Abstract
Background
Voxel-based morphometry (VBM) studies have demonstrated that grey matter abnormalities are involved in the pathophysiology of late-life depression (LLD), but the findings are inconsistent and have not been quantitatively reviewed. The aim of the present study was to conduct a meta-analysis that integrated the reported VBM studies, to determine consistent grey matter alterations in individuals with LLD.
Methods
A systematic search was conducted to identify VBM studies that compared patients with LLD and healthy controls. We performed a meta-analysis using the effect size signed differential mapping method to quantitatively estimate regional grey matter abnormalities in patients with LLD.
Results
We included 9 studies with 11 data sets comprising 292 patients with LLD and 278 healthy controls in our meta-analysis. The pooled and subgroup meta-analyses showed robust grey matter reductions in the right lentiform nucleus extending into the parahippocampus, the hippocampus and the amygdala, the bilateral medial frontal gyrus and the right subcallosal gyrus as well as a grey matter increase in the right lingual gyrus. Meta-regression analyses showed that mean age and the percentage of female patients with LLD were not significantly related to grey matter changes.
Limitations
The analysis techniques, patient characteristics and clinical variables of the studies included were heterogeneous, and most participants were medicated.
Conclusion
The present meta-analysis is, to our knowledge, the first to overcome previous inconsistencies in the VBM studies of LLD and provide robust evidence for grey matter alterations within fronto–striatal–limbic networks, thereby implicating them in the pathophysiology of LLD. The mean age and the percentage of female patients with LLD did not appear to have a measurable impact on grey matter changes, although we cannot rule out the contributory effects of medication.
Introduction
Late-life depression (LLD) is a common and disabling disorder, which is typically defined as depression in individuals older than 60 years. Estimates of the prevalence of clinically relevant depressive symptoms in older adults typically range from 10% to 15%, and rates of major depression range from 1% to 5%.1–3 Late-life depression is characterized by diverse etiological factors that remain poorly understood.4,5 Successes in delineating the neurobiology of LLD have closely paralleled progress in neuroimaging and have provided evidence that LLD is associated with underlying brain anatomic and functional abnormalities.
Structural neuroimaging findings have revealed grey matter volume reductions in multiple fronto–striatal–limbic regions of older depressed patients, including the anterior cingulate cortex (ACC), the prefrontal cortices, the striatum, the hippocampus and the amygdala,4,6,7 and these regions are therefore hypothesized to play a key role in the pathophysiology of the disorder. Most studies investigating grey matter changes in patients with LLD have used a seed-based region of interest (ROI) approach, in which investigators decide a priori which regions to investigate, and have compared volumes in patients and healthy controls. Recently, Sexton and colleagues6 conducted a systematic review and meta-analysis of the seed-based ROI studies on LLD and identified grey matter volume reductions in the hippocampus, orbitofrontal cortex, putamen and thalamus. Although their meta-analysis of ROI studies produced intriguing results, the conclusions reached may clearly be biased in favour of the relatively small number of seed regions chosen in the different individual studies.8
Voxel-based morphometry (VBM) is an automated whole brain–based analysis that can be used to perform a voxel-wise comparison of the local concentration of grey matter between groups without a priori specification of ROIs. Thus, it can identify potentially unexpected brain structure abnormalities,9 thereby overcoming the drawbacks of seed-based approaches, and provide a powerful tool for the unbiased study of brain-wide changes in grey matter volume in patients with LLD. Although VBM studies have also revealed abnormal grey matter alterations in patients with LLD compared with healthy controls, there have been a number of nonreplications and contradictory results reported. For example, grey matter reductions in the hippocampus of patients with LLD relative to healthy controls have been reported in 2 studies,10,11 but not in others.12,13 One study reported grey matter reductions in the frontal lobe, amygdala and hippocampus,11 whereas another reported reductions mainly in the caudate, thalamus and parietal and occipital lobes.12 Three additional studies did not detect any significant differences in grey matter volumes between LLD and healthy control groups.14–16 There is considerable variation across published studies in terms of the sample sizes, the demographic and clinical characteristics of the patients and the imaging protocols used. Small sample sizes in particular may result in false-positive or false-negative findings. Overall, these variations among studies may account for the inconsistent findings. Therefore, identifying consistent results from VBM studies of grey matter volume in patients with LLD through a meta-analysis is of particular importance.
Effect size signed differential mapping (ES-SDM) is a newly developed voxel-based meta-analytic technique that has been successfully applied to neuroimaging studies of several neurologic and psychiatric disorders, such as Alzheimer disease,17 attention-deficit/hyperactivity disorder18 and first-episode psychosis.19 The ES-SDM technique has been shown to be superior, in some respects, to previous coordinate-based meta-analytical methods, such as activation likelihood estimation (ALE; for more details, please see the Methods section).20 However, to our knowledge, ES-SDM has not been used in a meta-analysis of VBM studies comparing patients with LLD and healthy controls. Given the inconsistencies in previous reports of grey matter abnormalities in patients with LLD, we quantitatively reviewed the published VBM studies on LLD using this ES-SDM analysis approach to identify consistent regional grey matter abnormalities.
Methods
Study selection
Studies considered for inclusion in the meta-analysis were identified from exhaustive searches using PubMed, Web of Knowledge, Embase and Science Direct, and the keywords “geriatric,” “elder,” “elderly,” “late-life,” “old age,” “old,” “early-onset,” “late-onset” or “older,” plus “major depressive disorder,” “depressive,” “depressed” or “depression,” plus “voxel-based morphometry,” “voxel-based,” “morphometry” or “VBM.” Studies were also identified by consulting review articles and the reference lists in the articles identified. We considered studies to be eligible for inclusion if they reported a VBM comparison between patients with LLD and healthy controls and reported whole-brain results of grey matter alterations in a stereotactic space in 3 coordinates (x, y, z; Montreal Neurological Institute or Talairach).
Quality assessment
We assessed the quality of the included studies using a 12-point checklist that focused on both the clinical and demographic aspects of individual study samples and on the imaging-specific methodology. The checklist was based on previous meta-analytic studies,21–23 which included structural measures from MRI, but were modified to reflect critical variables that are important to assess in VBM studies. This assessment included the quality of the diagnostic procedures, the demographic and clinical characterization, the sample size, the MRI acquisition parameters, the analysis technique and the quality of the reported results (see the Appendix, Table S1, available at jpn.ca). This checklist was not designed as an assessment tool; however, it provided an objective indication of the rigor of individual studies. At least 2 authors reviewed every paper and independently determined a completeness rating. These ratings were compared; any paper for which the 2 ratings disagreed was discussed, and a consensus score was obtained. The quality scores for each study are presented in Table 1.
Table 1.
Demographic and clinical characteristics of the participants in 9 voxel-based morphometry studies (11 data sets) included in the meta-analysis
| Patients with LLD | Healthy controls | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Study | No. (% female) | Mean age, yr | % Medicated | Illness duration, yr | Severity (scale type), state | Age at onset, yr (EO or LO) | Quality scores (out of 12) | Comorbidity | No. (% female) | Mean age, yr |
| Colloby et al.16 | 38 (71.0) | 74.1 | NA | 23 | 11.6 (GDS) 12.9 (MADRS) depressed or remitted |
51.8 (EO or LO) | 11 | Negative | 30 (66.7) | 74.4 |
| Delaloye et al.27* | 11 (63.6) | 75.82 | 73 | 1.73 | 2.36 (GDS) 11/11 remitted |
74.09 (LO) | 11 | Negative | 30 (73.3) | 70.83 |
| Delaloye et al.27† | 30 (80.0) | 65 | 50 | 29.3 | 2.47 (GDS) 30/30 remitted |
35.67 (EO) | 11 | Negative | 30 (73.3) | 70.83 |
| Egger et al.11 | 14 (71.4) | 71.4 | 71.4 | NA | 21.14 (GDS) 14/14 depressed |
> 60 (LO) | 11.5 | Negative | 20 (65) | 72.3 |
| Hwang et al.28 | 70 (0.00) | 79.4 | NA | 8.34 | 29.2 (HDRS) 70/70 depressed |
68.8 (LO) | 10 | Negative | 26 (0.00) | 79.5 |
| Koolschijn et al.15 | 28 (100) | 64.04 | 78.6 | 7.79 | 18.32 (MADRS) 8/28 depressed |
33.04 (EO) | 12 | Negative | 38 (100) | 61.89 |
| Smith et al.12 | 16 (62.5) | 65.3 | 25 | NA | 26 (HDRS) 20.1 (GDS) 16/16 depressed |
NA (EO or LO) | 11.5 | Negative | 13 (61.5) | 67.4 |
| Sexton et al.14 | 36 (66.7) | 71.83 | 97.2 | NA | 4.19 (HDRS) 3.83 (GDS) 27/36 remitted |
45.39 (EO or LO) | 10.5 | Some with controlled hypertension and diabetes | 25 (64.0) | 71.76 |
| Xie et al.29‡ | 18 (77.8) | 68.61 | 88.9 | NA | 17.28 (GDS) 18/18 depressed |
NA (EO or LO) | 11.5 | Negative | 25 (48.0) | 74.28 |
| Xie et al.29§ | 12 (58.3) | 68.33 | 83.3 | NA | 14.25 (GDS) 12/12 depressed |
NA (EO or LO) | 11.5 | MCI | 25 (48.0) | 74.28 |
| Yuan et al.13 | 19 (52.6) | 67.1 | Washout period > 3 mo | 3.7 | 3.1 (HDRS) 19/19 remitted |
64.6 (LO) | 11.5 | Negative | 16 (50) | 67.7 |
EO = early onset; GDS = Geriatric Depression Scale; HDRS = Hamilton Rating Scale for Depression; LLD = late-life depression; LO = late onset; MADRS = Montgomery-Åsberg depression rating scale; MCI = mild cognitive impairment; NA = not available.
Subgroup of patients with early-onset LLD.
Subgroup of patients with late-onset LLD.
Subgroup of patients with LLD without amnestic mild cognitive impairment.
Subgroup of patients with LLD and comorbid amnestic mild cognitive impairment.
Voxel-wise meta-analysis
Regional differences in grey matter volume between the LLD and healthy control groups were analyzed using ES-SDM (http://www.sdmproject.com), which involved a voxel-based meta-analytic approach.20 The ES-SDM approach has previously been used in the meta-analysis of studies across several neuropsychiatric disorders17–19 and incorporates useful features from previous methods, such as ALE24 and multilevel kernel density analysis.25 The approach also embodies some improvements and new features. For example, in ES-SDM, unlike other coordinate-based meta-analytic methods,24 both positive (i.e., increased volume) and negative (i.e., decreased volume) coordinates are reconstructed in the same map to prevent a particular voxel from erroneously appearing to be positive and negative at the same time.26 Second, rather than assigning voxels a conventional value (e.g., “0” or “1”), ES-SDM assigns each voxel a measure of the effect size, namely, the standardized mean (for 1-sample designs) and the standardized mean difference (for 2-sample designs), referred to as Hedges d at the sample level. The use of effect sizes allows the combination of reported peak coordinates with statistical parametric maps, thereby allowing more exhaustive and accurate meta-analyses. Third, complementary analyses, such as jack-knife and meta-regression analyses, were used to assess the robustness and heterogeneity of the results.20 Finally, the ES-SDM takes into account null findings, which would cause a number of voxels to cease to be detected as significant.
First, we first performed a pooled meta-analysis of all included studies. Next, we performed 2 subgroup meta-analyses, including studies of patients with LLD but without comorbidity and studies in which all patients were currently depressed. Strategically, recruiting patients with LLD but without comorbidity helps to reduce the influence of comorbidity, which may dilute the neuropathological findings in LLD. The ES-SDM methods have been described in detail elsewhere.18–20 Briefly, we selected the reported peak coordinates of all structural differences that were statistically significant at the whole brain level. We checked that each included study used the same statistical threshold throughout the whole brain to avoid potential bias toward liberally thresholded regions. Second, we separately recreated a standard Talairach map of the differences in grey matter for each study by means of a Gaussian kernel. For peak coordinates, the recreation was based on converting the peak t value to Hedges effect size and then applying a non-normalized Gaussian kernel to the voxels near the peak, which assigns higher values to the voxels closer to peaks. Null findings from studies were recreated with effect sizes as normal, with the only difference that all voxels in the effect size map were estimated to have a null effect size. These null effect sizes were included in the random-effects meta-analytic models similar to other effect sizes, thus modifying the meta-analytic effect size. Third, the mean map was obtained by a voxel-wise calculation of the mean of the study maps, which was weighted by the square root of the sample size of each study, so that studies with larger sample sizes had a larger contribution. Finally, we determined statistical significance using standard randomization tests, thus creating null distributions from which the p values were directly obtained. Default ES-SDM kernel size and thresholds were used (full-width at half-maximum = 20 mm, voxel p = 0.005, peak height Z = 1, cluster extent = 10 voxels).20
Reliability analysis
A systematic whole-brain voxel-based jack-knife sensitivity analysis was also conducted to test the robustness of the results.20 The reliability analysis of the pooled analysis results consisted of repeating the main statistical analysis 11 times, but discarding 1 different study each time (i.e., removing 1 study and repeating the analysis, then putting that study back and removing another study and repeating the analysis). Similarly, the reliability estimate of the subgroup analysis results consisted of repeating the main statistical analysis 9 times, but discarding 1 different study each time. The rationale of this test is that if a previously significant brain region remains significant in all or most of the combinations of the studies, it can be concluded that this finding is highly replicable.20,26
Meta-regression analysis
The potential effects of several relevant sociodemographic and clinical variables listed in Table 1 were examined by means of simple linear regression, weighted by the square root of the sample size and restricted to predict only possible SDM values (i.e., from −1 to 1) in the observed range of values of the variable. The main output for each variable was a map of the regression slope.26 As in previous meta-analyses, to minimize the detection of spurious associations, we decreased the probability threshold to 0.0005, required abnormalities to be detected both in the slope and in 1 of the extremes of the regressor, and discarded findings in regions other than those detected in the main analyses. Finally, regression plots were visually inspected to discard fittings driven by too few studies.19,26
Results
Included studies and sample characteristics
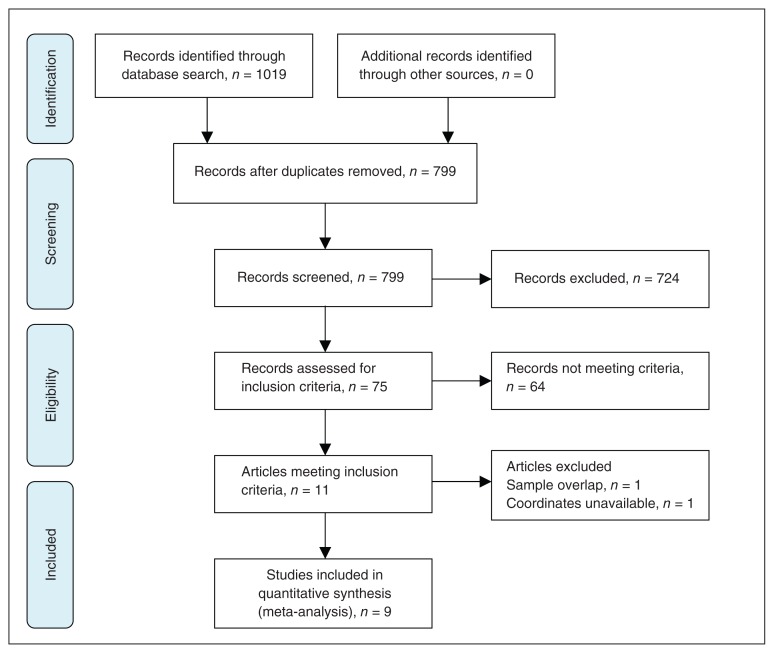
The search strategy identified a total of 799 documents, and 11 VBM studies10–16,27–30 met the inclusion criterion of comparing grey matter volume alterations between patients with LLD and healthy controls. No additional articles were found in the reference lists of the selected studies. Of these 11 VBM studies, we excluded studies if they reanalyzed previously published data30 and if they authors did not report coordinates for the relevant contrasts and did not or could not supply these when we contacted them by email.10 This left a total of 9 articles for our meta-analysis, including 2 studies that recruited patients with LLD and with comorbidities, which are commonly encountered in clinical practice (Table 1).14,29 In 2 studies,27,29 the analyses were performed based on 2 different subgroups of patients with LLD, who we then compared with the same healthy control groups. Therefore, we treated these studies as 2 unique studies, with each patient subgroup included independently in the meta-analysis; a total of 11 data sets were ultimately included in the meta-analysis. Our final sample comprised 292 patients with LLD and 278 healthy controls. Figure 1 shows the identification and attrition of the studies. The clinical and demographic data of the participants from all recruited studies are presented in Table 1. In each study, no significant difference was found in age or sex between the LLD and control groups. There was also no significant difference in age or sex between the 2 groups when considering the entire data set: the mean age was 71.61 years in the patient group versus 71.27 years in the control group, and there were 161 (55.14%) women in the patient group versus 171 (61.51%) women in the control group.
Fig. 1.
Meta-analysis of voxel-based morphometry studies in patients with late-life depression.
Pooled meta-analysis of all studies
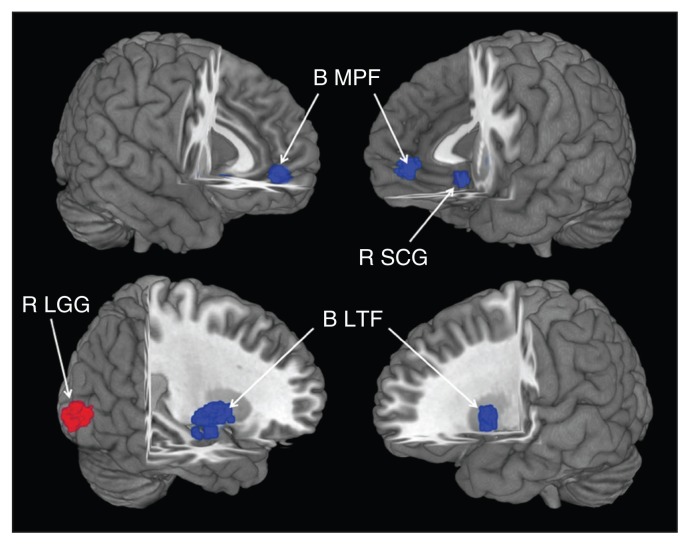
In the pooled meta-analysis, patients with LLD had grey matter reductions in 5 clusters relative to healthy controls (Fig. 2 and Table 2). The largest cluster had a peak located in the right lentiform nucleus and extended into the parahippocampus, hippocampus and amygdala. The second largest cluster consisted of bilateral reductions in the medial frontal gyrus. Grey matter reductions in the left putamen and the right subcallosal gyrus were also detected. A grey matter volume increase was observed in the right lingual gyrus.
Fig. 2.
The areas of decreased (blue) and increased (red) grey matter volumes in patients with late-life depression (LLD) compared with healthy controls in the pooled meta-analysis. Grey matter volume changes in patients with LLD are displayed on a 3-dimensionally rendered brain, with part of the left or right hemisphere removed.
Table 2.
Regional differences in grey matter volume between patients with LLD and healthy controls in the pooled meta-analysis
| Maximum | Cluster | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Brain regions* | Talairach coordinates, x, y, z | SDM value | p value | No. of voxels | Breakdown (no. of voxels) |
| LLD < control | |||||
| R lentiform nucleus, putamen | 30, −6, −6 | −1.991 | < 0.001 | 385 | R lentiform nucleus, putamen (144) R claustrum (42) R parahippocampalgyrus, amygdala (80) R lentiform nucleus, globuspallidus (76) R parahippocampalgyrus, hippocampus (19) R parahippocampalgyrus, BA 28,34 (22) R insula (2) |
| L lentiform nucleus, putamen | −26, 8, 0 | −1.310 | 0.002 | 77 | L lentiform nucleus, putamen (61) L claustrum (16) |
| R medial frontal gyrus | 4, 50, −2 | −1.305 | 0.002 | 85 | R medial frontal gyrus, BA 10 (41) R anterior cingulate, BA 10,32 (16) L medial frontal gyrus, BA 10 (19) L anterior cingulate, BA 10,32 (9) |
| R subcallosal gyrus | 4, 18, −12 | −1.265 | 0.002 | 36 | R subcallosal gyrus, BA 25 (16) R anterior cingulate, BA 25,32 (17) R medial frontal gyrus, BA 25 (3) |
| LLD > control | |||||
| R lingual gyrus | 12, −98, −8 | 1.141 | < 0.001 | 217 | R lingual gyrus, BA 17,18 (176) R cuneus, BA 17,18 (30) R inferior occipital gyrus, BA 17 (10) R fusiform gyrus, BA 18 (1) |
BA = Brodmann area; L = left; LLD = late-life depression; R = right; SDM = signed differential mapping.
Regions identified by meta-analysis of coordinates from 11 data sets (voxel-wise p < 0.005 and full-width at half-maximum 20 mm).
Subgroup meta-analysis of studies including patients without comorbidities
We analyzed 8 studies that included patients with LLD but without comorbidities as a subgroup. This analysis included 9 data sets that compared 244 patients without comorbidities and 228 healthy controls (Table 1). The subgroup analysis revealed grey matter volume decreases in patients relative to controls in the right lentiform nucleus extending into the parahippocampus, hippocampus, amygdala, right subcallosal gyrus and bilateral medial frontal gyrus. We observed a grey matter volume increase in the right lingual gyrus (Table 3).
Table 3.
Regional differences in grey matter volume between patients with LLD and healthy controls in the 2 subgroup meta-analyses*
| Maximum | Cluster | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Brain regions | Talairach coordinates, x, y, z | SDM value | p value | No. of voxels | Breakdown (no. of voxels) |
| Subgroup meta-analysis of studies without comorbidity | |||||
| LLD < control | |||||
| R lentiform nucleus, putamen | 30, −2, 0 | −1.551 | < 0.001 | 105 | R claustrum (49) R insula (29) R parahippocampalgyrus, amygdala (28) R lentiform nucleus, globus pallidus (19) R parahippocampal gyrus, hippocampus (4) |
| R subcallosal gyrus | 4, 18, −12 | −1.336 | 0.001 | 102 | R subcallosal gyrus, BA 25 (26) R anterior cingulate, BA 24,25,32 (38) R medial frontal gyrus, BA 11,25 (18) L anterior cingulate, BA 25,32 (16) L medial frontal gyrus, BA 11,25 (4) |
| LLD > control | |||||
| R lingual gyrus | 14, −98, −10 | 1.255 | < 0.001 | 326 | R lingual gyrus, BA 17,18 (235) R inferior occipital gyrus, BA 17,18 (20) R fusiform gyrus, BA 18 (18) R cuneus, BA 17,18 (25) R declive (28) |
| Subgroup meta-analysis of studies with currently depressed patients | |||||
| LLD < control | |||||
| R lentiform nucleus, putamen | 26, 2, −4 | −2.411 | < 0.001 | 388 | R lentiform nucleus, putamen (161) R lentiform nucleus, globus pallidus (91) R parahippocampalgyrus, amygdala (89) R parahippocampal gyrus, BA 28,34 (22) R claustrum (25) |
| L lentiform nucleus, putamen | −24, 8, 2 | −1.948 | < 0.001 | 196 | L lentiform nucleus, putamen (166) L claustrum (30) |
| R medial frontal gyrus | 2, 50, 0 | −1.567 | 0.003 | 111 | R medial frontal gyrus, BA 10 (71) L medial frontal gyrus, BA 10 (40) |
| R subcallosal gyrus | 4, 20, −12 | −1.555 | 0.003 | 40 | R subcallosal gyrus, BA 25 (36) R medial frontal gyrus, BA 25 (4) |
| LLD > control | |||||
| R lingual gyrus | 14, −94, −2 | 1.459 | < 0.001 | 228 | R lingual gyrus, BA 17,18 (169) R cuneus, BA 17,18 (59) |
BA = Broadmann area; L = left; LLD = late-life depression; R = right; SDM = signed differential mapping.
Voxel-wise p < 0.005 and full-width at half-maximum 20 mm.
Subgroup meta-analysis of patients with depressive episodes
We analyzed 4 studies in which all patients were currently depressed at the time of the scan, including 5 data sets that compared 130 patients with 109 healthy controls (Table 1). The subgroup analysis revealed grey matter volume decreases in patients relative to controls in the right lentiform nucleus extending into the parahippocampus, hippocampus, amygdala, left putamen, right subcallosal gyrus and bilateral medial frontal gyrus. We also observed a grey matter volume increase in the right lingual gyrus (Table 3).
Reliability analysis
As shown in Table 4, a whole-brain jack-knife sensitivity analysis of the pooled meta-analysis showed that the findings of grey matter volume reductions in patients with LLD in the right lentiform nucleus extending into the hippocampus, parahippocampus and amygdala were highly replicable, as these findings were preserved throughout all 11 combinations of the data sets. The results in the left putamen and the bilateral medial frontal gyrus were also significant in all but 1 combination of the data sets. The reduced volume in the right subcallosal gyrus remained significant in all but 2 combinations of the data sets. The higher grey matter volume in the right lingual gyrus was also significant in all but 1 combination of the data sets.
Table 4.
Sensitivity analyses of voxel-based morphometry studies of grey matter in patients with LLD in the pooled meta-analysis
| Decreased grey matter | Increased grey matter | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Discarded study | R putamen | R hippo | R parahippo | R amygdala | L putamen | Bilateral ACC | Bilateral MFG | R subcallosal gyrus | R lingual gyrus |
| Colloby et al.16 | Y¶ | Y | Y | Y | Y | Y | Y | Y | Y |
| Delaloye et al.27* | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Delaloye et al. 27† | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Egger et al.11 | Y | Y | Y | Y | Y | Y | Y | N** | Y |
| Hwang et al.28 | Y | Y | Y | Y | Y | Y | Y | Y | N |
| Koolschijn et al.15 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Smith et al.12 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Sexton et al.14 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Xie et al.29‡ | Y | Y | Y | Y | Y | N | N | N | Y |
| Xie et al.29§ | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Yuan et al.13 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
ACC = anterior cingulate cortex; hippo = hippocampus; L = left; LLD = late-life depression; MFG = medial frontal gyrus; N = no; parahippo = parahippocampus; R = right; VBM = voxel-based morphometry; Y = yes.
Subgroup of patients with early-onset LLD.
Subgroup of patients with late-onset LLD.
Subgroup of patients with LLD but without amnestic mild cognitive impairment.
Subgroup of patients with LLD and comorbid amnestic mild cognitive impairment.
Remained significantly increased/decreased after exclusion of the study in the jack-knife analysis.
No longer significantly increased/decreased after exclusion of the study in the jack-knife analysis.
Whole-brain jack-knife sensitivity analyses of the subgroup meta-analysis of studies involving patients without comorbidities showed that the findings of grey matter volume reductions in patients with LLD in the right lentiform nucleus extending to the hippocampus, parahippocampus and amygdala were highly replicable, as they were preserved throughout all 9 combinations of the data sets. The results in the right subcallosal gyrus and the bilateral medial frontal gyrus remained significant in all but 2 combinations of the data sets. The higher grey matter volume in the right lingual gyrus was also significant in all but 1 combination of the data sets.
Whole-brain jack-knife sensitivity analysis of the subgroup meta-analysis of patients with LLD who were currently depressed showed that the findings of grey matter volume reductions in patients with LLD in the right lentiform nucleus and left putamen were highly replicable, as this finding was preserved throughout all 5 combinations of the data sets. The results in the bilateral medial frontal gyrus and right subcallosal gyrus were also significant in all but 1 combination of the data sets. The higher grey matter volume in the right lingual gyrus was also significant in all but 1 combination of the data sets.
Meta-regression
We explored the information on the mean age and the percentage of female patients with LLD, which was available for all 292 participants in the 11 data sets, using meta-regression. The analysis revealed that the mean age and the percentage of female patients with LLD were not linearly associated with grey matter volume changes. The following variables could not be studied because data were available for fewer than 9 studies: mean age at onset, illness duration and percentage of medicated participants.26 We were also unable to assess whether LLD symptom severity was associated with the reported structural changes because the included studies used a wide range of different measures.
Discussion
To our knowledge, this is the first study to pool VBM studies for a meta-analysis of grey matter differences between patients with LLD and healthy controls. The study is timely given that a sufficient number of high-quality studies have only recently become available. The meta-analysis identified grey matter reductions localized in the fronto–striatal–limbic network, which plays key roles in memory and emotion processing, and a grey matter increase in the right lingual gyrus in patients with LLD. Meta-regression analyses showed the mean age and the percentage of female patients with LLD were not significantly related to grey matter changes, which illustrates that age and sex did not influence the results. Although we conducted a subgroup meta-analysis of patients during depressive episodes, the subgroup analysis included only 4 studies (5 data sets) and had limited power. Moreover, most patients in the present meta-analysis had previously taken, or were currently taking, antidepressant medication. Thus, our findings may be confounded by medication effect and/or current mood state. Therefore, further studies taking these variables into account are needed for a more complete understanding of brain abnormalities in patients with LLD.
Both the pooled meta-analysis and the subgroup meta-analysis revealed grey matter reductions in the right putamen, hippocampal gyrus, parahippocampal gyrus and amygdala. Decreased grey matter volume in these areas might suggest neuronal apoptosis or a loss of neuropil.31 Our findings are compatible with those of previous postmortem studies of patients with mood disorders, which reported reduced grey matter volumes in the putamen of patients with LLD patients compared with healthy controls32 and abnormal neuronal densities in these regions.33 Evidence has shown that the dorsal striatum (caudate nucleus and putamen) plays a key role in learning and memory;34 the hippocampus, parahippocampal gyrus and amygdala, in particular, comprise the key neural circuitry whereby emotions modulate memory function. Therefore, grey matter damage in these brain regions may contribute to memory function impairment, which is one of the clinical manifestations of LLD.13 Our findings are also in line with those of a recent meta-analysis of ROI volumetric MRI studies6 that reported reduced striatal and limbic volumes in patients with LLD. In addition, functional neuroimaging studies in patients with LLD have shown abnormal activations in the putamen35,36 and hippocampus37 relative to healthy controls, and these activations were correlated with memory deficits. Furthermore, an epigenetic interaction of catechol-O-methyltransferase Val158Met and methyl-enetetrahydrofolate reductase C677T polymorphisms may contribute to putamen volume differences between depressed and nondepressed elderly individuals.38
The subgenual areas (Brodmann area [BA] 25, also called the subgenual cingulate gyrus) have been found to play key roles in regulating visceral and autonomic responses to stressful events, assigning emotional valence to internal and external stimuli, and in emotional expression by virtue of strong connections with limbic and paralimbic structures.39,40 Moreover, converging evidence has indicated that the medial frontal gyrus is involved in emotion and mood disorders.41,42 Therefore, grey matter reductions in the bilateral medial frontal gyrus and the right subcallosal gyrus, which were identified both by the pooled and subgroup meta-analyses, may contribute to disrupted memory function and emotion processing in patients with LLD. Our findings are in line with those of a postmortem study of patients with major depressive disorder that reported decreases in the density or number of glia and the density and size of neurons in the ACC43 in patients with LLD. Functional neuroimaging studies have also reported decreased spontaneous neural activity in the right ACC and the bilateral medial prefrontal gyrus44–46 in patients with LLD relative to controls. It is striking that the sub-callosal gyrus, which was identified in this meta-analysis, is an important target for deep brain stimulation treatment for treatment-resistant depression, with good long-term safety and antidepressant efficacy.47–50 Furthermore, a recent neuropathological study found that cytoarchitecture, metabolism and perfusion changes in the brain may be caused by reduced capillary diameters in the ACC in patients with LLD.51
Taken together, these converging lines of evidence suggest that grey matter abnormalities within the fronto–striatal–limbic networks are implicated in the pathophysiology of LLD. A recent systematic review of white matter hyperintensity (WMH) studies in patients with LLD identified LLD, particularly late-onset depression, as being associated with more frequent and intense WMH compared with healthy elderly controls.52 Many studies have shown that the WMH associations with LLD occur mainly in subcortical regions and their frontal white matter projections.44,53–56 Diffusion tensor imaging studies have also shown microstructural white matter abnormalities in fronto–striatal–limbic networks, including the lateral to anterior and posterior cingulate cortices and the prefrontal, insular and parahippocampal regions, are associated with executive dysfunction in patients with LLD.46,57
A grey matter volume increase was observed in the right lingual gyrus both in the pooled meta-analysis and the subgroup meta-analysis. This brain region is not typically implicated in mood disorders. However, recent studies of major depressive disorder have found that depressed patients showed increased brain activity during emotion recognition in the lingual gyrus58 and increased occipital delta dipole density using magnetoencephalography,59 which suggests that its importance may have been overlooked. We believe this finding warrants further investigation to clarify its significance. The present meta-analyses showed that decreased grey matter volume in the left putamen was only seen in the pooled meta-analysis, which might suggest that the decreased left putamen volume was associated with comorbidity.
Limitations
Our study has several limitations that must be highlighted. First, the included studies varied in terms of the data acquisition, analysis techniques, patient characteristics and clinical variables. For example, the patients with LLD included in our study had either early or late onset of illness. Early- and late-onset depression may have different etiologies, which could have influenced the results of our findings. However, we were unable to investigate most of these variables in our study because detailed information was not available for a sufficient number of variables to perform a separate subgroup or meta-regression analysis.26 Second, most patients in the present meta-analysis had previously taken, or were currently taking, antidepressant medication. There is evidence that antidepressants may affect brain structure.60 Thus, our findings may be confounded by a medication effect. Therefore, further studies taking these variables into account are important for a more complete understanding of brain abnormalities in patients with LLD. Third, although VBM is surprisingly well adapted for coordinate-based meta-analyses, the method does have limitations that should be considered when interpreting the results. Voxel-based morphometry analyses may overrepresent group differences in areas of high anatomic variability61,62 and may be biased toward detecting highly localized group differences and against detecting ones that are spatially more diverse. Finally, ES-SDM assumes that effect sizes originate from homogeneous t contrasts, but they might originate from different covariate models or from different raw statistics. However, this is a limitation of all coordinate-based methods (e.g., all combine peaks from different origins), which could be controlled by SDM covariate analyses, if relevant.
Conclusion
Taken together, the results from this meta-analysis suggest that patients with LLD have significantly and robustly lower grey matter volumes in the fronto–striatal–limbic networks. These findings integrate previous inconsistencies in the structural imaging literature on LLD, and they provide robust evidence that grey matter abnormalities within fronto–striatal–limbic networks are implicated in the pathophysiology of LLD. However, owing to the small number of unmedicated patients in the studies included in our analysis, we cannot rule out the potential contributory effects of medication. Our findings are somewhat different from those reported in a recent VBM meta-analysis on adults with major depressive disorder (MDD) using the SDM method, which identified a grey matter reduction in the ACC.63 This suggests that LLD may have a different pathophysiology from adult MDD, and both LLD and adult MDD may be different subtypes of MDD.
Acknowledgements
This study was supported by the National Natural Science Foundation (Grant Nos. 81030027, 81227002, 81220108013 and 91132720), National Key Technologies R&D Program (Program No. 2012BAI01B03) and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, Grant No.IRT1272) of China. Dr. Gong would also like to acknowledge his Visiting Adjunct Professor appointment in the Department of Radiology at the University of Illinois Hospital & Health Sciences System, USA.
Footnotes
Competing interests: None declared.
Contributors: Q. Gong designed the study. M. Du, J. Lui and K.M. Kendrick acquired the data, which all authors analyzed. M. Du, J. Liu, K.M. Kendrick and Q. Gong wrote the article, which Z. Chen, X. Huang, J. Li, W. Kwang, K. Yang, W. Zhang, D. Zhou, F. Bi and Q. Gong reviewed. All authors approved the final version for publication.
References
- 1.Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307–11. doi: 10.1192/bjp.174.4.307. [DOI] [PubMed] [Google Scholar]
- 2.Copeland JR, Beekman AT, Dewey ME, et al. Depression in Europe. Geographical distribution among older people. Br J Psychiatry. 1999;174:312–21. doi: 10.1192/bjp.174.4.312. [DOI] [PubMed] [Google Scholar]
- 3.Steffens DC, Fisher GG, Langa KM, et al. Prevalence of depression among older Americans: the Aging, Demographics and Memory Study. Int Psychogeriatr. 2009;21:879–88. doi: 10.1017/S1041610209990044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexopoulos GS. Frontostriatal and limbic dysfunction in late-life depression. Am J Geriatr Psychiatry. 2002;10:687–95. [PubMed] [Google Scholar]
- 5.Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961–70. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- 6.Sexton CE, Mackay CE, Ebmeier KP. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am J Geriatr Psychiatry. 2013;21:184–95. doi: 10.1016/j.jagp.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Naismith SL, Norrie LM, Mowszowski L, et al. The neurobiology of depression in later-life: clinical, neuropsychological, neuroimaging and pathophysiological features. Prog Neurobiol. 2012;98:99–143. doi: 10.1016/j.pneurobio.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein JM, Goodman JM, Seidman LJ, et al. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry. 1999;56:537–47. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- 9.Ashburner J, Friston KJ. Voxel-based morphometry — the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 10.Bell-McGinty S, Butters MA, Meltzer CC, et al. Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. Am J Psychiatry. 2002;159:1424–7. doi: 10.1176/appi.ajp.159.8.1424. [DOI] [PubMed] [Google Scholar]
- 11.Egger K, Schocke M, Weiss E, et al. Pattern of brain atrophy in elderly patients with depression revealed by voxel-based morphometry. Psychiatry Res. 2008;164:237–44. doi: 10.1016/j.pscychresns.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Smith GS, Kramer E, Ma Y, et al. The functional neuroanatomy of geriatric depression. Int J Geriatr Psychiatry. 2009;24:798–808. doi: 10.1002/gps.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan Y, Zhu W, Zhang Z, et al. Regional gray matter changes are associated with cognitive deficits in remitted geriatric depression: an optimized voxel-based morphometry study. Biol Psychiatry. 2008;64:541–4. doi: 10.1016/j.biopsych.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 14.Sexton CE, Allan CL, Le Masurier M, et al. Magnetic resonance imaging in late-life depression: multimodal examination of network disruption. Arch Gen Psychiatry. 2012;69:680–9. doi: 10.1001/archgenpsychiatry.2011.1862. [DOI] [PubMed] [Google Scholar]
- 15.Koolschijn PC, van Haren NE, Schnack HG, et al. Cortical thickness and voxel-based morphometry in depressed elderly. Eur Neuropsychopharmacol. 2010;20:398–404. doi: 10.1016/j.euroneuro.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Colloby SJ, Firbank MJ, Vasudev A, et al. Cortical thickness and VBM-DARTEL in late-life depression. J Affect Disord. 2011;133:158–64. doi: 10.1016/j.jad.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Pan P, Huang R, et al. A meta-analysis of voxel-based morphometry studies of white matter volume alterations in Alzheimer’s disease. Neurosci Biobehav Rev. 2012;36:757–63. doi: 10.1016/j.neubiorev.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–98. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- 19.Radua J, Borgwardt S, Crescini A, et al. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav Rev. 2012;36:2325–33. doi: 10.1016/j.neubiorev.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Radua J, Mataix-Cols D, Phillips ML, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. 2012;27:605–11. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Brambilla P, Hardan A, di Nemi SU, et al. Brain anatomy and development in autism: review of structural MRI studies. Brain Res Bull. 2003;61:557–69. doi: 10.1016/j.brainresbull.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Shepherd AM, Matheson SL, Laurens KR, et al. Systematic meta-analysis of insula volume in schizophrenia. Biol Psychiatry. 2012;72:775–84. doi: 10.1016/j.biopsych.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Strakowski SM, DelBello MP, Adler C, et al. Neuroimaging in bipolar disorder. Bipolar Disord. 2000;2:148–64. doi: 10.1034/j.1399-5618.2000.020302.x. [DOI] [PubMed] [Google Scholar]
- 24.Turkeltaub PE, Eden GF, Jones KM, et al. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–80. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- 25.Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Soc Cogn Affect Neurosci. 2007;2:150–8. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 27.Delaloye C, Moy G, de Bilbao F, et al. Neuroanatomical and neuropsychological features of elderly euthymic depressed patients with early- and late-onset. J Neurol Sci. 2010;299:19–23. doi: 10.1016/j.jns.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 28.Hwang JP, Lee TW, Tsai SJ, et al. Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. J Geriatr Psychiatry Neurol. 2010;23:171–84. doi: 10.1177/0891988710363713. [DOI] [PubMed] [Google Scholar]
- 29.Xie C, Li W, Chen G, et al. The co-existence of geriatric depression and amnestic mild cognitive impairment detrimentally affect gray matter volumes: voxel-based morphometry study. Behav Brain Res. 2012;235:244–50. doi: 10.1016/j.bbr.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sexton CE, Le Masurier M, Allan CL, et al. Magnetic resonance imaging in late-life depression: vascular and glucocorticoid cascade hypotheses. Br J Psychiatry. 2012;201:46–51. doi: 10.1192/bjp.bp.111.105361. [DOI] [PubMed] [Google Scholar]
- 31.Adler CM, Levine AD, DelBello MP, et al. Changes in gray matter volume in patients with bipolar disorder. Biol Psychiatry. 2005;58:151–7. doi: 10.1016/j.biopsych.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 32.Baumann B, Danos P, Krell D, et al. Reduced volume of limbic system-affiliated basal ganglia in mood disorders: preliminary data from a postmortem study. J Neuropsychiatry Clin Neurosci. 1999;11:71–8. doi: 10.1176/jnp.11.1.71. [DOI] [PubMed] [Google Scholar]
- 33.Khundakar AA, Thomas AJ. Morphometric changes in early- and late-life major depressive disorder: evidence from postmortem studies. Int Psychogeriatr. 2009;21:844–54. doi: 10.1017/S104161020999007X. [DOI] [PubMed] [Google Scholar]
- 34.Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25:563–93. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 35.Yuan Y, Zhang Z, Bai F, et al. Abnormal neural activity in the patients with remitted geriatric depression: a resting-state functional magnetic resonance imaging study. J Affect Disord. 2008;111:145–52. doi: 10.1016/j.jad.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Bobb DS, Jr, Adinoff B, Laken SJ, et al. Neural correlates of successful response inhibition in unmedicated patients with late-life depression. Am J Geriatr Psychiatry. 2012;20:1057–69. doi: 10.1097/JGP.0b013e318235b728. [DOI] [PubMed] [Google Scholar]
- 37.de Asis JM, Stern E, Alexopoulos GS, et al. Hippocampal and anterior cingulate activation deficits in patients with geriatric depression. Am J Psychiatry. 2001;158:1321–3. doi: 10.1176/appi.ajp.158.8.1321. [DOI] [PubMed] [Google Scholar]
- 38.Pan CC, McQuoid DR, Taylor WD, et al. Association analysis of the COMT/MTHFR genes and geriatric depression: an MRI study of the putamen. Int J Geriatr Psychiatry. 2009;24:847–55. doi: 10.1002/gps.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 40.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 41.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Harrison PJ. The neuropathology of primary mood disorder. Brain. 2002;125:1428–49. doi: 10.1093/brain/awf149. [DOI] [PubMed] [Google Scholar]
- 44.Firbank MJ, Lloyd AJ, Ferrier N, et al. A volumetric study of MRI signal hyperintensities in late-life depression. Am J Geriatr Psychiatry. 2004;12:606–12. doi: 10.1176/appi.ajgp.12.6.606. [DOI] [PubMed] [Google Scholar]
- 45.Liu F, Hu M, Wang S, et al. Abnormal regional spontaneous neural activity in first-episode, treatment-naive patients with late-life depression: a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:326–31. doi: 10.1016/j.pnpbp.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Murphy CF, Gunning-Dixon FM, Hoptman MJ, et al. White-matter integrity predicts stroop performance in patients with geriatric depression. Biol Psychiatry. 2007;61:1007–10. doi: 10.1016/j.biopsych.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holtzheimer PE, Kelley ME, Gross RE, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69:150–8. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 49.Hamani C, Mayberg H, Stone S, et al. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69:301–8. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 50.Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168:502–10. doi: 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- 51.Sinka L, Kovari E, Santos M, et al. Microvascular changes in late-life schizophrenia and mood disorders: stereological assessment of capillary diameters in anterior cingulate cortex. Neuropathol Appl Neurobiol. 2012;38:696–709. doi: 10.1111/j.1365-2990.2012.01263.x. [DOI] [PubMed] [Google Scholar]
- 52.Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79:619–24. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- 53.Tupler LA, Krishnan KR, McDonald WM, et al. Anatomic location and laterality of MRI signal hyperintensities in late-life depression. J Psychosom Res. 2002;53:665–76. doi: 10.1016/s0022-3999(02)00425-7. [DOI] [PubMed] [Google Scholar]
- 54.O’Brien J, Desmond P, Ames D, et al. A magnetic resonance imaging study of white matter lesions in depression and Alzheimer’s disease. Br J Psychiatry. 1996;168:477–85. doi: 10.1192/bjp.168.4.477. [DOI] [PubMed] [Google Scholar]
- 55.MacFall JR, Payne ME, Provenzale JE, et al. Medial orbital frontal lesions in late-onset depression. Biol Psychiatry. 2001;49:803–6. doi: 10.1016/s0006-3223(00)01113-6. [DOI] [PubMed] [Google Scholar]
- 56.MacFall JR, Taylor WD, Rex DE, et al. Lobar distribution of lesion volumes in late-life depression: the Biomedical Informatics Research Network (BIRN) Neuropsychopharmacology. 2006;31:1500–7. doi: 10.1038/sj.npp.1300986. [DOI] [PubMed] [Google Scholar]
- 57.Alexopoulos GS, Kiosses DN, Choi SJ, et al. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002;159:1929–32. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- 58.Scheuerecker J, Meisenzahl EM, Koutsouleris N, et al. Orbitofrontal volume reductions during emotion recognition in patients with major depression. J Psychiatry Neurosci. 2010;35:311–20. doi: 10.1503/jpn.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernández A, Rodriguez-Palancas A, Lopez-Ibor M, et al. Increased occipital delta dipole density in major depressive disorder determined by magnetoencephalography. J Psychiatry Neurosci. 2005;30:17–23. [PMC free article] [PubMed] [Google Scholar]
- 60.Lavretsky H, Roybal DJ, Ballmaier M, et al. Antidepressant exposure may protect against decrement in frontal gray matter volumes in geriatric depression. J Clin Psychiatry. 2005;66:964–7. doi: 10.4088/jcp.v66n0801. [DOI] [PubMed] [Google Scholar]
- 61.Tisserand DJ, Pruessner JC, Sanz Arigita EJ, et al. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–69. [PubMed] [Google Scholar]
- 62.Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images”. Neuroimage. 2001;14:1454–62. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- 63.Bora E, Fornito A, Pantelis C, et al. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]