Abstract
N- Glycosylation has long been linked to protein folding and quality control in the endoplasmic reticulum (ER). Recent work has shown that O-linked glycosylation and the corresponding glycosyltransferases also participate in this important function. Notably, Protein O-fucosyltransferase 1 (Ofut1/Pofut1), a soluble, ER localized enzyme that fucosylates Epidermal Growth Factor-like (EGF) repeats, functions as a chaperone involved in the proper localization of the Notch receptor in certain contexts. Pofut2, a related enzyme that modifies Thrombospondin type I repeats (TSRs), has also been hypothesized to play a role in the folding and quality control of TSR-containing proteins. Both enzymes only modify fully folded substrates suggesting that they are able to distinguish between folded and unfolded structures. Pofuts have known physiological relevance and are conserved across metazoans. Though consensus sequences for O-fucosylation have been established and structures of both Pofuts have been studied, the mechanism of how they participate in protein folding is not known. This article discusses past and recent advances made in novel roles for these protein O-glycosyltransferases.
Introduction
The structure of a protein is key to its identity and activity. This three-dimensional structure can be modified and regulated by the addition of a variety of moieties ranging from inorganic phosphates to sugars. Some modifications are reversible and are used as switches to regulate protein function while some others irreversibly alter the structure, and hence function, of proteins. Most types of glycosylation are irreversible and have structural influences on their target protein. Glycosylation can occur on side chains of several different amino acids and can be categorized based on the type of linkage between the sugar and amino acid. There are four types of glycosylation: N-linked, which occurs on the amide group of asparagines; O-linked, which occurs on hydroxyl groups of serines, threonines, hydroxyproline, hydroxylysine and tyrosines; C-linked, which occur on tryptophans, and S-linked, which occur on cysteines [1,2]. The first two types, N- and O-linked are the most common type of carbohydrate modifications found on proteins in mammals [3].
N-Linked glycosylation occurs on the majority of secreted and cell-surface proteins. In addition to having direct effects on the structure and function of proteins, it plays an important role in folding and quality control [4]. N-glycosylation is initiated by the co- or post-translational transfer of the oligosaccharide Glc3Man9GlcNAc2 from dolichol-pyrophosphate to asparagine residues in the sequence N-X-(S/T) (where X cannot be proline) by the multi-enzyme complex Oligosaccharyltransferase (OST) [4]. The terminal glucose residues are immediately removed by glucosidases I and II (GI/II) to generate a Glc1Man9GlcNAc2 glycan. This monoglucosylated glycan is recognized by ER chaperones calnexin (CNX, membrane associated) and/or calreticulin (CRT, soluble), which assist in folding of the protein. After a folding cycle, GII removes the remaining glucose residue to generate a Man9GlcNAc2 glycan. If the protein is properly folded, it exits the ER. If not, exposed hydrophobic patches on the misfolded protein are recognized by the enzyme UDP-glucose: glycoprotein glucosyltransferase (UGGT), which adds a glucose back to recreate the Glc1Man9GlcNAc2 glycan, allowing the protein to enter another folding cycle with CNX and/or CRT. UGGT has the unique property of being able to distinguish between properly and improperly folded proteins, a key characteristic for an enzyme involved in quality control. This cycle is iterated until the protein is properly folded or the protein cannot be folded and is targeted for degradation by the ERAD system [5]. Of course, there are several other chaperones, including disulfide isomerases, that are involved in this process and that intimately interact with the components of the N-glycan pathway. Once the folded proteins exit the ER, the N-glycans are further processed in the Golgi by other enzymes to generate complex or hybrid type structures.
The most common type of O-glycosylation is the addition of GalNAc to the hydroxyls of serine or threonine, forming the core structure for what is commonly called mucin-type glycosylation [6]. Several other forms of O-linked glycosylation exist, including O-GlcNAc, O-xylose, O-glucose, O-galactose, O-mannose, and O-fucose, [7] two of which (O-mannose and O-fucose) have been implicated in quality control or protein folding. Work by several groups suggests that protein O-fucosyltransferases have chaperone-like effects on their substrates. Here we summarize results from this work and propose a model for the action of these fucosyltansferases on their substrates. We also highlight the role of O-mannosylation in the clearance of misfolded proteins from the ER in yeast. However, this mechanism is specific to yeast and has not been demonstrated in higher organisms.
O-Fucosylation: An Overview
O-Fucosylation is the addition of a fucose moiety to the hydroxyl side chain of a serine or threonine residue. It was originally identified in 1975 as an amino acid glycoside, Glcβ1-3Fuc-O-Ser/Thr, isolated from human urine [8]. However, it was not until 1990 that the first protein modified with an O-linked fucose was identified: urokinase [9]. In the following years, O-fucose was observed on several proteins, including clotting Factors VII, IX and XII [10,11]. On human Factor IX, the O-fucose is elongated to a tetrasaccharide structure: NeuAcα2-6Galβ1-4GlcNAcβ1-3Fuc [12]. Since all these proteins were modified at the same location on a common cysteine-rich motif called an Epidermal Growth Factor-like repeat (EGF repeat), a consensus sequence for O-fucosylation was proposed [13], where the O-fucose could be elongated to the Glcβ1-3Fuc disaccharide or the NeuAcα2-6Galβ1-4GlcNAcβ1-3Fuc tetrasaccharide [14]. The subsequent discovery of the Glcβ1-3Fuc disaccharide on a different cysteine-rich motif, Thrombospondin Type 1 Repeats (TSRs) [15], suggested there may be two independent O-fucosylation pathways. Identification of the enzymes involved in addition of these sugars to EGF repeats and TSRs confirmed that there are two distinct pathways for O-fucosylation [16] (Figure 1).
Figure 1. Two distinct O-fucosylation pathways.
EGF repeats and TSRs are O-fucosylated by Pofut1 and 2, respectively [16]. Both motifs contain 6 conserved cysteine residues. The consensus sequence for each motif is shown. The modified residue occurs between the second and the third cysteine of the EGF repeat (C2 and C3), while it occurs between the first and second, or second and third cysteine of a TSR, depending on whether it is a Type II or Type I TSR, respectively [38]. The O-fucose on TSRs is elongated by β3-glucosyltransferase (β3GlcT) [40,41]. O-Fucose on EGF repeats is extended by Fringe, β4-galactosyltransferase 1 (β4GalT-1) and α2-3/6-sialytransferase (α3/6SiaT) [16].
EGF repeats are cysteine-rich motifs around 40 amino acids long and were first identified in Epidermal Growth Factor itself [17]. They are found in a wide variety of cell-surface and secreted proteins (nearly 200 in the Uniprot database). EGF repeats have six conserved cysteines that form three disulfide bonds, and the consensus sequence for O-fucosylation is found between the second and third cysteine (Figure 1). Database searches with this consensus in the context of an EGF repeat predict over 80 proteins in the human genome to be modified by Pofut1 (Table 1). Several proteins in this list have been demonstrated to be O-fucosylated, including transmembrane receptors such as Notch, extracellular matrix proteins such as agrin, and soluble clotting factors like Factor VII, IX and XII. The Notch family of receptors has more O-fucosylation sites than any other protein in the databases, and Notch O-fucosylation has been extensively studied [18].
Table 1.
Putative targets of Pofut1
| Agrin |
| AMACO |
| ATRAID |
| Brevican core protein |
| CD97 antigen |
| CELR 1, 2, 3 |
| Complement component C1q receptor |
| Crumbs 1, 2 |
| Cryptic protein |
| Cubulin |
| Delta and Notch-like EGF-related receptor 4 |
| Delta-like protein 1, 3, 4 |
| Dlk-1, 2 |
| EDIL3 |
| EGF-like protein 6, 7 |
| EMR1, 2, 4 |
| Factor VII, IX, XII |
| Fibrillin 1, 2 |
| Fibulin 7 |
| Heptatoctye growth factor activator |
| Jagged 1, 2 |
| Latent TFGβ-binding protein 2 |
| LRP 1, 1b |
| Multimerin 1 |
| Multiple EGF-like protein 6, 7, 8, 10, 11 |
| NELL 1 |
| Netrin G1, 2 |
| Neurocan core protein |
| Nidogen -2 |
| Notch 1, 2, 3, 4 |
| PAMR1 |
| PEAR1 |
| PGBM |
| Pikachurin |
| Protocadherin Fat 2, 3, 4 |
| Reelin |
| Slit 1–3 |
| SNED1 |
| SREC2 |
| Stabilin 1, 2 |
| SVEP1 |
| TDGF1 |
| Tenascin |
| Teneurin 2, 4 |
| Tie1 |
| Tissue plasminogen activator |
| TSP 3 |
| Urokinase |
| Uromodulin |
| Vasorin |
| Versican core protein |
| Vitamin K-dependent protein C, Z |
| Wnt inhibitory factor 1 |
O-Fucosylation on EGF repeats is catalyzed by Protein O-fucosyltransferase 1 (Pofut1 in mammals, Ofut1 in flies) [19,20]. This modification can be extended to a tetrasaccharide by the addition of an N-acetylglucosamine, galactose and sialic acid (in that order) by the enzymes Fringe (a β3-N-acetylglucosaminyltransferase), β4-galactosyltransferase-1 and α3/6sialytransferase, respectively (Figure 1), note that O-fucose is only extended to a disaccharide in flies [21]. Elimination of Pofut1/Ofut1 in mice or flies results in embryonic lethality with severe Notch-like phenotypes, suggesting O-fucosylation is required for proper Notch function [22–25]. Of the over 80 putative targets of Pofut1 (Table 1), the effects of O-fucosylation on Notch are the best studied, although O-fucosylation of agrin is also essential for clustering of acetylcholine receptors [26]. Elongation of O-fucose on Notch by the Fringe-family of β3-N-acetylglucosaminyltransferases modulates Notch activity, increasing or decreasing Notch response to ligand binding depending on context [27,28,18]. Addition of the galactose and sialic acid has additional subtle modulatory effects on Notch in mammalian systems [29–31]. In addition to O-fucosylation, EGF repeats on Notch can be modified with N-glycans, O-glucose, O-xylose and O-GlcNAc [32–35]. O-Glucosylation on EGF repeats is also essential for Notch function [36,37]. It also occurs within a consensus sequence (C1XSXPC2), and is catalyzed by Protein O-glucosyltransferase (Poglut1 in mammals, Rumi in flies) [36,35].
Interestingly, the originally identified Glc 1-3Fuc disaccharide in human urine, which prompted many of the studies in the field, was not found on an EGF repeat but rather a different cysteine-rich domain known as a Thrombospondin type I repeat (TSR). Thrombospondin type I repeats were originally named as one of the domains in the multi-domain matricellular protein Thrombospondin 1 (TSP1) [38]. They are found in many secreted or cell-surface proteins (~65 in humans) and are typically found as C-terminal tandem repeats. TSRs are about 60 amino acids in length and, similar to EGF repeats, are defined by six cysteines that form three disulfide bonds, although in a distinct pattern. O-Fucose is added to TSRs by Protein O-fucosyltransferase 2 (Pofut2), a distant homologue of Pofut1 [39,16]. The O-fucose on TSRs can be extended to a disaccharide by the addition of glucose by the enzyme β3-glucosyltransferase (β3GlcT) (Figure 1) [40,41]. Pofut2 knockout mice display early embryonic lethality with significant defects in gastrulation [42] whereas mutations in β3GlcT in humans (gene-name B3GALTL) cause a developmental disorder called Peters Plus syndrome [43]. Comparison of sites of O-fucosylation on TSRs has led to a proposed consensus sequence for modification (Figure 1). Database searches with this consensus predict that the majority of TSR-containing proteins (50 of 65 in humans) are targets for Pofut2 (Table 2). O-Fucosylation has also been mapped to specific sites on several TSR-containing proteins including TSP1 [15], TSP2 [44], F-spondin [45], Properdin [45], ADAMTS5 [46], ADAMTS13 [47], and ADAMTSL1 [48]. Most of these proteins have known biological roles and several have pathological associations. In addition to O-fucosylation, TSRs are frequently (96% of all known TSRs) modified with C-linked mannose residues [45,15]. C-Mannosylation occurs on tryptophan residues present within the consensus sequence Trp-X-X-Trp. The enzyme catalyzing C-mannosylation was recently identified in C. elegans, DPY19 [49]. Four human homologues exist (DPY19L1-4 [50]). C. elegans DPY19 and mouse DPY19L1 are known to play roles in neuronal migration [49]. Human DPY19L2 expression is linked to globozoospermia while deletions lead to infertility [49].
Table 2.
Putative targets of Pofut2
| ADAMTS 1–4, 5, 6–10, 12, 13, 14–20 |
| ADAMTSL 1, 2–6 |
| TSP1, 2 |
| CCN1, 2, 3–6 |
| BAI1-3 |
| THSD1, 7a, 7b |
| SEMA5a, b |
| F-spondin |
| SCO-spondin |
| Properdin |
| Hemicentin 1 |
| UNC5a |
| Papilin |
| CILP2 |
| C6 |
The consensus sequence for O-fucosylation by Pofut2 can be between either cysteine 1 and 2 or cysteine 2 and 3 of a TSR. Database searches of the human genome using the current consensus sequence (Figure 1) using ScanProsite predicts that Pofut2 has fifty potential targets in humans, and work by multiple groups has shown some of these to be modified with O-fucose (underlined) [45,15,44,47,46,48].
Ofut1 acts as a chaperone in the localization of the Notch receptor
Pofut1 was first purified from Chinese hamster ovary (CHO) cells in 1998 and demonstrated to transfer an O-fucose on to a bacterially expressed EGF repeat from Factor IX in vitro [20]. To evaluate the importance of residues in the consensus sequence, Wang et al. tested the activity of purified enzyme against several point mutants of the Factor IX EGF repeat. The consensus sequence for this particular EGF repeat is CLNGGSC and at the time, it was thought that the glycines were necessary for recognition by Pofut1. Mutating the glycines to alanines either singularly or in pairs resulted in formation of several folding isomers of the EGF repeat that could be separated by reverse phase HPLC, only one of which served as a substrate for Pofut1. These results suggested that while the glycines in EGF repeat may affect the efficiency of folding, they were not required for O-fucosylation (broadening the consensus sequence as shown in Figure 1). This study also provided the first piece of evidence that Pofut1 could distinguish between misfolded and properly folded structures. Subsequently, the gene encoding POFUT1 was cloned from the human genome [19] and homologues were identified in several species enabling studies in model organisms such as Drosophila and mice [22,24,25]. The sequence predicted that Pofut1 is a soluble protein with a C-terminal RDEF (KDEL-like) sequence. Cell biological studies confirmed that Pofut1/Ofut1 is a soluble ER-associated enzyme [51,52]. This unique localization of Pofut1 to a folding compartment and the data suggesting it can differentiate between folded and unfolded structures led to the suggestion that Pofut1 may play a role in protein folding or quality control [51].
A potential role of Pofut1/Ofut1 as a chaperone in Notch folding arose during studies examining how elimination or reduction in levels of the enzyme affects Notch activity in mice or flies [18,52,53]. Knockdown of Ofut1 in Drosophila wing discs or S2 cells resulted in loss of cell surface Notch and accumulation in the ER, supporting a chaperone-like activity for Ofut1 [52]. In an independent study, Sasamura et al. showed that Ofut1 deletion resulted in loss of endosome-mediated trafficking of Notch in flies [54]. Interestingly, both defects were rescued by expressing a catalytically inactive mutant of Ofut1, suggesting that the chaperone-like activity and the fucosyltransferase activity of Ofut1 are distinct. Consistent with this observation, deletion of an essential gene in the GDP-fucose biosynthetic pathway resulted in a Fringe-like phenotype in flies, suggesting that the addition of O-fucose to Notch is necessary as a substrate for Fringe but not for the proper localization of the Notch protein [55]. These results indicate that Ofut1 has a chaperone-like activity necessary for the folding and trafficking of Notch in Drosophila that does not require its fucosyltransferase activity. Different results have been obtained in studies on Pofut1 in mammalian systems. Stahl et al. observed that ES cells lacking Pofut1 had reduced Notch activity but normal cell-surface levels of Notch proteins, suggesting that in these cells, Pofut1 does not function as a chaperone. Similarly, while Lec13-CHO cells (bearing a mutation in the GDP-fucose biosynthetic pathway) showed reduced Notch signaling, the Notch receptor was properly localized to the cell surface [53]. They also demonstrated that catalytically inactive Pofut1 did not rescue Notch localization and signaling defects in Pofut1−/− ES cells. In another study, elimination of Pofut1 in lymphoid precursors caused a minor reduction in cell surface Notch but a nearly complete loss of ligand binding [56]. In contrast, cell surface Notch1 was significantly reduced in the presomitic mesoderm of Pofut1-null mice [57]. These contrasting observations suggest a more refined role for Pofut1 that maybe influenced by the ER environ of the cells in question. Different cells have distinct complements of chaperones, which may substitute for the chaperone effect on Pofut1/Ofut1 in certain contexts.
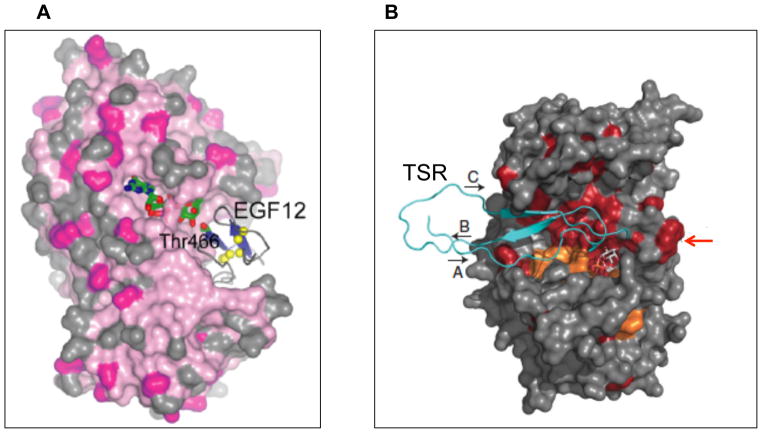
While the cell biology of Pofut1/Ofut1 indicates a chaperone function for the protein, the exact mechanism for this effect has not been described. The crystal structure of C. elegans Pofut1 showed the enzyme to have a classic GT-B fold formed by two Rossman domains [58]. GDP-fucose, the donor substrate in an O-fucosylation reaction, binds at the base of a large pocket nested between these two domains large enough to accommodate an EGF repeat (Figure 2A). This structure suggests a preferred order of substrate binding, with GDP-fucose binding first followed by the EGF repeat. Using a mutagenesis approach, Lira-Navarrette et al. were able to determine residues that are important for catalytic activity and substrate binding. Although the structure clearly shows that the enzyme recognizes a folded EGF repeat, it does not suggest an obvious mechanism for the chaperone effect of the enzyme.
Figure 2. Crystal structure of Pofuts docked with their respective substrates.
A) Pofut1 contains two Rossman-like folds with the GDP-fucose binding pocket nested deep between them [58]. The pocket is large enough to hold an EGF as well. Docking with human Notch EGF12 suggests a preferred order of substrate binding, with the GDP-fucose binding first. The modification site on EGF12 (Thr466) is indicated, demonstrating it is in close proximity to the active site. Used with permission from [58]. B) Pofut2, similar to Pofut1, has a substrate binding pocket large enough to hold GDP-fucose and a TSR [59]. In addition to the substrate-binding pocket, Pofut2 has a second hydrophobic patch (indicated by red arrow) that allows for binding to an additional (adjacent) TSR. Used with permission from [59].
Pofut2: a novel quality control mechanism for TSR folding?
Pofut2 was identified based on sequence homology with Pofut1 and shown to specifically modify TSRs but not EGF repeats [39]. Like Pofut1, Pofut2 is a soluble protein that localizes to the ER, but it is not known how it is retained in the ER since it lacks a C-terminal KDEL sequence [39]. Also like Pofut1, Pofut2 only modifies properly folded substrate, suggesting it can distinguish between folded and unfolded structures [39]. Two Pofut2 targets, ADAMTS13 and ADAMSL1, require O-fucosylation for efficient secretion [47,48]. ADAMTS13, the von Willebrand factor cleaving protease, has eight total TSRs, seven of which have consensus sequences for O-fucosylation [47]. In mature ADAMTS13, all seven TSRs are modified in high stoichiometries with the fucose-glucose disaccharide. Preventing fucosylation, either by mutating the hydroxyl amino acids in one or more of the tandem TSRs, reducing Pofut2 levels by siRNA-mediated knockdown, or reducing GDP-fucose levels, had similar effects on ADAMTS13 secretion [47]. Similar observations were made for ADAMTSL1, a secreted protein with unknown function [48]. Elimination of Pofut2 in mice results in embryonic lethality with severe defects during gastrulation [42]. A simple hypothesis for this lethality is that elimination of Pofut2 results in a global secretion defect for its targets (Table 2), and that one or more of these targets is essential for early stages of development. These results suggest that Pofut2 may function in quality control during folding of TSR-containing proteins.
A recently published crystal structure of Pofut2 offers more insight into substrate recognition, specificity, and catalytic activity [59]. Chen et al. were able to obtain crystal structures of the enzyme alone and the enzyme in a complex with GDP-fucose. Similar to Pofut1, Pofut2 has two large Rossman-like folds with a GDP-fucose binding pocket in between (Figure 2B). This pocket also binds TSRs and the docked structure of the enzyme and substrates suggests that GDP-fucose must bind to Pofut2 prior to TSR binding. The crystal structures provided important information about Pofut2 residues involved in donor and substrate binding, and mutations in several of these residues confirmed that they were involved in GDP-fucose binding (Asp297 and Arg294) and catalysis (Glu54). In order to understand how Pofut2 recognizes and binds to its substrates, key elements in a TSR interacting with the enzyme were identified. The docked TSR suggests that only a portion of the TSR fits in the binding cleft (Figure 2B). Chen et al. constructed a synthetic “mini-TSR” molecule with sufficient structural resemblance to the portion of the TSR that is predicted to bind to the enzyme and demonstrated it could be fucosylated by Pofut2 (Figure 3A). Of the thirty residues in this “mini-TSR”, ten are conserved (including the consensus sequence for fucosylation), nine are solvent exposed (hence are not predicted to interact with Pofut2) and the remaining show great variability across known TSRs (Figure 3B) [59]. They hypothesize that the substrate binding pocket accommodates the expected features of a TSR, but also has “large cavities” to make room for variable residues.
Figure 3. Schematic of the part of TSR that interacts with Pofut2.
A) Truncated TSR4 from F-spondin used in experiments to demonstrate essential structural elements required for substrate recognition by Pofut2 [59].
B) Distribution of various residues of a TSR involved in enzyme-substrate reaction. Of about 30 sites involved in enzyme interaction, 10 are conserved (amongst known Pofut2 substrates), 9 are solvent exposed, and the rest have highly variable side-chains. While specific residues in the active site interact with the conserved regions of the TSR, large cavities in the substrate-binding pocket can accommodate the variable side chains [59].
Based on the results that reduction in Pofut2 or GDP-fucose levels result in secretion defects of TSR-containing proteins like ADAMTS13 or ADAMTSL1 [47,48] and that Pofut2 recognizes one face of the TSR [59] a possible model describing the role of Pofut2 in the folding of TSRs can be drawn (Figure 4). This model is an extension of a previous model we have proposed [60] but takes into account the observations from the structural studies. TSRs have six cysteines that need to be paired for proper folding (Figure 4-i). An unfolded TSR can go through several folding isomers with various cysteines paired, correctly or incorrectly (Figure 4-ii). In the redox environment of the ER, we propose that these folding intermediates exist in equilibrium (reversible arrows, Figure 4-ii). Since Pofut2 can distinguish between folded and unfolded TSRs, once a partial but accurately folded structure if formed, Pofut2 may fucosylate this substrate (Figure 4-iii) [59,16]. The generation of a partially folded fucosylated substrate would cause a shift in the folding equilibrium, driving the folding reaction forward by mass action (thickened arrow, Figure 4-iv). Perhaps the most interesting structural feature of Pofut2 is the presence of a second substrate-binding patch (arrow in Figure 2B), which suggests the ability of the enzyme to interact with an adjacent protein motif. Since TSRs usually occur as tandem repeats, this may be especially important in the functioning of Pofut2 in folding of proteins such as ADAMTS13, which has 8 tandem TSRs.
Figure 4. A model for Pofut2 in the folding of TSRs.
Every unfolded TSR has six unpaired cysteines (orange circles) that must pair correctly (i) to be considered folded and ready for secretion. The unfolded TSR may exist as many different folding intermediates (ii) in equilibrium (double headed arrows). When an accurate, but only partially folded, structure is formed, Pofut2 recognizes this as a substrate and modifies it with a fucose (iii). The ability of Pofut2 to recognize and modify a mini-TSR supports this idea [59]. Since Pofut2 is an efficient enzyme, this reaction happens comparatively rapidly (thickened arrows) thus shifting the folding equilibrium. This drives the folding reaction forward leading to the formation of a fully folded, fucosylated TSR (iv).
Parts of the Pofut2 model may be true for Pofut1 as well since both enzymes recognize properly folded substrates, although this model suggests that both fucosyltransferase activity and GDP-fucose are necessary for the chaperone effect of Pofut2. This differs from the observations for Ofut1 in Drosophila where the fucosyltransferase activity appears to be independent of its chaperone activity [52,55]. Chang et al. have studied the folding of EGF, which bears strong structural resemblances to EGF repeats [61]. They propose that oxidative folding processed through a stable 2-disulfide intermediate, which results in a kinetic trap for the folding. Extrapolating these observations for EGF repeats, it is possible that Pofut1 relieves this stable intermediate through an unknown mechanism. In vitro analysis of EGF repeat and TSR folding coupled with single molecule studies should shed more light on this subject.
O-Mannosylation as a marker for terminating protein folding
The O-mannose modification has been known for over forty years [62]. A family of enzymes called Protein O-mannosyltransferases (Pmt), which are widely conserved between prokaryotes and eukaryotes, catalyze this modification on serines and threonines [63]. Unlike O-fucosylation, no strict consensus sequence for O-mannosylation has yet been identified. Pmts are ER-resident membrane proteins and most organisms have more than one homolog with redundant functionality [64]. They differ from most other glycosyltransferases in that they utilize dolichol-linked donor substrates rather than nucleotide sugars. Most organisms have multiple Pmts; S. cerevisiae has six family members (Pmt1p-6p) and mammals have two (Pomt1, 2). In yeast, lethality resulting from the deletion of particular combinations of Pmts is thought to be due to loss of cell wall integrity [65]. In mammals, the best characterized substrate for Pmts is α-Dystroglycan (α-DG). α-DG is a transmembrane protein that is part of the dystrophin glycoprotein complex in skeletal muscle. Mutations in the O-mannosylation pathway results in muscular dystrophies which are explained by impaired α-DG function [66].
In addition to its function as a structural modification on proteins, O-mannosylation occurs on misfolded proteins in the ER of yeast [67,68]. Mutant proteins with low folding efficiencies have been used as models to demonstrate that protein aggregates can be solubilized by modifying them with O-mannose. These misfolded proteins are eventually cleared by ER-associated degradation (ERAD) mechanisms. Recently, Xu et al. used engineered GFP to elegantly demonstrate that the amount of time spent by a mutant protein in the ER influences its O-mannosylation and clearance [69]. They also showed that modification with O-mannose reduced the ability of misfolded protein to associate with chaperones, thus terminating “futile protein folding cycles”. Collectively, these observations outline an Unfolded Protein O-mannosylation (UPOM) pathway for the clearance of misfolded protein in yeast. It remains to be investigated how Pmts recognize their unfolded substrates and mannosylate them.
Conclusions
The majority of work in protein folding is focused on chaperones and the N-glycosylation pathway that assist generic substrates. The discovery of chaperones like Pofut1 and Cosmc raises the possibility that there are domain-dedicated ER chaperones. Cosmc is a protein-specific molecular chaperone involved in the folding of the β3-galactosyltransferase, T-synthase [70,71]. Tissue specific deletion of Cosmc in mice results in misfolding of T-synthase, resulting in the loss of the T antigen and overproduction of the Tn antigen. The abnormalities observed in these mice correlate with human pathologies associated with Tn antigen defects [71]. Pofuts act on a longer list of targets, but are domain specific. Reduction or elimination of either Pofut1 or Pofut2 results in strong phenotypes in mice or flies with associated mislocalization of target proteins [57,47,54,48]. Together with the fact that the Pofuts can distinguish between folded and unfolded structures, these results suggest that at least in some contexts, Pofuts play key roles in protein folding. Nonetheless, little is known about the molecular mechanisms mediating this activity.
In addition to O-fucosylation, EGF repeats are modified by Protein O-glucosyltransferase (Poglut1) with an O-linked glucose [36,37,32]. Similar to Pofuts, Poglut1 only modifies properly folded EGF repeats [35]. This indicates the possibility that additional glycosyltransferases may be involved in the folding of EGF repeats, possibly performing somewhat redundant functions. The fact that TSRs and EGF repeats occur as tandem motifs increases the complexity of oxidative folding in the ER, warranting the existence of multiple dedicated chaperones or quality control mechanisms.
The emergence of novel and interesting roles for glycosylation in protein folding represent a change in perception of both post-translational modifications and chaperones. Structural and functional studies of Pofuts enable design of specific inhibitors, which could be therapeutically valuable in treatment of diseases caused by mutations in Pofut targets. Approaches combining new chemical tools such as azide-alkyne cycloaddition and genetics (e.g. tissue specific deletion, mosaic models) will yield better understanding and more discoveries in this field.
References
- 1.Stepper J, Shastri S, Loo TS, Preston JC, Novak P, Man P, Moore CH, Havlicek V, Patchett ML, Norris GE. Cysteine S-glycosylation, a new post-translational modification found in glycopeptide bacteriocins. FEBS Lett. 2011;585(4):645–650. doi: 10.1016/j.febslet.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 2.Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12(4):43R–56R. doi: 10.1093/glycob/12.4.43r. [DOI] [PubMed] [Google Scholar]
- 3.Varki A, Sharon N. Historical Background and Overview. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Cold Spring Harbor (NY): 2009. pp. 1–22. [PubMed] [Google Scholar]
- 4.Freeze HH, Esko JD, Parodi AJ. Glycans in Glycoprotein Quality Control. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Cold Spring Harbor (NY): 2009. [PubMed] [Google Scholar]
- 5.D’Alessio C, Caramelo JJ, Parodi AJ. UDP-GlC:glycoprotein glucosyltransferase-glucosidase II, the ying-yang of the ER quality control. Semin Cell Dev Biol. 2010;21(5):491–499. doi: 10.1016/j.semcdb.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brockhausen I, Schachter H, Stanley P. O-GalNAc Glycans. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Cold Spring Harbor (NY): 2009. [PubMed] [Google Scholar]
- 7.Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22(6):736–756. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallgren P, Lundblad A, Svensson S. A new type of carbohydrate-protein linkage in a glycopeptide from normal human urine. J Biol Chem. 1975;250(14):5312–5314. [PubMed] [Google Scholar]
- 9.Kentzer EJ, Buko A, Menon G, Sarin VK. Carbohydrate composition and presence of a fucose-protein linkage in recombinant human pro-urokinase. Biochem Biophys Res Commun. 1990;171(1):401–406. doi: 10.1016/0006-291x(90)91407-j. [DOI] [PubMed] [Google Scholar]
- 10.Bjoern S, Foster DC, Thim L, Wiberg FC, Christensen M, Komiyama Y, Pedersen AH, Kisiel W. Human plasma and recombinant factor VII. Characterization of O-glycosylations at serine residues 52 and 60 and effects of site-directed mutagenesis of serine 52 to alanine. J Biol Chem. 1991;266(17):11051–11057. [PubMed] [Google Scholar]
- 11.Harris RJ, Ling VT, Spellman MW. O-linked fucose is present in the first epidermal growth factor domain of factor XII but not protein C. J Biol Chem. 1992;267(8):5102–5107. [PubMed] [Google Scholar]
- 12.Harris RJ, van Halbeek H, Glushka J, Basa LJ, Ling VT, Smith KJ, Spellman MW. Identification and structural analysis of the tetrasaccharide NeuAc alpha(2-->6)Gal beta(1-->4)GlcNAc beta(1-->3)Fuc alpha 1-->O-linked to serine 61 of human factor IX. Biochemistry. 1993;32(26):6539–6547. doi: 10.1021/bi00077a007. [DOI] [PubMed] [Google Scholar]
- 13.Harris RJ, Spellman MW. O-linked fucose and other post-translational modifications unique to EGF modules. Glycobiology. 1993;3(3):219–224. doi: 10.1093/glycob/3.3.219. [DOI] [PubMed] [Google Scholar]
- 14.Moloney DJ, Lin AI, Haltiwanger RS. The O-linked fucose glycosylation pathway. Evidence for protein-specific elongation of o-linked fucose in Chinese hamster ovary cells. J Biol Chem. 1997;272(30):19046–19050. doi: 10.1074/jbc.272.30.19046. [DOI] [PubMed] [Google Scholar]
- 15.Hofsteenge J, Huwiler KG, Macek B, Hess D, Lawler J, Mosher DF, Peter-Katalinic J. C-mannosylation and O-fucosylation of the thrombospondin type 1 module. J Biol Chem. 2001;276(9):6485–6498. doi: 10.1074/jbc.M008073200. [DOI] [PubMed] [Google Scholar]
- 16.Luo Y, Nita-Lazar A, Haltiwanger RS. Two distinct pathways for O-fucosylation of epidermal growth factor-like or thrombospondin type 1 repeats. J Biol Chem. 2006;281(14):9385–9392. doi: 10.1074/jbc.M511974200. [DOI] [PubMed] [Google Scholar]
- 17.Rao Z, Handford P, Mayhew M, Knott V, Brownlee GG, Stuart D. The structure of a Ca(2+)-binding epidermal growth factor-like domain: its role in protein-protein interactions. Cell. 1995;82(1):131–141. doi: 10.1016/0092-8674(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 18.Rana NA, Haltiwanger RS. Fringe benefits: functional and structural impacts of O-glycosylation on the extracellular domain of Notch receptors. Curr Opin Struct Biol. 2011;21(5):583–589. doi: 10.1016/j.sbi.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Shao L, Shi S, Harris RJ, Spellman MW, Stanley P, Haltiwanger RS. Modification of epidermal growth factor-like repeats with O-fucose. Molecular cloning and expression of a novel GDP-fucose protein O-fucosyltransferase. J Biol Chem. 2001;276(43):40338–40345. doi: 10.1074/jbc.M107849200. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Spellman MW. Purification and characterization of a GDP-fucose:polypeptide fucosyltransferase from Chinese hamster ovary cells. J Biol Chem. 1998;273(14):8112–8118. doi: 10.1074/jbc.273.14.8112. [DOI] [PubMed] [Google Scholar]
- 21.Xu A, Haines N, Dlugosz M, Rana NA, Takeuchi H, Haltiwanger RS, Irvine KD. In vitro reconstitution of the modulation of Drosophila Notch-ligand binding by Fringe. J Biol Chem. 2007;282(48):35153–35162. doi: 10.1074/jbc.M707040200. [DOI] [PubMed] [Google Scholar]
- 22.Okajima T, Irvine KD. Regulation of notch signaling by o-linked fucose. Cell. 2002;111(6):893–904. doi: 10.1016/s0092-8674(02)01114-5. [DOI] [PubMed] [Google Scholar]
- 23.Okajima T, Xu A, Irvine KD. Modulation of notch-ligand binding by protein O-fucosyltransferase 1 and fringe. J Biol Chem. 2003;278(43):42340–42345. doi: 10.1074/jbc.M308687200. [DOI] [PubMed] [Google Scholar]
- 24.Sasamura T, Sasaki N, Miyashita F, Nakao S, Ishikawa HO, Ito M, Kitagawa M, Harigaya K, Spana E, Bilder D, Perrimon N, Matsuno K. neurotic, a novel maternal neurogenic gene, encodes an O-fucosyltransferase that is essential for Notch-Delta interactions. Development. 2003;130(20):4785–4795. doi: 10.1242/dev.00679. [DOI] [PubMed] [Google Scholar]
- 25.Shi S, Stanley P. Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc Natl Acad Sci U S A. 2003;100(9):5234–5239. doi: 10.1073/pnas.0831126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim ML, Chandrasekharan K, Glass M, Shi S, Stahl MC, Kaspar B, Stanley P, Martin PT. O-fucosylation of muscle agrin determines its ability to cluster acetylcholine receptors. Mol Cell Neurosci. 2008;39(3):452–464. doi: 10.1016/j.mcn.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruckner K, Perez L, Clausen H, Cohen S. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature. 2000;406(6794):411–415. doi: 10.1038/35019075. [DOI] [PubMed] [Google Scholar]
- 28.Moloney DJ, Panin VM, Johnston SH, Chen J, Shao L, Wilson R, Wang Y, Stanley P, Irvine KD, Haltiwanger RS, Vogt TF. Fringe is a glycosyltransferase that modifies Notch. Nature. 2000;406(6794):369–375. doi: 10.1038/35019000. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Lu L, Shi S, Stanley P. Expression of Notch signaling pathway genes in mouse embryos lacking beta4galactosyltransferase-1. Gene Expr Patterns. 2006;6(4):376–382. doi: 10.1016/j.modgep.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Moloney DJ, Stanley P. Fringe modulation of Jagged1-induced Notch signaling requires the action of beta 4galactosyltransferase-1. Proc Natl Acad Sci U S A. 2001;98(24):13716–13721. doi: 10.1073/pnas.241398098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou X, Tashima Y, Stanley P. Galactose differentially modulates lunatic and manic fringe effects on Delta1-induced NOTCH signaling. J Biol Chem. 2012;287(1):474–483. doi: 10.1074/jbc.M111.317578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rana NA, Nita-Lazar A, Takeuchi H, Kakuda S, Luther KB, Haltiwanger RS. O-glucose trisaccharide is present at high but variable stoichiometry at multiple sites on mouse Notch1. J Biol Chem. 2011;286(36):31623–31637. doi: 10.1074/jbc.M111.268243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakaidani Y, Furukawa K, Okajima T. O-GlcNAc modification of the extracellular domain of Notch receptors. Methods Enzymol. 2010;480:355–373. doi: 10.1016/S0076-6879(10)80016-3. [DOI] [PubMed] [Google Scholar]
- 34.Stanley P, Okajima T. Roles of glycosylation in Notch signaling. Curr Top Dev Biol. 2010;92:131–164. doi: 10.1016/S0070-2153(10)92004-8. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi H, Kantharia J, Sethi MK, Bakker H, Haltiwanger RS. Site-specific O-glucosylation of the epidermal growth factor-like (EGF) repeats of notch: efficiency of glycosylation is affected by proper folding and amino acid sequence of individual EGF repeats. J Biol Chem. 2012;287(41):33934–33944. doi: 10.1074/jbc.M112.401315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Acar M, Jafar-Nejad H, Takeuchi H, Rajan A, Ibrani D, Rana NA, Pan H, Haltiwanger RS, Bellen HJ. Rumi is a CAP10 domain glycosyltransferase that modifies Notch and is required for Notch signaling. Cell. 2008;132(2):247–258. doi: 10.1016/j.cell.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez-Valdivia R, Takeuchi H, Samarghandi A, Lopez M, Leonardi J, Haltiwanger RS, Jafar-Nejad H. Regulation of mammalian Notch signaling and embryonic development by the protein O-glucosyltransferase Rumi. Development. 2011;138(10):1925–1934. doi: 10.1242/dev.060020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams JC, Tucker RP. The thrombospondin type 1 repeat (TSR) superfamily: diverse proteins with related roles in neuronal development. Dev Dyn. 2000;218(2):280–299. doi: 10.1002/(SICI)1097-0177(200006)218:2<280::AID-DVDY4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 39.Luo Y, Koles K, Vorndam W, Haltiwanger RS, Panin VM. Protein O-fucosyltransferase 2 adds O-fucose to thrombospondin type 1 repeats. J Biol Chem. 2006;281(14):9393–9399. doi: 10.1074/jbc.M511975200. [DOI] [PubMed] [Google Scholar]
- 40.Kozma K, Keusch JJ, Hegemann B, Luther KB, Klein D, Hess D, Haltiwanger RS, Hofsteenge J. Identification and characterization of abeta1,3-glucosyltransferase that synthesizes the Glc-beta1,3-Fuc disaccharide on thrombospondin type 1 repeats. J Biol Chem. 2006;281(48):36742–36751. doi: 10.1074/jbc.M605912200. [DOI] [PubMed] [Google Scholar]
- 41.Sato T, Sato M, Kiyohara K, Sogabe M, Shikanai T, Kikuchi N, Togayachi A, Ishida H, Ito H, Kameyama A, Gotoh M, Narimatsu H. Molecular cloning and characterization of a novel human beta1,3-glucosyltransferase, which is localized at the endoplasmic reticulum and glucosylates O-linked fucosylglycan on thrombospondin type 1 repeat domain. Glycobiology. 2006;16(12):1194–1206. doi: 10.1093/glycob/cwl035. [DOI] [PubMed] [Google Scholar]
- 42.Du J, Takeuchi H, Leonhard-Melief C, Shroyer KR, Dlugosz M, Haltiwanger RS, Holdener BC. O-fucosylation of thrombospondin type 1 repeats restricts epithelial to mesenchymal transition (EMT) and maintains epiblast pluripotency during mouse gastrulation. Dev Biol. 2010;346(1):25–38. doi: 10.1016/j.ydbio.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lesnik Oberstein SA, Kriek M, White SJ, Kalf ME, Szuhai K, den Dunnen JT, Breuning MH, Hennekam RC. Peters Plus syndrome is caused by mutations in B3GALTL, a putative glycosyltransferase. Am J Hum Genet. 2006;79(3):562–566. doi: 10.1086/507567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leonhard-Melief C, Haltiwanger RS. O-fucosylation of thrombospondin type 1 repeats. Methods Enzymol. 2010;480:401–416. doi: 10.1016/S0076-6879(10)80018-7. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez de Peredo A, Klein D, Macek B, Hess D, Peter-Katalinic J, Hofsteenge J. C-mannosylation and o-fucosylation of thrombospondin type 1 repeats. Mol Cell Proteomics. 2002;1(1):11–18. doi: 10.1074/mcp.m100011-mcp200. [DOI] [PubMed] [Google Scholar]
- 46.Wang LW, Leonhard-Melief C, Haltiwanger RS, Apte SS. Post-translational modification of thrombospondin type-1 repeats in ADAMTS-like 1/punctin-1 by C-mannosylation of tryptophan. J Biol Chem. 2009;284(44):30004–30015. doi: 10.1074/jbc.M109.038059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ricketts LM, Dlugosz M, Luther KB, Haltiwanger RS, Majerus EM. O-fucosylation is required for ADAMTS13 secretion. J Biol Chem. 2007;282(23):17014–17023. doi: 10.1074/jbc.M700317200. [DOI] [PubMed] [Google Scholar]
- 48.Wang LW, Dlugosz M, Somerville RP, Raed M, Haltiwanger RS, Apte SS. O-fucosylation of thrombospondin type 1 repeats in ADAMTS-like-1/punctin-1 regulates secretion: implications for the ADAMTS superfamily. J Biol Chem. 2007;282(23):17024–17031. doi: 10.1074/jbc.M701065200. [DOI] [PubMed] [Google Scholar]
- 49.Buettner FF, Ashikov A, Tiemann B, Lehle L, Bakker H. C. elegans DPY-19 is a C-mannosyltransferase glycosylating thrombospondin repeats. Mol Cell. 2013;50(2):295–302. doi: 10.1016/j.molcel.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Carson AR, Cheung J, Scherer SW. Duplication and relocation of the functional DPY19L2 gene within low copy repeats. BMC Genomics. 2006;7:45. doi: 10.1186/1471-2164-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo Y, Haltiwanger RS. O-fucosylation of notch occurs in the endoplasmic reticulum. J Biol Chem. 2005;280(12):11289–11294. doi: 10.1074/jbc.M414574200. [DOI] [PubMed] [Google Scholar]
- 52.Okajima T, Xu A, Lei L, Irvine KD. Chaperone activity of protein O-fucosyltransferase 1 promotes notch receptor folding. Science. 2005;307(5715):1599–1603. doi: 10.1126/science.1108995. [DOI] [PubMed] [Google Scholar]
- 53.Stahl M, Uemura K, Ge C, Shi S, Tashima Y, Stanley P. Roles of Pofut1 and O-fucose in mammalian Notch signaling. J Biol Chem. 2008;283(20):13638–13651. doi: 10.1074/jbc.M802027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sasamura T, Ishikawa HO, Sasaki N, Higashi S, Kanai M, Nakao S, Ayukawa T, Aigaki T, Noda K, Miyoshi E, Taniguchi N, Matsuno K. The O-fucosyltransferase O-fut1 is an extracellular component that is essential for the constitutive endocytic trafficking of Notch in Drosophila. Development. 2007;134(7):1347–1356. doi: 10.1242/dev.02811. [DOI] [PubMed] [Google Scholar]
- 55.Okajima T, Reddy B, Matsuda T, Irvine KD. Contributions of chaperone and glycosyltransferase activities of O-fucosyltransferase 1 to Notch signaling. BMC Biol. 2008;6:1. doi: 10.1186/1741-7007-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao D, Huang Y, Huang X, Wang W, Yan Q, Wei L, Xin W, Gerson S, Stanley P, Lowe JB, Zhou L. Protein O-fucosyltransferase 1 (Pofut1) regulates lymphoid and myeloid homeostasis through modulation of Notch receptor ligand interactions. Blood. 2011;117(21):5652–5662. doi: 10.1182/blood-2010-12-326074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okamura Y, Saga Y. Pofut1 is required for the proper localization of the Notch receptor during mouse development. Mech Dev. 2008;125(8):663–673. doi: 10.1016/j.mod.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 58.Lira-Navarrete E, Valero-Gonzalez J, Villanueva R, Martinez-Julvez M, Tejero T, Merino P, Panjikar S, Hurtado-Guerrero R. Structural insights into the mechanism of protein O-fucosylation. PLoS One. 2011;6(9):e25365. doi: 10.1371/journal.pone.0025365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen CI, Keusch JJ, Klein D, Hess D, Hofsteenge J, Gut H. Structure of human POFUT2: insights into thrombospondin type 1 repeat fold and O-fucosylation. EMBO J. 2012;31(14):3183–3197. doi: 10.1038/emboj.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luther KB, Haltiwanger RS. Role of unusual O-glycans in intercellular signaling. Int J Biochem Cell Biol. 2009;41(5):1011–1024. doi: 10.1016/j.biocel.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang JY, Li L, Lai PH. A major kinetic trap for the oxidative folding of human epidermal growth factor. J Biol Chem. 2001;276(7):4845–4852. doi: 10.1074/jbc.M005160200. [DOI] [PubMed] [Google Scholar]
- 62.Sentandreu R, Northcote DH. The structure of a glycopeptide isolated from the yeast cell wall. Biochem J. 1968;109(3):419–432. doi: 10.1042/bj1090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lommel M, Strahl S. Protein O-mannosylation: conserved from bacteria to humans. Glycobiology. 2009;19(8):816–828. doi: 10.1093/glycob/cwp066. [DOI] [PubMed] [Google Scholar]
- 64.Manya H, Suzuki T, Akasaka-Manya K, Ishida HK, Mizuno M, Suzuki Y, Inazu T, Dohmae N, Endo T. Regulation of mammalian protein O-mannosylation: preferential amino acid sequence for O-mannose modification. J Biol Chem. 2007;282(28):20200–20206. doi: 10.1074/jbc.M702369200. [DOI] [PubMed] [Google Scholar]
- 65.Willer T, Brandl M, Sipiczki M, Strahl S. Protein O-mannosylation is crucial for cell wall integrity, septation and viability in fission yeast. Mol Microbiol. 2005;57(1):156–170. doi: 10.1111/j.1365-2958.2005.04692.x. [DOI] [PubMed] [Google Scholar]
- 66.Wells L. The o-mannosylation pathway: glycosyltransferases and proteins implicated in congenital muscular dystrophy. J Biol Chem. 2013;288(10):6930–6935. doi: 10.1074/jbc.R112.438978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harty C, Strahl S, Romisch K. O-mannosylation protects mutant alpha-factor precursor from endoplasmic reticulum-associated degradation. Mol Biol Cell. 2001;12(4):1093–1101. doi: 10.1091/mbc.12.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakatsukasa K, Okada S, Umebayashi K, Fukuda R, Nishikawa S, Endo T. Roles of O-mannosylation of aberrant proteins in reduction of the load for endoplasmic reticulum chaperones in yeast. J Biol Chem. 2004;279(48):49762–49772. doi: 10.1074/jbc.M403234200. [DOI] [PubMed] [Google Scholar]
- 69.Xu C, Wang S, Thibault G, Ng DT. Futile protein folding cycles in the ER are terminated by the unfolded protein O-mannosylation pathway. Science. 2013;340(6135):978–981. doi: 10.1126/science.1234055. [DOI] [PubMed] [Google Scholar]
- 70.Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci U S A. 2002;99(26):16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y, Ju T, Ding X, Xia B, Wang W, Xia L, He M, Cummings RD. Cosmc is an essential chaperone for correct protein O-glycosylation. Proc Natl Acad Sci U S A. 2010;107(20):9228–9233. doi: 10.1073/pnas.0914004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gebauer JM, Muller S, Hanisch FG, Paulsson M, Wagener R. O-glucosylation and O-fucosylation occur together in close proximity on the first epidermal growth factor repeat of AMACO (VWA2 protein) J Biol Chem. 2008;283(26):17846–17854. doi: 10.1074/jbc.M704820200. [DOI] [PubMed] [Google Scholar]
- 73.Harris RJ, Leonard CK, Guzzetta AW, Spellman MW. Tissue plasminogen activator has an O-linked fucose attached to threonine-61 in the epidermal growth factor domain. Biochemistry. 1991;30(9):2311–2314. doi: 10.1021/bi00223a004. [DOI] [PubMed] [Google Scholar]
- 74.Panin VM, Shao L, Lei L, Moloney DJ, Irvine KD, Haltiwanger RS. Notch ligands are substrates for protein O-fucosyltransferase-1 and Fringe. J Biol Chem. 2002;277(33):29945–29952. doi: 10.1074/jbc.M204445200. [DOI] [PubMed] [Google Scholar]
- 75.Shi S, Ge C, Luo Y, Hou X, Haltiwanger RS, Stanley P. The threonine that carries fucose, but not fucose, is required for Cripto to facilitate Nodal signaling. J Biol Chem. 2007;282(28):20133–20141. doi: 10.1074/jbc.M702593200. [DOI] [PubMed] [Google Scholar]