Abstract
Background
While many diffusion tensor imaging (DTI) investigations have noted disruptions to white matter integrity in individuals with chronic psychotic disorders, fewer studies have been conducted in young people at the early stages of disease onset. Using whole tract reconstruction techniques, the aim of this study was to identify the white matter pathology associated with the common clinical symptoms and executive function impairments observed in young people with psychosis.
Methods
We obtained MRI scans from young people with psychosis and healthy controls. Eighteen major white matter tracts were reconstructed to determine group differences in fractional anisotropy (FA), axial diffusivity (AD) and radial diffusivity (RD) and then were subsequently correlated with symptomatology and neurocognitive performance.
Results
Our study included 42 young people with psychosis (mean age 23 yr) and 45 healthy controls (mean age 25 yr). Compared with the control group, the psychosis group had reduced FA and AD in the left inferior longitudinal fasciculus (ILF) and forceps major indicative of axonal disorganization, reduction and/or loss. These changes were associated with worse overall psychiatric symptom severity, increases in positive and negative symptoms, and worse current levels of depression. The psychosis group also showed FA reductions in the left superior longitudinal fasciculus that were associated with impaired neurocognitive performance in attention and semantic fluency.
Limitations
Our analysis grouped 4 subcategories of psychosis together, and a larger follow-up study comparing affective and nonaffective psychoses is warranted.
Conclusion
Our findings suggest that impaired axonal coherence in the left ILF and forceps major underpin psychiatric symptoms in young people in the early stages of psychosis.
Introduction
While many diffusion tensor imaging (DTI) investigations have noted disruptions to white matter integrity in individuals with chronic psychotic disorders,1 fewer studies have been conducted in young people in the early stages of disease onset. Previous DTI investigations in young people with schizophrenia have shown white matter changes predominantly localized in the inferior longitudinal fasciculi (ILF), superior longitudinal fasciculi (SLF), cingulum, splenium, corpus callosum, minor forceps and corticospinal tracts.2,3 In a systematic review of white matter changes in patients with recent-onset schizophrenia,3 most studies used voxel-based whole brain (n = 25) or region of interest analysis (n = 12), but only 4 studies used tractography reconstruction techniques to characterize entire tracts.3 Tractography investigations have noted that, compared with controls, patients with first-episode psychosis (FEP) showed reduced fractional anisotropy (FA) within the uncinate,4,5 though another report found no difference.6 There was no difference in FA in the cingulum between healthy controls, patients with recent-onset schizophrenia and individuals with affective psychosis.4,5,7 Only 1 report5 has noted reduced FA in the SLF in patients with FEP compared with controls, and a more recent study8 found that compared with controls, patients with FEP had FA reductions within the left ILF. Hence, there are very few investigations into white matter tractography in patients with early psychosis, existing studies have modest sample sizes (average 27 patients with psychosis and 23 controls), and studies have yet to report on all major white matter tracts with more detailed white matter measurements.
Disruptions in white matter organization as reflected by reduced FA can be further described by calculating axial diffusivity (AD) and radial diffusivity (RD), which model water diffusion along or across axons, respectively. Decreases in AD are considered to reflect disorganization, damage or loss of axons, whereas increases in RD are indicative of disruptions to the myelin sheath.9 A recent advance in DTI tractography10 has combined detailed anatomic segmentation derived from Free-Surfer11 with the FSL probabilistic tractography approach12 to reconstruct 18 major white matter pathways. Using this method, Yendiki and colleagues10 reported that, compared with controls, patients with chronic psychosis had reduced FA and increased RD in a number of tracts, including the right SLF and major forceps, which is indicative of myelin-based pathology.
The association between the observed white matter alterations in young people with psychosis and their clinical and cognitive outcomes remains unclear. Individuals with recent-onset psychosis have shown reduced FA in the bilateral uncinate fasciculus correlating with worse verbal learning/memory functioning.13 Similarly, studies have reported that individuals with chronic psychosis have impairments in episodic memory and executive functions that correlate with reduced FA in the uncinate and cingulum bundle, respectively.14 A recent report15 showed that reduced FA in the splenium and posterior cingulum of young people with schizophrenia was associated with poorer performance in a working memory task, similar to findings in older individuals.16 Given recent evidence indicating that neuropsychological functioning is a better predictor of later socio-occupational outcome than symptomatology,17 it is important to understand the association between white matter integrity and neurocognitive outcomes in people in the early stages of psychosis to provide better insight into intervention strategies.
We sought to replicate the work of Yendiki and colleagues10 in a young group of people with psychotic disorders (i.e., schizophrenia, schizophreniform disorder, schizoaffective disorder, psychosis not otherwise specified [NOS]) and test the hypothesis that the same tracts would be affected. To our knowledge, this investigation was the first to use advanced tractography reconstruction techniques to characterize FA, AD and RD metrics of the entire length of these tracts in young people with psychosis. In addition, tracts found to have disruptions in white matter integrity would be subject to correlational analysis to identify any association with clinical symptoms and neurocognitive impairments. We hypothesized that disruptions of the left temporo-parietofrontal tracts would be associated with neurocognitive deficits in working memory. In accordance with our previous report in which we demonstrated that young people with psychosis have cortical thinning in the left temporoparietal junction that was correlated with deficits in visual sustained attention, semantic verbal fluency, verbal learning and verbal memory,18 we also hypothesized that these domains would be associated with disruptions to temporoparietal white matter tracts.
Methods
Participants
We recruited outpatients aged 15–34 years from specialist youth mental health clinics in Sydney, Australia,19 and healthy controls were recruited from the community in the same region and were screened for history of psychiatric disorders. As part of larger, ongoing youth mental health research, data on most of these participants have been previously reported in investigations of insula grey matter volume changes,20 cortical thinning18 and clinical staging of psychiatric illness.21
Exclusion criteria for the present study were medical instability (as determined by a psychiatrist), history of neurologic disease (e.g., tumour, head trauma, epilepsy), medical illness known to impact cognitive and brain function (e.g., cancer), intellectual and/or developmental disability, insufficient English for neuropsychological assessment and current substance dependence. Individuals with hazardous levels of alcohol consumption (as determined by the Alcohol Use Disorders Identification Test) or a history of excessive substance abuse (i.e., substance-induced psychotic disorder) were also excluded. All participants were asked to abstain from drug or alcohol use for 48 hours before testing and were informed about a drug screen protocol. The University of Sydney ethics committee approved the study, and participants gave written informed consent. During the recruitment process, individuals with psychosis were assessed by a psychiatrist to determine whether they had adequate competency to provide informed consent. All participants with psychosis were outpatients who were not floridly psychotic during any stage of this investigation and who were stabilized on their medication.
A psychiatrist assessed all patients to determine the nature and history of any mental health problems. By consensus of the senior investigators (I.B.H. and E.S.), participants were categorized according to DSM-IV-TR criteria.22 To capture information regarding young people with psychosis in the early stages of disease, we collectively analyzed schizophrenia, schizophreniform disorder, schizoaffective disorder and psychosis NOS.
Clinical assessment
A psychiatrist (I.B.H. or E.S.) or trained research psychologist conducted the clinical assessment (in a semistructured interview format) to determine the nature and history of any mental health problems using the Brain and Mind Research Institute Structured Interview for Neurobiological Studies.23 The assessment included the 17-item Hamilton Rating Scale for Depression (HAM-D) to quantify mood symptoms over the previous week and the Brief Psychiatric Rating Scale (BPRS) to quantify current general psychiatric symptom severity. In addition to a total score, the 24-item BPRS has sub-scales assessing depression, positive symptoms, negative symptoms, mania and disorientation. Participants’ educational level was assessed as the cumulative completed number of years in school, university and/or advanced diploma courses. We estimated premorbid intelligence (predicted IQ) using the Wechsler Test of Adult Reading.24
Self-report assessment
All participants completed a self-report assessment that included the Kessler-10 (K-10),25 which is a brief instrument designed to detect psychological distress, and the Depression Anxiety and Stress Scales (DASS),26 which measure the 3 related negative emotional states of depression, anxiety and tension/stress.
Neuropsychological assessment
To examine the association between white matter integrity and neuropsychological functioning, a trained research psychologist administered standardized tests as part of a broader battery (described previously27–29). Verbal learning and memory were assessed using the Rey Auditory Verbal Learning Test (RAVLT);30 verbal fluency was assessed using the Controlled Oral Word Association Test (COWAT);30 and we used the Trail-Making Test (TMT)31 to assess psycho-motor speed (TMT-A), attentional switching (TMT-B) and executive control (TMT-B-A). Tests derived from the Cam-bridge Automated Neuropsychological Testing Battery (CANTAB)30 included the Rapid Visual Information Processing task (RVP) to test visual sustained attention and the Spatial Span (SSP) task to assess working memory. The CANTAB internal normative database of 3000 healthy volunteers (www.cantab.com) was used to calculate the z score for each participant.32 Neuropsychological scores beyond a standard deviation of ± 3.0 from the centralized z score mean were curtailed to values of ± 3.0 (depending on the direction), so that the group analyses were not influenced by any extreme scores or artifacts as previously described.27
MRI acquisition and analysis
Participants underwent MRI scanning using a 3 T GE MR750 Discovery scanner (GE Medical Systems) at the Brain and Mind Research Institute, Camperdown, Australia. To enable neuroanatomical analysis at high resolution (0.9 mm isotropic resolution), we acquired a customized magnetization-prepared rapid acquisition gradient echo 3-dimensional T1-weighted sequence: repetition time (TR) 7264 ms, echo time (TE) 2784 ms, flip angle 15°, coronal orientation, field of view (FOV) 230 mm, matrix 256 × 256, 196 slices. Next, whole brain diffusion-weighted images were acquired using an echo planar imaging sequence: TR 7000 ms, TE 68 ms, slice thickness 2.0 mm, FOV 230 × 230 mm, acquisition matrix 256 × 256, axial orientation, 69 gradient directions. Eight images without gradient loading (b = 0 s/mm2) were acquired before the acquisition of 69 images (each containing 55 slices) with uniform gradient loading (b = 1159 s/mm2).
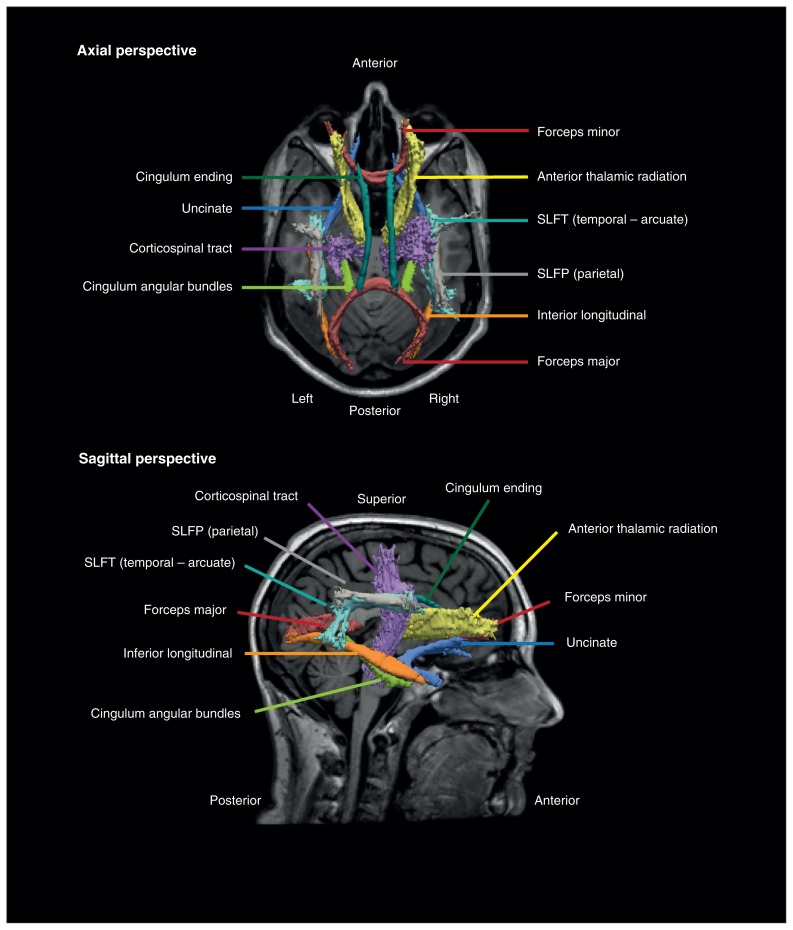
Surface-based neuroanatomical segmentation was performed using FreeSurfer version 5.1 (surfer.nmr.mgh.harvard.edu), as previously described.11 Inaccuracies in automated segmentations, typically involving the inclusion of dura within the pial surface adjacent to the superior sagittal sinus and temporal regions, were manually corrected by a trained neuroimaging analyst (S.N.H. or J.L.). Using this detailed neuroanatomical information, 18 major white matter pathways were reconstructed with the TRACULA process10 using FreeSurfer version 5.2, providing improved tract reconstruction over version 5.1. The preprocessing stage involved eddy current compensation (correcting eddy currents and head movements), intrasubject registration, intersubject registration to the FreeSurfer common template space, the creation of cortical and white matter masks from FreeSurfer reconstructions, tensor fitting for extract of tensor-based measures, and the computation of anatomic priors for white matter pathways based on the TRACULA atlas. A recent publication33 has highlighted that despite the routine use of comprehensive head motion–correction at the individual level, residual group-level differences in head motion can provide false-positive results. Thus we took each eddy-corrected diffusion-weighted image and aligned each volume within the 4-dimensional series with the non–diffusion-weighted image using affine registration34 to measure residual head movement artifacts. There were no significant differences in mean head displacement between the groups (absolute: t76 = 1.9, p = 0.07; relative: t85 = 0.34, p = 0.74). The next stage used the BedpostX ball-and-stick model of diffusion to estimate the probability distributions at each voxel, as implemented in the FSL software package.12,35 In the final stage, the white matter pathways are reconstructed by fitting the diffusion model to prior knowledge of the pathway anatomy derived from 33 training participants. We isolated the forceps major and minor and bilateral anterior thalamic radiations, uncinate fasciculi, ILF, corticospinal tracts, cingulate gyrus ending and cingulate angular bundle (Fig. 1). The bilateral SLF was split into 2 segments, the parietal segment (SLFP) and the temporal segment (SLFT), which corresponds with the arcuate fasciculi.36 Average FA, AD and RD measures along the entire reconstructed tract were used in subsequent statistical analysis.
Fig. 1.
Example reconstruction of major white matter pathways in a healthy control. The posterior distribution of each pathway is thresholded at 20% of its maximum and is displayed as an isosurface over the participant’s averaged T1-weighted image. The superior longitudinal fasciculi were subdivided into the parietal segment and temporal segment. SLFP = superior longitudinal fasciculus parietal segment; SLFT = superior longitudinal fasciculus temporal segment.
Statistical analysis
Statistical analyses were performed using SPSS version 20.0 for Mac. Between-groups analyses of demographic variables were conducted using independent-sample t tests or χ2 tests for categorical data. We considered results to be significant at p < 0.05, 2-tailed. As the BPRS disorientation subscore comprises only 2 items, the measure was not considered statistically robust and was omitted from this study.
A multivariate analysis of covariance examined average FA, AD and RD across all 18 tracts in the psychosis versus the control group, controlling for age and sex. Follow-up pairwise comparison examined average FA, AD and RD between cohorts per individual tract, controlling for age and sex. We considered results to be significant at p < 0.05, 2-tailed. Where data sets failed the Mann–Whitney U test of normality, we conducted a Monte Carlo simulation with 10 000 samples.
Pearson partial correlations (r) explored the linear correlation between white matter tract measurements (FA, AD, RD) and symptomatology, age at onset and duration of psychosis, and neurocognitive performance, controlling for age and sex — 2 factors shown to significantly influence both white matter and cognition in adolescence and young adulthood.37 We used Spearman correlation (ρ) when data violated the assumptions of normality. We set the significance threshold at p < 0.003 to control for the multiple comparison of 19 clinical and neurocognitive scores (i.e., p < [0.05/19 = 0.003]).
Results
Demographics, symptomatology and neurocognitive performance
We enrolled 42 outpatients with psychosis and 45 healthy controls in our study. The diagnoses and medication characteristics of patients are detailed in Table 1. Nine (21%) patients were not taking any psychotropic medications at the time of testing, whereas 12 (29%) were taking a second-generation antidepressant, 31 (74%) were taking an atypical antipsychotic and 4 (10%) were taking a mood stabilizer. Of the 11 (26%) patients taking multiple medications, 7 (64%) were taking antidepressants and antipsychotics, 1 (9%) was taking an antipsychotic and a mood stabilizer, and 3 (27%) were taking a combination of the three classes of medication. At the time of assessment no control participants were taking any psychotropic medications.
Table 1.
Medication usage by diagnosis based on DSM-IV-TR criteria22
| Medication, no. (%) | ||||
|---|---|---|---|---|
|
|
||||
| Diagnosis | Unmedicated | Antidepressants | Antipsychotics | Mood stabilizers |
| Schizophreniform disorder, n = 15 | 4 (33) | 5 (42) | 10 (83) | 0 (0) |
| Schizophrenia, n = 12 | 1 (8) | 1 (8) | 11 (92) | 1 (8) |
| Schizoaffective disorder, n = 8 | 2 (17) | 5 (42) | 6 (50) | 3 (25) |
| Psychosis NOS, n = 7 | 2 (17) | 1 (8) | 4 (33) | 0 (0) |
NOS = not otherwise specified.
There were no significant group differences in handedness, predicted IQ or intracranial volume between patients with psychosis and controls (Table 2). Compared with the control group, the psychosis group included significantly more men (controls 44.4% v. psychosis 71.4%, p = 0.011), and patients with psychosis were younger (24.8 ± 3.4 v. 22.7 ± 3.9 yr, p = 0.012) and had fewer years of education (14.5 ± 1.8 v. 12.5 ± 1.8, p < 0.001) than controls. All clinical symptom measures (i.e., HAM-D, K10, DASS) were significantly worse in the psychosis group than in the control group (Table 2). The mean age at onset of psychotic symptoms (e.g., hallucinations, delusions) was 20.3 ± 4.1 years, and the mean duration of psychosis was 2.4 ± 2.5 years.
Table 2.
Demographic and clinical characteristics and ratings scores of study participants
| Group; mean ± SD* | ||||
|---|---|---|---|---|
|
|
||||
| Variable | Control, n = 45 | Psychosis, n = 42 | Statistic† | p value |
| Male, % (male/female) | 44.4% (20/25) | 71.4% (30/12) | χ21 = 6.47 | 0.011 |
| Right handed, % (r/l/a) | 80.0% (36/9/0) | 88.1% (37/4/1) | χ22 = 2.84 | 0.24 |
| Age, yr | 24.8 ± 3.4 | 22.7 ± 3.9 | t85 = −2.57 | 0.012 |
| Predicted IQ | 103.9 ± 7.8 | 103.5 ± 7.4 | t48 = −0.22 | 0.83 |
| Years of education | 14.5 ± 1.8 | 12.5 ± 1.8 | t84 = −5.29 | < 0.001 |
| Intracranial volume, cm3 | 1530 ± 135 | 1569 ± 174 | t77.1 = 1.14 | 0.26 |
| HAM-D | 2.9 ± 2.5 | 14.8 ± 7.1 | t27.3 = 7.93 | < 0.001 |
| K10 | 15.7 ± 4.5 | 24.5 ± 8.6 | t48.7 = 5.45 | < 0.001 |
| DASS Depression scale | 4.9 ± 7.1 | 16.3 ± 11.5 | t60.5 = 5.31 | < 0.001 |
| DASS Anxiety scale | 3.9 ± 5.7 | 14.2 ± 10.6 | t53.3 = 5.32 | < 0.001 |
| DASS Stress scale | 7.8 ± 7.7 | 16.4 ± 9.1 | t79 = 4.57 | < 0.001 |
| BPRS Total | — | 43.8 ± 11.8 | — | — |
| BPRS Positive Symptoms | — | 12.9 ± 5.4 | — | — |
| BPRS Negative Symptoms | — | 8.7 ± 4.2 | — | — |
| RVP-A | 0.16 ± 1.05 | −0.55 ± 1.11 | t63 = −2.64 | 0.011 |
| RVP-B | −0.22 ± 1.11 | −0.19 ± 0.9 | t63 = 0.15 | 0.88 |
| RAVLT Sum | 0.00 ± 0.83 | −0.77 ± 1.09 | t64 = −3.21 | 0.002 |
| RAVLT A7 | 0.08 ± 0.95 | −0.92 ± 1.35 | t59.4 = −3.46 | < 0.001 |
| COWAT Letters | −0.17 ± 0.86 | −0.26 ± 0.94 | t63 = −0.37 | 0.71 |
| COWAT Animals | 0.56 ± 0.88 | −0.10 ± 0.84 | t63 = −3.09 | 0.003 |
| SSP | 0.68 ± 1.01 | −0.18 ± 1.08 | t62 = −2.97 | 0.004 |
| TMT-A | 0.66 ± 0.66 | 0.17 ± 1.03 | t64 = −2.28 | 0.026 |
| TMT-B | 0.39 ± 1.13 | −0.28 ± 1.34 | t63 = −2.14 | 0.036 |
BPRS = Brief Psychiatric Ratings Scale; COWAT = Controlled Word Association Test; DASS = Depression Anxiety and Stress Scales; HAM-D = Hamilton Rating Scale for Depression; K10 = Kessler-10; RAVLT = Rey Auditory Verbal Learning Test; RVP = Rapid Visual Processing; SSP = spatial span; TMT = Trail-Making Test.
Unless otherwise indicated.
Significant differences in sex and handedness were evaluated using a Pearson χ2 test. All other significance values were evaluated using independent samples t tests. Significance levels were set at p < 0.05 (2-tailed).
Neurocognitive performance between groups varied across tasks (Table 2). Relative to controls, the psychosis group exhibited impaired visual attention (RVP-A, t63 = −2.64, p = 0.011) but not impulsivity (RVP-B, t63 = 0.15, p = 0.88). The psychosis group was also impaired in the verbal learning and recall task (RAVLT Sum, t64 = −3.21, p = 0.002; RAVLT A7, t59.4 = −3.46, p < 0.001). There were no group differences in phonemic fluency (COWAT Letters, t63 = −0.37, p = 0.71); however, the psychosis group performed worse than the control group in semantic fluency (COWAT Animals, t63 = −3.09, p = 0.003), working memory (SSP, t62 = −2.97, p = 0.004), psychomotor speed (TMT-A, t64 = −2.28, p = 0.026) and attentional switching (TMT-B, t63 = −2.28, p = 0.026).
White matter tract integrity
Across the whole study, a total of 5 tracts were excluded from our analysis because they were inadequately reconstructed. Such errors can occur due to subtle focal imaging artifacts that skew or terminate a pathway, resulting in reconstructions that are not biologically representative of the tract. One participant with psychosis had the right ILF rejected, and 4 controls had a single tract rejected: namely, the right corticospinal tract, right uncinate, and the left and the right cingulate angular bundle.
Relative to controls, the psychosis group showed significant white matter abnormalities in 4 of the 18 white matter tracts investigated (Table 3). While a collective analysis of all white matter integrity measurements of all tracts was not able to separate participants into diagnostic groups, follow-up pairwise analysis showed that FA reductions in the left ILF (mean FA 0.542 ± 0.004 in the control group v. 0.527 ± 0.004 in the psychosis group, p = 0.035) were accompanied by reduced AD (mean AD 1.34 × 10−3 ± 7.40 × 10−6 in the control group v. 1.31 × 10−3 ± 7.69 × 10−6 in the psychosis group, p = 0.039), and this pattern approached significance in the right ILF (mean FA p = 0.09; mean AD p = 0.05). The FA was reduced in the forceps major (mean FA 0.670 ± 0.006 in the control group v. 0.649 ± 0.006 in the psychosis group, p = 0.022), with an associated reduction in AD (mean AD 1.51 × 10−3 ± 8.91 × 10−6 in the control group v. 1.48 × 10−3 ± 9.25 × 10−6 in the psychosis group, p = 0.028). The left SLF showed reduced FA in both the parietal segment (mean FA 0.490 ± 0.004 in the control group v. 0.473 ± 0.005 in the psychosis group, p = 0.014) and temporal segment (arcuate; mean FA 0.500 ± 0.004 in the control group v. 0.489 ± 0.004 in the psychosis group, p = 0.049). While there were no significant differences noted in the remaining tracts, the psychosis group had a trend indicating reduced AD within the bilateral cortical spinal tracts relative to controls (left p = 0.06; right p = 0.05).
Table 3.
White matter tract integrity in individuals with psychosis
| Left hemisphere* | Right hemisphere* | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Measure | Control | Psychosis | p value | Control | Psychosis | p value |
| Forceps major; forceps minor | ||||||
| FA | 0.669 | 0.649 | 0.022† | 0.555 | 0.558 | 0.75 |
| AD (×10−3) | 1.51 | 1.48 | 0.028† | 1.41 | 1.41 | 0.98 |
| RD (×10−4) | 4.07 | 4.21 | 0.14 | 5.17 | 5.13 | 0.67 |
| Inferior longitudinal fasciculi | ||||||
| FA | 0.542 | 0.527 | 0.035† | 0.530 | 0.519 | 0.09 |
| AD (×10−3) | 1.34 | 1.32 | 0.039† | 1.33 | 1.30 | 0.05 |
| RD (×10−4) | 5.20 | 5.29 | 0.21 | 5.30 | 5.36 | 0.40 |
| Superior longitudinal fasciculi (parietal segment) | ||||||
| FA | 0.490 | 0.473 | 0.014† | 0.482 | 0.475 | 0.30 |
| AD (×10−3) | 1.11 | 1.10 | 0.20 | 1.13 | 1.12 | 0.42 |
| RD (×10−4) | 5.09 | 5.17 | 0.23 | 5.12 | 5.17 | 0.43 |
| Superior longitudinal fasciculi (temporal segment — arcuate) | ||||||
| FA | 0.500 | 0.489 | 0.049† | 0.473 | 0.470 | 0.54 |
| AD (×10−3) | 1.15 | 1.15 | 0.73 | 1.12 | 1.11 | 0.45 |
| RD (×10−4) | 5.07 | 5.13 | 0.30 | 5.14 | 5.15 | 0.83 |
| Corticospinal tract | ||||||
| FA | 0.507 | 0.502 | 0.29 | 0.486 | 0.483 | 0.59 |
| AD (×10−3) | 1.16 | 1.13 | 0.06‡ | 1.14 | 1.12 | 0.05 |
| RD (×10−4) | 4.90 | 4.85 | 0.33 | 5.06 | 5.02 | 0.41 |
| Uncinate fasciculi | ||||||
| FA | 0.443 | 0.438 | 0.50 | 0.435 | 0.43 | 0.39 |
| AD (×10−3) | 1.20 | 1.19 | 0.06 | 1.23 | 1.22 | 0.10 |
| RD (×10−4) | 5.80 | 5.79 | 0.83 | 6.00 | 5.98 | 0.80 |
| Anterior thalamic radiation | ||||||
| FA | 0.450 | 0.445 | 0.35 | 0.427 | 0.425 | 0.73 |
| AD (×10−3) | 1.16 | 1.14 | 0.12 | 1.16 | 1.15 | 0.16 |
| RD (×10−4) | 5.50 | 5.50 | 0.95 | 5.77 | 5.73 | 0.37 |
| Cingulate gyrus ending | ||||||
| FA | 0.575 | 0.589 | 0.18 | 0.522 | 0.534 | 0.15 |
| AD (×10−3) | 1.32 | 1.34 | 0.35 | 1.25 | 1.26 | 0.32 |
| RD (×10−4) | 4.71 | 4.60 | 0.23 | 5.08 | 4.98 | 0.21 |
| Cingulate gyrus angular bundle | ||||||
| FA | 0.378 | 0.382 | 0.65 | 0.385 | 0.386 | 0.88 |
| AD (×10−3) | 1.17 | 1.15 | 0.19 | 1.16 | 1.16 | 0.84 |
| RD (×10−4) | 6.35 | 6.23 | 0.14 | 6.24 | 6.22 | 0.81 |
AD = axial diffusivity; FA = fractional anisotropy; RD = radial diffusivity.
Unless otherwise indicated.
Significance with Bonferroni correction was set at p < 0.05 (2-tailed).
Where data sets failed the Mann–Whitney U test of normality, the Monte Carlo simulation significance is shown.
Association of white matter tract integrity with symptomatology, illness duration and neurocognitive performance
There were significant associations between white matter integrity and symptomatology within the left ILF, forceps major and left SLF parietal segment (Table 4). Worse overall psychiatric symptom severity (BPRS total) was correlated with FA reductions in the left ILF (r66 = −0.34, p = 0.003) and forceps major (r66 = −0.37, p < 0.001), which was accompanied by significant AD reductions in the forceps major (r66 = −0.32, p = 0.004). An increase in negative symptoms (BPRS negative symptoms) was associated with decreased FA within the left ILF (r66 = −0.40, p < 0.001) and forceps major (r66 = −0.38, p < 0.001), with an associated decreased AD nearing significance within both tracts (left ILF: r66 = −0.22, p = 0.038; forceps major: r66 = −0.26, p = 0.017). More positive symptoms (BPRS positive symptoms) were associated with decreased FA within the forceps major (r70 = −0.33, p = 0.003) with a similar trend approaching significance within the left ILF (r70 = −0.21, p = 0.045). Finally, worse depression (HAM-D) was correlated with reduced FA in both tracts (left ILF: r52 = −0.43, p < 0.001; forceps major: r52 = −0.41, p < 0.001), with an associated reduction in AD nearing significance (left ILF: r52 = −0.22, p = 0.052; forceps major: r52 = −0.28, p = 0.020). Reductions in FA within the left SLF parietal segment neared statistical association with worse psychological distress (K10, r74 = −0.28, p = 0.008).
Table 4.
Associations between white matter tract integrity, symptomatology and neuropsychological performance
| Correlation with tract measurements, r/rho* | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Left ILF | Left ILF | Forceps | Forceps | Left SLFP | Left SLFT | |
| Variable | FA | AD | major FA | major AD | FA | FA |
| BPRS Total | −0.34‡ | −0.15 | −0.37‡ | −0.32‡ | −0.11 | −0.10 |
| BPRS Positive Symptoms† | −0.21§ | −0.06 | −0.33‡ | −0.14 | −0.12 | −0.18 |
| BPRS Negative Symptoms | −0.39‡ | −0.22§ | −0.38‡ | −0.26§ | −0.16 | −0.22§ |
| HAM-D | −0.43‡ | −0.22 | −0.41‡ | −0.28§ | −0.17 | −0.08 |
| K10 | −0.19 | −0.09 | −0.18 | −0.05 | −0.28§ | −0.21§ |
| DASS Anxiety scale | −0.15 | −0.06 | −0.18 | −0.12 | −0.14 | −0.14 |
| DASS Stress scale | −0.25§ | −0.11 | −0.19§ | −0.11 | −0.15 | −0.13 |
| RVP-A | 0.28§ | 0.11 | 0.26§ | 0.14 | 0.28§ | 0.35‡ |
| RVP-B | 0.04 | 0.07 | −0.04 | −0.11 | 0.06 | 0.08 |
| RAVLT Sum | 0.19 | 0.12 | 0.18 | 0.05 | 0.03 | 0.11 |
| RAVLT A7 | 0.16 | 0.13 | 0.12 | 0.04 | −0.03 | −0.02 |
| COWAT Letters | 0.03 | −0.03 | 0.08 | −0.12 | 0.18 | 0.14 |
| COWAT Animals | 0.25§ | 0.18 | 0.23§ | 0.10 | 0.40‡ | 0.28§ |
| SSP | 0.03 | −0.17 | 0.09 | −0.17 | 0.15 | 0.27§ |
| TMT-A | 0.02 | 0.09 | −0.04 | 0.17 | 0.04 | 0.03 |
| TMT-B | 0.01 | −0.01 | 0.08 | 0.09 | 0.16 | 0.23§ |
AD = axial diffusivity; BPRS = Brief Psychiatric Ratings Scale; COWAT = Controlled Word Association Test; DASS = Depression Anxiety and Stress Scales; FA = fractional anisotropy; HAM-D = Hamilton Rating Scale for Depression; ILF = inferior longitudinal fasciculus; K10 = Kessler-10; RAVLT = Rey Auditory Verbal Learning Test; RVP = Rapid Visual Processing; SSP = spatial span; SLFP = superior longitudinal fasciculus, parietal segment; SLFT = superior longitudinal fasciculus, temporal segment (arcuate); TMT = Trail-Making Test.
Partial correlation examined the association between tract measurements of interest in all participants with clinical scores and z scores of neurocognitive performance controlling for sex and age. Significance was set at p < 0.003, adjusting for multiple comparisons (1-tailed).
Spearman rho correlation analysis examined nonparametric distributions.
p < 0.003.
p < 0.05.
Older age at onset of psychosis was associated with lower FA in the left SLF parietal segment (r38 = −0.50, p < 0.001). In addition, older age at onset was also associated with lower FA (r38 = −0.47, p = 0.002) and increased AD in the right uncinate (r38 = 0.56, p < 0.001). After removing 2 outlying participants who had very long durations of illness (onset at age 14 yr, duration 8 yr and onset at age 15 yr, duration 11 yr) all results remained significant. Considering the strong correlation between age at the time of MRI and age at onset of psychosis (r42 = 0.80, p < 0.001), we interpret these results more as a function of normal brain maturation with age rather than a feature of psychotic illness. There were no significant associations between white matter measurements and duration of psychosis.
Within the neurocognitive domains tested, FA reductions in the left SLF parietal segment correlated with worse semantic fluency (COWAT Animals, r61 = 0.40, p < 0.001), with a similar trend toward significance seen within the left SLFT (r61 = 0.28, p = 0.012), left ILF (r61 = 0.25, p = 0.025) and forceps major (r61 = 0.23, p = 0.036). The FA reductions in the left SLFT correlated with worse attention (RVP-A, r61 = 0.35, p = 0.003), with a similar trend toward significance seen within the left SLF parietal segment (r61 = 0.28, p = 0.013), left ILF (r61 = 0.28, p = 0.012) and forceps major (r61 = 0.26, p = 0.022).
Discussion
The focus of this report was the reconstruction of 18 major white matter tracts in a large sample of young people with psychosis. Compared with the control group, the psychosis group exhibited reduced FA and AD in the left ILF and forceps major that were associated with increases in positive and negative symptoms, overall psychiatric symptom severity and depression. The left SLF also exhibited reduced FA that was associated with psychological distress. Abnormalities within the SLF correlated with neurocognitive deficits in sustained attention and verbal fluency, which is in line with our finding of similar neurocognitive deficits being associated with cortical thinning of the temporoparietal junction.18 Importantly, FA reductions in the left ILF and forceps major were accompanied by AD reduction, which has been shown to be associated with worse overall symptom severity. Compared with healthy controls, decreased AD in patients in the early stages of psychosis may reflect a reduction in the mean number of axons, reduced mean axonal diameter, the less efficient packing of axons, axonal damage or loss within these tracts from an early age. In contrast to the oligodendrocyte dysregulation hypothesis,38,39 our investigation suggests that axonal disorganization and/or loss within the left fronto-parieto-occipital tracts are an early pathobiological event in psychosis.
Changes in AD and RD must be interpreted with caution. A seminal investigation of AD and RD in a mouse model of dys-myelination40 found that reduced myelin was associated with increased water diffusion perpendicular to the axon direction (i.e., RD), but not water diffusion parallel to the axon direction (i.e., AD). Subsequent investigations have confirmed increased RD in models of dysmyelination41 and demyelination,42,43 with a few studies suggesting minor concomitant reductions in AD within these pathologies.41,42 Initial transgenic mice model investigations43,44 associated lower AD with histological evidence of reduced axonal diameter and overexpression of neurofilaments and III β-tubulin. Interestingly, within the corpus callosum, AD reductions (at 4 weeks) preceded RD increases (at 6 weeks44), a trajectory that could possibly explain how in our present study we have noted reduced AD in these early stages of illness as opposed to the prominent increased RD that characterizes chronic psychosis.10 Hence, we report that young people with psychosis have reduced water diffusion along the axons (i.e., reduced AD), which can be interpreted as a reduction in the number of axons, reduced axonal diameter, less efficient packing of axons, axonal damage or loss, although the true interpretation of this DTI change requires rare postmortem histological examination45 of young people in the early stages of illness.
Our results that young people with psychosis have reduced FA in the left ILF supports those of a previous report46 that used manual tractography construction, denoting that, compared with controls, adolescents with schizophrenia also have reduced FA in the left ILF. A recent study8 found that compared with controls, patients with FEP had FA reductions within the left ILF, and this FA change was intermediate in ultra-high risk individuals. To our knowledge, our study is the first to show that this FA reduction in the left ILF is accompanied by a reduction in AD, indicative of axonal disorganization or loss within this tract. By comparison, recent studies of patients with chronic schizophrenia have reported that reduced FA in the left middle temporal and inferior frontal regions47,48 and left ILF10 featured increased RD, which suggests myelin sheath disturbances. It is unclear if an axonal-dominated pathology in the early stages of psychosis progresses to a myelin-dominated pathology or whether this is a distinct subgroup from those reported in chronic psychosis.
Reports of the neurocognitive implications of white matter disruption to the ILF in psychosis are varied. A voxel-based analysis of medication-naive patients with chronic schizophrenia compared with healthy controls49 revealed significant FA reduction in the left ILF in the schizophrenia group, and this white matter disruption was associated with poorer neurocognitive performance in speed of processing, verbal learning and visual learning tasks. In contrast to the present study, Liu and colleagues49 found no significant between-groups differences in RD or AD or in white matter integrity markers of the left ILF and attention/vigilance or working memory. Another study on FEP50 found that reduced FA in the left ILF correlated with impairment in working memory/attention shifting (TMT-B) but not cognitive processing speed (TMT-A) or verbal learning and recall (RAVLT). Our finding that reduced FA in the left ILF was associated with worse semantic fluency corroborated the hypothesis that the ILF is involved in both language51 and visual semantics.52 Collectively, we add to the evidence that white matter abnormalities of the left ILF in the early stages of psychosis are concomitant with neurocognitive decline in tasks of attention and semantic fluency but not verbal learning, working memory or psychometric speed.
White matter abnormalities of the left ILF and forceps major were associated with depression and negative symptoms. The forceps major communicates somatosensory information between the parietal and occipital lobes via the splenium, a structure shown to be impaired in individuals at early and later stages of psychosis1 as well as ultra-high risk individuals.53 A report by Waltz and colleagues54 demonstrated that individuals with schizophrenia showed a selective deficit in the ability to learn from positive outcomes but no deficits in learning from negative outcomes. This motivational deficit correlated with negative symptoms but not with neuropsychological measures of working memory, suggesting that the observation was not a neuropsychological deficit. Notably, while reductions in FA in the left ILF and forceps major were associated with worse negative symptoms and depression, the tested domains of working memory (SSP) and cognitive flexibility (TMT) were not associated with these tracts. Further work is needed to elucidate the balance between motivational deficits and measures of cognitive dysfunction in white matter disruptions in psychosis.
In the present study, young people with psychosis have FA reductions within the left SLFT (arcuate) in keeping with reports of older patients with psychosis.1 Karlsgodt and colleagues55 reported that, compared with healthy controls, individuals with recent-onset schizophrenia showed FA reductions within the bilateral SLFT, and verbal working memory deficits correlated with reduced integrity of the left SLFT in both healthy controls and patients. We found no such association with the verbal learning task (RAVLT), nor did a similar study examining individuals with FEP.50 In summary, our findings suggest that the integrity of the left SLFT is impaired in the early stages of the disease and appears to be associated with attention rather than other neuropsychological functions, such as verbal learning and recall.
Reduced FA within the forceps major was associated with positive symptoms. Increased positive symptoms have been correlated with reduced fibre tract integrity in the left ILF and IFOF in medication-naive individuals with FEP,56 possibly indicating that white matter disruptions were present in the pretreatment phase and were likely to perpetuate after clinical treatment or symptom remission. Our data demonstrate that reductions in FA in the SLFT are associated with worse attention, a hallmark deficit linked with hallucinations in individuals with schizophrenia,57 and that this pathology is present in the early stages of psychosis onset.
Limitations
There are a number of limitations to this study that warrant discussion. Discrepancies between this work and other studies may be attributable to examining data in small cohorts (average of 24.3 patients with psychosis and 25.6 controls based on a review of DTI findings in recent-onset psychosis2) using voxel-based methods, which can be affected by registration confounds,58 and the lack of studies mapping specific tract anatomy within study participants.59 Our analysis grouped 4 subcategories of psychosis together (i.e., schizophrenia spectrum psychosis60), though there is argument as to whether schizoaffective disorders fall within or apart from this grouping. While this grouping enables the analysis of the shared components that give rise to the symptoms and neurocognitive deficits in emerging psychosis as a whole, larger studies should examine the white matter differences between patients with affective and nonaffective psychoses, taking into account the age at onset of affective, positive and negative symptoms. To obtain 69 diffusion directions within a reasonable scan time, we used an anisotropic voxel (in-plane resolution 0.9 mm, through-plane resolution 2 mm) that could have introduced a bias in diffusion estimates and partial volume estimates. The influence of medication on white matter remains contentious, as similar patterns of FA reductions have been noted in psychosis cohorts taking anti-psychotics61 and in medication-naive patients.62 Our study was cross-sectional in design, so no clear picture can be gained regarding the ways in which medication influences white matter integrity over time within the same individuals. Another potential limitation is that our family-wise α correction may have been too simplistic, and future investigations with larger sample sizes could control for other influential factors, such as hemispheric differences. Finally, while DTI tractography has the advantage of being able to quantify white matter tract integrity along the entire fibre bundle, the averaging of markers over large volumes may suppress focal pathologies. Future studies could use along-tract white matter statistics of the reconstructed tracts,63 though these investigations must take into consideration how normal white matter maturation over time influences neurocognition, and their association with medication and substance abuse.
While we reconstructed 18 major white matter tracts, future studies should investigate additional major long associational tracts that were not analyzed here, notably the inferior fronto-occipital fasciculi. Furthermore, short associational fibre pathology (“U-fibres” and superficial white matter) within the left temporal and occipital region have been noted in patients with chronic schizophrenia,64 and such white matter disruptions have been associated with neurocognitive deficits in processing speed, attention and working memory in this population.65 Future DTI studies of young people with psychosis should investigate the association between left temporal long and short associational fibres’ integrity and how disruptions of these pathways relate to neurocognition and symptomatology.
Conclusion
This study highlights that the core white matter pathology at the early stages of psychosis is accompanied by AD reductions within the forceps major and left ILF. Our results reinforce the notion that structural abnormalities in fronto-temporo-parietal connections are present at the early stages of psychosis, which is in line with DTI reports in patients with FEP.8,50,62 The left temporal nature of this pathology and its association with worse positive and negative symptoms, depression, symptom severity, semantic fluency and attention highlights that these are important features early in disease progression. Further DTI investigations into early psychosis need to clarify axonal coherence in these young people and how such white matter pathology progresses over time.
Acknowledgements
The authors thank Rico Sze Chun Lee and Django White for their assistance with data collection. They also thank individuals that participated in this study.
Footnotes
Funding and disclosure: This study was supported by the following National Health & Medical Research Council (NHMRC) funding sources: Program Grant (No. 566529), Centres of Clinical Research Excellence Grant (No. 264611), Australia Fellowship (No. 464914) and Clinical Research Fellowship (No. 402864). S. Hatton is supported by an Australian Postgraduate Award (APA). D. Hermens is supported by a grant from the NSW Health, Mental Health and Drug & Alcohol Office as well as an NHMRC Australia Fellowship (awarded to I.B. Hickie). He has received honoraria for educational seminars from Janssen-Cilag and Eli Lilly. I.B. Hickie is supported by a NHMRC Australia Fellowship (No. 464914). He was a director of Headspace: The National Youth Mental Health Foundation until January 2012. He is the executive director of the Brain and Mind Research Institute, which operates 2 early-intervention youth services under contract to Headspace. He has led a range of community-based and pharmaceutical industry–supported depression awareness and education and training programs. He has led depression and other mental health research projects that have been supported by a variety of pharmaceutical partners. Current investigator-initiated studies are supported by Servier and Pfizer. He has received honoraria for his contributions to professional educational seminars related to depression, youth mental health and circadian-rhythms research. No other competing interests declared.
Competing interests: S.N. Hatton, D.F. Hermens and I.B. Hickie are supported by a National Health and Medical Research Council Program Grant (566529), a Centres of Clinical Research Excellence Grant (264611), an Australia Fellowship (464914) and a Clinical Research Fellowship (402864). S.N. Hatton and D.F. Hermens have received personal fees for educational seminars from Janssen-Cilag and Eli Lilly, and S.N. Hatton and I.B. Hickie have received grants from Servier and Pfizer for investigator-led trials. No other competing interests declared.
Contributors: All authors designed the study. S.N. Hatton, J. Lagopoulos, D.F. Hermens, E. Scott and M.R. Bennett acquired the data, which S. Hatton, J. Lagopoulos, D.F. Hermens, I.B. Hickie and M.R. Bennett analyzed. S.N. Hatton, J. Lagopoulos, D.F. Hermens, I.B. Hickie and M.R. Bennett wrote the article, which all authors reviewed and approved for publication.
References
- 1.Fitzsimmons J, Kubicki M, Shenton ME. Review of functional and anatomical brain connectivity findings in schizophrenia. Curr Opin Psychiatry. 2013;26:172–87. doi: 10.1097/YCO.0b013e32835d9e6a. [DOI] [PubMed] [Google Scholar]
- 2.Peters BD, Blaas J, de Haan L. Diffusion tensor imaging in the early phase of schizophrenia: What have we learned? J Psychiatr Res. 2010;44:993–1004. doi: 10.1016/j.jpsychires.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Samartzis L, Dima D, Fusar-Poli P, et al. White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. J Neuroimaging. 2014;24:101–10. doi: 10.1111/j.1552-6569.2012.00779.x. [DOI] [PubMed] [Google Scholar]
- 4.Kawashima T, Nakamura M, Bouix S, et al. Uncinate fasciculus abnormalities in recent onset schizophrenia and affective psychosis: a diffusion tensor imaging study. Schizophr Res. 2009;110:119–26. doi: 10.1016/j.schres.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luck D, Buchy L, Czechowska Y, et al. Fronto-temporal disconnectivity and clinical short-term outcome in first episode psychosis: a DTI-tractography study. J Psychiatr Res. 2011;45:369–77. doi: 10.1016/j.jpsychires.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Price G, Cercignani M, Parker GJ, et al. White matter tracts in first-episode psychosis: a DTI tractography study of the uncinate fasciculus. Neuroimage. 2008;39:949–55. doi: 10.1016/j.neuroimage.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong X, Ouyang X, Tao H, et al. Complementary diffusion tensor imaging study of the corpus callosum in patients with first-episode and chronic schizophrenia. J Psychiatry Neurosci. 2011;36:120–5. doi: 10.1503/jpn.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carletti F, Woolley JB, Bhattacharyya S, et al. Alterations in white matter evident before the onset of psychosis. Schizophr Bull. 2012;38:1170–9. doi: 10.1093/schbul/sbs053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–29. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yendiki A, Panneck P, Srinivasan P, et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform. 2011;5:23. doi: 10.3389/fninf.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischl B. FreeSurfer. Neuroimage. 2012;62:774–81. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behrens TE, Berg HJ, Jbabdi S, et al. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144–55. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szeszko PR, Robinson DG, Ashtari M, et al. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33:976–84. doi: 10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- 14.Nestor PG, Kubicki M, Niznikiewicz M, et al. Neuropsychological disturbance in schizophrenia: a diffusion tensor imaging study. Neuropsychology. 2008;22:246–54. doi: 10.1037/0894-4105.22.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugranyes G, Kyriakopoulos M, Dima D, et al. Multimodal analyses identify linked functional and white matter abnormalities within the working memory network in schizophrenia. Schizophr Res. 2012;138:136–42. doi: 10.1016/j.schres.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlösser RG, Nenadic I, Wagner G, et al. White matter abnormalities and brain activation in schizophrenia: a combined DTI and fMRI study. Schizophr Res. 2007;89:1–11. doi: 10.1016/j.schres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Lee RS, Hermens DF, Redoblado-Hodge MA, et al. Neuropsychological and socio-occupational functioning in young psychiatric outpatients: a longitudinal investigation. PLoS ONE. 2013;8:e58176. doi: 10.1371/journal.pone.0058176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatton SN, Lagopoulos J, Hermens DF, et al. Cortical thinning in young psychosis and bipolar patients correlate with common neurocognitive deficits. Int J Bipolar Disord. 2013;1:3–13. doi: 10.1186/2194-7511-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott EM, Hermens DF, Glozier N, et al. Targeted primary care-based mental health services engage young Australians in treatment. Med J Aust. 2012;196:136–40. doi: 10.5694/mja11.10481. [DOI] [PubMed] [Google Scholar]
- 20.Hatton SN, Lagopoulos J, Hermens DF, et al. Correlating anterior in-sula gray matter volume changes in young people with clinical and neurocognitive outcomes: an MRI study. BMC Psychiatry. 2012;12:45. doi: 10.1186/1471-244X-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagopoulos J, Hermens DF, Hatton SN, et al. Microstructural white matter changes are correlated with the stage of psychiatric illness. Transl Psychiatry. 2013;3:e248. doi: 10.1038/tp.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. Washington (DC): The Association; 2000. p. 370. [Google Scholar]
- 23.Scott EM, Hermens DF, Naismith SL, et al. Distinguishing young people with emerging bipolar disorders from those with unipolar depression. J Affect Disord. 2013;144:208–15. doi: 10.1016/j.jad.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 24.Wechsler D. Wechsler Test of Adult Reading. San Antonio (TX): Psychological Corporation; 2001. [Google Scholar]
- 25.Kessler RC, Andrews G, Colpe LJ, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32:959–76. doi: 10.1017/s0033291702006074. [DOI] [PubMed] [Google Scholar]
- 26.Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33:335–43. doi: 10.1016/0005-7967(94)00075-u. [DOI] [PubMed] [Google Scholar]
- 27.Hermens DF, Redoblado Hodge MA, Naismith SL, et al. Neuropsychological clustering highlights cognitive differences in young people presenting with depressive symptoms. J Int Neuropsychol Soc. 2011;17:267–76. doi: 10.1017/S1355617710001566. [DOI] [PubMed] [Google Scholar]
- 28.Hermens DF, Ward PB, Hodge MA, et al. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:822–9. doi: 10.1016/j.pnpbp.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 29.Hermens DF, Naismith SL, Redoblado Hodge MA, et al. Impaired verbal memory in young adults with unipolar and bipolar depression. Early Interv Psychiatry. 2010;4:227–33. doi: 10.1111/j.1751-7893.2010.00194.x. [DOI] [PubMed] [Google Scholar]
- 30.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. 3rd ed. New York (NY): Oxford University Press; 2006. p. 1216. [Google Scholar]
- 31.Sánchez-Cubillo I, Perianez JA, Adrover-Roig D, et al. Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc. 2009;15:438–50. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- 32.Tombaugh TN, Kozak J, Rees L, editors. Normative data for the controlled oral word association test. New York (NY): Oxford University Press; 1996. [Google Scholar]
- 33.Yendiki A, Koldewyn K, Kakunoori S, et al. Spurious group differences due to head motion in a diffusion MRI study. Neuroimage. 2013;88C:79–90. doi: 10.1016/j.neuroimage.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 35.Jbabdi S, Woolrich MW, Andersson JL, et al. A Bayesian framework for global tractography. Neuroimage. 2007;37:116–29. doi: 10.1016/j.neuroimage.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 36.Wakana S, Jiang H, Nagae-Poetscher LM, et al. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 37.Schmithorst VJ, Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 2010;72:16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Haroutunian V, Davis KL. Introduction to the special section: myelin and oligodendrocyte abnormalities in schizophrenia. Int J Neuropsychopharmacol. 2007;10:499–502. doi: 10.1017/S1461145706007449. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi N, Sakurai T, Davis KL, et al. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol. 2011;93:13–24. doi: 10.1016/j.pneurobio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 41.Tyszka JM, Readhead C, Bearer EL, et al. Statistical diffusion tensor histology reveals regional dysmyelination effects in the shiverer mouse mutant. Neuroimage. 2006;29:1058–65. doi: 10.1016/j.neuroimage.2005.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–40. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 43.Harsan LA, Poulet P, Guignard B, et al. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res. 2006;83:392–402. doi: 10.1002/jnr.20742. [DOI] [PubMed] [Google Scholar]
- 44.Sun SW, Liang HF, Trinkaus K, et al. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med. 2006;55:302–8. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- 45.Wheeler-Kingshott CA, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61:1255–60. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- 46.Ashtari M, Cottone J, Ardekani BA, et al. Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch Gen Psychiatry. 2007;64:1270–80. doi: 10.1001/archpsyc.64.11.1270. [DOI] [PubMed] [Google Scholar]
- 47.Leroux E, Delcroix N, Alary M, et al. Functional and white matter abnormalities in the language network in patients with schizophrenia: a combined study with diffusion tensor imaging and functional magnetic resonance imaging. Schizophr Res. 2013;150:93–100. doi: 10.1016/j.schres.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 48.Scheel M, Prokscha T, Bayerl M, et al. Myelination deficits in schizophrenia: evidence from diffusion tensor imaging. Brain Struct Funct. 2013;218:151–6. doi: 10.1007/s00429-012-0389-2. [DOI] [PubMed] [Google Scholar]
- 49.Liu X, Lai Y, Wang X, et al. Reduced white matter integrity and cognitive deficit in never-medicated chronic schizophrenia: a diffusion tensor study using TBSS. Behav Brain Res. 2013;252:157–63. doi: 10.1016/j.bbr.2013.05.061. [DOI] [PubMed] [Google Scholar]
- 50.Pérez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, et al. White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry. 2010;167:451–8. doi: 10.1176/appi.ajp.2009.09050716. [DOI] [PubMed] [Google Scholar]
- 51.Mandonnet E, Nouet A, Gatignol P, et al. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130:623–9. doi: 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- 52.Catani M, Jones DK, Donato R, et al. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- 53.Clemm von Hohenberg C, Pasternak O, Kubicki M, et al. White matter microstructure in individuals at clinical high risk of psychosis: a whole-brain diffusion tensor imaging study. Schizophr Bull. 2013 Jun. doi: 10.1093/schbul/sbt079. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waltz JA, Frank MJ, Robinson BM, et al. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62:756–64. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karlsgodt KH, van Erp TG, Poldrack RA, et al. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry. 2008;63:512–8. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 56.Cheung V, Chiu CP, Law CW, et al. Positive symptoms and white matter microstructure in never-medicated first episode schizophrenia. Psychol Med. 2011;41:1709–19. doi: 10.1017/S003329171000156X. [DOI] [PubMed] [Google Scholar]
- 57.Bennett AO MR. Consciousness and hallucinations in schizophrenia: the role of synapse regression. Aust N Z J Psychiatry. 2008;42:915–31. doi: 10.1080/00048670802419253. [DOI] [PubMed] [Google Scholar]
- 58.Alexander DC, Pierpaoli C, Basser PJ, et al. Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans Med Imaging. 2001;20:1131–9. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- 59.Kubicki M, McCarley R, Westin CF, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baldwin P, Browne D, Scully PJ, et al. Epidemiology of first-episode psychosis: illustrating the challenges across diagnostic boundaries through the Cavan-Monaghan study at 8 years. Schizophr Bull. 2005;31:624–38. doi: 10.1093/schbul/sbi025. [DOI] [PubMed] [Google Scholar]
- 61.Kuroki N, Kubicki M, Nestor PG, et al. Fornix integrity and hippocampal volume in male schizophrenic patients. Biol Psychiatry. 2006;60:22–31. doi: 10.1016/j.biopsych.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheung V, Cheung C, McAlonan GM, et al. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol Med. 2008;38:877–85. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- 63.Colby JB, Soderberg L, Lebel C, et al. Along-tract statistics allow for enhanced tractography analysis. Neuroimage. 2012;59:3227–42. doi: 10.1016/j.neuroimage.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phillips OR, Nuechterlein KH, Asarnow RF, et al. Mapping corticocortical structural integrity in schizophrenia and effects of genetic liability. Biol Psychiatry. 2011;70:680–9. doi: 10.1016/j.biopsych.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nazeri A, Chakravarty MM, Felsky D, et al. Alterations of superficial white matter in schizophrenia and relationship to cognitive performance. Neuropsychopharmacology. 2013;38:1954–62. doi: 10.1038/npp.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]