. Mortensen, A.D. Børglum, D.M. Hougaard, J. Frank, S.H. Witt, M. Rietschel, A. Zimmer and X. Miró acquired the data. A.J. Forstner, F.B. Basmanav, M. Mattheisen, L. Priebe, F. Degenhardt, P. Hoffmann, S. Herms, A. Zimmer, M.M. Nöthen, X. Miró and S. Cichon analyzed the data. A.J. Forstner, F.B. Basmanav, A. Zimmer, M.M. Nöthen, X. Miró and S. Cichon wrote the article, which all other authors reviewed. All authors approved the final version for publication.
GROUP Investigators: René S. Kahn, Don H. Linszen, Jim van Os, Durk Wiersma, Richard Bruggeman, Wiepke Cahn, Lieuwe de Haan, Lydia Krabbendam and Inez Myin-Germeys. See the Appendix, available at jpn.ca for affiliations and contributor information. These authors declare no competing interests.
References
- 1.Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–93. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 2.Levinson DF, Duan J, Oh S, et al. Copy number variants in schizophrenia: confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. Am J Psychiatry. 2011;168:302–16. doi: 10.1176/appi.ajp.2010.10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11:402–16. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–5. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- 5.Bassett AS, Chow EW. Schizophrenia and 22q11.2 deletion syndrome. Curr Psychiatry Rep. 2008;10:148–57. doi: 10.1007/s11920-008-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Earls LR, Fricke RG, Yu J, et al. Age-Dependent MicroRNA Control of Synaptic Plasticity in 22q11 Deletion Syndrome and Schizophrenia. J Neurosci. 2012;32:14132–44. doi: 10.1523/JNEUROSCI.1312-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelmann L, Pandita RK, Morrow BE. Low-copy repeats mediate the common 3-Mb deletion in patients with velo-cardio-facial syndrome. Am J Hum Genet. 1999;64:1076–86. doi: 10.1086/302343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaikh TH, Kurahashi H, Saitta SC, et al. Chromosome 22-specific low copy repeats and the 22q11.2 deletion syndrome: genomic organization and deletion endpoint analysis. Hum Mol Genet. 2000;9:489–501. doi: 10.1093/hmg/9.4.489. [DOI] [PubMed] [Google Scholar]
- 9.Fineberg SK, Kosik KS, Davidson BL. MicroRNAs potentiate neural development. Neuron. 2009;64:303–9. doi: 10.1016/j.neuron.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Schratt G. microRNAs at the synapse. Nat Rev Neurosci. 2009;10:842–9. doi: 10.1038/nrn2763. [DOI] [PubMed] [Google Scholar]
- 11.Xu B, Karayiorgou M, Gogos JA. MicroRNAs in psychiatric and neurodevelopmental disorders. Brain Res. 2010;1338:78–88. doi: 10.1016/j.brainres.2010.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beveridge NJ, Tooney PA, Carroll AP, et al. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum Mol Genet. 2008;17:1156–68. doi: 10.1093/hmg/ddn005. [DOI] [PubMed] [Google Scholar]
- 13.Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–76. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stark KL, Xu B, Bagchi A, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40:751–60. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- 15.Benetti S, Mechelli A, Picchioni M, et al. Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain. 2009;132:2426–36. doi: 10.1093/brain/awp098. [DOI] [PubMed] [Google Scholar]
- 16.Xu B, Hsu PK, Stark KL, et al. Derepression of a neuronal inhibitor due to miRNA dysregulation in a schizophrenia-related microdeletion. Cell. 2013;152:262–75. doi: 10.1016/j.cell.2012.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu M, Lang N, Chen X, et al. miR-185 targets RhoA and Cdc42 expression and inhibits the proliferation potential of human colorectal cells. Cancer Lett. 2011;301:151–60. doi: 10.1016/j.canlet.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11:557–66. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- 19.Ide M, Lewis DA. Altered cortical CDC42 signaling pathways in schizophrenia: implications for dendritic spine deficits. Biol Psychiatry. 2010;68:25–32. doi: 10.1016/j.biopsych.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson PT, Wilfred BR. In situ hybridization is a necessary experimental complement to microRNA (miRNA) expression profiling in the human brain. Neurosci Lett. 2009;466:69–72. doi: 10.1016/j.neulet.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miró X, Zhou X, Boretius S, et al. Haploinsufficiency of the murine polycomb gene Suz12 results in diverse malformations of the brain and neural tube. Dis Model Mech. 2009;2:412–8. doi: 10.1242/dmm.001602. [DOI] [PubMed] [Google Scholar]
- 22.Diez-Roux G, Banfi S, Sultan M, et al. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol. 2011;9:e1000582. doi: 10.1371/journal.pbio.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 24.Børglum AD, Demontis D, Grove J, et al. Genome-wide study of association and interaction with maternal cytomegalovirus infection suggests new schizophrenia loci. Mol Psychiatry. 2014;19:325–33. doi: 10.1038/mp.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedroso I, Lourdusamy A, Rietschel M, et al. Common genetic variants and gene-expression changes associated with bipolar disorder are over-represented in brain signaling pathway genes. Biol Psychiatry. 2012;72:311–7. doi: 10.1016/j.biopsych.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treutlein J, Cichon S, Ridinger M, et al. Genome-wide association study of alcohol dependence. Arch Gen Psychiatry. 2009;66:773–84. doi: 10.1001/archgenpsychiatry.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stefansson H, Rujescu D, Cichon S, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–6. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colella S, Yau C, Taylor JM, et al. QuantiSNP: an Objective Bayes Hidden-Markov Model to detect and accurately map copy number variation using SNP genotyping data. Nucleic Acids Res. 2007;35:2013–25. doi: 10.1093/nar/gkm076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kent WJ, Sugnet CW, Furey TS, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pruitt KD, Tatusova T, Klimke W, et al. NCBI reference sequences: current status, policy and new initiatives. Nucleic Acids Res. 2009;37:D32–6. doi: 10.1093/nar/gkn721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abecasis GR, Auton A, Brooks LD, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat. 2000;15:7–12. doi: 10.1002/(SICI)1098-1004(200001)15:1<7::AID-HUMU4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 34.Desmet FO, Hamroun D, Lalande M, et al. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmermund A, Mohlenkamp S, Stang A, et al. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL Study. Risk factors, evaluation of coronary calcium and lifestyle. Am Heart J. 2002;144:212–8. doi: 10.1067/mhj.2002.123579. [DOI] [PubMed] [Google Scholar]
- 36.Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–93. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- 37.Olenik C, Barth H, Just I, et al. Gene expression of the small GTP-binding proteins RhoA, RhoB, Rac1, and Cdc42 in adult rat brain. Brain Res Mol Brain Res. 1997;52:263–9. doi: 10.1016/s0169-328x(97)00270-2. [DOI] [PubMed] [Google Scholar]
- 38.Chow EW, Mikulis DJ, Zipursky RB, et al. Qualitative MRI findings in adults with 22q11 deletion syndrome and schizophrenia. Biol Psychiatry. 1999;46:1436–42. doi: 10.1016/s0006-3223(99)00150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerkes EH, Hordijk R, Dijkhuizen T, et al. Bilateral polymicrogyria as the indicative feature in a child with a 22q11.2 deletion. Eur J Med Genet. 2010;53:344–6. doi: 10.1016/j.ejmg.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Koolen DA, Veltman JA, Renier WO, et al. Chromosome 22q11 deletion and pachygyria characterized by array-based comparative genomic hybridization. Am J Med Genet A. 2004;131:322–4. doi: 10.1002/ajmg.a.30377. [DOI] [PubMed] [Google Scholar]
- 41.Robin NH, Taylor CJ, McDonald-McGinn DM, et al. Polymicrogyria and deletion 22q11.2 syndrome: window to the etiology of a common cortical malformation. Am J Med Genet A. 2006;140:2416–25. doi: 10.1002/ajmg.a.31443. [DOI] [PubMed] [Google Scholar]
- 42.Lynch DR, McDonald-McGinn DM, Zackai EH, et al. Cerebellar atrophy in a patient with velocardiofacial syndrome. J Med Genet. 1995;32:561–3. doi: 10.1136/jmg.32.7.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gamazon ER, Ziliak D, Im HK, et al. Genetic architecture of microRNA expression: implications for the transcriptome and complex traits. Am J Hum Genet. 2012;90:1046–63. doi: 10.1016/j.ajhg.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shida T, Cueva JG, Xu Z, et al. The major alpha-tubulin K40 acetyl-transferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci U S A. 2010;107:21517–22. doi: 10.1073/pnas.1013728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L, Wei D, Wang Q, et al. MEC-17 deficiency leads to reduced alpha-tubulin acetylation and impaired migration of cortical neurons. J Neurosci. 2012;32:12673–83. doi: 10.1523/JNEUROSCI.0016-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi J, Levinson DF, Duan J, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–7. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stefansson H, Ophoff RA, Steinberg S, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–7. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Courtneidge SA. Cell migration and invasion in human disease: the Tks adaptor proteins. Biochem Soc Trans. 2012;40:129–32. doi: 10.1042/BST20110685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy DA, Diaz B, Bromann PA, et al. A Src-Tks5 pathway is required for neural crest cell migration during embryonic development. PLoS ONE. 2011;6:e22499. doi: 10.1371/journal.pone.0022499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Owen MJ, O’Donovan MC, Thapar A, et al. Neurodevelopmental hypothesis of schizophrenia. Br J Psychiatry. 2011;198:173–5. doi: 10.1192/bjp.bp.110.084384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamballe F, Smeyne RJ, Barbacid M. Developmental expression of trkC, the neurotrophin-3 receptor, in the mammalian nervous system. J Neurosci. 1994;14:14–28. doi: 10.1523/JNEUROSCI.14-01-00014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schramm M, Falkai P, Feldmann N, et al. Reduced tyrosine kinase receptor C mRNA levels in the frontal cortex of patients with schizophrenia. Neurosci Lett. 1998;257:65–8. doi: 10.1016/s0304-3940(98)00807-6. [DOI] [PubMed] [Google Scholar]
- 54.Weickert CS, Ligons DL, Romanczyk T, et al. Reductions in neurotrophin receptor mRNAs in the prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2005;10:637–50. doi: 10.1038/sj.mp.4001678. [DOI] [PubMed] [Google Scholar]
- 55.Athanasiu L, Mattingsdal M, Melle I, et al. Intron 12 in NTRK3 is associated with bipolar disorder. Psychiatry Res. 2011;185:358–62. doi: 10.1016/j.psychres.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Otnaess MK, Djurovic S, Rimol LM, et al. Evidence for a possible association of neurotrophin receptor (NTRK-3) gene polymorphisms with hippocampal function and schizophrenia. Neurobiol Dis. 2009;34:518–24. doi: 10.1016/j.nbd.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 57.Guo L, Lu Z. The fate of miRNA* strand through evolutionary analysis: implication for degradation as merely carrier strand or potential regulatory molecule? PLoS ONE. 2010;5:e11387. doi: 10.1371/journal.pone.0011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hildebrand MS, Witmer PD, Xu S, et al. miRNA mutations are not a common cause of deafness. Am J Med Genet A. 2010;152A:646–52. doi: 10.1002/ajmg.a.33299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams NM. Molecular mechanisms in 22q11 deletion syndrome. Schizophr Bull. 2011;37:882–9. doi: 10.1093/schbul/sbr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bansal V, Libiger O, Torkamani A, et al. Statistical analysis strategies for association studies involving rare variants. Nat Rev Genet. 2010;11:773–85. doi: 10.1038/nrg2867. [DOI] [PMC free article] [PubMed] [Google Scholar]