Abstract
Objective
Computation of a pre-articulatory phonological representation (phonological access, or phonological retrieval) is an essential process in speech production whose neural localization is not clear. This study combined a specific behavioral measure of phonological access and multivariate voxel-based lesion-symptom mapping (VLSM) in a series of left hemisphere stroke patients to identify brain regions critical for this process.
Methods
Phonological access was assessed in 40 chronic ischemic stroke patients using a silent rhyming task to avoid confounds with motor planning and articulation deficits. Additional covariates were incorporated in the VLSM analysis to control for orthographic and working memory demands of the rhyming task, and for age, education, and total lesion volume. The resulting t-statistic maps were thresholded at voxel-wise p < .001 and cluster-corrected at a family-wise error of p < .05.
Results
Phonological access impairment was correlated with damage to a focal region of cortex and white matter caudal to the posterior sylvian fissure, which included the posterior supramarginal gyrus and adjacent anterior angular gyrus, planum temporale, and posterior superior temporal gyrus. No correlation was observed with Broca’s area, insula, or sensorimotor cortex. An additional VLSM showed no correlation between damage in this posterior perisylvian region and spoken word comprehension.
Interpretation
This is the first demonstration of a specific lesion correlate for phonological access impairment. Although this posterior perisylvian region overlaps with some versions of the classical Wernicke area, the present results demonstrate its involvement in pre-articulatory phonological production rather than speech perception or lexical-semantic processes.
Keywords: Aphasia, VLSM, phonological access, phonology
Introduction
Spoken language production depends on the ability to retrieve from memory a sequence of speech sounds, or phonological form, for each word one wishes to say. This phonological representation must be accessed mentally before articulatory movements can be activated to overtly produce the word.1,2 As an illustration of this process, consider that it is possible to determine that the word trough rhymes with cough but not with dough. This determination can be made without overt articulation, and it cannot be the result of a purely visual analysis, since the three word endings are spelled identically. Wernicke referred to this pre-articulatory phonologic representation as the “word sound form” (Wortklangbilden),3 and modern theorists have used similar terms such as “phonological lexicon” or “phonological word form”.4–8 All speech production tasks, including repetition, reading aloud, naming, and natural conversation, require phonologic access. Impairment of this process typically manifests as errors in selecting or ordering phonemes during production, known as phonemic paraphasia. Wernicke proposed that the same auditory word image used during production is also used for word comprehension, though many modern theories posit separate phonological representations for input and output tasks.2,9–11
Various left perisylvian structures have been activated in fMRI studies of phonological access,12–16 yet the neural localization of this process is not entirely clear. While some results implicate the posterior superior temporal gyrus (STG) and adjacent supramarginal gyrus (SMG),11 others point to more inferior temporal cortex, particularly the posterior middle temporal gyrus (MTG),2 and still others implicate inferior frontal cortex.17,18
Localization data from patients with aphasia are also inconclusive. Attempts to localize phonological access have focused mainly on the lesion correlates of phonemic paraphasia, particularly in patients with conduction aphasia. A limitation in many of these studies is that they relied on simple lesion overlap methods, which lack controls for nonspecific overlap due to common vascular supply patterns.19 Due to its central location in the middle cerebral artery (MCA) territory, the perisylvian region is commonly damaged in left hemisphere ischemic stroke, thus a degree of non-specific overlap can be expected across patients. A second limitation is the reliance on overt production tasks to assess phonological access. Although the presence of phonemic paraphasia is a strong indication that phonological access is impaired, similar and sometimes indistinguishable errors can result from more distal articulatory disturbances, as in speech apraxia.20,21
We used voxel-based lesion symptom mapping (VLSM) to identify the lesion correlates of phonological access impairment across an unselected sample of left hemisphere ischemic stroke patients. To avoid confounds with motor planning and articulation deficits, phonological access was tested using a silent rhyme-matching task. Because performance on this task could be affected by letter recognition and general executive deficits, we incorporated covariates in the VLSM analysis to control for orthographic processing, working memory, age, education, and total lesion volume, thereby isolating a specific lesion correlate of phonological access impairment. Additional analyses incorporating measures of articulatory agility, oral reading, and spoken word comprehension were carried out to test alternative interpretations of this lesion-deficit correlation.
Materials and Methods
Participants
Participants were 40 chronic left hemisphere ischemic stroke patients (21 women, 19 men). Participants were included regardless of lesion location or behavioral profile. All participants were at least 180 days post-stroke, native English speakers, and premorbidly right-handed according to the Edinburgh Handedness Inventory handedness quotient (M = 89.8, SD = 19.4).22 Lesions included 34 MCA infarcts, 1 anterior cerebral artery (ACA) infarct, 2 combined MCA/ACA infarcts, 2 posterior cerebral artery (PCA) infarcts, and 1 combined MCA/PCA infarct. Other participant data are listed in Table 1 and Supplementary Table 1. The study was approved by the Medical College of Wisconsin Institutional Review Board and undertaken in accord with the Declaration of Helsinki. Patients were enrolled prospectively and provided informed consent to participate.
Table 1.
Demographic, clinical, and performance data.
|
Correlation with Rhyme Matching
|
|||||
|---|---|---|---|---|---|
| Variable | Mean | SD | Range | r | p |
| Age | 58.8 | 13.1 | 28.1 – 84.4 | −.21 | .19 |
| Education | 14.2 | 2.7 | 8 – 20 | .25 | .12 |
| Days Post Onset | 1032 | 1316 | 180 – 6732 | .12 | .45 |
| Lesion Size (ml) | 67.56 | 52.52 | 0.83 – 226.98 | −.53 | <.001 |
| Semantic Matching | 84.8 | 11.2 | 54.2 – 98.3 | .65 | <.001 |
| Auditory WPM | 92.0 | 13.5 | 42.5 – 100.0 | .55 | <.001 |
| Articulatory Agility | 4.8 | 1.6 | 1 – 7 | .45 | .004 |
| Oral Reading | 61.5 | 31.0 | 0 – 96.8 | .76 | <.001 |
Testing Procedure
All tasks were administered on a laptop computer connected to a touch-sensitive LCD monitor (ELO model 1522L). Tasks were programmed in the “Runtime Revolution” environment (www.runrev.com). Testing occurred in a quiet clinic environment. Participants wore a headphone set with a built-in microphone (Sennheiser model PC 166 USB). Participants initiated each trial by touching a green square on the computer screen. Manual and vocal responses were automatically recorded.
Rhyme Matching
To measure phonologic impairment independent of speech articulation, a 40-trial silent rhyme matching task was given (Fig 1, top). On each trial, a sample word (e.g., shoe) was presented in the center of the computer display with two choice items (e.g., crow and knew) presented side by side below the sample. Participants were asked to indicate, by touching the screen, which of the two choice items rhymed with the sample. An orthographic matching strategy was precluded by using either alternative spellings of the same rhyme, such as shoe and knew, or pairing target and foil items with alternative pronunciations of the same rime, such as south and youth.
Figure 1.
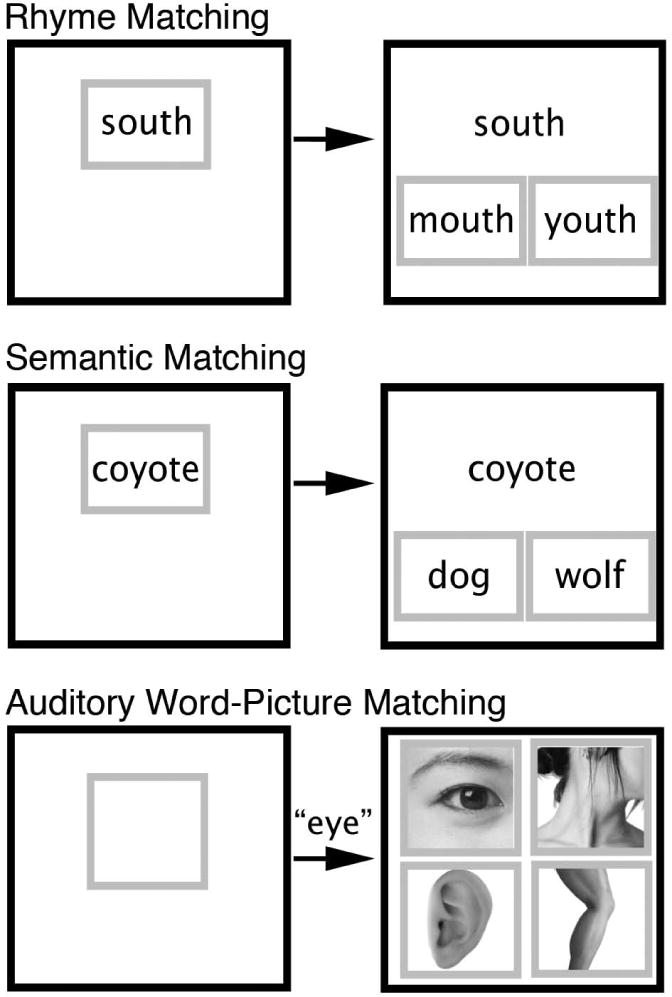
Example trials from the behavioral tasks (actual trials were in color). Participants see a prompt in a green box signaling the start of a trial (left column). Pressing the prompt causes the choices to appear, each surrounded by a green box (right column). This display remains on the screen until the participant selects one of the choices. The top row shows an example of the Rhyme Matching task. The middle row shows an example of the Semantic Matching task. The bottom row shows an example of the Auditory Word-Picture Matching (AWPM) task. In the AWPM task, pressing the prompt causes a spoke word sound file to be played simultaneously with the appearance of the picture choices.
Semantic Matching
A 120-trial semantic matching task (Fig 1, middle) was used as a control for orthographic, non-specific visual, attention, working memory, and motor response components of the Rhyme Matching task. This task used a 2-alternative choice format identical to the Rhyme Matching task, except that participants selected from two choices (e.g., lamb and swan) the item that is most similar in meaning to the sample word (e.g., goose). Half of the trials involved concrete concepts and half involved abstract concepts, such that overall word imageability was matched between the Rhyme Matching and Semantic Matching tests.
Articulatory Agility
Although the Rhyme Matching task does not require speech articulation, it is possible that phonological access automatically engages and is aided by internal motor representations.23 To assess the degree to which articulatory deficits account for phonological access deficits, a measure of articulatory agility was included as a covariate in a supplementary analysis. Participants were shown the Cookie Theft Picture from the Boston Diagnostic Aphasia Examination (BDAE) and asked to verbally describe the picture.24 Articulatory agility was rated according to the BDAE on a 1–7 scale, where 1 indicates complete inability to produce speech sounds, and 7 indicates normal agility (4 = sometimes clumsy and effortful). Ratings were made with regard only to speed and agility of articulation, ignoring word-finding pauses as well as phoneme selection and sequence errors. Four participants who were not administered the Cookie Theft Picture were assessed using overt responses made during clinical interactions and recorded during other tasks. Two independent raters scored articulatory agility across all patients, with an inter-rater reliability of r = .84. On patients for whom the two raters disagreed, an average was computed between the raters’ scores.
Oral Reading
Not all reading tasks require phonological access. The Semantic Matching task does not require phonological access because meaning can be accessed directly from the orthographic form. In contrast, phonological access is a process common to all speech production tasks, regardless of whether the phonological form is overtly articulated. As a further test of this model, a measure of reading aloud was included in a second supplementary analysis to demonstrate that the lesion correlate of phonological access impairment is not specific to “inner speech” tasks.25 Because reading aloud and silent rhyme matching depend on the same phonological access process, including the oral reading task as a covariate should remove any shared variance contributing to the VLSM results. Participants read aloud 188 words ranging in length from 4–6 letters. Vocal responses were recorded and scored off-line.
Auditory Word-Picture Matching
A third supplementary analysis was done to determine whether the areas correlated with phonological access impairment also supported speech comprehension. An 80-trial auditory word-to-picture matching (AWPM) task evaluated the mapping from input phonological word-forms to word meanings. Participants heard a digitally recorded object name and had to choose the correct picture from four semantically related choices (Fig 1, bottom).
Lesion Tracing
High-resolution T1-weighted MRI images were obtained in the chronic stage in all patients. MRI was performed at 3T in 38 patients and 1.5T in 2 patients. Voxel size was approximately 1×1×1 mm3 in all studies.
Lesioned areas were labeled using a semi-automated procedure, beginning with 6-way segmentation of the MRI volume using FSL’s Automatic Segmentation Tool (FMRIB Software Library, www.fmrib.ox.ac.uk/fsl).26 The purpose of the automated segmentation step was to identify tissue boundaries in the volume as objectively as possible. Boundaries between damaged and normal tissue are often indistinct, resulting in a degree of subjectivity in placing these boundaries manually. The segmentation step locates local 3-dimensional boundaries based on objective changes in image intensity. However, this process is not capable of distinguishing normal from damaged tissue because of the extreme heterogeneity of intensity values within lesions, which can contain cerebrospinal fluid, cystic tissue, ischemic remnants of grey or white matter, and residual iron products. This heterogeneity combined with the variation present in normal tissue produces considerable overlap between the overall intensity distributions of normal and lesioned tissue. Thus, any given segment produced by the algorithm typically contains a number of bounded volumes, some of which contain damaged tissue and others of which contain normal tissue. Each segment was therefore visually inspected and manually edited by an experienced stroke neurologist (JRB) to include only bounded volumes within the lesioned tissue. The six edited segments were then combined to make a complete lesion map (Fig 2). Each patient’s lesion map was then registered to a stereotaxic template (“Colin n27”) using a constrained cost-function masking approach in ANTS (Advanced Normalization Tools),27 with resampling to a nominal 1×1×1 mm3 voxel grid. This nonlinear registration process corrects for anatomical distortions, particularly ventricular enlargement. Normalized total lesion size (in template voxels) was obtained in each patient from the template-registered lesion map.
Figure 2.
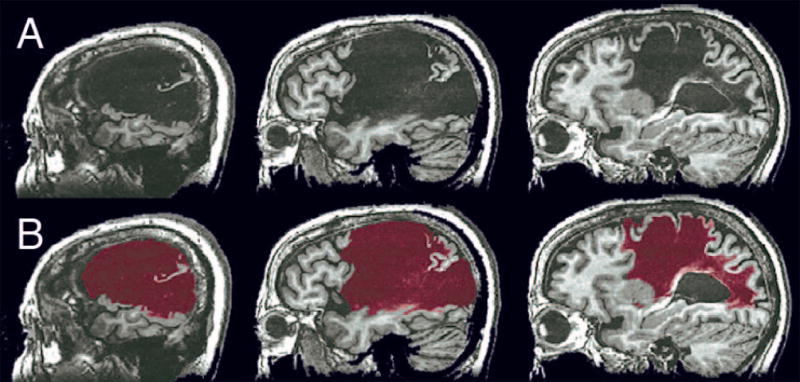
(A) T1-weighted anatomical MRI showing the lesion in one participant. (B) Lesion segment (red) obtained using the semi-automated segmentation method.
VLSM
Five separate analyses were completed. Only voxels lesioned in at least 5 patients were included. Using a custom Matlab script, VLSM analyses were done using lesion status at each voxel as a grouping variable. Analysis of covariance (ANCOVA) was used to remove variance related to Semantic Matching (controlling for low-level visual, orthographic, attention, working memory, and motor response components of the task), total lesion volume, age, and education for each of the five analyses. The first, and primary, analysis identified a specific lesion correlate for phonological access impairment using Rhyme Matching performance as the dependent variable. A second analysis included Articulatory Agility as an additional covariate to remove any shared variance associated with covert engagement of articulatory mechanisms during the Rhyme task. The third analysis included performance on the Oral Reading task as a covariate to demonstrate that phonological access is a critical component of overt speech production tasks. The fourth analysis included AWPM performance as an additional covariate to remove any shared variance between speech comprehension and phonological access processes. For all four of these analyses using Rhyme Matching as the dependent variable, the resulting t-statistic maps were thresholded at a voxel-wise p < .001 and cluster-corrected at a family-wise error of p < .05 using a minimum cluster size criterion of 650 μl (i.e., 650 template voxels), as determined by randomization testing with 10,000 permutations.
The final analysis assessed whether lesions producing phonological access impairment were also associated with speech comprehension impairment. In this final VLSM, AWPM performance was used as the dependent variable to determine whether lesions impairing speech comprehension showed any spatial overlap with lesions impairing phonological access. A more relaxed threshold of p < .005 and cluster criterion of 500 μl was used to increase sensitivity and the likelihood of detecting any overlap.
Results
Phonological Access Deficits
Mean performance on the Rhyme Matching task was 74.6 (SD 19.1, range 40 to 100), with a relatively even distribution across the range from chance to perfect performance. Relative to a group of 25 age-matched controls without stroke, 29 of 40 patients (72.5%) showed impaired Rhyme Matching performance, as defined by a score ≥2 SD below the control mean. Summary data for the other tasks are given in Table 1.
Scores on Semantic Matching, Articulatory Agility, Oral Reading, and AWPM were all correlated with Rhyme Matching. Lesion size was also correlated with Rhyme Matching, as larger lesions were associated with poorer performance. Age and education showed trends toward correlation in the expected direction, as older and less educated participants performed less well on the Rhyme Matching task. There was no significant relationship between Rhyme Matching performance and time from stroke onset. Individual patient performances are reported in Supplementary Table 1.
Lesion Coverage
Fig 3A shows the overlap of lesions across all 40 patients, thresholded to include only voxels that were lesioned in at least 5 patients. Coverage was good throughout the left MCA territory. The highest lesion count for any single voxel was 27. Frequently involved structures (defined using the TT_Desai_MPM atlas included with AFNI; http://afni.nimh.nih.gov/sscc/dglen/AFNIAtlases) included the following, with the number of patients (n) showing at least partial damage within each structure given in parentheses: SMG (33), insula (32), STG (31), inferior frontal gyrus (28), planum temporale (27), precentral gyrus (26), middle frontal gyrus (23), putamen (22), MTG (21), angular gyrus (20), postcentral gyrus (19), superior parietal lobe (14), caudate (13), inferior temporal gyrus (11), and superior frontal gyrus (7).
Figure 3.
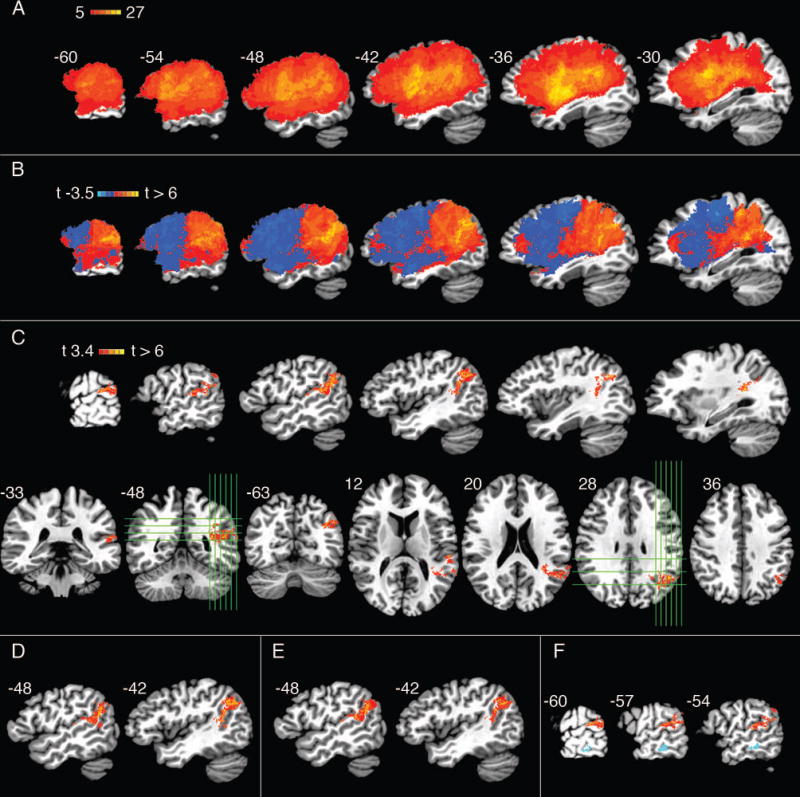
(A) Lesion overlap across all 40 patients, thresholded to include only voxels that were lesioned in at least 5 patients. (B–E) Primary VLSM analysis using Rhyme Matching performance as the dependent measure. (B) Unthresholded t-map, shown in serial sagittal sections through the left hemisphere. (C) T-map thresholded at p < .001, cluster-corrected at FWE of p < .05, shown in serial left sagittal, coronal (lower left), and axial (lower right) sections. Green lines indicate the locations of orthogonal sections. (D) Articulatory Agility included as an additional covariate in the VLSM of Rhyme Matching. (E) Auditory Word-Picture Matching (AWPM) included as an additional covariate in the VLSM of Rhyme Matching. (F) Composite image showing results of the final supplementary analysis with AWPM performance as the dependent measure, shown in light blue, and the primary analysis with Rhyme Matching performance as the dependent measure in red. AWPM performance is shown with relaxed voxel-wise threshold of p < .005 and cluster size threshold of 500 μl.
Primary VLSM Analysis: Phonological Access
The unthresholded VLSM relating lesion location with impairment on the Rhyme Matching task (controlling for orthographic processing, working memory, age, education, and total lesion volume) is shown in Figure 3B. Positive correlations were observed throughout the inferior parietal and posterior superior temporal region. Damage in the posterior frontal lobe (i.e., Broca’s area) did not impair phonological access, and in fact there were trends toward a negative correlation between damage in this region and phonological access impairment. Negative correlations indicate that lesions in these regions are actually predictive of better phonological access ability. This pattern likely arises from the fact that lesions tend to be somewhat focal, thus damage farther away from the critical zone is less likely to involve the critical zone. The abrupt transition near the central sulcus may arise from the fact that the MCA typically bifurcates into upper (anterior) and lower (posterior) divisions, and lesions affecting one division are less likely to affect the other. After thresholding with correction for family-wise error, the only region where damage correlated significantly with phonological access impairment was a contiguous volume of cortex and white matter inferior and posterior to the posterior sylvian fissure (Fig 3C). This cluster was centered on the junction of the posterior SMG and anterior AG (center of mass at x = −44, y = −50, z = 22), extending anteriorly into the posterior STG and planum temporale. White matter beneath the SMG, AG, and posterior STG was also part of the main cluster and included the voxel with the highest t-value (6.35; x = −32, y = −48, z = 16). Notably, there were no voxel clusters in the frontal lobe, insula, or MTG that showed a significant association with phonological access impairment.
Including Articulatory Agility as an additional covariate (with otherwise the same covariates as in the primary analysis) did not significantly alter the primary results (Figure 3D), supporting our hypothesis that the correlation observed in the primary analysis is independent of articulatory deficits.
The third analysis included performance on the Oral Reading task as an additional covariate (with otherwise the same covariates as in the primary analysis). As predicted, inclusion of this variable removed all voxels that were correlated with phonological access ability in the primary analysis.
Finally, the inclusion of AWPM performance as an additional covariate (with otherwise the same covariates as in the primary analysis) had no effect on voxels that were correlated with phonological access impairment (Figure 3E).
Lesion Correlates of Auditory Word Comprehension Impairment
The aim of the final supplementary analysis was to test more directly whether lesions associated with phonological access impairment were also associated with speech comprehension impairment. VLSM was conducted using AWPM performance as the dependent variable and the same covariates that were used in the primary analysis. Given the more limited power of this analysis due to the smaller amount of variance in AWPM scores, a more relaxed voxel-wise threshold of p < .005 and minimum cluster size of 500 μl was used. A cluster in the MTG was associated with impaired AWPM performance. There was no overlap between this cluster and the cluster identified in the primary analysis (Figure 3F). This absence of overlap indicates that lesion sites associated with phonological access impairment did not correlate with auditory word comprehension impairment.
Discussion
This study used multivariate voxel-based correlation to identify lesions responsible for phonological access impairment in chronic left hemisphere stroke. Lesions to posterior infrasylvian structures including the posterior STG, posterior SMG, anterior AG, and underlying white matter are correlated with phonological access impairment, even when orthographic, executive, articulatory, and speech comprehension ability, and age, education, and total lesion size are taken into consideration. Following precedent, we defined phonological access as a specific stage of speech production in which a sound-based word form is computed prior to articulation.6 Defined in this way, phonological access depends on a relatively focal temporoparietal region inferior and posterior to the posterior sylvian fissure. In contrast to several prior studies, there was no correlation with damage in the frontal lobe, insula, or MTG.2,28,29,25 Despite the relatively focal neural representation of this process, phonological access impairment was common in our sample, reflecting the frequent involvement of this brain region by ischemic events in the MCA territory.
To our knowledge, this is the first study to specifically localize phonological access processes using VLSM. A related study by Geva et al.25 used VLSM to examine the neural correlates of impaired “inner speech”. Deficits on a rhyme judgment task, similar to the task we used, were localized to the left IFG and anterior SMG. Inner speech, as defined by Geva et al., refers to the “active ‘use’ of inner speech, in a way that one has to monitor, or listen to, one’s own inner speech in order to successfully perform the task.” Critically, they included ability to read aloud as a covariate of no interest in the VLSM, and state explicitly in their Table 2 (pg. 3077) that reading aloud does not, according to their theory, require “inner speech”. However, the inclusion of reading aloud as a covariate, a task that requires pre-articulatory phonological access, would have masked the lesion correlates of phonological access impairment and shifted the focus of the analysis to post-access “monitoring” processes. When a similar reading aloud measure was included as an additional covariate in the present study, regions correlated with phonological access impairment were masked in exactly this way. These results indicate that phonological access is a process common to speech production tasks whether the phonological representation is subsequently articulated or not.
Another important difference between the two studies may explain why Geva et al.25 observed correlations between impaired rhyme judgment and IFG and anterior SMG damage, whereas we did not (in fact our analysis showed trends toward negative correlations in the IFG). The rhyming task makes considerable demands on working memory, as the phonological codes for multiple words need to be maintained throughout each trial. Reading aloud makes no equivalent demand on maintenance in working memory. Geva et al. did not include a control for this processing confound (or, alternatively, this process is equivalent to their “inner speech”), whereas our analysis controls for general working memory demands using a silent semantic matching task with a similar three-word stimulus array and response procedure. We replicated the Geva et al. analysis, using oral reading as a control task but no control for general working memory demands. The negative correlations we had observed in the frontal lobe disappeared completely, and several positively-correlated IFG foci (centered at Talairach coordinates −27, 27, 11) survived the p <.05 threshold used by Geva et al. The entire SMG, including anterior SMG, also survived this threshold. Thus, the apparent discrepancy between the two studies largely disappears when the same analysis and controls are used. The fact that the IFG and anterior SMG correlations disappear when a non-phonological working memory control (semantic matching) is included suggests that the deficit associated with damage in these areas is not specific to phonological tasks and is more likely to be a general working memory or sustained attention deficit.
Other previous VLSM studies examined aspects of the phonological access process when naming objects and repeating words30,23. The use of overt production tasks, however, introduces a source of variance that makes it difficult to distinguish between phonological access and articulatory mapping impairments. Schwartz and colleagues23 reported that phonological paraphasia during picture naming was correlated with lesions in the anterior SMG and pre- and postcentral gyri. These frontoparietal regions have been postulated to comprise a dorsal pathway for sensory-motor mapping specifically during overt articulation.31 Schwartz et al. proposed that phonological access actually depends on the same sensory-motor mapping processes that support speech articulation. However, their use of an overt naming task necessarily confounds phonological access with articulatory processes. Our study separates these processes by using a dependent measure that does not require articulation and, in the second analysis, by incorporating an articulatory agility score as a covariate of no interest. The regions associated with this more specific definition of phonological access were clearly posterior to those reported by Schwartz et al. These results show that phonological access can be impaired, independent of articulation, by posterior lesions involving infrasylvian temporoparietal cortex. The more anterior regions identified previously are more likely involved in articulatory planning or phonological-articulation mapping rather than phonological code retrieval per se.
In Wernicke’s influential model of fluent aphasia, the same phonological representations (“word sound forms”) are used during word comprehension and word production.3 This conceptualization provided a powerful explanation for the co-occurrence of word comprehension impairment and paraphasic speech production in the syndrome known as Wernicke aphasia. The existence of patients with paraphasic speech and intact comprehension (conduction aphasia) was explained as due to a lesion outside of the phonological representation, in the pathway linking this representation with the motor articulation center.32 The current results lend support to a very different model, in which the phonological representations used for speech comprehension and speech production are largely separate and functionally independent.9,33–35 This argument rests on two findings. First, damage to the posterior infrasylvian region impairs phonological access on a task that does not require articulation, and this correlation was independent of articulatory ability, thus the deficit is in generating a phonological code, not in “conducting” phonological information to an articulatory system. Second, we found no evidence that damage to this region is associated with an impairment of speech comprehension. The final VLSM, in which AWPM performance served as the dependent variable, may have been limited in terms of sensitivity due to the fact that many of the patients performed fairly well on this task. However, even at a more lenient threshold, no overlap was observed between the lesion sites associated with phonological access and speech comprehension deficits. Instead, a separate region in the MTG showed a specific correlation with AWPM performance. Furthermore, adding AWPM performance as a covariate in the Rhyme Matching VLSM had no effect on the temporoparietal correlation with phonological access impairment. Though far from definitive, these findings cast further doubt on one central feature of the widely held “classical” model of fluent aphasia. They are consistent with previous evidence suggesting that speech comprehension depends on a widely distributed network of brain regions that includes bilateral auditory association areas, temporal lobe lexical-semantic representations, and prefrontal cognitive control systems36–39 rather than on a highly localized phonological module.
The AG is more closely associated with semantic processing than with phonological processing in healthy controls,36 thus involvement of the anterior AG in phonological access was unexpected. Recent cytoarchitectonic and functional imaging studies suggest that the AG is comprised of several distinct regions.40,41 It is possible that anterior AG, labeled PGa by Caspers et al.,41 is functionally distinct from more posterior regions involved in semantic processing. An alternative possibility is that the VLSM method is limited in its ability to distinguish posterior SMG from anterior AG because of the shared vascular supply to these regions. The inferior terminal branch of the M2 division of the left MCA typically supplies both of these regions via either a single angular artery or a posterior parietal branch from the angular artery. Occlusion of these branches often results in a combined lesion to both regions, and this close cortical proximity may result in a spurious correlation. Two patients in the current sample had relatively isolated lesions of the AG (patients 11 and 30 in Supplementary Table 1). Both of these patients showed normal phonological access (97.5% accuracy on Rhyme Matching), suggesting that involvement of the anterior AG in the group VLSM may be a proximity effect due to a shared vascular supply.
The methods used in the present study do not allow conclusions to be drawn concerning regions outside the left MCA territory, which are not well represented in unselected ischemic stroke patient samples. This limits the ability to test the contribution of structures such as the medial frontal and parietal lobe, ventral and medial temporal lobe, occipital lobe, and thalamus. These brain regions, however, have not been implicated in phonological processing in previous neuropsychological or functional imaging studies. Future studies on this topic should include a wider variety of neurological patients with more varied lesion locations to allow more complete assessment of areas outside the MCA territory.
Supplementary Material
Acknowledgments
This study was supported by grants from the NIH/NINDS (ROI NS033576, ROI DC003681, RO3 NS054958) to J.R.B, and by an award from the American Heart Association (13PRE16510003) to S.B.P. We thank William L. Gross for helpful discussions about data analysis, Megan E. Rozman for help testing patients, and the patients for their generous participation.
Footnotes
Authorship: Sara Pillay participated in the conceptualization of the study, patient recruitment, collection and analysis of data, and writing and editing of the manuscript. Benjamin Stengel and Colin Humphries participated in the conceptualization of the study and analysis of data. Diane Book participated in the design of the study and patient recruitment. Jeffrey Binder participated in the design and conceptualization of the study, patient recruitment, collection and analysis of data, and writing and editing of the manuscript.
References
- 1.Foygel D, Dell GS. Models of impaired lexical access in speech production. J Mem Lang. 2000;43(2):182–216. [Google Scholar]
- 2.Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92(1–2):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Wernicke . Der aphasische symptomencomplex: eine psychologische studie auf anatomischer basis. [The symptom complex of aphasia: A psychological study on an anatomical basis] In: Eggert GH, editor. Wernicke’s works on aphasia: a sourcebook and review. The Hague: Mouton; 1874. pp. 91–145. [Google Scholar]
- 4.Coltheart M, Curtis B, Atkins P, Haller M. Models of reading aloud: Dual-route and parallel-distributed-processing approaches. Psychol Rev. 1993;100(4):589–608. [Google Scholar]
- 5.Coltheart M, Rastle K, Perry C, et al. DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychol Rev. 2001;108(1):204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- 6.Levelt WJ. Accessing words in speech production: stages, processes and representations. Cognition. 1992;42(1–3):1–22. doi: 10.1016/0010-0277(92)90038-j. [DOI] [PubMed] [Google Scholar]
- 7.Perry C, Ziegler JC, Zorzi M. Nested incremental modeling in the development of computational theories: the CDP+ model of reading aloud. Psychol Rev. 2007;114(2):273–315. doi: 10.1037/0033-295X.114.2.273. [DOI] [PubMed] [Google Scholar]
- 8.Roelofs A. The WEAVER model of word-form encoding in speech production. Cognition. 1997;64(3):249–284. doi: 10.1016/s0010-0277(97)00027-9. [DOI] [PubMed] [Google Scholar]
- 9.Dell GS, Martin N, Saffran EM, et al. Lexical access in aphasic and nonaphasic speakers. Psychol Rev. 1997;104:801–838. doi: 10.1037/0033-295x.104.4.801. [DOI] [PubMed] [Google Scholar]
- 10.Martin N, Saffran EM. The relationship of input and output phonological processing: An evaluation of models and evidence to support them. Aphasiology. 2002;16(1–2):107–150. [Google Scholar]
- 11.Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 12.Hickok G, Erhard P, Kassubek J, et al. A functional magnetic resonance imaging study of the role of left posterior superior temporal gyrus in speech production: implications for the explanation of conduction aphasia. Neurosci Lett. 2000;287(2):156–160. doi: 10.1016/s0304-3940(00)01143-5. [DOI] [PubMed] [Google Scholar]
- 13.Graves WW, Desai R, Humphries C, et al. Neural systems for reading aloud: A multiparametric approach. Cereb Cortex. 2010;20(8):1799–1815. doi: 10.1093/cercor/bhp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graves WW, Grabowski TJ, Mehta S, Gordon JK. A neural signature of phonological access: distinguishing the effects of word frequency from familiarity and length in overt picture naming. J Cogn Neurosci. 2007;19(4):617–631. doi: 10.1162/jocn.2007.19.4.617. [DOI] [PubMed] [Google Scholar]
- 15.Buchsbaum BR, Olsen RK, Koch PF, et al. Reading, hearing, and the planum temporale. NeuroImage. 2005;24(2):444–454. doi: 10.1016/j.neuroimage.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Price CJ, Wise RJ, Warburton EA, et al. Hearing and saying. The functional neuroanatomy of auditory word processing. Brain J Neurol. 1996;119:919–931. doi: 10.1093/brain/119.3.919. [DOI] [PubMed] [Google Scholar]
- 17.Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362(6418):342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- 18.Poldrack RA, Temple E, Protopapas A, et al. Relations between the neural bases of dynamic auditory processing and phonological processing: evidence from fMRI. J Cogn Neurosci. 2001;13(5):687–697. doi: 10.1162/089892901750363235. [DOI] [PubMed] [Google Scholar]
- 19.Bates E, Wilson SM, Saygin AP, et al. Voxel-based lesion–symptom mapping. Nat Neurosci. 2003;6(5):448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- 20.Baum SR. Fricative production in aphasia: Effects of speaking rate. Brain Lang. 1996;52(2):328–341. doi: 10.1006/brln.1996.0015. [DOI] [PubMed] [Google Scholar]
- 21.Blumstein SE. Phonological aspects of aphasia. In: Sarno MT, editor. Acquired Aphasia. Academic Press; 1998. pp. 157–185. [Google Scholar]
- 22.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz MF, Faseyitan O, Kim J, Coslett HB. The dorsal stream contribution to phonological retrieval in object naming. Brain. 2012;135(12):3799–3814. doi: 10.1093/brain/aws300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodglass H, Kaplan E, Barresi B. BDAE: The Boston Diagnositc Aphasia Examination. Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 25.Geva S, Jones PS, Crinion JT, et al. The neural correlates of inner speech defined by voxel-based lesion–symptom mapping. Brain. 2011;134(10):3071–3082. doi: 10.1093/brain/awr232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 27.Avants BB, Tustison N, Song G. Advanced Normalization Tools (ANTS) Penn Image Computing and Science Laboratory; 2011. [Google Scholar]
- 28.Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc. 2006;12(6):896–900. doi: 10.1017/S1355617706061078. [DOI] [PubMed] [Google Scholar]
- 29.Damasio H, Damasio AR. Localization of lesion in conduction aphasia. In: Kertesz A, editor. Localization in Neuropsychology. New York: Academic Press; 1983. pp. 231–243. [Google Scholar]
- 30.Dell GS, Schwartz MF, Nozari N, et al. Voxel-based lesion-parameter mapping: Identifying the neural correlates of a computational model of word production. Cognition. 2013;128(3):380–396. doi: 10.1016/j.cognition.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hickok G. Computational neuroanatomy of speech production. Nat Rev Neurosci. 2012;13(2):135–145. doi: 10.1038/nrn3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lichtheim L. On aphasia. Brain. 1885;7:433–484. [Google Scholar]
- 33.Howard D, Nickels L. Separating input and output phonology: semantic, phonological, and orthographic effects in short-term memory impairment. Cogn Neuropsychol. 2005;22(1):42–77. doi: 10.1080/02643290342000582. [DOI] [PubMed] [Google Scholar]
- 34.Martin RC, Lesch MF, Bartha MC. Independence of input and output phonology in word processing and short-term memory. J Mem Lang. 1999;41(1):3–29. [Google Scholar]
- 35.Hickok G. The architecture of speech production and the role of the phoneme in speech processing. Lang Cogn Neurosci. 2014;29(1):2–20. doi: 10.1080/01690965.2013.834370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends Cogn Sci. 2000;4(4):131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- 38.Dronkers NF, Wilkins DP, Van Valin RD, Jr, et al. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92(1–2):145–177. doi: 10.1016/j.cognition.2003.11.002. [cited 2014 Jan 10] [DOI] [PubMed] [Google Scholar]
- 39.Jefferies E. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129(8):2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- 40.Seghier ML, Fagan E, Price CJ. Functional subdivisions in the left angular gyrus where the semantic system meets and diverges from the default network. J Neurosci. 2010;30(50):16809–16817. doi: 10.1523/JNEUROSCI.3377-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caspers S, Geyer S, Schleicher A, et al. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. NeuroImage. 2006;33(2):430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


