Abstract
Objective
The aim was to develop a method for the purpose of localizing epilepsy related hemodynamic foci for patients suffering intractable focal epilepsy using task-free fMRI alone.
Methods
We studied three groups of subjects: patients with intractable focal epilepsy, healthy volunteers performing motor tasks, and healthy volunteers in resting state. We performed spatial independent component analysis (ICA) on the fMRI alone data and developed a set of IC selection criteria to identify epilepsy related ICs. The method was then tested in the two healthy groups.
Results
In seven out of the nine surgery patients, identified ICs were concordant with surgical resection. Our results were also consistent with presurgical evaluation of the remaining one patient without surgery and may explain why she was not suitable for resection treatment. In the motor task study of ten healthy subjects, our method revealed components with concordant spatial and temporal features as expected from the unilateral motor tasks. In the resting state study of seven healthy subjects, the method successfully rejected all components in four out of seven subjects as non-epilepsy related components.
Conclusion
These results suggest the lateralization and localization value of fMRI alone in presurgical evaluation for patients with intractable unilateral focal epilepsy.
Significance
The proposed method is noninvasive in nature and easy to implement. It has the potential to be incorporated in current presurgical workup for treating intractable focal epilepsy patients.
Keywords: fMRI, ICA, epilepsy, lateralization, default mode network
Introduction
For patients with drug resistant epilepsy, surgical resection is among the well-established methods for seizure control. During presurgical planning, if non-invasive methods such as structural MRI, semiology, single photon emission computed tomography (SPECT) and positron emission tomography (PET) etc. are not adequate in localizing the epileptic foci, invasive procedures including electrocorticography (ECoG) and depth electrodes are currently employed to define the seizure onset zone. However these methods are not only invasive in nature, they may also fail to provide additional information needed for surgery due to the relatively limited spatial coverage (Rodionov et al., 2007).
EEG/ MEG source imaging approaches (Fukushima et al. 2012; Wang et al. 2012; Wu et al. 2012) have also been investigated from noninvasive measurements during interictal (He et al., 1987; Hämäläinen et al. 1993; Baillet et al. 2001; Lantz et al., 2003; Baumgartner & Pataraia, 2006; Koessler et al., 2010; Holmes et al., 2010; Wang et al., 2010; He et al., 2011; Lai et al., 2011) and ictal stages (Ding et al., 2007; Yang et al., 2011; Lu et al., 2012) by solving the EEG/MEG inverse source imaging problem. While such noninvasive imaging techniques have improved substantially over the past decades, source imaging approaches in general are still relatively insensitive toward deep brain structures.
As a noninvasive imaging method, functional MRI (fMRI) has shown promises in the evaluation of epileptic foci. FMRI is currently used in presurgical assessment to identify eloquent cortex that affect visual, language, motor functions to be spared during surgery (Thornton et al., 2009). In addition, fMRI may also offer values as a useful tool to localize epileptic foci. It is commonly used in combination with simultaneously collected scalp EEG (Liu et al., 2006; Liu & He, 2008; He & Liu, 2008). Temporal information of epileptic events identified from EEG can be used to correlate with hemodynamic changes in blood oxygen level dependent (BOLD) signal, to study the areas in the brain with epileptic activities (Hamandi et al., 2004; Gotman et al., 2006; Laufs & Duncan, 2007; Lopes et al., 2012). In standard EEG-fMRI analysis, timing of interictal epileptiform discharges (IED) on scalp EEG is first identified. Each occurrence is treated as an impulse function which is then convolved with the hemodynamic response function (HRF) to obtain a general linear model (GLM). The model is then statistically fitted to the fMRI data, with appropriate thresholding to arrive at an activation map (Lemieux et al., 2001; Benar et al., 2002; Hamandi et al., 2004; Rodionov et al., 2007). However, EEG recorded in the scanner is often heavily contaminated with artifacts that are difficult to remove completely. EEG is also known for its limited sensitivity towards deep brain structures. Another challenge in using the GLM approach is that an accurate model of the HRF is often needed, as it serves as the linkage that represents the neurovascular coupling. A standard canonical shaped HRF has been widely used but there is a growing consensus about the variability among subjects, or within the same individual but among different brain areas (Jacobs et al., 2009; Bai et al., 2010; LeVan 2010). Additionally, recording EEG and fMRI simultaneously requires a complicated setup system that is not easy to use in a clinical setting. Moreover, some researchers using EEG informed fMRI have demonstrated the value of fMRI in epilepsy, as it may be able to identify patients with widespread epileptic networks (Zijlmans et al., 2007).
Several previous studies have described the possibility of utilizing model-free, data driven methods on the BOLD signal alone to delineate epileptic activities (Rodionov et al., 2007; LeVan & Gotman 2009; Moeller et al., 2011; Lopez et al., 2012; Oikonomou et al. 2012; Barbé et al. 2012; Deng et al. 2013). Such approaches may offer an alternative to circumvent some of the aforementioned challenges associated with simultaneous EEG and fMRI. Independent component analysis (ICA) is a widely used blind source separation method. In the context of fMRI signals, it extracts regions of activities with corresponding time course based on spatial independence, which does not impose any constraint on the prior knowledge of HRF or the timing of interictal epileptiform discharges (IED) on EEG (Rodionov et al., 2007; LeVan & Gotman 2009). However, the process to select the independent components (ICs) that are related to epileptic activity often requires human supervision to a large extent. Therefore, we sought to develop an easy to implement framework that requires minimal subjective input to extract clinically relevant components.
Normally, focal epilepsy is highly treatable by surgery due to the focality of isolated epileptogenic zone. On the other hand, patients with diffused or distributed epileptogenic zones may not be suitable for surgical consideration. We intended to develop a tool to aid surgical planning for surgical candidates. Therefore, in the present study, we focused on the focal epilepsy population. To assess the sensitivity of the method, we simulated focal cortical activity in healthy subjects performing a lateralized motor task. We then evaluated the results of the proposed method by comparing selected components to expected motor activation area in healthy subjects and to surgical resection in patients respectively. To assess the specificity of the method of component selection, we also included a study of healthy volunteers during resting state.
Methods
Data Acquisition
Patients
Data were collected from 10 consecutive patients (ages 20-58 years, 35.3±15.9 years, 5 males) with intractable epilepsy who underwent presurgical evaluation at Mayo Clinic (Rochester, MN). Clinical information was listed in Table 1. Resting state functional images were acquired using a General Electric 3T Signa HDx (Waukesha, Wisconsin) scanner. Each set of data was 20 min using a T2*-weighted EPI sequence. TR=3000 ms, flip angle =90, 3 mm isotropic voxel, 30±2 slices. A spoiled gradient recalled T1 weighted anatomical image before and after operation was acquired for coregistration with functional data (1 mm isotropic voxel, 120 or 190 slices). The study was conducted according to a protocol approved by the Institutional Review Boards (IRB) of Mayo Clinic and the University of Minnesota respectively.
Table 1.
# ICs concordant: the number of components that are in concordant with surgical resection area.
| Patient | Age | Gender | Clinical Diagnosis | Resection | #ICs concordant (total # ICs selected) | Surgical Outcome |
|---|---|---|---|---|---|---|
| 1 | 27 | Male | Left frontal | Left frontal focal resection | 2 (3) | -- |
| 2 | 25 | Female | Left parietal | Left frontoparietal focal resection | 1 (2) | ILAE-1 |
| 3 | 58 | Female | Left temporal | Left temporal lobectomy | 1 (1) | ILAE-4 |
| 4 | 29 | Male | Right temporal | Right temporal lobectomy | 1 (1) | ILAE-4 |
| 5 | 20 | Female | Left temporal | Left temporal lobectomy | 0 (1) | ILAE-2 |
| 6 | 20 | Male | Left temporal | Left temporal lobectomy | 2 (2) | ILAE-1 |
| 7 | 47 | Female | Left temporal | Left temporal lobectomy | 1 (1) | ILAE-3 |
| 8 | 20 | Female | Right parietal | -- | -- | -- |
| 9 | 58 | Male | Right temporal | Optic laser ablation of hippocampus | 1 (3) | ILAE-1 |
| 10 | 49 | Male | Left temporal | Left temporal lobectomy | 0 (1) | -- |
The total number of components selected by the algorithm is indicated in the parenthesis. Follow-ups in Pt #1 and 10 are not available. Pt #8 did not receive surgery.
Healthy subjects with motor tasks
In order to evaluate the application of our method in localizing lateralized focal activity with known activity pattern, we performed an experiment where subjects performed hand movement tasks. Ten healthy volunteers (ages 20-36 years, 27.5±5.6 years, 5 males) participated in this study with written consent according to a protocol approved by the Institutional Review Board of the University of Minnesota. The experiment followed a block design where task blocks were interleaved with resting blocks. Each block lasted for 20 s. Within a task block, subjects performed randomized left and right hand movement tasks. The tasks included either finger tapping or hand clenching of a given hand, one hand at a time. Each task block included only one type of task. Each individual anatomical MRI data set consisted of 176 contiguous sagittal slices with 1 mm slice thickness (matrix size: 256 * 256; FOV: 256 mm * 256 mm; TR/TE=20 ms/3.3 ms) on a 3T MRI system (Siemens Skyra, Siemens, Erlangen, Germany). Whole-brain functional images with BOLD contrast were acquired using gradient echo planar imaging sequence (32 axial 3-mm thick interleaved slices with 0.3-mm gap; TR/TE = 2000 ms/30 ms; flip angle = 90°; matrix size: 64 * 64; FOV: 192 mm * 192 mm). Each functional run started with a rest block and contained 160 volumes.
Healthy subjects during Resting State
In order to test the specificity of the algorithm, we also recruited a group of healthy subjects for resting state recording. Seven healthy volunteers (ages 24-31 years, 26±2.4 years, 5 males) participated in this study with written consent according to a protocol approved by the Institutional Review Board of the University of Minnesota. Each subject was instructed to lie quietly in the scanner for two scans, each lasting for six minutes. Additionally, individual anatomical MRI data were collected which were consisted of 176 contiguous sagittal slices with 1 mm slice thickness (matrix size: 256 * 256; FOV: 256 mm * 256 mm; TR/TE=20 ms/3.3 ms) on a 3T MRI system (Siemens Trio, Siemens, Erlangen, Germany). Whole-brain functional images with BOLD contrast were acquired using gradient echo planar imaging sequence (32 axial 3-mm thick interleaved slices with 0.3-mm gap; TR/TE = 2000 ms/30 ms; flip angle = 90°; matrix size: 64 * 64; FOV: 192 mm * 192 mm).
Preprocessing
First, the pre-surgical structural MRI was segmented into two parts: regions within the boundary of the brain volume and those outside of the boundary. FMRI data were then spatially corregistered to the structural MRI. The boundary of the brain from the segmented structural MRI was used as a marker to distinguish voxels from the fMRI that corresponded to areas inside vs. outside of the brain in a later step after ICA decomposition. All fMRI data were pre-processed for slice scan time correction, 3-D motion correction and temporal filtering using BrainVoyager QX software (Brain Innovation, Maastricht, Netherlands).
Independent Component Analysis of fMRI data
Independent component analysis (ICA) in the spatial domain was performed using Brain Voyager QX. Detailed methodological principles of ICA decomposition implemented in Brain Voyager QX were previously described (Formisano et al., 2004; De Martino et al., 2007). Briefly, ICA decomposition of fMRI signal can be written as:
where Y is the fMRI signal, S is the spatial maps of the components and T is the time course defining the weights of the spatial maps in the time domain. S and T were obtained using the hierarchical (deflation) mode of the FastICA algorithm (Hyvärinen, 1999; Calhoun et al. 2001, 2005). Thirty components were computed and the voxel intensities of each IC maps were converted to z-scores. The spatial maps were color coded to reflect the absolute value and sign. It should be noted that the sign of each voxel value does not correspond to BOLD activation or deactivation. A positive value represents that the time course of the particular voxel is positively correlated with the time course of the IC. A higher z-score represents a higher correlation coefficient. As pointed out previously, the z-values have no statistical significance, as no hypothesis was tested (McKeown et al., 1998; De Martino et al., 2007).
Classification of components
We proposed a set of data driven criteria to identify epilepsy related independent components (ICs). The criteria and rationales are described as following:
Criterion 1: Biophysical constraints of neurological sources
The BOLD signal of our interest indirectly measures neurological activity in the brain (Arthurs & Boniface 2002). Neurological activities are known to be generated by neurons residing in the grey matter of the cortex. However the BOLD signal measured is often confounded with other sources caused by physiological activities such as breathing, pulsation, or abrupt motion artifacts (Mitra et al., 1997; McKeown et al., 1998; Jiang et al., 2002). Some of these noisy components tend to have majority of the activity outside of the cortex in areas such as brainstem, eyes or the periphery of the cortex, which is usually due to residual motion artifacts (Mitra et al., 1997; De Martino et al., 2007). As described in the pre-processing section, the boundary of the brain obtained from segmenting the structural MRI was used to mark fMRI voxels as inside vs. outside of the brain. FMRI voxels outside of the brain, which are clearly caused by noise, were not excluded before performing ICA. ICA was performed before exclusion of any voxel because we aimed to find and reject noisy activities within the brain that have statistical dependence with the noisy voxels outside of the brain. Therefore, by retaining obviously noisy voxels outside of the brain, it can help identify noisy activities that are within the brain in subsequent processing steps. To quantify this feature, we used the index Ri/o, where
Ni denotes the number of voxels inside the brain, and No is the number of voxels outside of the brain. Components with Ri/o value below a cut-off value will be excluded from further analysis (Fig. 1, Step 2, Criterion 1). This was to separate cortical components from noisy components with signals concentrated predominantly outside of the brain volume. The default cut-off value was set to be the median of Ri/o values of all thirty components. This particular cut-off value was adopted to be inclusive rather exclusive. In this way, half of all the components will remain to be considered based on the next criterion.
Fig. 1.
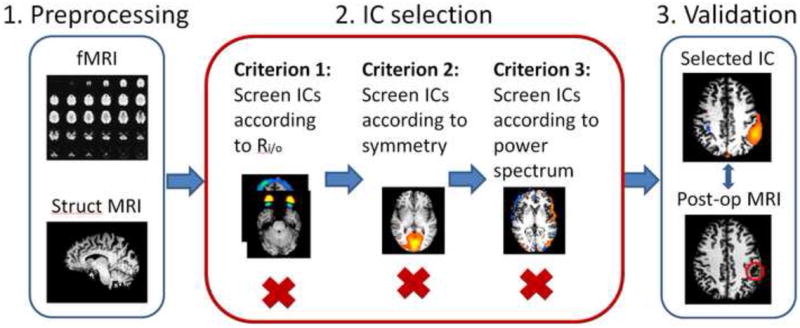
Data analysis procedures. Step 1. Preprocessing of fMRI and MRI images. Step 2. Selection of the components basing on proposed three criteria. Step 3. Evaluation of the method. In the patient group, selected component was compared with post-surgical MRI.
Criterion 2: Spatial lateralization of the epilepsy related components in suitable surgical candidates
The concept of lateralization has traditionally been used in EEG to initially lateralize epileptogenic zone and to guide placement of intracranial recording. We now applied a similar concept in fMRI data analysis. As mentioned earlier, the patient population is surgical candidates with focal epilepsy. We assumed the epileptic activity is lateralized to one hemisphere. On the other hand, other common resting state activities in the brain are usually symmetrical. Such components include signals arising from major blood vessels, auditory activities, or default mode network (Raichle et al., 2001; Seifritz et al., 2002; Beckmann et al., 2005; Fox et al., 2005; Fransson, 2005; Aragri et al., 2006; De Martino et al., 2007; Rodionov et al., 2007; Greicius et al., 2009). To quantify the symmetricity of the signal distribution of each component, we used the index Corr to denote the correlation of activities among mirroring voxels about the anterior commissure – posterior commissure (ACPC) plane. The three-dimensional (3D) distribution of voxel intensity about on either side of the ACPC plane was first reduced to a one-dimensional series. It was arranged so that the ith entry of both series corresponds to the mirroring voxels in the 3D space. The correlation value was calculated using Pearson’s correlation coefficient.
where L(i) denotes the ith entry of the activity of the ith voxel in the left half of the brain and R(i) denote the mirroring ith voxel on the right side. Then the components were ranked according to their Corr values, from the smallest to the largest. Asymmetrical components are thus the ones with low Corr values. A cut-off threshold was set as one standard deviation smaller than the mean of the remaining components which passed the first level screening. This cutoff was used to identify components which would be considered for subsequent Criterion 3.
Criterion 3: Temporal features of the components
Components that passed the two aforementioned spatially based criteria were subjected to a third temporally based criterion to remove any additional noise. Components with dominant power outside of the range of 0.01 to 0.1 Hz were excluded. As described by De Martino et al (2007), neurophysiologically meaningful components are expected to have certain temporal structure, which often fall within the range of 0.01 to 0.1 Hz (Cordes et al. 2001; van de Ven et al. 2004). Components with dominant frequency outside of this range are often a reflection of aliasing of cardiac and respiration artifacts (>0.1 Hz) or scanner susceptibility artifacts (<0.01 Hz). Power contribution of each component at each frequency band was computed as part of the BrainVoyager QX ICA ‘Fingerprint’ function. Briefly, the power spectrum density of each IC was first computed. Relative contribution of each frequency band ([0, 0.008 Hz], [0.008, 0.02 Hz], [0.02, 0.05 Hz], [0.05, 0.1 Hz] and [0.1, 0.25 Hz]) was captured by calculating the weight of each frequency bands over the entire spectrum. The metric of each component at any given frequency was normalized first within the component then cross all components to arrange from 0 to 1 (De Martino et al 2007). If a component has a metric of 1 for a specific frequency band, that frequency band is considered as a dominant frequency of the component. Components with dominant frequency above 0.1 Hz or below 0.008 Hz were removed.
Evaluation
In the patient study, the spatial patterns of identified components were compared to the co-registered postoperative MRI. If the area of activity in the identified component falls within or well overlaps with the resected area, the component was considered as concordant. In the motor task experiment, the accuracy of identified components was evaluated both temporally and spatially. Temporally, the expected time course of motor response was obtained by convolving the block design time and the canonical HRF. The time course of identified components was then compared with the expected time course to compute the correlation coefficient. Spatially, a general linear model was used with expected time course as a regressor to obtain the activation maps corresponds to the motor tasks. The activation maps were compared to maps of identified components. Group averages of the maps from both GLM and ICA were computed and compared in the Talairach space. In the resting state experiment with healthy subjects, the specificity of the algorithm was assessed by examining if there were any components that passed the three-criterion algorithm.
Results
General component screening
Fig. 2 shows representative components taken from a subject in the motor-task simulation, where the subjects were asked to perform hand movement tasks to simulate a unilateral focal neural activity. Components removed by Criterion 1 included a pattern that is consistent with activity in eye areas (Fig. 2A). This eye component has low in-brain vs. out-brain ratio, Ri/o, of 0.02, which is significantly lower than the average of all the 30 ICs (1.60±0.9). The second criterion of lateralization rejected a component consistent with fMRI activity in visual cortex (Fig. 2B). This component has Ri/o=1.6 and Corr= 0.4. It survived Criterion 1 but rejected by Criterion 2 due to high symmetry between left and right visual cortex. The component shown in Fig. 2C passed both Criterion 1 and 2 but was rejected by Criterion 3 as this component has dominant frequency in the >0.1 Hz range. The spatial pattern of this component matches the pattern of typical residual motion artifacts as reported in previous studies (Mitra et al., 1997; McKeown et al., 1998; Thomas et al., 2002). The only component which passed all three criteria showed activity in left motor cortex (Fig. 2D). It is consistent with the right finger tapping activity of the subject during the fMRI recording.
Fig. 2.
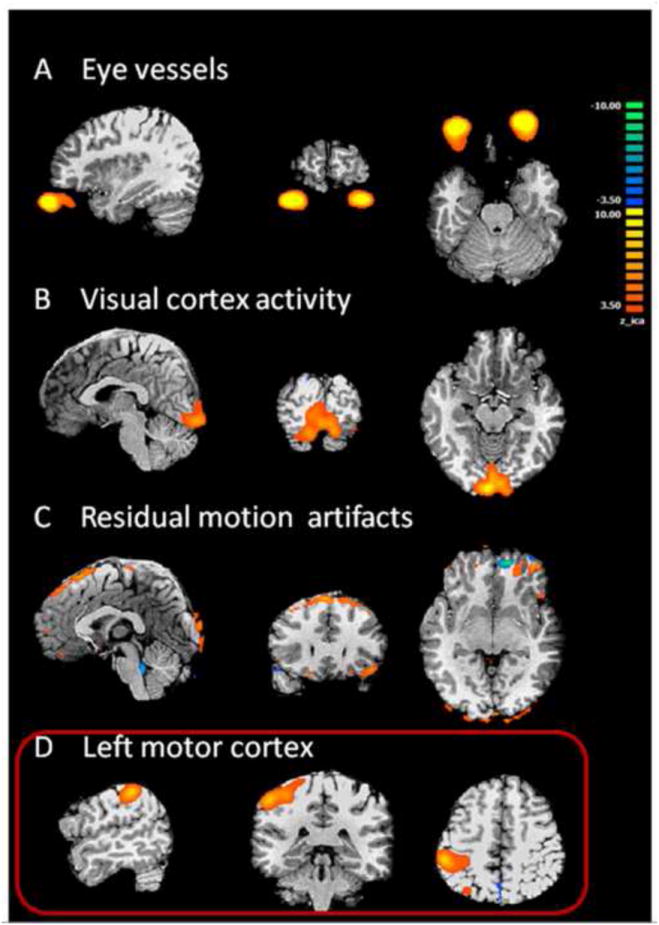
Illustration of different components from motor simulation in a healthy subject. A. Eye movements. The BOLD activities are located outside of the brain volume. This component has a low Ri/o value and was screened out by Criterion 1. B. Visual activity. This BOLD pattern is highly symmetrical and was rejected based on Criterion 2 because of the high symmetry between left and right mirroring voxels. C. Residual motion artifacts, located at the periphery of the brain. This component was rejected by Criterion 3. D. Selected component that passed all 3 screening criteria. The spatial distribution of this component is concentrated in the left motor cortex. This is an example of contralateral activation, as expected from right hand movement. In the patient group, components rejected by Criterion 1-3 have similar patterns as shown in A-C.
Patients with focal epilepsy
Patient 1 was diagnosed with frontal lobe epilepsy and underwent left frontal craniotomy. In our component selection algorithm, the cut-off value of Ri/o used in Criterion 1 is 0.9 and the cut-off value of Corr used in Criterion 2 is 0.16. Three out of fifteen components passed Criterion 2.The first component (Fig. 3A, Ri/o=2.62, Corr = 0.12) shows activity in left frontopolar cortex, which falls within the surgical resected zone as indicated by the red arrow in postoperative MRI (Fig. 3B). Component 2 (Ri/o=0.90, Corr = 0.16) has two areas of activities (Fig. 3C). One is located in the midline along the longitudinal fissure, and another near to the left middle frontal gyrus, which coincides with another region of resection as shown in Fig. 3D. The third component (Fig. 3E) survived the screening process but was not considered epilepsy-related by visual evaluation. This is because it has a ring shaped distribution of activity around the peripheral of the cortex, which is a typical pattern for residual movement artifact.
Fig. 3.
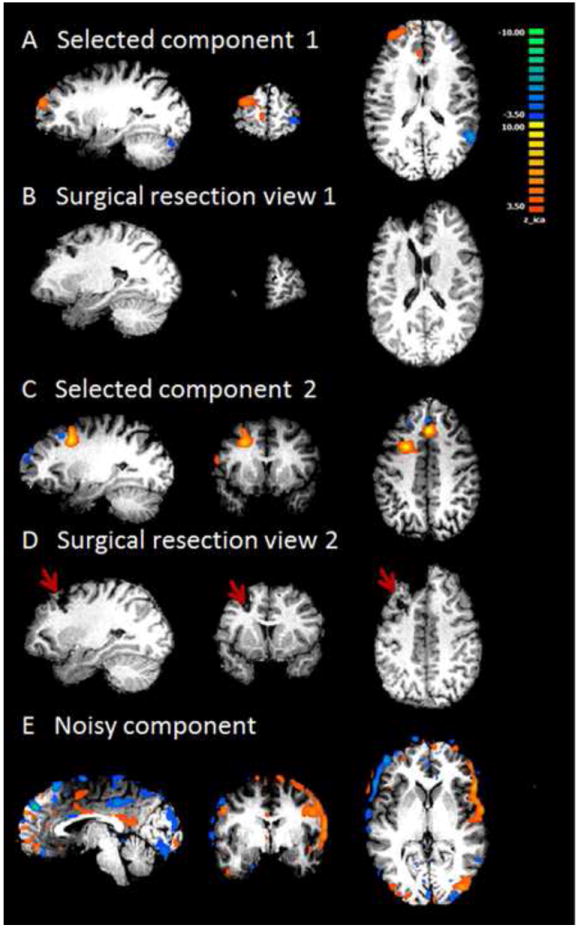
Results from Patient #1. A and C are the two components selected by the proposed algorithm. They both have high lateralization values. This patient had left frontal lobe epilepsy and received surgical resection in left frontal lobe. B and D are post-operation MRI, showing two different surgical locations corresponding to the two identified components. E shows a typical noisy component that survived the three criteria but was rejected by visual insepection. This component has voxels within the brain volume and is highly asymmetrical. But the spatial pattern of this component is of a typical residual movement artifact.
Patient 2 had left parietal epilepsy. Presurgical EEG showed frequent epileptogenic abnormalities over the left central region, which was consistent with a partial seizure disorder. In this patient, the cut-off value of Ri/o in Criterion 1 is 1.2 and the mean of Ri/o and cut-off value of Corr used in Criterion 2 is 0.29. Two out of the fifteen components passed Criterion 3 and were examined by visual inspection. One first component (Fig. 4A, Ri/o= 2.2, Corr=0.27) localized in the close vicinity of the surgical resected parietal cortex (Fig. 4B). This patient received intra-cranial recording and left parietal cortical resection. The largest cluster with the highest z-sore of the selected component falls in the right parietal cortex, which is in concordance with the resected region. The other component was considered not epilepsy-related by visual evaluation because it has a similar ring-shaped pattern as shown in Fig. 3E in Patient 1.
Fig. 4.
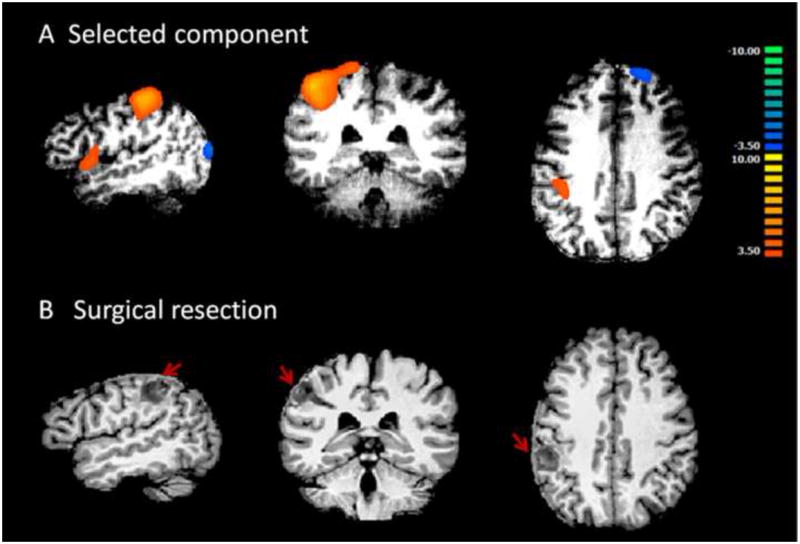
Results from patient #2. A shows the only component selected by the algorithm and accepted by visual inspection. This component has high lateralization value and is localized in the left parietal lobe. This patient had left parietal lobe epilepsy. B shows surgical resection in left parietal lobe, indicated by red arrows. The orange cluster in left parietal region shown in A agrees well with the surgical resection in B.
Patient 3 was diagnosed with left temporal epilepsy and underwent left temporal lobectomy. In this patient, the cut-off value of Ri/o in Criterion 1 is 0.50 and the cut-off value of Corr used in Criterion 2 is 0.12. Only one component was identified by our first two criteria (Fig. 5A). This component also passed the additional Criterion 3. It has activity in left anterior temporal area, which agrees with spikes and sharp waves observed from anterior temporal electrodes on ECoG. It also co-localizes to the surgical resected zone as indicated by the red arrow in postoperative MRI (Fig. 5B). The Ri/o and Corr of this component are 0.54 and 0.01 respectively.
Fig. 5.
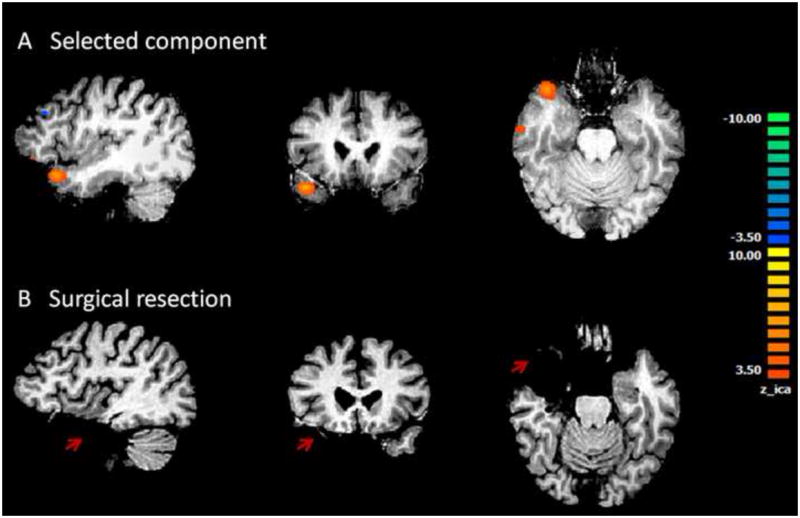
Results from Patient #3. A shows the only component selected by the algorithm and accepted by visual inspection. This component is located at the anterior portion of the left temporal lobe. This patient had left temporal lobe epilepsy. B shows surgical resection in left temporal lobe. Orange cluster in left temporal lobe in A falls well within the surgical resection indicated in B.
Patient 8 was diagnosed with right parietal epilepsy. However, no component was identified from the fMRI basing our 3-criterion algorithm. We carefully examined all the components from the patient, and noticed that two components had abnormal spatial distributions (Fig. 6A and 6B). Both components show distributed activity in the bilateral inferior parietal lobules and posterior cingulate cortex, which resemble typical ’default-mode-network’ pattern. Additionally, as shown in Fig 6B, this component also has a dorsal/ventral medial prefrontal cortex node which is typical in default mode network. However, several extra-network clusters in the right frontal and parietal regions were found to be temporally correlated with the default mode network. These clusters occur unilaterally in the right hemisphere. According to the clinical report of video EEG, ECoG and SPECT, this patient had diffuse seizure onset in frontal central as well as right central head regions. The observations of the altered network property and diffuse epileptogenesis may suggest the interplay between the two phenomena. Consistent with our inconclusive findings, presurgical evaluation of the patient conducted independently in the hospital resulted in a conclusion that the patient was not a good surgical candidate.
Fig 6.
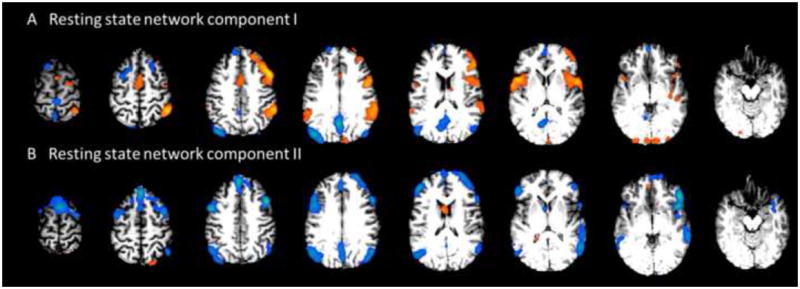
Results from Patient #8. A shows one component associated with the bilateral fronto-parietal association cortex, which represents a typical resting state network. Additionally, there are also clusters on the right lateral frontal and parietal regions. B shows another component associated with the bilateral network, with an additional cluster on the right fronto-parietal region. This patient was initially diagnosed with right parietal epilepsy. Presurgical ECoG revealed diffuse seizure onset involving a large region simultaneously. This patient was not selected to receive surgical resection.
Information and results of all patients were summarized in Table 1 with additional information provided in Supplementary Table S1. Out of ten patients studied, nine received surgery. Epilepsy related components found in seven patients were highly concordant with surgical resection. For the patient who did not receive surgery, our analysis showed activity lateralized to right parietal lobe, however, overlapping with widespread resting state network in bilateral parietal and frontal areas. Diffused epileptic activity is not suitable for surgical resection treatment. This finding is consistent with the surgeon’s decision not to operate on this particular patient. The algorithm did not identify any epilepsy related components in two other patients.
Healthy subjects with motor tasks
Results from healthy subjects performing motor tasks served as a preliminary evaluation of the sensitivity of the proposed algorithm. The method detected lateralized motor-task-related components from ICA with minimal supervision and high accuracy across all subjects. We examined the spatial and temporal features of the selected components to the timing of the task design. The temporal feature of the selected component has time course that correlated with the expected time course based on the known stimulus onset convolved with canonical hemodynamic function (HRF). The identified components have BOLD activities in the sensorimotor areas correspond to the left or right hand. Fig. 7 shows group-level average activation maps in the left motor area that corresponds to right hand movement across all healthy subjects. Fig. 7A shows the averaged map obtained from maps of identified motor task related ICs. Fig. 7B shows the averaged map obtained from GLM using expected time course derived from experiment design as the main regressor. Temporally, the Pearson’s correlation coefficient between the identified IC time course and the expected time course specified by the experiment is 0.72 ±0.08.
Fig. 7.
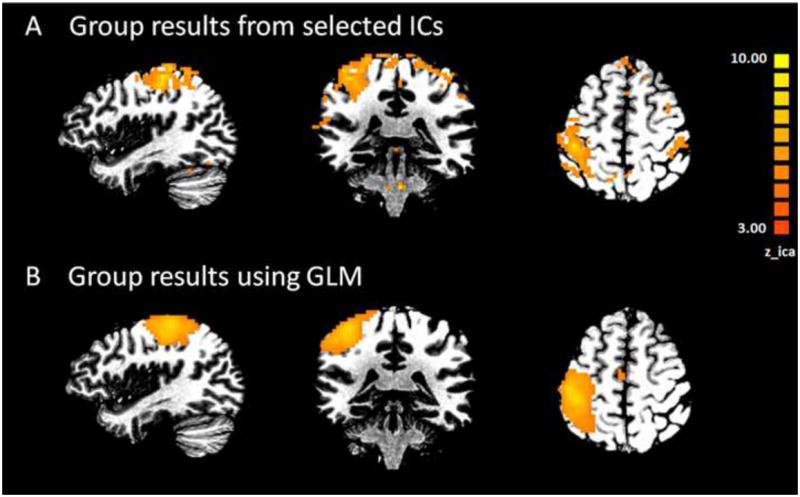
Group results in healthy subjects performing right hand motor tasks. A) Averaged map of selected independent components(IC) from all subjects. B) Group averaged activation map obtained from GLM analysis. Expected time course derived from experiment design was used as the main regressor.
Healthy subjects during Resting State
Results from seven healthy subjects during resting state served as a preliminary evaluation of the specificity of the proposed algorithm. Identical parameters were applied to this data set, and the data were processed to examine whether there were any components selected by the algorithm as epilepsy related. In four out of the seven subjects, the three objective criteria ruled out all the components, which reflected true negative results as expected. In the other three subjects, between one to three components were identified by the algorithm as potentially epilepsy related. They represented either additional noisy component (Fig. 8A), similar to that seen in Fig. 3E, that the algorithm was not able to screen out or lateralized physiological activities, for example in sensorimotor area (Fig. 8B). In one out of the three false positive cases, the only component identified was associated with noise. In two other cases, both a noisy component and a lateralized physiological component were identified. The nature and frequency of occurrence of such non-epilepsy component identified by the method is similar to what we observed in the patient group. Findings from this study can be used to guide further refinement of the algorithm in the future.
Fig. 8.
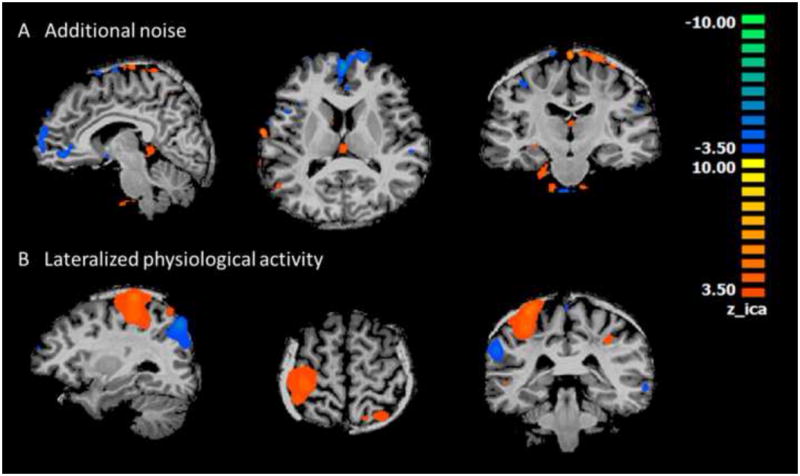
Examples of components that survived the three criteria in resting state healthy subjects. A) Typical noisy component with distributed pattern in small clusters through the brain but mostly near to the peripheral or along the midline. B) Unilateral physiological activity in sensorimotor area.
Discussion
For the purpose of both lateralizing and localizing hemodynamic foci in focal epilepsy patients, we proposed an automated algorithm that detects epilepsy related components from ICA with minimal supervision and high accuracy. Our algorithm was evaluated by comparing the identified components to surgical resection. In the current patient group we were able to lateralize and localize hemodynamic foci using proposed method reasonably well. We also tested the algorithm in healthy subjects with and without tasks and the results showed reasonable sensitivity and specificity of the method.
Our proposed method can be easily implemented in the current presurgical workup to provide additional information for guiding the surgical resection. The benefits are two folds. Firstly of all, the data currently used were collected as part of clinical routine for mapping of eloquent brain to preserve during the surgery. Therefore, to implement this method, no additional scans need to be prescribed. Secondly, the algorithm is automated and does not require subjective input or excessive training from the clinicians. However, at this stage, it is not meant to replace any aspect of the presurgical planning process, but rather to further inform each step of presurgical planning.
Method Applications
The spatial ICA algorithm allows for the computation of as many components as the number of time points. We explored a range of different numbers of components from twenty to forty five. The overall patterns of the components remained similar to the case of thirty components. But when a larger number of components were used, there were occasional splits of components in a few subjects. This will result in false positives by using the symmetry measure, as split components can be assymetrical but symmetrical when combined. Therefore, the number of components used in the ICA decomposition was set to thirty, as it represented a good tradeoff between computation efficiency and separation of components in our data set. The optimal number may vary among subjects and different groups, but we used thirty for all the subjects studied.
In the patients we studied, the surgical resection sizes are typically large, except in the case of one patient, #9, who received focal ablation. In the six patients where we found ‘overlapping’ clusters with surgical resection, four had temporal lobectomy, where the resection size is approximately 3.5 cm in length. The identified clusters in the four temporal cases are much smaller and fall within the surgical resection area. In patient #1, the clusters identified are comparable in size with the two separate resection areas. The degree of overlap is about 80%. In the case of patient #2, the identified cluster is roughly two times greater than the actual resection but covers the resection completely.
Method Assumptions
In this study, we aimed to identify epilepsy related components from components produced by spatial ICA of resting state fMRI. Characteristics of task related fMRI independent components (ICs) of healthy subjects were previously discussed by De Martino et al. (2007) and applied in the context of focal epilepsy (Rodionov et al., 2007). In our approach, instead of comprehensively studying all different types of ICs, we focused mainly on extracting epilepsy related components by applying three basic assumptions.
The first assumption is that neurological activity dominant components should locate mainly inside the brain volume. This was assessed by calculating the ratio between the numbers of voxels with activity inside vs. outside of the brain volume. Secondly, we focused on focal epilepsy patients and assumed that this group of patients have unilateral, epilepsy related BOLD foci. Most successful surgeries in epilepsy often involve patients with focal epilepsy because of the isolated epileptogenic zone. Therefore, it is clinically important to noninvasively identify suitable candidates as well as to localize epilepsy foci for resection. We thus targeted this group of patients with unilateral, instead of bilateral epilepsy related BOLD activities. With this condition, we could easily separate epilepsy related components from other neurophysiological components associated with auditory or occipital activation, resting-state networks, and major endovascular activities. These non-epilepsy components often have a symmetrical spatial distribution, which will lead to a high correlation score, Corr, when the signals between mirroring voxels of the left and right hemispheres were compared. The third assumption is that BOLD fluctuations due to neurological activity have a frequency range near to 0.01 to 0.1 Hz. Some noisy components may fall within the perimeter of the brain and have asymmetrical distribution, but these components often have dominant frequency fall below 0.01 Hz or above 0.1 Hz due to artifacts from aliasing of cardiac and respiration activity or scanner susceptibility.
In the first noise reduction step we used median Ri/o as cut-off, which represents thresholding at 50%. This may appear to be too aggressive. At this cut-off, the actual cut-off value of Ri/owas 0.74±0.28 among all patients. This means there are still a relatively large number of voxels outside of the brain comparing to inside of the brain at the cut-off level. Such a component may still be relatively noise dominant. Certainly an even more stringent cut-off value may further control the specificity of the algorithm, but it may also result in less sensitivity in detecting actual epilepsy related components. The current cut-off level, therefore, seems to be appropriate for this group studied.
The concept of lateralization has traditionally been used in epilepsy diagnosis. For example, scalp EEG is often used in clinic to initially lateralize epileptogenic zone and to guide placement of intracranial electrodes. In the application of fMRI, Negishi and colleagues (2011) found lateralized fMRI connectivity could serve as a predictor of the surgical outcome. When a patient has lateralized connectivity pattern, they found their surgical outcome is likely to be seizure free. In this study, we applied the same concept to the fMRI data analysis. By taking advantage of the spatial resolution of fMRI, we aimed to not only lateralize but also to localize epileptic activities in patients with focal epilepsy.
Model-free approach
A number of previous studies have explored the utility of fMRI alone, both ictal and interictal, using data driven approaches. Ictal fMRI studies reported have shown concordance between identified IC and seizure onset (LeVan et al., 2010; Thornton et al., 2010), but ictal events are hard to capture in a limited time window of the scanning time. Several other studies have reported using interictal resting state fMRI and compared the accuracy using simultaneously acquired EEG-fMRI in a general linear model (GLM) (Rodionov et al., 2007; LeVan et al., 2009, 2010; Moeller et al., 2011; Lopez et al., 2012). Unlike the aforementioned studies, we focused on the localization value of interictal fMRI without simultaneously acquired EEG in focal epilepsy. We compared our results with surgical resection and follow-up. Resection with seizure free outcome is considered as the ground truth for evaluation of the localization accuracy.
This data-driven, exploratory approach using ICA has several advantages. First of all, knowledge of the precise timing of interictal epileptic discharges (IEDs) is not required. Scalp EEG has a long history in the diagnosis of epilepsy. Abnormalities observed on EEG can be used to classify epilepsy types and lateralize epileptic area. However, in cases when EEG is absent, or when clear epileptiform discharges are not well formed, our results suggested that it is still possible to extract epilepsy related activities using fMRI analysis alone. Secondly, motion residual artifacts or various physiological noises do not need to be modeled explicitly. In conventional GLM-based fMRI analysis, movement parameters and other physiological noises need to be accounted for to improve the effects caused by epileptic activity. However, using ICA analysis, these noisy components are often well separated automatically. Thirdly, the complexity and variability of HRF can be circumvented. The HRF is the link between electrophysiological events and hemodynamic responses in the brain. There has been growing evidence suggesting variability of the HRF in different areas of the brain and among individuals. Simultaneously acquired EEG and fMRI can provide insight into the nature of the HRF, but for our specific purpose of assessing the localization value of fMRI-alone recording without EEG, the knowledge of HRF is not required. Furthermore, if simultaneously acquired EEG is available, this method can offer a way to study epilepsy specific HRF characteristics.
Resting-state network
Components representing the resting-state networks including default mode network (DMN) were found in all patients. Previous studies also showed that networks including the default mode network are involved in epileptic activity (Archer et al., 2003; Blumenfeld et al., 2004, 2009; Gotman et al., 2005; Laufs et al., 2007; Zijlmans et al., 2007) and other pathological brain diseases such as Alzheimer’s (Buckner et al., 2002; Greicius et al., 2004). However, the primary goal of our proposed method is to lateralize and localize the hemodynamic foci for presurgical evaluation purpose. Regions in the resting state network are hypothesized to be involved in multiple cognitive functions (Raichle et al., 2007) and are rarely the target for surgical removal. Therefore, we design the algorithm to detect areas that can be surgical targets instead of other brain networks that are not suitable for current surgical treatment, although those regions might also be impacted or involved in complicated epileptic activities. Our method identified the DMN components as neurological sources with high spatial signal to noise ratio, i.e. high Ri/o values. But the correlation coefficient indices of the DMN components are much higher than the selected component due to the symmetric nature, Corr=0.51± 0.10 for DMN components vs. 0.18 ± 0.09 for epilepsy foci components. As reported previously, DMN may be altered by epileptic events in both focal as well as generalized epilepsy (Blumenfeld et al., 2009; Gotman et al., 2005; Laufs et al., 2007; Voets et al., 2012; Zhang et al., 2010, 2011). Interestingly, in the case of Patient 8 we also found two components representing altered DMN or resting-state networks. In addition to activities in the typical bilateral fronto-parietal association cortex, there were also unilateral clusters in the right frontal and central parietal regions (Fig. 6A and 6B). This statistical dependency between the right central parietal and frontal clusters with the resting state network may shed some light in explaining this patient’s seizure characteristics. In the surgical report of this patient, it was mentioned that she had diffuse onset involving a large region simultaneously on ECoG and was therefore not suitable for operation. No abnormal pattern was found in other patients DMN components. The spatial and temporal features of DMN in focal and generalized epilepsy will be examined more thoroughly in future studies.
Study limitations
Although the proposed method worked reasonably well in the current patient group, where patients only had unilateral focal epilepsy, this method was not intended to be an all-encompassing approach that will detect all epilepsy foci in all focal epilepsy cases. The current method was designed to detect epileptic activities with unilateral origin. If we know ahead of time that the epileptic activities originate bilaterally, the method will not provide additional insight. Fortunately in partial epilepsy, there are a good portion of surgical patients with epileptogenic foci located unilaterally. It may also be able to detect bilateral multifocal epileptic activity if the distribution of the foci is not symmetrical. However, it may not be suitable for detecting epileptogenic foci located near to the midline or symmetrically in both hemispheres. Sometimes, the BOLD response of unilateral spike activity may appear to be bilateral. In these cases, our method will not capture such a component, which may explain why we did not detect an epilepsy related unilateral component in patients #5 and #10.
This method is also not perfect in rejecting all non-epilepsy components. As seen in both patient and healthy subject resting state results, there are a small number of non-epilepsy related components selected by the algorithm. These components can be largely summarized into two categories: 1) Additional noise that was not captured by the noise-reduction procedure. Such components often have distributed small clusters (Fig. 8A) often near to the peripheral of the brain boundary (Fig. 3E). A further improvement in the algorithm could potentially exclude such components. For example, the brain boundary can be slightly shrunk so that activities of the voxels in close vicinity of the boundary can be disregarded from evaluation, since they are often prone to residual movement artifacts. 2) Unilateral physiological activities that are mislabeled as epilepsy related (Fig. 8B). Such components may be resulted from unilateral activation in eloquent cortex. In patient #2 for example (Fig. 4A), the identified epilepsy component was in close vicinity to the left sensorimotor area. Fortunately in this case, additional evidence from ECoG, surgical resection and the seizure free outcome indicated the accuracy of the selection. But for future application, if the algorithm identifies an area overlapping with eloquent cortex, as we saw in two of the healthy subjects, additional care should be given to exclude false detection of other physiological activities that are not related to epilepsy.
One additional implicit assumption of this method is the symmetry of the anatomical structure. Because the symmetricity calculation was based on the relative location to the ACPC plane specified on the anatomical MRI. The current method may not be sensitive to slight contoured ACPC plane or small imbalance of anatomical sizes between the left and right hemispheres. But if the brain is largely asymmetrical due to prior surgery, a large lesion or congenital distortions, this method will not be applicable.
Conclusion
In the present study we proposed an ICA-based automated method to lateralize and localize hemodynamic foci in focal epilepsy patients for presurgical evaluation. Focal activities identified by our method were in concordant with surgical resection in majority cases studied. Our findings suggest the possibility of noninvasively and accurately localizing hemodynamic epileptic foci using fMRI alone in presurgical planning. Overall, this is a feasibility study to demonstrate the value of the proposed method. Additional features can be incorporated in the algorithm to improve reliability and performance. A larger patient population needs to be studied to test the broad applicability of this method. Our proposed method can be easily implemented in the current presurgical workup to provide additional information for guiding the surgical resection.
Supplementary Material
Highlights.
Spatial ICA on resting state fMRI revealed hemodynamic foci that are concordant with surgical resection.
A novel IC selection algorithm basing on the concept of lateralization was proposed.
Method was verified in healthy subjects with motor tasks and during resting state.
Acknowledgments
This work was supported in part by NIH EB006433, EB007920, EY023101, HL117664, and NS076408.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aragri A, Scarabino T, Seifritz E, Comani S, Cirillo S, Tedeschi G, et al. How does spatial extent of fMRI dataset affect independent component analysis decomposition? Hum. Brain Mapp. 2006;27:736–746. doi: 10.1002/hbm.20215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthurs OJ, Boniface S. How well do we understand the neural origins of the fMRI BOLD signal? Trends Neurosci. 2002;25:27–31. doi: 10.1016/s0166-2236(00)01995-0. [DOI] [PubMed] [Google Scholar]
- Bai X, Vestal M, Berman R, Negishi M, Spann M, Vega C, et al. Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. J Neurosci. 2010;30:5884–5893. doi: 10.1523/JNEUROSCI.5101-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillet S, Mosher JC, Leahy RM. Electromagnetic brain mapping. IEEE Signal Process Mag. 2001:14–30. [Google Scholar]
- Barbé K, Van Moer W, Nagels G. Fractional-Order Time Series Models for Extracting the Haemodynamic Response From Functional Magnetic Resonance Imaging Data. IEEE Trans Biomed Eng. 2012;59:2264–2272. doi: 10.1109/TBME.2012.2202117. [DOI] [PubMed] [Google Scholar]
- Baumgartner C, Pataraia E. Revisiting the role of magnetoencephalography in epilepsy. Curr Opin Neurol. 2006;19:181–186. doi: 10.1097/01.wco.0000218236.44969.67. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond, B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benar CG, Gross DW, Wang YH, Petre V, Pike B, Dubeau F, et al. The BOLD response to interictal epileptiform discharges. NeuroImage. 2002;17:1182–1192. doi: 10.1006/nimg.2002.1164. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, McNally KA, Vanderhill SD, Paige AL, Chung R, Davis K, et al. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 2004;14:892–902. doi: 10.1093/cercor/bhh048. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Varghese G, Purcaro M, Motelow J, Enev M, et al. Cortical and subcortical networks in human secondarily generalized tonic–clonic seizures. Brain. 2009;132:999–1012. doi: 10.1093/brain/awp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. Spatial and temporal independent component analysis of functional MRI data containing a pair of task-related waveforms. Hum Brain Mapp. 2001;13:43–53. doi: 10.1002/hbm.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Stevens MC, Kiehl KA, Pekar JJ. Semi-blind ICA of fMRI: a method for utilizing hypothesis-derived time courses in a spatial ICA analysis. NeuroImage. 2005;25:527–538. doi: 10.1016/j.neuroimage.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting state” data. Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- De Martino F, Gentile F, Esposito F, Balsi M, Di Salle F, Goebel R, et al. Classification of fMRI independent components using IC-fingerprints and support vector machine classifiers. NeuroImage. 2007;34:177–194. doi: 10.1016/j.neuroimage.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Deng F, Zhu D, Lv J, Guo L, Liu T. FMRI signal analysis using empirical mean curve decomposition. IEEE Trans Biomed Eng. 2013;60:42–54. doi: 10.1109/TBME.2012.2221125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Worrell GA, Lagerlund TD, He B. Ictal source analysis: localization and imaging of causal interactions in humans. NeuroImage. 2007;34:575–86. doi: 10.1016/j.neuroimage.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formisano E, Esposito F, Di Salle F, Goebel R. Cortex-based independent component analysis of fMRI time-series. Magn Reson Imaging. 2004;22:1493–1504. doi: 10.1016/j.mri.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima M, Yamashita O, Kanemura A, Ishii S, Kawato M, Sato MA. A state-space modeling approach for localization of focal current sources from MEG. IEEE Trans Biomed Eng. 2012;59:1561–1571. doi: 10.1109/TBME.2012.2189713. [DOI] [PubMed] [Google Scholar]
- Gotman J, Kobayashi E, Bagshaw AP, Benar CG, Dubeau F. Combining EEG and fMRI: a multimodal tool for epilepsy research. Magn Reson Imaging. 2006;23:906–920. doi: 10.1002/jmri.20577. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV. Magnetoencephalography—Theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys. 1993;65:413–497. [Google Scholar]
- Hamandi K, Salek-Haddadi A, Fish DR, Lemieux L. EEG/ functional MRI in epilepsy: the Queen Square experience. Clin Neurophysiol. 2004;21:241–248. doi: 10.1097/00004691-200407000-00002. [DOI] [PubMed] [Google Scholar]
- He B, Musha T, Okamoto Y, Homma S, Nakajima Y, Sato T. Electric dipole tracing in the brain by means of the boundary element method and its accuracy. IEEE Trans Biomed Eng. 1987;34:406–414. doi: 10.1109/tbme.1987.326056. [DOI] [PubMed] [Google Scholar]
- He B, Liu Z. Multimodal functional neuroimaging: integrating functional MRI and EEG/MEG. IEEE Rev Biomed Eng. 2008;1:23–40. doi: 10.1109/RBME.2008.2008233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Yang L, Wilke C, Yuan H. Electrophysiological imaging of brain activity and connectivity – challenges and opportunities. IEEE Trans Biomed Eng. 2011;58:1918–31. doi: 10.1109/TBME.2011.2139210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MD, Tucker DM, Quiring JM, Hakimian S, Miller JW, Ojemann JG. Comparing noninvasive dense array and intracranial electroencephalography for localization of seizures. Neurosurgery. 2010;66:354–362. doi: 10.1227/01.NEU.0000363721.06177.07. [DOI] [PubMed] [Google Scholar]
- Hyvärinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw. 1999;10:626–634. doi: 10.1109/72.761722. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Levan P, Moeller F, Boor R, Stephani U, Gotman J, Siniatchkin M. Hemodynamic changes preceding the interictal EEG spike in patients with focal epilepsy investigated using simultaneous EEG-fMRI. NeuroImage. 2009;45:1220–1231. doi: 10.1016/j.neuroimage.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Jiang H, Golay X, van Zijl PC, Mori S. Origin and minimization of residual motion-related artifacts in navigator-corrected segmented diffusion-weighted EPI of the human brain. Magn Reson Med. 2002;47:818–822. doi: 10.1002/mrm.10102. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ, et al. Motion correction and the use of motion covariates in multiple-subject fMRI analysis. Hum Brain Mapp. 2006;27:779–788. doi: 10.1002/hbm.20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koessler L, Benar C, Maillard L, Badier JM, Vignal JP, Bartolomei F, et al. Source localization of ictal epileptic activity investigated by high resolution EEG and validated by SEEG. Neuroimage. 2010;51:642–653. doi: 10.1016/j.neuroimage.2010.02.067. [DOI] [PubMed] [Google Scholar]
- Lai Y, Zhang X, van Drongelen W, Korhman M, Hecox K, Ni Y, et al. Noninvasive cortical imaging of epileptiform activities from interictal spikes in pediatric patients. Neuroimage. 2011;54:244–52. doi: 10.1016/j.neuroimage.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz G, Grave de Peralta R, Spinelli L, Seeck M, Michel C. Epileptic source localization with high density EEG: how many electrodes are needed? Clin Neurophysiol. 2003;114:63–69. doi: 10.1016/s1388-2457(02)00337-1. [DOI] [PubMed] [Google Scholar]
- Laufs H, Duncan JS. Electroencephalography/functional MRI in human epilepsy: what it currently can and cannot do. Curr Opin Neurol. 2007;20:417–423. doi: 10.1097/WCO.0b013e3282202b92. [DOI] [PubMed] [Google Scholar]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, et al. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci U S A. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Hamandi K, Salek-Haddadi A, Kleinschmidt AK, Duncan JS, Lemieux L. Temporal lobe interictal epileptic discharges affect cerebral activity in “default mode” brain regions. Hum Brain Mapp. 2006;28:1923–1932. doi: 10.1002/hbm.20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux L, Salek-Haddadi A, Josephs O, Allen P, Toms N, Scott C, et al. Event-related fMRI with simultaneous and continuous EEG: description of the method and initial case report. Neuroimage. 2001;14:780–787. doi: 10.1006/nimg.2001.0853. [DOI] [PubMed] [Google Scholar]
- LeVan P, Gotman J. Independent component analysis as a model-free approach for the detection of BOLD changes related to epileptic spikes: a simulation study. Hum Brain Mapp. 2009;30:2021–2031. doi: 10.1002/hbm.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVan P, Tyvaert L, Moeller F, Gotman J. Independent component analysis reveals dynamic ictal BOLD responses in EEG-fMRI data from focal epilepsy patients. Neuroimage. 2010;49:366–378. doi: 10.1016/j.neuroimage.2009.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVan P, Tyvaert L, Gotman J. Modulation by EEG features of BOLD responses to interictal epileptiform discharges. NeuroImage. 2010;50:15–26. doi: 10.1016/j.neuroimage.2009.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, He B. fMRI-EEG integrated cortical source imaging by use of time-variant spatial constraints. Neuroimage. 2008;39:1198–214. doi: 10.1016/j.neuroimage.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Kecman F, He B. Effects of fMRI-EEG mismatches in cortical current density estimation integrating fMRI and EEG Noninvasive cortical imaging of epileptiform activities from interictal spikes in pediatric patients: a simulation study. Clin Neurophysiol. 2006;117:1610–22. doi: 10.1016/j.clinph.2006.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes R, Lina JM, Fahoum F, Gotman J. Detection of epileptic activity in fMRI without recording the EEG. Neuroimage. 2012;60:1867–1879. doi: 10.1016/j.neuroimage.2011.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Yang L, Worrell GA, Brinkmann B, Nelson C, He B. Dynamic imaging of seizure activity in pediatric epilepsy patients. Clin Neurophysiol. 2012;123:2122–2129. doi: 10.1016/j.clinph.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown MJ, Jung T-P, Makeig S, Brown GG, Kindermann SS, Lee T-W, et al. Spatially independent activity patterns in functional magnetic resonance imaging data during the Stroop color-naming task. Proc Natl Acad Sci U S A. 1998;95:803–810. doi: 10.1073/pnas.95.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra PP, Ogawa S, Hu X, Ugurbil K. The nature of spatiotemporal changes in cerebral hemodynamics as manifested in functional magnetic resonance imaging. Magn Reson Med. 1997;37:511–518. doi: 10.1002/mrm.1910370407. [DOI] [PubMed] [Google Scholar]
- Moeller F, LeVan P, Gotman J. Independent component analysis (ICA) of generalized spike wave discharges in fMRI: comparison with general linear model based EEG-fMRI. Hum Brain Mapp. 2011;32:209–217. doi: 10.1002/hbm.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher JC, Leahy RM, Lewis PS. EEG and MEG: Forward solutions for inverse methods. IEEE Trans Biomed Eng. 1999;46:245–259. doi: 10.1109/10.748978. [DOI] [PubMed] [Google Scholar]
- Negishi M, Martuzzi R, Novotny E, Spencer DD, Constable RT. Functional MRI connectivity as a predictor of the surgical outcome of epilepsy. Epilepsia. 2011;52:1733–1740. doi: 10.1111/j.1528-1167.2011.03191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonomou VP, Blekas K, Astrakas L. A sparse and spatially constrained generative regression model for fMRI data analysis. IEEE Trans Biomed Eng. 2012;59:58–67. doi: 10.1109/TBME.2010.2104321. [DOI] [PubMed] [Google Scholar]
- Ray A, Tao JX, Hawes-Ebersole SM, Ebersole JS. Localizing value of scalp EEG spikes: a simultaneous scalp and intracranial study. Clin Neurophysiol. 2007;118:69–79. doi: 10.1016/j.clinph.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Rodionov R, De Martino F, Laufs H, Carmichael DW, Formisano E, Walker M, et al. Independent component analysis of interictal fMRI in focal epilepsy: comparison with general linear model-based EEG-correlated fMRI. Neuroimage. 2007;38:488–500. doi: 10.1016/j.neuroimage.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Hennel F, Mustovic H, Neuhoff JG, Bilecen D, et al. Spatiotemporal pattern of neuronal processing in the human auditory cortex. Science. 2002;297:1706–1708. doi: 10.1126/science.1074355. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Richard A, Menon RS. Noise reduction in BOLD based fMRI using component analysis. NeuroImage. 2002;17:1521–1537. doi: 10.1006/nimg.2002.1200. [DOI] [PubMed] [Google Scholar]
- Thornton R, Powell R, Lemieux L. fMRI in Epilepsy. In: Filippi M, editor. fMRI Techniques and protocols Neuromethods 41. Springer; 2009. pp. 681–735. [Google Scholar]
- Thornton RC, Rodionov R, Laufs H, Vulliemoz S, Vaudano A, Carmichael D, et al. Imaging haemodynamic changes related to seizures: comparison of EEG-based general linear model, independent component analysis of fMRI and intracranial EEG. NeuroImage. 2010;53:196–205. doi: 10.1016/j.neuroimage.2010.05.064. [DOI] [PubMed] [Google Scholar]
- van de Ven VG, Formisano E, Röder CH, Prvulovic D, Bitter RA, Dietz MG, et al. The spatiotemporal pattern of auditory cortical responses during verbal hallucinations. NeuroImage. 2005;27:644–655. doi: 10.1016/j.neuroimage.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Voets NL, Beckmann CF, Cole DM, Hong S, Bernasconi A, Bernasconi N. Structural substrates for resting network disruption in temporal lobe epilepsy. Brain. 2012;135:2350–2357. doi: 10.1093/brain/aws137. [DOI] [PubMed] [Google Scholar]
- Wang G, Worrell G, Yang L, Wilke C, He B. Interictal spike analysis of high-density EEG in patients with partial epilepsy. Clin Neurophysiol. 2010;122:1098–105. doi: 10.1016/j.clinph.2010.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tang Q, Zheng W. L1-norm-based common spatial patterns. IEEE Trans Biomed Eng. 2012;59:653–662. doi: 10.1109/TBME.2011.2177523. [DOI] [PubMed] [Google Scholar]
- Wu S, Swindlehurst A, Wang P, Nenadic Z. Efficient dipole parameter estimation in EEG systems with near-ML performance. IEEE Trans Biomed Eng. 2012;59:1339–1348. doi: 10.1109/TBME.2012.2187336. [DOI] [PubMed] [Google Scholar]
- Yang L, Wilke C, Brinkmann B, Worrell GA, He B. Dynamic imaging of ictal oscillations using non-invasive high-resolution EEG. Neuroimage. 2011;56:1908–17. doi: 10.1016/j.neuroimage.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lu G, Zhong Y, Tan Q, Chen H, Liao W, et al. fMRI study of mesial temporal lobe epilepsy using amplitude of low-frequency fluctuation analysis. Hum Brain Mapp. 2010;31:1851–1861. doi: 10.1002/hbm.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liao W, Chen H, Mantini D, Ding JR, Xu Q, et al. Altered functional-structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain. 2011;134:2912–2928. doi: 10.1093/brain/awr223. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Huiskamp G, Hersevoort M, Seppenwoolde J-H, van Huffelen AC, Leijten FSS. Brain. 2007;130:2343–2353. doi: 10.1093/brain/awm141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


