Abstract
Background
Maternal-fetal transfer of antiretroviral drugs contributes to prevention of vertical transmission of HIV.
Objective
This systematic review discusses published studies containing data pertaining to the pharmacokinetics of placental transfer in humans, including paired cord and maternal plasma samples collected at the time of delivery as well as ex vivo placental perfusion models.
Methods
Articles pertaining to placental transfer of antiretrovirals were identified from PubMed, from references of included articles, and from U.S. Department of Health and Human Services Panel on Treatment of HIV-infected Pregnant Women and Prevention of Perinatal Transmission guidelines. Articles from non-human animal models or that had no original maternal-fetal transfer data were excluded. PRISMA guidelines were followed.
Results
A total of 103 published studies were identified. Data across studies appeared relatively consistent for the nucleoside reverse transcriptase inhibitors (NRTIs) and the non-nucleotide reverse transcriptase inhibitors (NNRTIs), with cord to maternal ratios approaching 1 for many of these agents. The protease inhibitors atazanavir and lopinavir exhibited consistent maternal-to-fetal transfer across studies, although the transfer may be influenced by variations in drug-binding proteins. The protease inhibitors indinavir, nelfinavir, and saquinavir exhibited unreliable placental transport, with cord blood concentrations that were frequently undetectable. Limited data, primarily from case reports, indicate that darunavir and raltegravir provide detectable placental transfer.
Conclusion
These findings appear consistent with current guidelines of using two NRTIs plus an NNRTI, atazanavir/ritonavir, or lopinavir/ritonavir to maximize placental transfer as well as to optimally suppress maternal viral load. Darunavir/ritonavir and raltegravir may reasonably serve as second-line agents.
Introduction
The necessity of combination antiretroviral therapy (cART) for HIV-infected pregnant women has been well-established. Such regimens are important both for maternal wellbeing and to prevent perinatal transmission of HIV to the fetus. This decrease in perinatal transmission is thought to occur both by decreasing maternal viral load and by providing the infant with pre-exposure prophylaxis via placental transfer [1]. Cases have been reported, albeit rarely, of perinatal transmission from mothers on cART who had undetectable or very low levels of plasma HIV RNA [2, 3]. HIV viral shedding in the genital tract has been documented in women whose plasma HIV RNA was undetectable [4]. Such cases indicate that maternal HIV plasma RNA levels may not be fully indicative of transmission risk and that placental transfer of antiretroviral agents (ARVs) may be another necessary component in protecting the fetus from infection. Since even the most rigorous neonatal regimen cannot be expected to “cure” an infant already infected in utero, prevention of transmission should always be the highest priority.
The most recent, March 2014, United States Department of Health and Human Services (HHS) guidelines recommend that women already taking a cART regimen who have undetectable plasma HIV RNA viral load continue on their current regimens during pregnancy [1]. HHS recommends that treatment-naïve pregnant patients undergo resistance testing and then commence a cART regimen consistent with those recommended for non-pregnant adults [1]. That is, the regimen should contain two nucleoside reverse transcriptase inhibitors (NRTIs) plus either a ritonavir-boosted protease inhibitor (PI) or a non-nucleoside reverse transcriptase inhibitor (NNRTI) [1]. Further, recognizing the importance of transplacental transfer of ARVs to the fetus, HHS guidelines recommend that at least one of the NRTIs selected has “high” placental transfer [1]. The HHS guidelines also suggest that certain ARVs be given in higher doses during the second and/or third trimesters of pregnancy in order to maintain adequate maternal concentrations [1]. While not specifically studied, it is reasonable to expect that these higher doses may also lead to higher absolute placental transfer.
Several types of studies address the issue of transplacental transfer, and each approach has its benefits and drawbacks. One approach is a measure of human cord blood and maternal plasma ARV concentrations at the time of delivery. This approach is relatively safe but only provides transfer information at a single time that is typically at or near term. Further, the time between the patient's last dose and delivery is variable. A recent variation of this approach is to include these data into a broader population pharmacokinetic model for the pregnant patient that contains a fetal compartment; this provides a more comprehensive (though theoretical) model of fetal exposure. Another approach utilizes ex vivo perfusion of recently-delivered human placentas; this enables collection of transfer data for known concentrations of ARVs but is typically limited to full or near-term placentas in non-infected women. A final approach involves animal studies of fetal transfer; this enables fetal transfer measurements in vivo at multiple time points, sometimes extended throughout gestation to include times preceding fetal viability. The largest downside to this approach is that it is unclear whether these findings can be extrapolated to humans due to various interspecies differences in the placenta composition as well as timing of the establishment of the maternal-fetal circulation [5]. While non-human primate models appear more representative than mouse models [5], further work would still need to be completed prior to drawing conclusions based on any animal model. Given that animal models cannot be applied reliably to humans, they are not further addressed in this review.
The objective of this review is to present the available pharmacokinetic data regarding transplacental transfer of ARVs. When selecting an ARV regimen and dosing for a pregnant woman, clinicians must consider pharmacokinetic data both for this transplacental transfer and for treatment of the pregnant woman herself. While this review focuses only on the transplacental component, clinicians need to consider this information only in the context of the additional maternal pharmacokinetic and treatment components.
Methods
The aim was to include all studies containing pharmacokinetic data pertaining to placental transfer of ARVs. The search strategy was conducted according to PRISMA guidelines. The primary search was conducted in PubMed on March 29, 2014 and using the following search terms: (HIV AND (pregnancy OR placenta) AND (pharmacokinetics OR pharmacokinetic OR “placental transfer”)). We kept the search terms broad enough to include any studies of pharmacokinetics of ARVs in pregnancy; many studies are focused on maternal pharmacokinetics but include a single paired cord blood and maternal plasma ARV concentration measurement at the time of delivery. This broader search strategy allowed us to capture such studies that contained placental transfer data despite not having an overall focus on placental transfer. Publication types designated as “review” were automatically excluded from the search. Articles were not restricted based on year of publication or language.
Articles identified by the PubMed search were further screened manually by review of the abstract and, frequently, the full article text. Articles were then only deemed eligible if they included original data pertaining to transfer of ARVs from mother to fetus in humans (e.g. review articles and consensus guidelines that were not excluded by PubMed were manually excluded at this point). Articles without readily-apparent transfer data, e.g. maternally-focused pharmacokinetic studies, were electronically searched using Adobe Acrobat's “Find” function. Specifically the article text was searched for the terms “cord,” “amniotic,” “placenta” (also detects the words “placental” and “transplacental”), “fet” (to detect “fetus” or “fetal”), and “neonat” (to detect “neonate” or “neonatal”). Occurrences of these terms were manually evaluated for presence of maternal-fetal ARV transfer data. If after this thorough process no transfer data were detected the article was excluded.
Finally, the references of included articles were reviewed for potentially relevant articles missed by the PubMed strategy above. Also, “Appendix B” of the HHS guidelines pertaining to ARVs in pregnancy [1], which contains a listing of studies for each available ARV, was reviewed for any other relevant studies not otherwise detected above.
Given the diversity of studies included, both in terms of ARVs and study designs, no summary measures of data were formally calculated (i.e. meta-analysis). For studies with paired cord and maternal plasma ARV concentrations, ranges of transfer ratios and cord levels were presented for studies with similar results and any outlying results described separately. For other study designs each study's results are briefly described separately.
Results
1. Study Selection
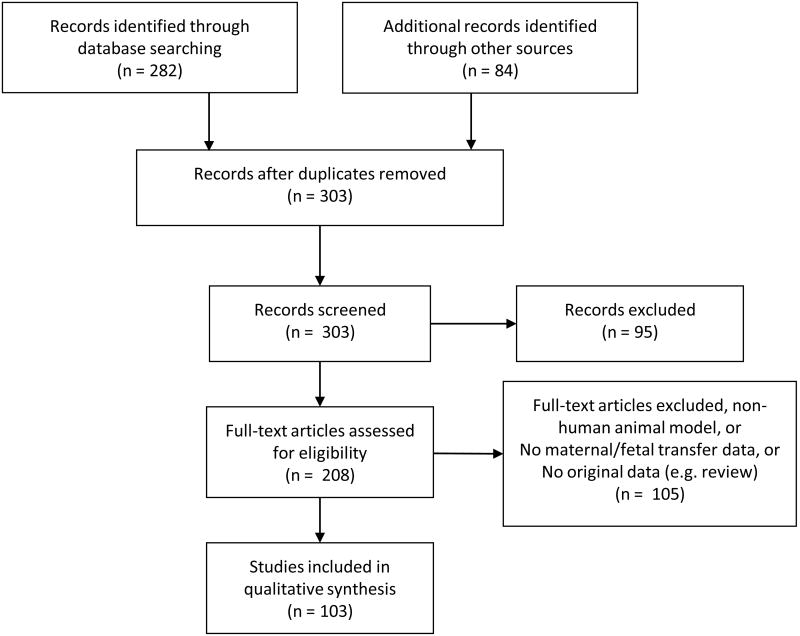
The study selection process is displayed in Figure 1. In summary, the initial PubMed search yielded 282 articles for additional screening. Eighty-four articles were identified from the HHS guidelines Appendix B[1]. No additional eligible articles were discovered by reviewing the reference sections of the included articles. After removing duplicate records, 303 articles were screened. Based on the title or abstract, 95 records were excluded, leaving 208 full-text articles that were assessed for eligibility. Of those articles, 103 met our eligibility criteria and the remaining 105 were excluded either for being a non-human placental transfer study, for not containing maternal-fetal transfer data for ARVs in pregnancy, and/or for not containing original data (e.g. review articles and consensus guidelines).
Figure 1. Overview of search strategy and included studies.
This yielded 103 articles for inclusion. Their study designs can be stratified as follows: 71 paired maternal-fetal transfer studies in humans, 7 population pharmacokinetic models of maternal-fetal transfer (most were part of the same larger study), 19 ex vivo human placental perfusion models, and 8 studies with miscellaneous approaches including mechanistic transporter and protein binding studies and studies of cord or fetal cells for drug incorporation. Of note, the sum of the study design types is greater than 103 as two articles incorporated more than one approach.
2. Paired Maternal-Fetal Human Transfer Data
An overview of the paired maternal-fetal transfer data at the time of delivery is shown in Table 1.
Table 1. Summary of paired human maternal-fetal transfer data at time of delivery.
The range of average cord (or neonatal) ARV levels and average cord (or neonatal) to maternal ARV plasma ratios are included for each ARV. The “averages” reported from each article are typically medians but also include means. All ARVs were assumed to have reached steady-state prior to measurement unless otherwise noted.
| ARV | Number of Studies* | Total Number Paired Samples** | Range of Average§ Cord Levels (mg/L) | Range of Average§ C:M | Comments |
|---|---|---|---|---|---|
| NRTIs | |||||
| Abacavir [6, 7] | 2 | 14 | 0.26 - 0.83 | 1.03-1.06 | |
| Didanosine [7] | 1 | 10 | < 0.05 | 0.38 | 14 additional excluded from C:M as maternal plasma levels were < LOQ |
| Emtricitabine [13-15] | 3 | 22 | 0.25 – 0.26 | 1.2 – 1.7 | |
| Lamivudine [7-11] | 5 | 258 | 0.22 – 0.4 | 0.93 – 1.22 | |
| Stavudine [7, 12] | 2 | 22 | 0.04 – 0.14 | 1.0 – 1.3 | Median of 0.04 for n=32, but 20 of these were excluded from study's C:M (1.3) as maternal levels were < LOQ [7] |
| Tenofovir | |||||
| Steady-state [10, 14, 15] | 3 | 18 | 0.03, 0.05 [10, 15] | 0.82 (n=14) [14] 1.1 (n = 1) [15] 6.0 (n=3) [10] |
|
| Labor only [16] | 1 | 99 | 0.08 (600mg) 0.12 (900mg) |
0.60 | n=63 on 600mg, n=36 on 900mg |
| Vaginal [17] | 1 | 16 | 0.002 (IQR 0.001 – 0.003) | 0.5 (IQR 0.5-0.6) | Single dose of 1% vaginal gel (40mg tenofovir) 2 hours prior to scheduled Cesarean |
| Zidovudine [7, 8, 10, 18-24] | 10 | 153 | 0.12 – 0.70 (9 studies) | 0.81 – 1.6 (8 studies) | All studies assumed steady-state prior to labor but increased and/or switched to IV at start of labor |
|
| |||||
| NNRTIs | |||||
| Efavirenz | |||||
| Cord data [28] | 1 | 23 | 1.1 (0.47-4.5) | 0.49 (0.37-0.74) | |
| Neonate plasma [29] | 1 | 56 | 1.7 (0.05-7.9) | 0.1 | C:M median estimated from figure (average not reported) |
| Etravirine | 3 case reports | Singleton cases [30, 15]: | 0.11, 0.22 | 0.33, 0.51 | |
| Twin case [31]: | 0.41 (twin 1), 0.35 (twin 2) | - | No maternal plasma levels at delivery; dizygotic | ||
| Nevirapine | |||||
| Steady-state [10, 32-37] | 7 | 70 | 1.5-3.4 (for studies with n > 2, minimum = 2.4) | 0.59-1.0 | C:M values evenly dispersed across wide range |
| Labor only [38-41] | 4 | 74 | 0.76 – 1.4 | 0.71-0.92 (3 studies) | In all 3 studies, average 4-5 hours from dose to delivery. Average of 0.92 as weighted mean from 3 subgroups reported in 1 study [38]. |
| Rilpivirine [42] | 1 | 1 | 0.016 | 0.74 | |
|
| |||||
| Protease Inhibitors | |||||
| Atazanavir [35, 43-48] | 7 | 158 | 300mg: 0.13-0.22 400mg: 0.18-0.200 |
13 – 0.24 | 3 studies with 300mg daily [43-45], 1 with 400mg [47], 1 with both [46], 2 unspecified [35, 48]; all with 100mg RTV |
| Darunavir | All doses with 100mg RTV | ||||
| Study, 600mg BID [70] | 1 | 9 | 0.38 | 0.15 | |
| Case Reports, 600mg BID [15, 31, 67, 69] | 4 | 5 | Single: 0.21-1.4 Twins: 0.58, 1.0 |
0.15 – 0.32 (excludes twins) | Includes 3 case reports (4 patients) of single gestation [15, 67, 69] and 1 case report of dizygotic twins (for which no paired maternal concentration provided) [31] |
| Case Report, 800mg once daily [68] | 1 | 1 | 0.43 | 0.11 | |
| Fosamprenavir [71] | 1 | 7 | 0.11 | 0.27 | For amprenavir active metabolite. |
| Indinavir | 3 | n = 21 [56] n = 4 [57] n = 19 [63] |
All cords < LOQ 0.00 0.12 |
0.00 0.01 0.12 |
GMR of 0.12 excluded 7/26 cords with ARV < LOQ [63] |
| Lopinavir | |||||
| 400mg (+ 100 RTV) BID [10, 33, 49-53] | 7 | 63 | 0.45 – 0.70 (4 studies) | 0.16 – 0.22 (6 studies) | One study did not provide cord levels. Two studies had outlying levels: 1) 1.3 mg/L (n=1) with C:M in range [50], 2) 0.08 mg/L and C:M 0.57 (n=6) [10] |
| 533mg (+ 133 RTV) BID [54] | 1 | 23 | 0.89 mg/L | 0.23 | |
| 600mg (+150 RTV) BID [29, 53, 55] | 3 | 83 | 0.88 - 1.0 | 0.16 – 0.20 | One study [29] with neonate plasma instead of cord |
| Dose not specified [32, 35, 36] | 3 | 54 | 0.00 - 0.96 | 0.00 - 0.24 | Detectable in study with 42 patients [36]; not detectable in any cord samples in study with 11 patients [35] and case report [32] |
| Nelfinavir [10, 32, 33, 35, 36, 56-62] | 12 | 217 | 0.00 – 0.27 (10 studies) | 0 – 0.49 | Many cord samples < LOQ; studies reporting higher values for cord and C:M typically excluded the LOQ samples. Median values for studies fairly evenly distributed throughout the ranges specified. |
| Saquinavir [32, 33, 56, 57, 64-66] | 7 | 37 | < LOQ in 5/6 reporting cord levels | 0.00 – 0.04 (6 studies) | 1 study with median 0.128 mg/L in cord (n = 7) but no ratio reported [66] |
| Tipranavir [72] | 1 | 1 | 15.6 | 0.41 | |
|
| |||||
| Ritonavir (booster only, 100-150mg) [10, 29, 33, 51, 53, 57, 70] | 7 | 132 | 0 – 35.8 | 0.00 – 0.55 | 1 study (n=6) with ratio 0.55 [10], otherwise highest ratio was 0.24 [53] |
|
| |||||
| Other ARVs | |||||
| Enfuvirtide [31, 72-74] | 4 | 11 | Undetectable in | all cord samples. | 3 of these records are case reports (n ≤ 2) |
| Maraviroc [15] | 1 | 1 | 0.069 | 0.37 | |
| Raltegravir [50, 75, 76] | 3 | 5 | 0.19 (cord) 0.21-3.8 (neonate) |
C:M 1.00 N:M 1.63-15.5 |
All case reports. N:M concentrations evenly distributed throughout range reported. |
Number of studies, ranging from case reports to large trials.
Sum of patients included in each study for the paired C:M reading; occasionally more patients were included in each study's report of average cord ARV level (unpaired).
Reported as median by most articles but reported by some as means.
Abbreviations in order of appearance: ARV = antiretroviral drug; C:M = cord to maternal plasma ARV ratio; N:M = neonatal to maternal plasma ARV ratio (prior to neonatal ARV dosing); NRTI = nucleoside reverse transcriptase inhibitor; NNRTI = non-nucleoside reverse transcriptase inhibitor; GM(R) = geometric mean (ratio); LOQ, limit of quantification of assay; IQR = interquartile range; RTV, ritonavir
2.1 Nucleoside/Nucleotide Reverse Transcriptase Inhibitors (NRTIs)
The reported average (median or mean, as specified in each study) cord to maternal plasma concentration ratios at the time of delivery were approximately 1 for abacavir, lamivudine, and stavudine [6-12]. The observed ratio was lower for didanosine, at 0.38, but only included a single study with 10 patients [7]. Of the three emtricitabine studies, the geometric mean ratio of cord to maternal plasma concentration was 1.2 in one study (n = 11); for this study the median time between dosing and delivery was 19 hours [13]. For the other emtricitabine study (n = 10), the median cord to maternal ratio was 1.6 but the median time between dosing and delivery was 8.5 hours [14]. For an emtricitabine case report (n = 1) the ratio was 1.7 at 13 hours after dosing [15].
The five included records for tenofovir varied in study design. Two studies and a case report all included patients at steady-state, yet the median cord to maternal plasma concentration ratio varied from 0.82 (range 0.64-1.1, n=14) [14] in one study to 6.0 (range 3.5-7.2, n =3) [10] for the other; the case report (n = 1) was in between with a ratio of 1.1 [15]. Data for differences in time elapsed post-dose were not available for comparison. The third study included patients administered tenofovir at the onset of labor, excluding any patients who had previously received tenofovir. Patients received a dose of either 600mg (n = 63) or 900mg (n = 36); for both groups the median cord to maternal plasma concentration ratio was 0.6 [16].
The final tenofovir study included 16 patients administered a single dose of vaginal tenofovir 1% gel (containing 40mg tenofovir). While the cord blood concentrations were lower than in the oral single-dose tenofovir study (Table 1), the median cord to maternal plasma concentration ratio was similar at 0.5 (interquartile range (IQR) of 0.5 to 0.6) [17].
Zidovudine has been studied the most frequently, with 10 total included records from studies at the time of viable delivery. The average cord to maternal plasma concentration ratios were evenly dispersed from 0.81 to 1.6 [7, 8, 10, 18-24]. While for all of these studies patients were taking zidovudine throughout pregnancy, the dose was frequently increased and/or switched to an intravenous formulation during labor. The studies varied widely in these dosing protocols, so a wide range of cord concentrations was expected; further, since maternal levels were likely drawn at various points in time before, during, or after the zidovudine infusion and not necessarily at the immediate time of delivery a wide range in the cord to maternal ratios is not surprising.
Zidovudine placental transfer was studied in three additional studies in earlier-stage pregnancies, prior to elective termination. A study of 26 HIV-negative pregnant women in their first trimester (median 11 weeks), given 400mg of zidovudine, found a median ratio of zidovudine in fetal tissue to maternal serum of 0.92 (IQR 0.40-1.36) [25]. The second study included six HIV-positive patients given 1000mg zidovudine each (divided into five doses) prior to elective termination at 14-26 weeks (mean 18 weeks); it was not stated whether any of these patients were taking zidovudine prior to the study. Maternal zidovudine was not detected in 1 patient; for the remaining five patients, based on the raw data provided in the article, the median concentration ratio for fetal tissue to maternal serum was 1.3 (range 0.95 – 3.2) [26]. The third early placental transfer study included two HIV-positive women at 18 and 21 weeks of gestation prior to elective termination; they were administered zidovudine over three days (total 3200mg) prior to sampling of umbilical venous blood in utero under ultrasound guidance. One patient had a cord to maternal concentration ratio of 1.1 with umbilical vein concentration of 0.62 mg/L while the other had a ratio of 6.0 but with a lower cord concentration of 0.12 mg/L [27].
2.2 Non-nucleoside Reverse Transcriptase Inhibitors (NNRTIs)
Only two placental transfer studies of efavirenz have been published. In one (n=23), the average cord to maternal ratio was 0.5 [28]; the other (n=56) used neonatal plasma with an estimated median ratio of 0.1 [29]. Three case reports of etravirine [30, 31] have been published, two (n=1 each) with cord to maternal plasma ratios of 0.3 [30] and 0.5 [15]; the other report did not include maternal plasma sampling [31] (see Table 1 for cord values). Nevirapine is the NNRTI that has been most studied in pregnancy, both throughout pregnancy and in patients presenting in labor. Average cord to maternal plasma concentration ratios were dispersed from 0.6 to 1.0 in patients on chronic nevirapine [10, 32- 37] and from 0.7 to 0.9 in patients starting nevirapine at labor onset [38-41]. Finally, cord and maternal plasma concentrations of rilpivirine have been measured in just one case report [n = 1] with a cord to maternal ratio of 0.74 [42].
2.3 Protease Inhibitors
Ritonavir-boosted atazanavir and lopinavir are currently the first-line protease inhibitors in pregnancy [1] and they appear to have the highest reliable cord to maternal plasma concentration ratios in this drug class. In the seven included atazanavir studies, the average cord to maternal ratios ranged from 0.13 to 0.24 [35, 43-48].
In the ten lopinavir studies with a specified dosage (ranging from 400mg to 600mg twice daily) the average cord to maternal plasma concentration ratios for total lopinavir ranged from 0.16 to 0.23 [29, 33, 49-55] except for one study (n=6) with a median ratio of 0.57 [10]. In three other studies the dose was not specified: in one, the ratio was 0.24 [36] but in a small study [35] and in a case report [32] the cord concentrations were undetectable. Two studies of 400mg lopinavir additionally measured free cord and maternal lopinavir concentrations. The mean free lopinavir cord to maternal ratios were 0.43 (n = 16) [52] and 0.31 (n = 7) [51]. These ratios are higher than those for total lopinavir, consistent with the lower binding protein concentrations in the cord versus in maternal plasma. Accordingly, the mean α-1-acid glycoprotein (AAG) level was found to be 0.15 g/L in the cord and 0.47 g/L in the maternal plasma in one study (n = 16) [52].
Nelfinavir has also been well-studied in pregnancy, but is no longer recommended in patients able to tolerate other agents [1]. In the twelve included studies [10, 32, 33, 35, 36, 56-62], reported average cord to maternal concentration ratios fluctuated widely from 0.0 to 0.49; likewise, cord blood nelfinavir concentration averages varied from 0.0 to 0.27 mg/L. Concentrations of nelfinavir were frequently undetectable in both maternal and cord samples, and the studies that reported higher averages tended to exclude these undetectable concentrations from their summary measure. In cases of undetectable nelfinavir in maternal plasma this may be a result of either complete noncompliance or, more likely, poor bioavailability from failing to take the drug with a high-fat meal. Hence while in some patients the placental transfer appears similar to atazanavir and lopinavir, in others the nelfinavir is undetectable even in maternal plasma and thus does not reach the cord blood.
Indinavir [56, 57, 63] and saquinavir [32, 33, 56, 57, 64-66] both had low and unpredictable placental transfer ratios. Limited data for darunavir [31, 67-70], fosamprenavir [71], and tipranavir [72] reveal potential for moderate placental transfer (see Table 1 for details). In particular, darunavir has been studied in 5 case reports and 1 small (n = 9) study with cord to maternal plasma concentration ratios ranging from 0.11 to 0.32 [15, 31, 67, 68, 69, 70]
Finally, some of the studies of protease inhibitors included paired cord and maternal plasma sampling for the ritonavir booster. Most of these transfer ratios ranged from 0 to 0.2 [29, 33, 51, 53, 57, 70] although one smaller study reported a value of 0.55 [10]. It is not clear that placental transfer of ritonavir is necessary as its purpose is to increase the systemic exposure of its accompanying protease inhibitor, hence increasing the amount of those agents available for placental transfer.
2.4 Fusion/Entry Inhibitors
The fusion inhibitor enfuvirtide has been undetectable in all cord samples in a total of 11 patients observed to date [31, 72-74]. Placental transfer of the CCR5 receptor antagonist maraviroc has only been studied in one case report (n = 1) in which the cord to maternal plasma concentration ratio was 0.37 [15].
2.5 Integrase Inhibitor
Raltegravir is the only integrase inhibitor for which placental transfer data are published. These data are only from limited case reports for a total of five patients on raltegravir at steady-state and two not at steady-state. A case report of 1 patient reported a cord to maternal concentration ratio of 1.0 [50]. A report of 3 patients utilized neonatal plasma levels instead and reported high neonatal concentrations as well as high neonatal to maternal concentration ratios of 7.37, 9.5, and 15.5 [75]. The third set of cases focused on pre-term deliveries. One patient was at steady state prior to delivery at 33 + 2/7 weeks and had a neonatal plasma concentration of 3.8 mg/L with a neonatal to maternal ratio of 1.63 [76]. The other two patients in this report were not at steady-state, having started raltegravir only 14 to 23 hours prior to delivery. One of these patients delivered at 30 + 3/7 weeks with a neonatal concentration of 0.12 mg/L and a neonatal to maternal ratio of 1.9; the other delivered at 29 + 5/7 weeks with a neonatal concentration of 0.60 mg/L and a ratio of 2.0 [76].
3. Population Pharmacokinetic Models of Maternal-Fetal Transfer
A few studies of ARVs in pregnancy have utilized maternal, cord, and/or neonatal plasma ARV concentrations to construct a population-based model, describing the placental transfer process in the context of the maternal model. These models estimate a fetal to maternal exposure ratio, often expressed as a ratio of areas under the plasma concentration versus time curves (AUCs).
A population pharmacokinetic study of lamivudine in pregnancy included 228 total women receiving lamivudine throughout pregnancy, including 123 with cord samples at time of delivery [11]. The predicted fetal to maternal AUC ratio was 0.86 [11], slightly below the observed concentration ratios reported for the lamivudine studies in Table 1.
Two articles from a single research study described placental transfer of emtricitabine on a population level. In both steps of this study patients previously on zidovudine monotherapy were given 400mg emtricitabine, 600mg tenofovir, and 200mg nevirapine at the onset of labor. The first step of the study included 38 HIV-infected pregnant women and described a median predicted cord level at delivery of 0.72 mg/L with a transfer ratio for cord to maternal plasma of 0.76 and an AUC ratio of 0.80 [77]. According to a figure provided in that article, the AUC ratio appears to peak at close to 1 for patients with 5 hours elapsed between the dose and delivery and gradually decreases to a plateau around 0.7 for patients delivering at 15-25 hours post-dose [77]. While the predicted cord concentrations were higher than those observed in the individual pharmacokinetic studies cited in Table 1, the predicted total placental transfer ratio (AUC ratio) appears a bit lower. The second article on this emtricitabine population pharmacokinetic study reported on the active, phosphorylated intracellular form of emtricitabine. It measured this metabolite in cord blood from 36 deliveries using liquid chromatography-tandem mass spectrometry and compared with literature that described concentrations of this metabolite in non-pregnant adults [78]. From this study the median predicted exposure to the active intracellular phosphorylated emtricitabine was 5.9 times higher in the cord blood than in adults [78] despite the lower exposure to the parent drug reported in the first part of the study [77]. This is relevant clinically since it implies that measurement of the parent drug may underestimate the extent of placental transfer of active drug and hence measurement of the active form in future studies may provide more meaningful results.
The population pharmacokinetic study of emtricitabine also described tenofovir placental transfer in the same patients with the same study design as described above. In the first part of this study the median predicted cord concentration at delivery for tenofovir was 0.10 mg/L with a median predicted cord to maternal ratio of 0.71 and an AUC ratio of 0.60 [79]. From a figure provided the AUC ratio appears consistently around 0.6 beginning at 5 hours post-dose [79]. This ratio is consistent with the observed concentration ratio in the study of tenofovir given during labor [16] as shown in Table 1. The intracellular phosphorylation component of the study included 20 cord samples in which the intracellular metabolite was measured [80]. Unlike in the emtricitabine study, these levels were below the limit of detection in 6/20 of the subjects and below the limit of quantification in another 12/20 despite the parent drug being detectable in all of these samples [80]. The authors attributed this to possibly either low rates of cell transfer or a delay prior to phosphorylation in the fetus [80].
Two population pharmacokinetic studies described placental transfer for single-dose, 200mg, nevirapine at the onset of labor. The first study, involving the same 38 patients and design as the emtricitabine and tenofovir studies described above, predicted a median cord concentration at delivery of 1.35mg/L and a median fetal to maternal AUC ratio of 0.75 [41]. These values are consistent with the observed values reported for single-dose nevirapine in Table 1. A study of 113 patients predicted much lower transfer ratios of 0.11 to 0.25 despite having the same median time to delivery of around 5 hours [81]. This study, focused on neonatal dosing, provided these ratios without providing their actual observed or predicted cord concentrations for comparison.
No population pharmacokinetic studies calculated predictions for fetal to maternal AUCs of protease inhibitors. One population pharmacokinetic study modeled transplacental transfer of nelfinavir but provides predictions of inter-compartmental rate constants only; the fraction transferred was expressed only as observed, not predicted, values and hence included with the studies shown in Table 1 [60].
4. Ex Vivo Placental Perfusion Models
Ex vivo models of human placentas, immediately following delivery, have been utilized as another means of quantifying maternal-fetal transfer. Transplacental transfer results in the maternal-to-fetal direction are discussed below. Such results reflect the amount of the ARV transferred from the maternal side of the placenta to the fetal side relative to the transfer of antipyrine, a freely-diffusible molecule. These studies generally allowed recirculation of the ARV for up to 2 hours.
Earlier ex vivo models (1992-1998) measured maternal-to-fetal clearance indices for each ARV at low and high infused concentrations to reflect expected maternal trough and peak concentrations. Such indices were reported for abacavir [82], didanosine [83-85], lamivudine [86], stavudine [87], zidovudine [83], amprenavir [82], and ritonavir [88] and are summarized in Table 2. For most of the NRTIs the clearance indices did not appear to differ between the two concentration extremes whereas for the two protease inhibitors tested the transfer index was higher with higher plasma ARV concentrations. Two additional zidovudine models did not specifically calculate clearance indices. However, one of these models exposed the placenta to zidovudine over a longer period of time (14 hours) and showed that zidovudine equilibrated in the fetal compartment within 60 minutes of each of the three dosing exposures [89]. The other reported fetal and maternal concentrations at each time point in two placentas, without calculated AUCs; the ratios of each of these concentrations appeared to vary from 0.2 to 0.5 over 45 to 90 minutes [90].
Table 2. Maternal-to-fetal clearance indices from ex vivo placental perfusion models for antiretroviral agents infused at both expected trough and peak in vivo concentrations.
Low (trough) concentrations were 0.2 to 2 mcg/mL and high (peak) concentrations were 6-15 mcg/mL, except where otherwise specified.
| Antiretroviral Drug | Maternal-to-Fetal Clearance Index (Mean ± Standard Deviation) | |
|---|---|---|
| Low ARV Concentration | High ARV Concentration | |
| Abacavir [82] | 0.47 ± 0.19 | 0.50 ± 0.07 |
| Didanosine [83-85] | 0.14 ± 0.03 to 0.35 ± 0.1 | 0.15 ± 0.04 to 0.39 ± 0.16a |
| Lamivudine [86] | 0.23 ± 0.14 | 0.14 ± 0.06 |
| Stavudine [87] | 0.24 ± 0.07 | 0.24 ± 0.05 |
| Zidovudine [83] | 0.31 (n = 1) | 0.29 ± 0.04 |
| Amprenavir [82] | 0.14 ± 0.08 | 0.39 ± 0.09 |
| Ritonavir (with 10mcg/mL ZDV) [88] | 0.085 ± 0.05b | 0.30 ± 0.11b |
The index of 0.39 was from a study in which a high concentration, 118 μg/mL (500μM) didanosine was infused;however, this study used a low concentration of 0.24 μg/mL (1μM) with similar results [84].
For ritonavir the “low” plasma concentration was 20 mcg/mL and the “high” concentration was 100 mcg/mL. Concentrations of 1-2 mcg/mL were also attempted but yielded undetectable ritonavir on the fetal side.
ARV = antiretroviral drug, ZDV = zidovudine
Later ex vivo models often utilized a single perfusate concentration but additionally utilized these models to study influence of protein binding or transporters on placental perfusion in a manner not feasible in vivo. Two studies found a mean saquinavir maternal-to-fetal transfer index of 0.05 [91, 92]. One of these found that the amount crossing the placenta corresponded to the fraction not bound to serum albumin [91]. The other investigated two polymorphisms that affect P-glycoprotein expression, but this study had a small sample size (n = 15 across all genotypic permutations) and was unable to demonstrate an effect of genotype on transplacental transfer [92]. A third study found a mean clearance index of 0.04 ± 0.05 for saquinavir that increased to 0.14 ± 0.08 and 0.12 ± 0.04 with addition of P-glycoprotein inhibitors PSC833 and GG918 respectively [93]. These authors note that this indicates potential for increased teratogenicity when drugs that are P-glycoprotein substrates are administered with P-glycoprotein inhibitors during pregnancy [93]. A final saquinavir study found a mean maternal-to-fetal transfer index of 0.27 ± 0.1 in a control group, 0.23 ± 0.1 with MK-571 (an MRP1 transporter inhibitor), and 0.22 ± 0.2 with probenecid (an OAT transporter inhibitor) [94]. Based on these results the maternal-fetal transfer of saquinavir does not rely on MRP1 or OAT transporters. However, the authors also acknowledge that the results are questionable given that the transfer index for their control group is higher than the index of 0.05 in their previous study [92] despite using the same methods and experiencing similar rates of antipyrine transport [94].
One group published two reports of indinavir models; in the earlier study the mean ± standard deviation clearance index was 0.39 ± 0.09 (n=5) [95]. This is perhaps surprising given the poor transfer in vivo as shown in Table 1. However, these clearance indices may vary between placentas, as later models using the same methods reported indices of 0.25 ± 0.03 (n=7) and 0.34 ± 0.14 (n=5) for identically-treated subgroups [96]. In the subgroup with the initial mean clearance index of 0.25, upon addition of PSC833, a P-glycoprotein inhibitor, the clearance index significantly increased (p = 0.047) to 0.37 ± 0.14 [96]. In the subgroup with the initial mean clearance index of 0.34, addition of ritonavir did not significantly alter this index (p = 0.31) and resulted in a mean index of 0.39 ± 0.13 [96].
An ex vivo model of lopinavir transport revealed mean maternal-to-fetal clearance indices of 0.68 ± 0.12, 0.57 ± 0.07, and 0.10 ± 0.01 when infused with albumin concentrations of 2 g/L, 10 g/L, and 40 g/L respectively [97]. These results are consistent with the in vivo placental transport data in the presence of typical plasma albumin concentrations (e.g. 40 g/L). That is, at this higher, physiologic, albumin concentration the lopinavir is mostly bound and unable to cross the placenta as freely.
A model of nelfinavir transport revealed a mean clearance index of 0.085 ± 0.05 for maternal perfusate nelfinavir concentrations of less than 500 mcg/L (n = 3) and a mean index of 0.39 ± 0.1 for the remaining 10 placentas, with perfusate concentrations ranging from 1256 to 4436 mcg/L [98]. These results are consistent with the poor placental perfusion in the presence of lower maternal concentrations in in vivo models, as discussed above.
An ex vivo model of maraviroc transport had an overall mean maternal-to-fetal clearance index of 0.26 ± 0.07 [99]. No in vivo placental transfer studies have been published for comparison. This study found a significant inverse correlation between the clearance index and the gene expression levels of ABCC2, ABCC10, and ABCC11 transport proteins but not for the other ABC transport proteins tested, indicating that those 3 efflux transporters may hinder maternal-to-fetal transport of maraviroc [99].
Finally, enfuvirtide was studied in three ex vivo placentas with 18 serial samples each [100]. The mean concentration in the maternal perfusate was 12,400 mcg/L (2.5 times the goal maximum concentration in vivo of 5000 mcg/L) [100]. Consistent with in vivo data (Table 1), enfuvirtide was undetectable (< 50mcg/L) in all 54 fetal venous samples [100].
5. Miscellaneous Studies
The remaining ARV studies focused on mechanisms of the transplacental transport rather than the extent to which transport occurs. While these are not technically pharmacokinetic studies they are pertinent to the interpretation of the pharmacokinetic data. Four such studies focused on zidovudine. One in vitro study investigated zidovudine uptake by syncytiotrophoblasts, finding that the uptake affinity was increased in the presence of dehydroepiandrosterone sulfate (DHEA-S) although the actual transporter involved was not identified [101]. The rest of the studies looked at consequences of the zidovudine uptake. One found that trophoblast cells exposed to zidovudine did not convert it to the toxic catabolite 3′-amino-3′-deoxythymidine (AMT), and hence this was unlikely to be a mechanism of toxicity to the fetus [102]. A study of incorporation of zidovudine into cord blood leukocyte DNA discovered such incorporation in 70% of samples, although interindividual variability was high in the extent of this incorporation that did not appear correlated with the length of zidovudine treatment [103]. A karyotypic study of exposed cord blood cells was consistent with this finding of zidovudine-induced mutagenicity, finding a higher proportion (p < 0.001) of aneuploid cells in the exposed group (median 18.8%) versus controls (median 6.6%); these alterations were randomly distributed across all chromosomes [104]. However, the clinical ramifications of these alterations are unknown.
One study suggested a significantly higher, 3.3-fold (p < 0.0009) mean increase in the expression of the MDR1 gene in the placentas of HIV-infected versus uninfected women [105]. This gene encodes P-glycoprotein, which can result in efflux transport of many protease inhibitors [105]. The increased expression did not appear to be confounded by protease inhibitor administration to the HIV-infected group, as a similar extent of increased expression was observed whether the women were treated with zidovudine alone or in combination with nelfinavir and lamivudine [105]. This finding may account in part for difficulty in transplacental transport of protease inhibitors in HIV-infected women.
An in vitro study of the ATP-binding cassette (ABC) efflux transporters revealed that the prodrug tenofovir disoproxil fumarate was actively effluxed from trophoblast cells by ABCB1 and ABCG2 transporters but not by ABCC2 [106]. However, tenofovir itself was not effluxed by these transporters [106].
Two protein binding studies were conducted involving protease inhibitors, which as a class are highly-bound to AAG. Both collected cord blood and maternal plasma from HIV negative women, subsequently spiking these samples with a protease inhibitor. The first study found the unbound fraction of both indinavir and saquinavir (p ≤ 0.001) to be significantly higher in cord samples than in maternal samples [107]. Accordingly, the cord samples were found to have significantly lower levels of AAG compared to maternal samples (p < 0.05) [107]. A similar study of lopinavir found that the unbound fraction actually did not differ significantly between cord and maternal samples; this was attributed to saturable binding at higher concentrations of lopinavir [108]. However, presence of ritonavir did not appear to interfere with the fraction of lopinavir that was protein-bound [108]. The measured fractions unbound are consistent with those discussed above from studies of total and free lopinavir concentrations in cord and maternal plasma of HIV-infected women who were chronically administered lopinavir in vivo [51, 52].
Discussion
The published pharmacokinetic data for maternal-fetal transfer of ARVs still leave many clinical questions unanswered. Vertical transmission outcomes cannot feasibly or ethically be studied in fetuses exposed to ARVs in utero in absence of maternal ARV exposure or vice versa. Hence, clinical outcomes due to transplacental transfer of ARVs cannot be rigorously studied separately from maternal administration of ARVs.
As discussed in the introduction, vertical transmission from mothers with low or undetectable viral loads, although rare, can occur [2, 3]. This provides reasonable evidence for administering ARVs that are transferred across the placenta as a form of prophylaxis for the fetus. This also raises the question of what cord to maternal ratio is needed to be considered adequate placental transfer. Alternatively, perhaps the ratio is not important but rather the absolute concentration of the ARV in the fetal circulation is the key factor. While most of the studies to date have measured ARV concentrations at the time of term or near-term delivery, the fetal concentrations earlier in pregnancy are unknown. Finally, studies have been unable to assess how many drugs that cross the placenta are necessary for fetal prophylaxis – for instance, in a patient on a three-drug regimen, must all three cross the placenta or would only one or two drugs crossing the placenta be sufficient?
Of the various methods for determining the extent of placental transfer, paired cord to maternal ratios is most common and can conveniently be completed in the context of studies of third trimester pharmacokinetics. However, this ratio only provides evidence of exposure at one point in time and can vary greatly depending on timing of the most recent maternal dose relative to time of delivery, missed doses at the time of delivery, and timing of the maternal plasma sample. Ex vivo perfusion models allow for strict control of the drug concentration to which the system is exposed as well as duration of this exposure and appear to yield similar results to those found in vivo. As new drugs enter the market, this method is unlikely to replace the in vivo methods since studies during pregnancy itself are necessary for determining the pharmacokinetics in that population; once such a study is underway it is fairly simple to then obtain the paired maternal and cord samples at delivery in these same patients. However, the ex vivo models have the advantage that they could be used for new drugs before any data for safety in pregnancy are available. For instance if a new drug is thought to have similar clinical efficacy to other drugs already on the market and the ex vivo model shows limited placental penetration perhaps researchers could decide to not pursue studying this drug in pregnancy and focus their efforts on drugs with higher placental transfer. In the future, perhaps placentas from pregnancy terminations could be used as a unique opportunity to study placental transfer of antiretrovirals from earlier in pregnancy. This could provide more information on second trimester placental transfer than can be achieved from in vivo studies.
In determining target ARV concentrations for the cord blood, comparison with each ARV's IC50 value for the wild-type HIV virus is a reasonable starting point. Data for these values from published studies [109, 110, 111], but converted to units of mg/L, are shown in Table 3. In comparing average cord ARV concentrations from Table 1 with the IC50 values in Table 3, all of the ranges of average cord concentrations for NRTIs except for zidovudine included values less than the IC50. The cord concentrations for nevirapine and efavirenz were more than 100 times greater than the IC50 and the cord concentration for raltegravir was fifty times the IC50. The cord concentrations for lopinavir and atazanavir were greater than the IC50, whereas the cord concentrations of nelfinavir, indinavir, and saquinavir were frequently undetectable and hence well below the IC50. This approach has clear drawbacks as achieving a concentration equal to the IC50 is only effectively suppressing half of the viral replication. Further, the cord concentrations measured in studies may be artificially low depending on time elapsed since the patient's last medication dose, which may often be unintentionally omitted during labor. The extent to which the target concentration should be increased above the IC50 is unclear. Further, fetuses may not require the same ARV concentrations as HIV-positive adult patients because 1) intent of ARV exposure in the fetus is prophylactic, 2) fetal circulation contains decreased levels of AAG so lower total concentrations of protein-bound drugs (e.g. protease inhibitors) are required to achieve similar free concentrations, and 3) fetuses at various times throughout pregnancy can be expected to have vastly different pharmacokinetic parameters from adults and even from neonates.
Table 3. IC50 values for wild-type HIV for select antiretroviral agents [109, 110, 111].
Converted from original molar concentrations to mg/L for ease of comparison with cord data.
| Antiretroviral Drug | IC50 (mg/L) [109]* | |
|---|---|---|
| Zidovudine | 0.0053 | |
| Lamivudine | 0.55 | |
| Didanosine | 1.18 | |
| Stavudine | 0.11 | |
| Abacavir | 0.46 | |
| Tenofovir | 0.20 | |
| Nevirapine | 0.024 | |
| Efavirenz | 0.00051 | |
| Indinavir | 0.0037 | |
| Nelfinavir | 0.0032 | |
| Saquinavir | 0.0017 | |
| Lopinavir | 0.0019 | |
| Atazanavir [110] | No AAG: | 0.0049 |
| With AAG: | 0.047 | |
| Raltegravir [111] | 0.0036 | |
From this reference except where otherwise specified.
AAG = α-1-acid glycoprotein, a drug-binding protein
Limitations to this systematic review include the possibility of incomplete retrieval and inclusion of all identified research in these areas. These types of studies are observational and descriptive, often with small sample sizes, which could lead to challenges in obtaining acceptance for publication in biomedical journals. The descriptive results themselves, however, should not lead to reporting bias in either direction.
Overall, both the in vivo studies and ex vivo placental models mostly demonstrate a moderate (> 0.5) extent of maternal-fetal transfer for both the NRTIs and NNRTIs, although data are sparse for NNRTIs besides nevirapine. However, the maternal-to-fetal transfer is less for the protease inhibitors. It appears highest for ritonavir-boosted atazanavir and lopinavir based on many studies, as well as possibly for darunavir and tipranavir based on limited case reports. Placental transfer of indinavir and saquinavir is extremely limited (< 0.2) and transfer of nelfinavir is often extremely limited, though in many cases nelfinavir is not even entering the maternal plasma at a predictable level. Decreased levels of AAG in the fetal circulation relative to the maternal circulation may account for the lessened transfer of protease inhibitors that are highly protein-bound. Raltegravir provides substantial transfer and while it is not currently recommended as first-line in pregnancy due to limited data, perhaps it may be recommended in combination with two NRTIs in the future. Enfuvirtide appears to have absolutely no maternal-to-fetal transfer. Consistent with national guidelines, recommended treatment with two NRTIs plus an NNRTI or ritonavir-boosted atazanavir or lopinavir should provide consistent ARV transplacental transfer. The extent of transfer of these recommended agents across the placenta at term varies by individual ARV administered, and each is expected to contribute to fetal prophylaxis against wild-type HIV1 infection.
Key Points.
Nucleoside reverse transcriptase inhibitors and non-nucleotide reverse transcriptase inhibitors have reliable maternal-to-fetal transfer across in vivo studies and ex vivo human placental studies.
Protease inhibitors, except for atazanavir/ritonavir and lopinavir/ritonavir, appear to have inconsistent maternal-to-fetal transfer.
The maternal-to-fetal transfer of protease inhibitors may be affected by differences in drug-binding proteins between the maternal and fetal circulations.
Acknowledgments
Author S. A. McCormack was supported by the National Institutes of Health, Grant TL1TR00098.
References
- 1.U.S. Department of Health and Human Services & NIH; 2014. [Accessed 10 April 2014]. Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/perinatalgl.pdf. [Google Scholar]
- 2.Tubiana R, Le Chenadec J, Rouzioux C, Mandelbrot L, Hamrene K, Dollfus C, et al. Factors associated with mother-to-child transmission of HIV-1 despite a maternal viral load <500 copies/ml at delivery: a case-control study nested in the French perinatal cohort (EPF-ANRS CO1) Clin Infect Dis. 2010;50(4):585–96. doi: 10.1086/650005. [DOI] [PubMed] [Google Scholar]
- 3.European Collaborative S. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40(3):458–65. doi: 10.1086/427287. [DOI] [PubMed] [Google Scholar]
- 4.Launay O, Tod M, Tschope I, Si-Mohamed A, Belarbi L, Charpentier C, et al. Residual HIV-1 RNA and HIV-1 DNA production in the genital tract reservoir of women treated with HAART: the prospective ANRS EP24 GYNODYN study. Antivir Ther. 2011;16(6):843–52. doi: 10.3851/IMP1856. [DOI] [PubMed] [Google Scholar]
- 5.Carter AM. Animal models of human placentation--a review. Placenta. 2007;28(Suppl A):S41–7. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Best BM, Mirochnick M, Capparelli EV, Stek A, Burchett SK, Holland DT, et al. Impact of pregnancy on abacavir pharmacokinetics. AIDS. 2006;20(4):553–60. doi: 10.1097/01.aids.0000210609.52836.d1. [DOI] [PubMed] [Google Scholar]
- 7.Chappuy H, Treluyer JM, Jullien V, Dimet J, Rey E, Fouche M, et al. Maternal-fetal transfer and amniotic fluid accumulation of nucleoside analogue reverse transcriptase inhibitors in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother. 2004;48(11):4332–6. doi: 10.1128/AAC.48.11.4332-4336.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moodley D, Pillay K, Naidoo K, Moodley J, Johnson MA, Moore KH, et al. Pharmacokinetics of zidovudine and lamivudine in neonates following coadministration of oral doses every 12 hours. J Clin Pharmacol. 2001;41(7):732–41. doi: 10.1177/00912700122010636. [DOI] [PubMed] [Google Scholar]
- 9.Mandelbrot L, Peytavin G, Firtion G, Farinotti R. Maternal-fetal transfer and amniotic fluid accumulation of lamivudine in human immunodeficiency virus-infected pregnant women. Am J Obstet Gynecol. 2001;184(2):153–8. doi: 10.1067/mob.2001.108344. [DOI] [PubMed] [Google Scholar]
- 10.Yeh RF, Rezk NL, Kashuba AD, Dumond JB, Tappouni HL, Tien HC, et al. Genital tract, cord blood, and amniotic fluid exposures of seven antiretroviral drugs during and after pregnancy in human immunodeficiency virus type 1-infected women. Antimicrob Agents Chemother. 2009;53(6):2367–74. doi: 10.1128/AAC.01523-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benaboud S, Treluyer JM, Urien S, Blanche S, Bouazza N, Chappuy H, et al. Pregnancy-related effects on lamivudine pharmacokinetics in a population study with 228 women. Antimicrob Agents Chemother. 2012;56(2):776–82. doi: 10.1128/AAC.00370-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wade NA, Unadkat JD, Huang S, Shapiro DE, Mathias A, Yasin S, et al. Pharmacokinetics and safety of stavudine in HIV-infected pregnant women and their infants: Pediatric AIDS Clinical Trials Group protocol 332. J Infect Dis. 2004;190(12):2167–74. doi: 10.1086/425903. [DOI] [PubMed] [Google Scholar]
- 13.Stek AM, Best BM, Luo W, Capparelli E, Burchett S, Hu C, et al. Effect of pregnancy on emtricitabine pharmacokinetics. HIV Med. 2012;13(4):226–35. doi: 10.1111/j.1468-1293.2011.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colbers AP, Hawkins DA, Gingelmaier A, Kabeya K, Rockstroh JK, Wyen C, et al. The pharmacokinetics, safety and efficacy of tenofovir and emtricitabine in HIV-1-infected pregnant women. AIDS. 2013;27(5):739–48. doi: 10.1097/QAD.0b013e32835c208b. [DOI] [PubMed] [Google Scholar]
- 15.Calcagno A, Trentini L, Marinaro L, Montrucchio C, D'Avolio A, Ghisetti V, et al. Transplacental passage of etravirine and maraviroc in a multidrug-experienced HIV-infected woman failing on darunavir-based HAART in late pregnancy. J Antimicrob Chemother. 2013;68(8):1938–9. doi: 10.1093/jac/dkt095. [DOI] [PubMed] [Google Scholar]
- 16.Mirochnick M, Taha T, Kreitchmann R, Nielsen-Saines K, Kumwenda N, Joao E, et al. Pharmacokinetics and safety of tenofovir in HIV-infected women during labor and their infants during the first week of life. J Acquir Immune Defic Syndr. 2014;65(1):33–41. doi: 10.1097/QAI.0b013e3182a921eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beigi R, Noguchi L, Parsons T, Macio I, Kunjara Na Ayudhya RP, Chen J, et al. Pharmacokinetics and placental transfer of single-dose tenofovir 1% vaginal gel in term pregnancy. J Infect Dis. 2011;204(10):1527–31. doi: 10.1093/infdis/jir562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watts DH, Brown ZA, Tartaglione T, Burchett SK, Opheim K, Coombs R, et al. Pharmacokinetic disposition of zidovudine during pregnancy. J Infect Dis. 1991;163(2):226–32. doi: 10.1093/infdis/163.2.226. [DOI] [PubMed] [Google Scholar]
- 19.Sperling RS, Roboz J, Dische R, Silides D, Holzman I, Jew E. Zidovudine pharmacokinetics during pregnancy. Am J Perinatol. 1992;9(4):247–9. doi: 10.1055/s-2007-994781. [DOI] [PubMed] [Google Scholar]
- 20.O'Sullivan MJ, Boyer PJ, Scott GB, Parks WP, Weller S, Blum MR, et al. The pharmacokinetics and safety of zidovudine in the third trimester of pregnancy for women infected with human immunodeficiency virus and their infants: phase I acquired immunodeficiency syndrome clinical trials group study (protocol 082). Zidovudine Collaborative Working Group. Am J Obstet Gynecol. 1993;168(5):1510–6. doi: 10.1016/s0002-9378(11)90791-1. [DOI] [PubMed] [Google Scholar]
- 21.Moodley J, Moodley D, Pillay K, Coovadia H, Saba J, van Leeuwen R, et al. Pharmacokinetics and antiretroviral activity of lamivudine alone or when coadministered with zidovudine in human immunodeficiency virus type 1-infected pregnant women and their offspring. J Infect Dis. 1998;178(5):1327–33. doi: 10.1086/314431. [DOI] [PubMed] [Google Scholar]
- 22.Rodman JH, Flynn PM, Robbins B, Jimenez E, Bardeguez AD, Rodriguez JF, et al. Systemic pharmacokinetics and cellular pharmacology of zidovudine in human immunodeficiency virus type 1-infected women and newborn infants. J Infect Dis. 1999;180(6):1844–50. doi: 10.1086/315152. [DOI] [PubMed] [Google Scholar]
- 23.Bhadrakom C, Simonds RJ, Mei JV, Asavapiriyanont S, Sangtaweesin V, Vanprapar N, et al. Oral zidovudine during labor to prevent perinatal HIV transmission, Bangkok: tolerance and zidovudine concentration in cord blood. Bangkok Collaborative Perinatal HIV Transmission Study Group. AIDS. 2000;14(5):509–16. doi: 10.1097/00002030-200003310-00006. [DOI] [PubMed] [Google Scholar]
- 24.Mirochnick M, Rodman JH, Robbins BL, Fridland A, Gandia J, Hitti J, et al. Pharmacokinetics of oral zidovudine administered during labour: a preliminary study. HIV Med. 2007;8(7):451–6. doi: 10.1111/j.1468-1293.2007.00495.x. [DOI] [PubMed] [Google Scholar]
- 25.Siu SS, Yeung JH, Pang MW, Chiu PY, Lau TK. Placental transfer of Zidovudine in first trimester of pregnancy. Obstet Gynecol. 2005;106(4):824–7. doi: 10.1097/01.AOG.0000178160.38042.04. [DOI] [PubMed] [Google Scholar]
- 26.Gillet JY, Bongain A, Abrar D, Garraffo R, Lapalus P. Preliminary study on the transport of AZT (Retrovir-zidovudine) through the placenta. J Gynecol Obstet Biol Reprod (Paris) 1990;19(2):177–80. [PubMed] [Google Scholar]
- 27.Pons JC, Taburet AM, Singlas E, Delfraissy JF, Papiernik E. Placental passage of azathiothymidine (AZT) during the second trimester of pregnancy: study by direct fetal blood sampling under ultrasound. Eur J Obstet Gynecol Reprod Biol. 1991;40(3):229–31. doi: 10.1016/0028-2243(91)90122-2. [DOI] [PubMed] [Google Scholar]
- 28.Cressey TR, Stek A, Capparelli E, Bowonwatanuwong C, Prommas S, Sirivatanapa P, et al. Efavirenz pharmacokinetics during the third trimester of pregnancy and postpartum. J Acquir Immune Defic Syndr. 2012;59(3):245–52. doi: 10.1097/QAI.0b013e31823ff052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gandhi M, Mwesigwa J, Aweeka F, Plenty A, Charlebois E, Ruel TD, et al. Hair and plasma data show that lopinavir, ritonavir, and efavirenz all transfer from mother to infant in utero, but only efavirenz transfers via breastfeeding. J Acquir Immune Defic Syndr. 2013;63(5):578–84. doi: 10.1097/QAI.0b013e31829c48ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izurieta P, Kakuda TN, Feys C, Witek J. Safety and pharmacokinetics of etravirine in pregnant HIV-1-infected women. HIV Med. 2011;12(4):257–8. doi: 10.1111/j.1468-1293.2010.00874.x. [DOI] [PubMed] [Google Scholar]
- 31.Furco A, Gosrani B, Nicholas S, Williams A, Braithwaite W, Pozniak A, et al. Successful use of darunavir, etravirine, enfuvirtide and tenofovir/emtricitabine in pregnant woman with multiclass HIV resistance. AIDS. 2009;23(3):434–5. doi: 10.1097/QAD.0b013e32832027d6. [DOI] [PubMed] [Google Scholar]
- 32.Marzolini C, Rudin C, Decosterd LA, Telenti A, Schreyer A, Biollaz J, et al. Transplacental passage of protease inhibitors at delivery. AIDS. 2002;16(6):889–93. doi: 10.1097/00002030-200204120-00008. [DOI] [PubMed] [Google Scholar]
- 33.Gingelmaier A, Kurowski M, Kastner R, Eberle J, Mylonas I, Belohradsky BH, et al. Placental transfer and pharmacokinetics of lopinavir and other protease inhibitors in combination with nevirapine at delivery. AIDS. 2006;20(13):1737–43. doi: 10.1097/01.aids.0000242820.67001.2c. [DOI] [PubMed] [Google Scholar]
- 34.Capparelli EV, Aweeka F, Hitti J, Stek A, Hu C, Burchett SK, et al. Chronic administration of nevirapine during pregnancy: impact of pregnancy on pharmacokinetics. HIV Med. 2008;9(4):214–20. doi: 10.1111/j.1468-1293.2008.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanovic J, Nicastri E, Anceschi MM, Ascenzi P, Signore F, Pisani G, et al. Transplacental transfer of antiretroviral drugs and newborn birth weight in HIV-infected pregnant women. Curr HIV Res. 2009;7(6):620–5. doi: 10.2174/157016209789973628. [DOI] [PubMed] [Google Scholar]
- 36.van Hoog S, Boer K, Nellen J, Scherpbier H, Godfried MH. Transplacental passage of nevirapine, nelfinavir and lopinavir. Neth J Med. 2012;70(2):102–3. [PubMed] [Google Scholar]
- 37.Taylor GP, Lyall EGH, Back D, Ward C, Tudor-Williams G. Pharmacological implications of lengthened in-utero exposure to nevirapine. Lancet. 2000;355(9221):2134–5. doi: 10.1016/s0140-6736(00)02383-7. [DOI] [PubMed] [Google Scholar]
- 38.Mirochnick M, Fenton T, Gagnier P, Pav J, Gwynne M, Siminski S, et al. Pharmacokinetics of nevirapine in human immunodeficiency virus type 1-infected pregnant women and their neonates. Pediatric AIDS Clinical Trials Group Protocol 250 Team. J Infect Dis. 1998;178(2):368–74. doi: 10.1086/515641. [DOI] [PubMed] [Google Scholar]
- 39.Musoke P, Guay LA, Bagenda D, Mirochnick M, Nakabiito C, Fleming T, et al. A phase I/II study of the safety and pharmacokinetics of nevirapine in HIV-1-infected pregnant Ugandan women and their neonates (HIVNET 006) AIDS. 1999;13(4):479–86. doi: 10.1097/00002030-199903110-00006. [DOI] [PubMed] [Google Scholar]
- 40.Mirochnick M, Dorenbaum A, Blanchard S, Cunningham CK, Gelber RD, Mofenson L, et al. Predose infant nevirapine concentration with the two-dose intrapartum neonatal nevirapine regimen: association with timing of maternal intrapartum nevirapine dose. J Acquir Immune Defic Syndr. 2003;33(2):153–6. doi: 10.1097/00126334-200306010-00006. [DOI] [PubMed] [Google Scholar]
- 41.Benaboud S, Ekouevi DK, Urien S, Rey E, Arrive E, Blanche S, et al. Population pharmacokinetics of nevirapine in HIV-1-infected pregnant women and their neonates. Antimicrob Agents Chemother. 2011;55(1):331–7. doi: 10.1128/AAC.00631-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colbers A, Gingelmaier A, van de Ende M, Rijnders B, Burger D. Pharmacokinetics, safety and transplacental passage of rilpivirine in pregnancy: two cases. AIDS. 2014;28(2):288–90. doi: 10.1097/QAD.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 43.Ripamonti D, Cattaneo D, Maggiolo F, Airoldi M, Frigerio L, Bertuletti P, et al. Atazanavir plus low-dose ritonavir in pregnancy: pharmacokinetics and placental transfer. AIDS. 2007;21(18):2409–15. doi: 10.1097/QAD.0b013e32825a69d1. [DOI] [PubMed] [Google Scholar]
- 44.Mirochnick M, Best BM, Stek AM, Capparelli EV, Hu C, Burchett SK, et al. Atazanavir pharmacokinetics with and without tenofovir during pregnancy. J Acquir Immune Defic Syndr. 2011;56(5):412–9. doi: 10.1097/QAI.0b013e31820fd093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandelbrot L, Mazy F, Floch-Tudal C, Meier F, Azria E, Crenn-Hebert C, et al. Atazanavir in pregnancy: impact on neonatal hyperbilirubinemia. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):18–21. doi: 10.1016/j.ejogrb.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Conradie F, Zorrilla C, Josipovic D, Botes M, Osiyemi O, Vandeloise E, et al. Safety and exposure of once-daily ritonavir-boosted atazanavir in HIV-infected pregnant women. HIV Med. 2011;12(9):570–9. doi: 10.1111/j.1468-1293.2011.00927.x. [DOI] [PubMed] [Google Scholar]
- 47.Kreitchmann R, Best BM, Wang J, Stek A, Caparelli E, Watts DH, et al. Pharmacokinetics of an increased atazanavir dose with and without tenofovir during the third trimester of pregnancy. J Acquir Immune Defic Syndr. 2013;63(1):59–66. doi: 10.1097/QAI.0b013e318289b4d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lechelt M, Lyons F, Clarke A, Magaya V, Issa R, de Ruiter A. Human placental transfer of atazanavir: a case report. AIDS. 2006;20(2):307. doi: 10.1097/01.aids.0000202653.49020.dd. [DOI] [PubMed] [Google Scholar]
- 49.Stek AM, Mirochnick M, Capparelli E, Best BM, Hu C, Burchett SK, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20(15):1931–9. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 50.Croci L, Trezzi M, Allegri MP, Carli T, Chigiotti S, Riccardi MP, et al. Pharmacokinetic and safety of raltegravir in pregnancy. Eur J Clin Pharmacol. 2012;68(8):1231–2. doi: 10.1007/s00228-012-1250-5. [DOI] [PubMed] [Google Scholar]
- 51.Else LJ, Douglas M, Dickinson L, Back DJ, Khoo SH, Taylor GP. Improved oral bioavailability of lopinavir in melt-extruded tablet formulation reduces impact of third trimester on lopinavir plasma concentrations. Antimicrob Agents Chemother. 2012;56(2):816–24. doi: 10.1128/AAC.05186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fayet-Mello A, Buclin T, Guignard N, Cruchon S, Cavassini M, Grawe C, et al. Free and total plasma levels of lopinavir during pregnancy, at delivery and postpartum: implications for dosage adjustments in pregnant women. Antivir Ther. 2013;18(2):171–82. doi: 10.3851/IMP2328. [DOI] [PubMed] [Google Scholar]
- 53.Santini-Oliveira M, Estrela Rde C, Veloso VG, Cattani VB, Yanavich C, Velasque L, et al. Randomized clinical trial comparing the pharmacokinetics of standard- and increased-dosage lopinavir-ritonavir coformulation tablets in HIV-positive pregnant women. Antimicrob Agents Chemother. 2014;58(5):2884–93. doi: 10.1128/AAC.02599-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mirochnick M, Best BM, Stek AM, Capparelli E, Hu C, Burchett SK, et al. Lopinavir exposure with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2008;49(5):485–91. doi: 10.1097/QAI.0b013e318186edd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Best BM, Stek AM, Mirochnick M, Hu C, Li H, Burchett SK, et al. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2010;54(4):381–8. doi: 10.1097/qai.0b013e3181d6c9ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mirochnick M, Dorenbaum A, Holland D, Cunningham-Schrader B, Cunningham C, Gelber R, et al. Concentrations of protease inhibitors in cord blood after in utero exposure. Pediatr Infect Dis J. 2002;21(9):835–8. doi: 10.1097/01.inf.0000027591.04920.c7. [DOI] [PubMed] [Google Scholar]
- 57.Chappuy H, Treluyer JM, Rey E, Dimet J, Fouche M, Firtion G, et al. Maternal-fetal transfer and amniotic fluid accumulation of protease inhibitors in pregnant women who are infected with human immunodeficiency virus. Am J Obstet Gynecol. 2004;191(2):558–62. doi: 10.1016/j.ajog.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 58.van Heeswijk RP, Khaliq Y, Gallicano KD, Bourbeau M, Seguin I, Phillips EJ, et al. The pharmacokinetics of nelfinavir and M8 during pregnancy and post partum. Clin Pharmacol Ther. 2004;76(6):588–97. doi: 10.1016/j.clpt.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 59.Villani P, Floridia M, Pirillo MF, Cusato M, Tamburrini E, Cavaliere AF, et al. Pharmacokinetics of nelfinavir in HIV-1-infected pregnant and nonpregnant women. Br J Clin Pharmacol. 2006;62(3):309–15. doi: 10.1111/j.1365-2125.2006.02669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirt D, Urien S, Jullien V, Firtion G, Chappuy H, Rey E, et al. Pharmacokinetic modelling of the placental transfer of nelfinavir and its M8 metabolite: a population study using 75 maternal-cord plasma samples. Br J Clin Pharmacol. 2007;64(5):634–44. doi: 10.1111/j.1365-2125.2007.02885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bryson YJ, Mirochnick M, Stek A, Mofenson LM, Connor J, Capparelli E, et al. Pharmacokinetics and safety of nelfinavir when used in combination with zidovudine and lamivudine in HIV-infected pregnant women: Pediatric AIDS Clinical Trials Group (PACTG) Protocol 353. HIV Clin Trials. 2008;9(2):115–25. doi: 10.1310/hct0902-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Read JS, Best BM, Stek AM, Hu C, Capparelli EV, Holland DT, et al. Pharmacokinetics of new 625 mg nelfinavir formulation during pregnancy and postpartum. HIV Med. 2008;9(10):875–82. doi: 10.1111/j.1468-1293.2008.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cressey TR, Best BM, Achalapong J, Stek A, Wang J, Chotivanich N, et al. Reduced indinavir exposure during pregnancy. Br J Clin Pharmacol. 2013;76(3):475–83. doi: 10.1111/bcp.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Acosta EP, Zorrilla C, Van Dyke R, Bardeguez A, Smith E, Hughes M, et al. Pharmacokinetics of saquinavir-SGC in HIV-infected pregnant women. HIV Clin Trials. 2001;2(6):460–5. doi: 10.1310/PUY3-5JWL-FX2B-98VU. [DOI] [PubMed] [Google Scholar]
- 65.Vithayasai V, Moyle GJ, Supajatura V, Wattanatchariya N, Kanshana S, Sirichthaporn P, et al. Safety and efficacy of saquinavir soft-gelatin capsules + zidovudine + optional lamivudine in pregnancy and prevention of vertical HIV transmission. J Acquir Immune Defic Syndr. 2002;30(4):410–2. doi: 10.1097/00042560-200208010-00006. [DOI] [PubMed] [Google Scholar]
- 66.Acosta EP, Bardeguez A, Zorrilla CD, Van Dyke R, Hughes MD, Huang S, et al. Pharmacokinetics of saquinavir plus low-dose ritonavir in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother. 2004;48(2):430–6. doi: 10.1128/AAC.48.2.430-436.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ripamonti D, Cattaneo D, Cortinovis M, Maggiolo F, Suter F. Transplacental passage of ritonavir-boosted darunavir in two pregnant women. Int J STD AIDS. 2009;20(3):215–6. doi: 10.1258/ijsa.2008.008515. [DOI] [PubMed] [Google Scholar]
- 68.Ivanovic J, Bellagamba R, Nicastri E, Signore F, Vallone C, Tempestilli M, et al. Use of darunavir/ritonavir once daily in treatment-naive pregnant woman: pharmacokinetics, compartmental exposure, efficacy and safety. AIDS. 2010;24(7):1083–4. doi: 10.1097/QAD.0b013e32833653b2. [DOI] [PubMed] [Google Scholar]
- 69.Pinnetti C, Tamburrini E, Ragazzoni E, De Luca A, Navarra P. Decreased plasma levels of darunavir/ritonavir in a vertically infected pregnant woman carrying multiclass-resistant HIV type-1. Antivir Ther. 2010;15(1):127–9. doi: 10.3851/IMP1473. [DOI] [PubMed] [Google Scholar]
- 70.Zorrilla CD, Wright R, Osiyemi OO, Yasin S, Baugh B, Brown K, et al. Total and unbound darunavir pharmacokinetics in pregnant women infected with HIV-1: results of a study of darunavir/ritonavir 600/100 mg administered twice daily. HIV Med. 2014;15(1):50–6. doi: 10.1111/hiv.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cespedes MS, Castor D, Ford SL, Lee D, Lou Y, Pakes GE, et al. Steady-state pharmacokinetics, cord blood concentrations, and safety of ritonavir-boosted fosamprenavir in pregnancy. J Acquir Immune Defic Syndr. 2013;62(5):550–4. doi: 10.1097/QAI.0b013e318285d918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weizsaecker K, Kurowski M, Hoffmeister B, Schurmann D, Feiterna-Sperling C. Pharmacokinetic profile in late pregnancy and cord blood concentration of tipranavir and enfuvirtide. Int J STD AIDS. 2011;22(5):294–5. doi: 10.1258/ijsa.2009.009166. [DOI] [PubMed] [Google Scholar]
- 73.Brennan-Benson P, Pakianathan M, Rice P, Bonora S, Chakraborty R, Sharland M, et al. Enfurvitide prevents vertical transmission of multidrug-resistant HIV-1 in pregnancy but does not cross the placenta. AIDS. 2006;20(2):297–9. doi: 10.1097/01.aids.0000200535.02232.1b. [DOI] [PubMed] [Google Scholar]
- 74.Jeantils V, Alloui C, Rodrigues A, Bentata M, Peytavin G, Carbillon L. Use of enfurvitide in pregnancy in HIV positive women in seven cases. Gynecol Obstet Fertil. 2009;37(5):396–400. doi: 10.1016/j.gyobfe.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 75.McKeown DA, Rosenvinge M, Donaghy S, Sharland M, Holt DW, Cormack I, et al. High neonatal concentrations of raltegravir following transplacental transfer in HIV-1 positive pregnant women. AIDS. 2010;24(15):2416–8. doi: 10.1097/QAD.0b013e32833d8a50. [DOI] [PubMed] [Google Scholar]
- 76.Hegazi A, Mc Keown D, Doerholt K, Donaghy S, Sadiq ST, Hay P. Raltegravir in the prevention of mother-to-child transmission of HIV-1: effective transplacental transfer and delayed plasma clearance observed in preterm neonates. AIDS. 2012;26(18):2421–3. doi: 10.1097/QAD.0b013e32835a9aeb. [DOI] [PubMed] [Google Scholar]
- 77.Hirt D, Urien S, Rey E, Arrive E, Ekouevi DK, Coffie P, et al. Population pharmacokinetics of emtricitabine in human immunodeficiency virus type 1-infected pregnant women and their neonates. Antimicrob Agents Chemother. 2009;53(3):1067–73. doi: 10.1128/AAC.00860-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hirt D, Pruvost A, Ekouevi DK, Urien S, Arrive E, Kone M, et al. Very high concentrations of active intracellular phosphorylated emtricitabine in neonates (ANRS 12109 trial, step 2) Antimicrob Agents Chemother. 2011;55(6):2953–60. doi: 10.1128/AAC.01376-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hirt D, Urien S, Ekouevi DK, Rey E, Arrive E, Blanche S, et al. Population pharmacokinetics of tenofovir in HIV-1-infected pregnant women and their neonates (ANRS 12109) Clin Pharmacol Ther. 2009;85(2):182–9. doi: 10.1038/clpt.2008.201. [DOI] [PubMed] [Google Scholar]
- 80.Hirt D, Ekouevi DK, Pruvost A, Urien S, Arrive E, Blanche S, et al. Plasma and intracellular tenofovir pharmacokinetics in the neonate (ANRS 12109 trial, step 2) Antimicrob Agents Chemother. 2011;55(6):2961–7. doi: 10.1128/AAC.01377-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frank M, Harms G, Kunz A, Kloft C. Population pharmacokinetic analysis of a nevirapine-based HIV-1 prevention of mother-to-child transmission program in Uganda to assess the impact of different dosing regimens for newborns. J Clin Pharmacol. 2013;53(3):294–304. doi: 10.1177/0091270012448397. [DOI] [PubMed] [Google Scholar]
- 82.Bawdon RE. The ex vivo human placental transfer of the anti-HIV nucleoside inhibitor abacavir and the protease inhibitor amprenavir. Infect Dis Obstet Gynecol. 1998;6(6):244–6. doi: 10.1155/S1064744998000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bawdon RE, Sobhi S, Dax J. The transfer of anti-human immunodeficiency virus nucleoside compounds by the term human placenta. Am J Obstet Gynecol. 1992;167(6):1570–4. doi: 10.1016/0002-9378(92)91742-s. [DOI] [PubMed] [Google Scholar]
- 84.Dancis J, Lee JD, Mendoza S, Liebes L. Transfer and metabolism of dideoxyinosine by the perfused human placenta. J Acquir Immune Defic Syndr. 1993;6(1):2–6. [PubMed] [Google Scholar]
- 85.Henderson GI, Perez AB, Yang Y, Hamby RL, Schenken RS, Schenker S. Transfer of dideoxyinosine across the human isolated placenta. Br J Clin Pharmacol. 1994;38(3):237–42. doi: 10.1111/j.1365-2125.1994.tb04347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bloom SL, Dias KM, Bawdon RE, Gilstrap LC., 3rd The maternal-fetal transfer of lamivudine in the ex vivo human placenta. Am J Obstet Gynecol. 1997;176(2):291–3. doi: 10.1016/s0002-9378(97)70487-3. [DOI] [PubMed] [Google Scholar]
- 87.Bawdon RE, Kaul S, Sobhi S. The ex vivo transfer of the anti-HIV nucleoside compound d4T in the human placenta. Gynecol Obstet Invest. 1994;38(1):1–4. doi: 10.1159/000292432. [DOI] [PubMed] [Google Scholar]
- 88.Casey BM, Bawdon RE. Placental transfer of ritonavir with zidovudine in the ex vivo placental perfusion model. Am J Obstet Gynecol. 1998;179(3 Pt 1):758–61. doi: 10.1016/s0002-9378(98)70078-x. [DOI] [PubMed] [Google Scholar]
- 89.Boal JH, Plessinger MA, van den Reydt C, Miller RK. Pharmacokinetic and toxicity studies of AZT (zidovudine) following perfusion of human term placenta for 14 hours. Toxicol Appl Pharmacol. 1997;143(1):13–21. doi: 10.1006/taap.1996.8046. [DOI] [PubMed] [Google Scholar]
- 90.Olivero OA, Parikka R, Poirier MC, Vahakangas K. 3′-azido-3′-deoxythymidine (AZT) transplacental perfusion kinetics and DNA incorporation in normal human placentas perfused with AZT. Mutat Res. 1999;428(1-2):41–7. doi: 10.1016/s1383-5742(99)00030-7. [DOI] [PubMed] [Google Scholar]
- 91.Forestier F, de Renty P, Peytavin G, Dohin E, Farinotti R, Mandelbrot L. Maternal-fetal transfer of saquinavir studied in the ex vivo placental perfusion model. Am J Obstet Gynecol. 2001;185(1):178–81. doi: 10.1067/mob.2001.113319. [DOI] [PubMed] [Google Scholar]
- 92.Rahi M, Heikkinen T, Hakkola J, Hakala K, Wallerman O, Wadelius M, et al. Influence of adenosine triphosphate and ABCB1 (MDR1) genotype on the P-glycoprotein-dependent transfer of saquinavir in the dually perfused human placenta. Hum Exp Toxicol. 2008;27(1):65–71. doi: 10.1177/0960327108088971. [DOI] [PubMed] [Google Scholar]
- 93.Molsa M, Heikkinen T, Hakkola J, Hakala K, Wallerman O, Wadelius M, et al. Functional role of P-glycoprotein in the human blood-placental barrier. Clin Pharmacol Ther. 2005;78(2):123–31. doi: 10.1016/j.clpt.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 94.Rahi MM, Heikkinen TM, Hakala KE, Laine KP. The effect of probenecid and MK-571 on the feto-maternal transfer of saquinavir in dually perfused human term placenta. Eur J Pharm Sci. 2009;37(5):588–92. doi: 10.1016/j.ejps.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 95.Sudhakaran S, Ghabrial H, Nation RL, Kong DC, Gude NM, Angus PW, et al. Differential bidirectional transfer of indinavir in the isolated perfused human placenta. Antimicrob Agents Chemother. 2005;49(3):1023–8. doi: 10.1128/AAC.49.3.1023-1028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sudhakaran S, Rayner CR, Li J, Kong DC, Gude NM, Nation RL. Inhibition of placental P-glycoprotein: impact on indinavir transfer to the foetus. Br J Clin Pharmacol. 2008;65(5):667–73. doi: 10.1111/j.1365-2125.2007.03067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gavard L, Gil S, Peytavin G, Ceccaldi PF, Ferreira C, Farinotti R, et al. Placental transfer of lopinavir/ritonavir in the ex vivo human cotyledon perfusion model. Am J Obstet Gynecol. 2006;195(1):296–301. doi: 10.1016/j.ajog.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 98.Gavard L, Beghin D, Forestier F, Cayre Y, Peytavin G, Mandelbrot L, et al. Contribution and limit of the model of perfused cotyledon to the study of placental transfer of drugs. Example of a protease inhibitor of HIV: nelfinavir. Eur J Obstet Gynecol Reprod Biol. 2009;147(2):157–60. doi: 10.1016/j.ejogrb.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 99.Vinot C, Gavard L, Treluyer JM, Manceau S, Courbon E, Scherrmann JM, et al. Placental transfer of maraviroc in an ex vivo human cotyledon perfusion model and influence of ABC transporter expression. Antimicrob Agents Chemother. 2013;57(3):1415–20. doi: 10.1128/AAC.01821-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ceccaldi PF, Ferreira C, Gavard L, Gil S, Peytavin G, Mandelbrot L. Placental transfer of enfuvirtide in the ex vivo human placenta perfusion model. Am J Obstet Gynecol. 2008;198(4):433 e1–2. doi: 10.1016/j.ajog.2007.10.802. [DOI] [PubMed] [Google Scholar]
- 101.Nishimura T, Seki Y, Sato K, Chishu T, Kose N, Terasaki T, et al. Enhancement of zidovudine uptake by dehydroepiandrosterone sulfate in rat syncytiotrophoblast cell line TR-TBT 18d-1. Drug Metab Dispos. 2008;36(10):2080–5. doi: 10.1124/dmd.108.021345. [DOI] [PubMed] [Google Scholar]
- 102.Plessinger MA, Boal JH, Miller RK. Human placenta does not Reduce AZT (zidovudine) to 3′-amino-3′-deoxythymidine. Proc Soc Exp Biol Med. 1997;215(3):243–7. doi: 10.3181/00379727-215-44134. [DOI] [PubMed] [Google Scholar]
- 103.Olivero OA, Shearer GM, Chougnet CA, Kovacs AA, Baker R, Stek AM, et al. Incorporation of zidovudine into cord blood DNA of infants and peripheral blood DNA of their HIV-1-positive mothers. Ann N Y Acad Sci. 2000;918:262–8. doi: 10.1111/j.1749-6632.2000.tb05495.x. [DOI] [PubMed] [Google Scholar]
- 104.Andre-Schmutz I, Dal-Cortivo L, Six E, Kaltenbach S, Cocchiarella F, Le Chenadec J, et al. Genotoxic signature in cord blood cells of newborns exposed in utero to a Zidovudine-based antiretroviral combination. J Infect Dis. 2013;208(2):235–43. doi: 10.1093/infdis/jit149. [DOI] [PubMed] [Google Scholar]
- 105.Camus M, Delomenie C, Didier N, Faye A, Gil S, Dauge MC, et al. Increased expression of MDR1 mRNAs and P-glycoprotein in placentas from HIV-1 infected women. Placenta. 2006;27(6-7):699–706. doi: 10.1016/j.placenta.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 106.Neumanova Z, Cerveny L, Ceckova M, Staud F. Interactions of tenofovir and tenofovir disoproxil fumarate with drug efflux transporters ABCB1, ABCG2, and ABCC2; role in transport across the placenta. AIDS. 2014;28(1):9–17. doi: 10.1097/QAD.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 107.Sudhakaran S, Rayner CR, Li J, Kong DC, Gude NM, Nation RL. Differential protein binding of indinavir and saquinavir in matched maternal and umbilical cord plasma. Br J Clin Pharmacol. 2007;63(3):315–21. doi: 10.1111/j.1365-2125.2006.02766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gulati A, Boudinot FD, Gerk PM. Binding of lopinavir to human alpha1-acid glycoprotein and serum albumin. Drug Metab Dispos. 2009;37(8):1572–5. doi: 10.1124/dmd.109.026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Parkin NT, Hellmann NS, Whitcomb JM, Kiss L, Chappey C, Petropoulos CJ. Natural variation of drug susceptibility in wild-type human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2004;48(2):437–43. doi: 10.1128/AAC.48.2.437-443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Drusano GL, Bilello JA, Preston SL, O'Mara E, Kaul S, Schnittman S, et al. Hollow-fiber unit evaluation of a new human immunodeficiency virus type 1 protease inhibitor, BMS-232632, for determination of the linked pharmacodynamic variable. J Infect Dis. 2001;183(7):1126–9. doi: 10.1086/319281. [DOI] [PubMed] [Google Scholar]
- 111.Acosta EP, Limoli KL, Trinh L, Parkin NT, King JR, Weidler JM, et al. Novel method to assess antiretroviral target trough concentrataions using in vitro susceptibility data. Antimicrob Agents Chemother. 2012;56(11):5938–5945. doi: 10.1128/AAC.00691-12. [DOI] [PMC free article] [PubMed] [Google Scholar]