Abstract
Combining multiple genetic variants related to obesity into a genetic risk score (GRS) might improve identification of individuals at risk of developing obesity. Moreover, characterizing gene-diet interactions is a research challenge to establish dietary recommendations to individuals with higher predisposition to obesity. Our objective was to analyze the association between an obesity GRS and BMI in the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) population, focusing on gene-diet interactions with total fat and saturated fatty acid (SFA) intake and to replicate findings in Multi-Ethnic Study of Atherosclerosis (MESA) population. Cross-sectional analyses included 783 US Caucasian participants from GOLDN and 2035 from MESA. Dietary intakes were estimated with validated food frequency questionnaires. Height and weight were measured. A weighted GRS was calculated on the basis of 63 obesity-associated variants. Multiple linear regression models adjusted by potential confounders were used to examine gene-diet interactions between dietary intake (total fat and SFA) and the obesity GRS in determining BMI. Significant interactions were found between total fat intake and the obesity GRS using these variables as continuous for BMI (P for interaction=0.010, 0.046, and 0.002 in GOLDN, MESA and meta-analysis, respectively). These association terms were stronger when assessing interactions between SFA intake and GRS for BMI (P for interaction=0.005, 0.018, and <0.001 in GOLDN, MESA and meta-analysis, respectively). SFA intake interacts with an obesity GRS in modulating BMI in two US populations. Although to determine the causal direction requires further investigation, these findings suggest that potential dietary recommendations to reduce BMI effectively in populations with high obesity GRS would be to reduce total fat intake mainly by limiting SFAs.
Keywords: body mass index, genetic risk score, saturated fat, saturated fatty acids, obesity
INTRODUCTION
The worldwide obesity epidemic has become a tremendous health challenge. Susceptibility to obesity is determined by a combination of genetic factors with dietary and other behavioral factors. The genetic contribution to interindividual variation in common obesity has been estimated at 40–70%.1 Diet is generally recognized to be one of the most important vehicles for obesity prevention and treatment. Although investigation continues into dietary contributors to obesity, excess energy intake and, particularly, increased amounts of fat may promote obesity because fat is the most energy dense and, according to some studies, the least satiating macronutrient.2,3 For the time being, current dietary recommendations alone to reduce obesity are failing to provide effective long-term solutions to this epidemic. Since 2007 genome-wide association studies (GWAS) have identified several genetic variants that are frequently associated with obesity and its related traits (BMI, weight, waist, hip and waist-hip ratio),4 but few gene-diet interactions for these traits have been described.5 Thus, characterizing gene-diet interactions for obesity is an important research challenge that may facilitate the identification and eventual application of targeted dietary recommendations to individuals with higher predisposition to obesity.
Although a number of genes have obesity associations, few have been shown definitively to interact with diet for the outcome of BMI. For instance, a variant in the FTO gene showed significant interactions for BMI with intake of total dietary fat,6,7 SFAs,7,8 MUFAs7 and PUFAs to SFAs ratio.8 A variant in the PPARG gene displayed significant interactions with dietary fat9 and MUFA intake10, 11 on BMI and weight change.11 The influence of the APOA2 -265T>C polymorphism on body-weight-related measures was modulated by SFA intake in different populations.12,13
The gene-diet interactions described above are each limited by their consideration of a single genetic variant examined in isolation in relation to obesity. Each obesity locus identified by empirical studies of GWAS explains only a small fraction of the variation in BMI. Thus, combining multiple loci with modest effects into a global GRS is thought to improve identification of individuals at risk of developing obesity. Although a GRS-by-sugar-sweetened beverages interaction in relation to BMI and obesity risk was published recently,14 no prior studies have observed significant interactions between an obesity GRS and dietary fat on BMI.15 Based on previously reported individual gene-diet interactions, we hypothesized that dietary fat (particularly SFAs) may be important in modulating the effects of aggregated single nucleotide polymorphisms (SNPs) using an obesity GRS. Therefore, the objective of this study was to analyze the association between an obesity GRS and BMI in the GOLDN study, with a focus on gene-diet interactions with total fat and SFA intake and to study the replication of these gene-diet interactions in another US population from the Multi-Ethnic Study of Atherosclerosis (MESA).
SUBJECTS AND METHODS
Study population
2817 participants from two US populations were studied. All participants provided written informed consent.
The GOLDN study population comprised 782 participants (aged 49 ± 16 years) recruited from three-generational pedigrees from two National Heart, Lung, and Blood Institute Family Heart Study field centers (Minneapolis, MN, and Salt Lake City, UT). The study included entirely individuals of European origin. The detailed design and methodology of the study are published.16 The protocol was approved by the Institutional Review Boards at the University of Alabama at Birmingham, the University of Minnesota, the University of Utah, and Tufts University.
The MESA study population consisted of 2035 Caucasian participants (aged 63 ± 10 years) recruited from six US communities (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan, New York; and St. Paul, Minnesota). Detailed descriptions of the study design and methods are published.17 The protocol was approved by the institutional review board of each study center.
Anthropometric and laboratory measurements
Anthropometric data including height, weight, waist circumference, and waist-to-hip ratio were measured by standard techniques.16,17 BMI was calculated as weight in kilograms divided by height in meters squared. Obesity was defined as a BMI ≥30kg/m2. Blood samples were drawn after fasting overnight. Detailed laboratory methods have been described.16,18
Dietary intake, physical activity and other lifestyle variables
Dietary intake was estimated by the validated Diet History Questionnaire in GOLDN19,20 and a food frequency questionnaire modified from the Insulin Resistance Atherosclerosis Study instrument in MESA.21,22 GOLDN participants with total daily energy intake outside the range 800-5500 kcal in men and 600-4500 kcal in women were excluded from analysis.16 MESA participants with implausible energy intakes, defined by consuming >6000 kcal/day or <600 kcal/day, were excluded.23 3.5% and 8.4% of GOLDN and MESA participants, respectively, had energy intakes outside the indicated range. Physical activity in GOLDN was assessed with a nonvalidated questionnaire.16 In MESA, physical activity was measured using a detailed, semiquantitative questionnaire adapted from the Cross-cultural Activity Participation Study,24 which captured total intentional exercise in MET-minutes/week. Questionnaires were administered to assess lifestyle and demographic information, medication use, and medical history in both populations. Lifestyle data also included the number of hours per day spent viewing television or using a computer (“screen time”) in GOLDN and television viewing time in MET-minutes/week in MESA.24,25
Genetic analysis
Genomic DNA was extracted from peripheral blood lymphocytes by standard methods. We selected 60 single nucleotide polymorphisms (SNPs) that showed significant genome-wide association with obesity phenotypes4 and four SNPs on the basis of data from empirical evidence with gene-diet interactions for BMI or obesity related measures or that were strongly associated with BMI: rs4073054 (APOA2) (proxy for rs5082; r2=0.93),12,13 rs1801282 (PPARG),9-11 rs2972162 (PPARG) (proxy for rs2938392; r2=1.00)26 and rs4580704 (CLOCK). 27 Proxy SNPs were obtained from the SNP Annotation and Proxy Search tool employing 1000 Genomes Pilot 1 data.28 Genotype data were produced using the Affymetrix 6.0 platform in both GOLDN and MESA. SNP descriptions are presented in Table 1. Genotype frequency distributions were consistent with Hardy-Weinberg equilibrium (HWE) (P>0.01).
Table 1.
Genotypic information for the obesity GRS calculation (Continued)
|
GOLDN study
|
MESA study
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nearest Gene | Chr | SNP | Allele major/minor | Risk allele | β coefficient ± SEa | P-valueb | MAF | SNP | r2 (proxy SNP) | Allele major/minor | Risk allele | β coefficient ± SEa | P-valueb | MAF |
| CPEB4 | 5 | rs6861681 | G/A | G | 0.2536 ± 0.2749 | 0.358 | 0.35 | rs966544 | 1.000 | A/G | G | 0.1794 ± 0.1699 | 0.291 | 0.30 |
| PCSK1 | 5 | rs6234 | C/G | C | 0.3749 ± 0.3318 | 0.266 | 0.28 | rs7713317 | 1.000 | A/G | G | 0.3190 ± 0.1773 | 0.072 | 0.28 |
| ZNF608 | 5 | rs4836133 | A/C | A | 0.1450 ± 0.2785 | 0.607 | 0.50 | rs6864049 | 1.000 | G/A | G | 0.2153 ± 0.1535 | 0.161 | 0.46 |
| FLJ35779 | 5 | rs2112347 | T/G | T | 0.4647 ± 0.3149 | 0.147 | 0.37 | rs3797580 | 1.000 | T/C | T | 0.0153 ± 0.1677 | 0.927 | 0.37 |
| PCSK1 | 5 | rs6232 | A/G | G | 0.9962 ± 0.7755 | 0.223 | 0.06 | - | - | - | - | - | - | - |
| A1F1 | 6 | rs2844479 | T/G | T | 0.1107 ± 0.3416 | 0.746 | 0.36 | - | - | - | - | - | - | - |
| VEGFA | 6 | rs6905288 | A/G | A | 0.1332 ± 0.2542 | 0.601 | 0.43 | rs6905288 | A/G | A | 0.1038 ± 0.1587 | 0.513 | 0.41 | |
| RSPO3 | 6 | rs9491696 | G/C | C | 0.2345 ± 0.2805 | 0.409 | 0.49 | rs9482771 | 1.000 | C/G | G | 0.1114 ± 0.1565 | 0.477 | 0.47 |
| HMGA1 | 6 | rs206936 | A/G | G | 0.2715 ± 0.3394 | 0.416 | 0.16 | rs3798560 | 1.000 | T/C | C | 0.0661 ± 0.1959 | 0.736 | 0.21 |
| PRL | 6 | rs4712652 | A/G | G | 0.3233 ± 0.3015 | 0.286 | 0.43 | rs9366426 | 0.834 | C/T | T | 0.1372 ± 0.1579 | 0.385 | 0.45 |
| LY86 | 6 | rs1294421 | G/T | G | 0.3515 ± 0.2609 | 0.184 | 0.39 | rs1294421 | - | G/T | G | 0.0287 ± 0.1630 | 0.860 | 0.39 |
| TFAP2B | 6 | rs987237 | A/G | G | 0.7510 ± 0.3346 | 0.024 | 0.19 | rs987237 | - | A/G | G | 0.0717 ± 0.2138 | 0.738 | 0.17 |
| NFE2L3 | 7 | rs1055144 | G/A | A | 0.6590 ± 0.4040 | 0.102 | 0.20 | rs1055144 | - | G/A | A | 0.1140 ± 0.2100 | 0.587 | 0.17 |
| TNKS | 8 | rs17150703 | G/A | G | 0.4493 ± 0.5057 | 0.372 | 0.10 | rs17150703 | - | G/A | A | 0.2414 ± 0.2723 | 0.376 | 0.09 |
| LRRN6C | 9 | rs10968576 | A/G | G | 0.1105 ± 0.2968 | 0.710 | 0.28 | rs16912921 | 1.000 | C/A | A | 0.0750 ± 0.1700 | 0.659 | 0.31 |
| PTER | 10 | rs10508503 | C/T | C | 1.1912 ± 0.4354 | 0.014 | 0.10 | rs10508503 | - | C/T | C | 0.0291 ± 0.2653 | 0.913 | 0.10 |
| BDNF | 11 | rs6265 | G/A | G | 0.2110 ± 0.3573 | 0.554 | 0.22 | rs6265 | - | G/A | G | 0.1539 ± 0.1904 | 0.419 | 0.20 |
| MTCH2 | 11 | rs10838738 | A/G | A | 0.2473 ± 0.2919 | 0.406 | 0.37 | rs4752856 | 1.000 | G/A | A | 0.0302 ± 0.1703 | 0.859 | 0.35 |
| BDNF | 11 | rs925946 | G/T | T | 0.3625 ± 0.3343 | 0.283 | 0.30 | rs7124442 | 1.000 | T/C | C | 0.1769 ± 0.1751 | 0.313 | 0.29 |
| TUB | 11 | rs4929949 | C/T | C | 0.4956 ± 0.3032 | 0.111 | 0.50 | rs9300092 | 0.966 | A/G | G | 0.0715 ± 0.1615 | 0.658 | 0.47 |
| HOXC13 | 12 | rs1443512 | C/A | C | 0.0772 ± 0.3090 | 0.803 | 0.23 | rs1822438 | 0.947 | C/A | C | 0.0300 ± 0.1841 | 0.871 | 0.25 |
| FA1M2 | 12 | rs7138803 | G/A | G | 0.1046 ± 0.3640 | 0.774 | 0.40 | rs7138803 | - | G/A | A | 0.3076 ± 0.1577 | 0.051 | 0.40 |
| ITPR2/SSPN | 12 | rs718314 | T/C | T | 0.1407 ± 0.3603 | 0.697 | 0.26 | rs10842708 | 1.000 | A/G | G | 0.0224 ± 0.1779 | 0.900 | 0.26 |
Abbreviations: Chr, chromosome; SNP; single nucleotide polymorphism; MAF, minor allele frequency.
Effect sizes in kg/m2 for BMI obtained from the GOLDN population (n=782) and from MESA population (n=2035).
P-value indicates association between the SNP and BMI using generalized linear regression model for GOLDN (GENMOD procedure) adjusted by family relationships, gender, age and center; and general linear regression model for MESA studies adjusted by population structure, gender, age and center.
Obesity genetic risk score calculation
The obesity GRS was calculated on the basis of 59 of 60 established SNPs associated with obesity phenotypes4 and four previously described SNPs. From 60 established SNPs,4 rs2074356 (C12orf51) was omitted from the GRS calculation because it was monomorphic. Therefore, the GRS was calculated with 63 SNPs related to obesity phenotypes using a weighting method described previously.14 GRS may reflect the effect of epistasis, which may be specific to each population. Thus, the effect size of each risk allele was calculated within each population. Scores ranged from 0 to 126 and higher scores indicated a greater genetic risk of obesity. Each SNP genotype was coded as 0, 1, and 2 according to the number of risk alleles and weighted by its relative effect size (β-coefficient), obtained for each SNP on BMI in the GOLDN population (Table 1). The GRS was calculated by multiplying each β-coefficient by the number of corresponding risk alleles and then summing the products. This produced a possible maximum score of 47.56 (twice the sum of the reported β-coefficients), and thus all values were divided by 47.56 and multiplied by 126 so that the GRS closely approximates one point for each risk allele. In MESA, the GRS was calculated on the basis of 55 of 60 established SNPs associated with obesity phenotypes4 and four other SNPs: rs4073054 (APOA2), rs1801282 (PPARG), rs2972162 (PPARG) and rs4580704 (CLOCK). Multiplying each β-coefficient by the number of corresponding risk alleles and then summing the products produced a score of 19.34. Therefore, all values were divided by 19.34 and multiplied by 118.
Statistical analysis
Continuous variables were examined for normality. The relationship between obesity GRS, fat intake and biochemical measures were evaluated using analysis of variance techniques. We used the generalized estimating equation approach with exchangeable correlation structure implemented in the SAS GENMOD procedure to adjust for familial relationships in GOLDN. R2 was calculated to estimate the proportion of variation in BMI using GENMOD procedure for GOLDN adjusted by family relationships, gender, age and center; and general linear regression model for MESA study adjusted by population structure (using 10 principal components), gender, age and center. To study gene-diet interactions in determining BMI and other obesity phenotypes (waist circumference and waist-to-hip ratio), we used a multivariate interaction model using GENMOD procedure adjusted by family relationships, gender, age, center, tobacco smoking (current vs. former/never), alcohol consumption (current vs. former/never), hyperlipidemia medication use, presence of diabetes, physical activity and total energy intake in the GOLDN study, and a general linear regression model adjusted by population structure and the same covariates mentioned above, except family relationships, in MESA. The GRS, BMI (and waist circumference) and dietary variables were evaluated continuously and SFA intake was also evaluated categorically. A prior study29 found no significant population structure in GOLDN, allowing this term to be omitted from the model as a potential confounder. To construct the categorical variable, SFA intakes expressed as percent total daily energy intake were classified into two groups (low or high) according to the median score of the population. SAS software (version 9.2 of the SAS System for Windows, 2008, SAS Institute Inc, Cary, NC, USA) was used to analyze data. The combined analyses were performed using a weighted inverse normal method via the function “metagen”, with a fixed effect, using the META R package (version 2.15.3 for Windows, 2013, R Foundation for Statistical Computing, Vienna, Austria). A P value of 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Genotypic information
The genotypic information for the obesity GRS calculation is described in Table 1. Variation in BMI explained by each SNP ranged from 0.13 to 1.89% and 0.01 to 0.56% in GOLDN and MESA populations, respectively; whereas the variation by the obesity GRS was 11.09% in GOLDN and 3.72% in MESA. The difference in heritability between the two populations can be attributed to the family structure in GOLDN vs. the unrelated individuals in MESA. It is well established that heritability estimated in family-based populations is generally higher than that based on unrelated individuals.30 The reason for this difference is that genetic variance estimated using a family-based population contains (co)variances of gene x gene and gene x environment interactions (reflecting epigenetic contribution as well). These covariances are less likely to occur in populations of unrelated individuals.
Anthropometric, lifestyle and dietary measurements
Anthropometric, lifestyle and dietary characteristics in the entire population and stratified according to obesity status for both populations are shown in Table 2. In GOLDN (N=782), the mean BMI was 28.5 kg/m2 and 34.5% were obese, whereas in MESA (N=2035) the mean BMI was 27.9 kg/m2 and 28.0% were obese. In both populations percentages of total fat, SFA, MUFA and PUFA intakes were higher and percentage of carbohydrate intake was lower in obese compared to non-obese participants. Obese participants showed higher screen time than non-obese.
Table 2.
Anthropometric, biochemical, lifestyle and dietary characteristics in the entire population and according to obesity statusa
|
GOLDN study
|
MESA study
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Entire population |
Non-obese (BMI<30 kg/m2) |
Obese (BMI≥30 kg/m2) |
P-valueb | Entire population |
Non-obese (BMI<30 kg/m2) |
Obese (BMI≥30 kg/m2) |
P-valuec | |
| mean ± SE | mean ± SE | mean ± SE | mean ± SE | mean ± SE | mean ± SE | |||
| N for population (men/women) | 782 (383/399) | 512 (253/259) | 270 (130/140) | 2035 (1026/1009) | 1465 (740/725) | 570 (286/284) | ||
| Age, y | 48.9 ± 0.6 | 46.7 ± 0.7 | 53.5 ± 1.1 | <0.001 | 62.9 ± 0.2 | 63.4 ± 0.3 | 61.2 ± 0.5 | <0.001 |
| Weight, kg | 83.7 ± 0.6 | 76.1 ± 0.6 | 99.5 ± 1.0 | <0.001 | 80.2 ± 0.4 | 73.3 ± 0.3 | 97.3 ± 0.5 | <0.001 |
| Waist, cm | 97.1 ± 0.6 | 90.0 ± 0.6 | 112.0 ± 0.9 | <0.001 | 98.3 ± 0.3 | 92.4 ± 0.3 | 114.1 ± 0.5 | <0.001 |
| Waist-to-hip ratio | 0.90 ± 0.01 | 0.87 ± 0.01 | 0.95 ± 0.01 | <0.001 | 0.92 ± 0.01 | 0.91 ± 0.01 | 0.97 ± 0.01 | <0.001 |
| BMI, kg/m2 | 28.5 ± 0.2 | 25.6 ± 0.2 | 34.3 ± 0.3 | <0.001 | 27.9 ± 0.1 | 25.3 ± 0.1 | 34.1 ± 0.1 | <0.001 |
| Systolic blood pressure, mmHg | 115.6 ± 0.6 | 112.1 ± 0.8 | 121.9 ± 1.2 | <0.001 | 123.8 ± 0.5 | 121.7 ± 0.5 | 128.3 ± 0.8 | <0.001 |
| Diastolic blood pressure, mmHg | 68.4 ± 0.3 | 67.1 ± 0.4 | 70.9 ± 0.6 | <0.001 | 70.2 ± 0.2 | 69.8 ± 0.3 | 71.4 ± 0.4 | <0.001 |
| Cholesterol, mg/dLd | 192.3 ± 1.5 | 189.6 ± 2.3 | 200.5 ± 2.7 | <0.001 | 0195.8 ± 0.8 | 195.0 ± 1.2 | 196.2 ± 1.5 | 0.507 |
| LDL-cholesterol, mg/dLd | 123.5 ± 1.2 | 120.4 ± 1.5 | 132.4 ± 1.9 | <0.001 | 117.3 ± 0.8 | 116.1 ± 0.8 | 117.3 ± 1.5 | 0.448 |
| HDL-cholesterol, mg/dLd | 46.4 ± 0.4 | 48.0 ± 0.8 | 43.3 ± 0.8 | <0.001 | 52.2 ± 0.4 | 54.6 ± 0.4 | 47.6 ± 0.8 | <0.001 |
| Triglycerides, mg/dLe | 140.9 ± 3.5 | 131.1 ± 4.4 | 165.7 ± 8.0 | 0.001 | 133.8 ± 1.8 | 120.5 ± 2.7 | 158.6 ± 4.4 | <0.001 |
| Fasting glucose, mg/dLf | 101.9 ± 0.7 | 98.6 ± 0.7 | 108.5 ± 1.6 | <0.001 | 91.4 ± 0.5 | 88.6 ± 0.5 | 98.1 ± 0.9 | <0.001 |
| Total energy intake, kcal/d | 2051.1 ± 30.5 | 2038.1 ± 39.1 | 2067.7 ± 51.2 | 0.615 | 1600.7 ± 15.6 | 1541.0 ± 19.5 | 1708.0 ± 29.8 | <0.001 |
| Total fats, %g | 35.6 ± 0.2 | 35.0 ± 0.3 | 36.5 ± 0.4 | 0.005 | 32.2 ± 0.2 | 31.5 ± 0.2 | 33.3 ± 0.3 | <0.001 |
| SFAs, %g | 11.8 ± 0.1 | 11.7 ± 0.1 | 12.1 ± 0.2 | 0.031 | 10.8 ± 0.1 | 10.5 ± 0.1 | 11.4 ± 0.1 | <0.001 |
| MUFAs, %g | 13.4 ± 0.1 | 13.2 ± 0.1 | 13.7 ± 0.2 | 0.006 | 12.3 ± 0.1 | 12.1± 0.1 | 12.7 ± 0.1 | <0.001 |
| PUFAs, %g | 7.7 ± 0.1 | 7.6 ± 0.1 | 8.0 ± 0.1 | 0.024 | 6.3 ± 0.1 | 6.3 ± 0.1 | 6.4 ± 0.1 | 0.205 |
| Proteins, %g | 15.8 ± 0.1 | 15.7 ± 0.1 | 16.1 ± 0.2 | 0.093 | 15.6 ± 0.1 | 15.6 ± 0.1 | 15.8 ± 0.1 | 0.261 |
| Carbohydrates, %g | 48.9 ± 0.3 | 49.7 ± 0.4 | 47.6 ± 0.5 | 0.002 | 50.8 ± 0.2 | 51.1 ± 0.3 | 50.0 ± 0.4 | 0.016 |
| Screen timeh | 2.8 ± 0.1 | 2.7 ± 0.1 | 3.0 ± 0.1 | 0.032 | 822.2 ± 13.5 | 769.5 ± 17.3 | 983.7 ± 26.5 | <0.001 |
| Physical activityi | 34.0 ± 0.2 | 34.0 ± 0.3 | 33.9 ± 0.4 | 0.813 | 1687.5 ± 50.3 | 1927.6 ± 66.5 | 1283.4 ± 101.5 | <0.001 |
| Current smoking, n (%) | 63 (8.1) | 48 (9.4) | 15 (5.6) | 0.172 | 208 (10.2) | 157.0 (10.7) | 51 (9.0) | 0.166 |
| Current drinking, n (%) | 393 (50.3) | 270 (52.7) | 123 (45.6) | 0.207 | 1456 (77.9) | 1081.0 (80.0) | 375 (72.5) | 0.004 |
| Lipid medication use, n (%) | 34 (4.4) | 18 (3.5) | 16 (5.9) | 0.900 | 367 (18.0) | 238.0 (16.3) | 129 (22.6) | <0.001 |
| Diabetes mellitus, n (%) | 60 (7.7) | 22 (4.3) | 38 (14.1) | 0.002 | 89 (4.4) | 37.0 (2.5) | 52 (9.1) | <0.001 |
| Obesity GRS | 68.9 ± 0.2 | 67.7 ± 0.4 | 71.0 ± 0.4 | <0.001 | 59.2 ± 0.2 | 58.4 ± 0.2 | 60.6 ± 0.3 | <0.001 |
Abbreviations: BMI, body mass index; LDL-cholesterol, low-density lipoprotein cholesterol; HDL-cholesterol, high-density lipoprotein cholesterol; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; GRS, genetic risk score.
Values are expressed as mean ± SE or n (%).
P-value in GOLDN study was calculated by using the generalized estimating equation approach with exchangeable correlation structure implemented in the SAS GENMOD procedure adjusted by familial relationships, gender, age and center.
cP-value in MESA study was calculated by using a general linear regression model adjusted by population structure, gender, age and center.
To convert mg/dL cholesterol to mmol/L, multiply mg/dL by 0.026. To convert mmol/L cholesterol to mg/dL, multiply mmol/L by 38.7.
To convert mg/dL triglyceride to mmol/L, multiply mg/dL by 0.0113. To convert mmol/L triglyceride to mg/dL, multiply mmol/L by 88.6.
To convert mg/dL glucose to mmol/L, multiply mg/dL by 0.0555. To convert mmol/L glucose to mg/dL, multiply mmol/L by 18.0.
%“ refers to total daily energy intake.
“Screen time” refers to the number of hours/day spent viewing television or using a computer in GOLDN study and light leisure TV (MET-minutes/week) in MESA study.
“Physical activity” was measured by a physical activity score in the GOLDN study and self-reported intentional physical activity (MET-minutes/week) in MESA study (as described in Methods).
Nutrient-obesity GRS interactions
Significant interactions were found between total fat intake and the obesity GRS using these variables as continuous for BMI (P for interaction=0.010, 0.046, and 0.002 in GOLDN, MESA, and meta-analysis, respectively) (Table 3). These association terms were stronger when assessing interactions between SFA intake and GRS for BMI (P for interaction=0.005, 0.018, and <0.001 in GOLDN, MESA, and meta-analysis, respectively). For the meta-analysis, there was no evidence of statistical heterogeneity between studies (Cochran's Q statistic P>0.05). Significant interactions were observed between total fat and SFA intake and the GRS for waist circumference (P for interaction=0.003 and 0.019, respectively) and waist-to-hip ratio (P for interaction=0.046 and 0.027, respectively) in GOLDN but not MESA (data not shown). As intake of SFAs (percent total daily energy intake) correlated positively with MUFA intakes in GOLDN (r=0.66, P<0.001) and MESA (r=0.75, P<0.001), significant interactions between MUFA intake and the GRS were also observed for BMI (P for interaction=0.005 and 0.047 in GOLDN and MESA, respectively).
Table 3.
Dietary fat interactions with the obesity GRS for BMI (kg/m2) in GOLDN and MESA populations and in both populations meta-analyzed
|
GOLDN study (n=782)
|
MESA study (n=2035)
|
Meta-analysisa
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Difference in BMI |
Difference in BMI |
Difference in BMI |
||||||||
| β coefficient | SE | P for interactionb | β coefficient | SE | P for interactionc | β coefficient | SE | P for interaction | Q | |
| Total fats, % | 0.0127 | 0.0046 | 0.010 | 0.0048 | 0.0024 | 0.046 | 0.0065 | 0.0057 | 0.002 | 0.128 |
| SFAs, % | 0.0325 | 0.0108 | 0.005 | 0.0126 | 0.0053 | 0.018 | 0.0165 | 0.0047 | <0.001 | 0.098 |
| MUFAs, % | 0.0279 | 0.0120 | 0.021 | 0.0117 | 0.0059 | 0.047 | 0.0149 | 0.0053 | 0.005 | 0.226 |
| PUFAs, % | 0.0179 | 0.0136 | 0.194 | 0.0020 | 0.0100 | 0.845 | 0.0076 | 0.0081 | 0.347 | 0.346 |
Abbreviations: BMI, body mass index; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
Estimated from the combined analyses using a weighted inverse normal method via the function “metagen”, with a fixed effect, using META R package; Q = Cochran‘s Q statistic.
Multivariate interaction model using SAS GENMOD procedure adjusted by familial relationships, gender, age, center, tobacco smoking (current vs. former/never), alcohol consumption (current vs. former/never), hyperlipidemia medication use, presence of diabetes, physical activity score and total energy intake.
General linear regression model adjusted by population structure, gender, age, center, tobacco smoking (current vs. former/never), alcohol consumption (current vs. former/never), hyperlipidemia medication use, presence of diabetes, physical activity and total energy intake.
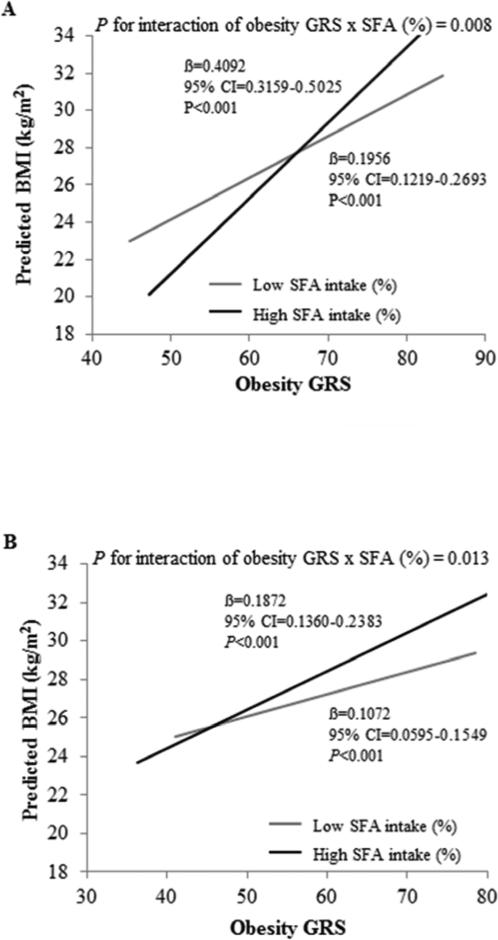
To understand the effect of the interaction between SFA intake and the GRS, SFA intake was evaluated categorically according to low and high intake based on the population median (interquartile range): 11.83 (9.96-13.51) and 10.68 (8.34-13.01) in GOLDN and MESA, respectively; and significant interactions were observed with the GRS for BMI (P for interaction=0.008 in GOLDN; 0.013 in MESA). Participants with a high SFA intake (≥11.8 and ≥10.7%, in GOLDN and MESA, respectively) and high GRS were associated with higher BMI compared to participants with low SFA intake (Figure 1A and Figure 1B). When SFA intake was low, a 0.20 versus 0.11 unit increase in BMI (in GOLDN versus MESA) was predicted for every unit increase in the GRS (P<0.001) (in both populations); whereas when SFA intake was high, a 0.41 and 0.19 unit increase in BMI was predicted (P<0.001). Examining the impact of the GRS on BMI according to SFA intake level, we observed that for a GRS of 62, high SFA intake was predicted to be 0.72 versus 0.94 units lower in BMI than low SFA intake in GOLDN versus MESA. However, for a GRS of 75, high SFA intake was predicted to be 1.50 versus 2.08 units in BMI higher than low SFA intake in GOLDN versus MESA. Similar results were observed for waist circumference but only in GOLDN (data not shown).
Figure 1.
Interaction between the obesity GRS and SFA intake (% total daily energy intake) for BMI. (A) Interaction between the obesity GRS and SFA intake (% total daily energy intake) for BMI in GOLDN. Predicted values of BMI and waist circumference by the obesity GRS are shown according to SFA intake (as a dichotomous variable). Predicted values were calculated from the regression models that contain SFA intake (as a categorical variable, 2 levels based on the population median (interquartile range): 11.82 (9.96-13.51), the obesity GRS (as continuous), their interaction term, and the potential confounders including familial relationships, gender, age, center, tobacco smoking (current vs. former/never), alcohol consumption (current vs. former/never), hyperlipidemia medication use, presence of diabetes, physical activity and total energy intake (in both studies). P-value for interaction indicates the statistical significance of the interaction term for SFA intake and the obesity GRS in the adjusted regression model. P-values for low and high SFA intake models indicate the statistical significance of the regression coefficients for the obesity GRS in the adjusted regression model. (B) Interaction between the obesity GRS and SFA intake (% total daily energy intake) for BMI in MESA. Predicted values of BMI by the obesity GRS are shown according to SFA intake (as a dichotomous variable). Predicted values were calculated from the regression models that contain SFA intake (as a categorical variable, 2 levels based on the population median (interquartile range): 10.68 (8.34-13.01), the obesity GRS (as continuous), their interaction term, and the potential confounders including population structure, gender, age, center, tobacco smoking (current vs. former/never), alcohol consumption (current vs. former/never), hyperlipidemia medication use, presence of diabetes, physical activity and total energy intake (in both studies). P-value for interaction indicates the statistical significance of the interaction term for SFA intake and the obesity GRS in the adjusted regression model. P-values for the low and high SFA intake models indicate the statistical significance of the regression coefficients for the obesity GRS in the adjusted regression model. BMI, body mass index; GRS, genetic risk score; SFA, saturated fatty acid.
Our findings are consistent with previous results obtained from single locus gene-diet interactions for obesity in which dietary SFA intake represented an important environmental modulator of genetically based obesity risk. For example, genes encoding FTO (highly expressed in the hypothalamus) and APOA2 (hypothesized to act as a satiety signal) appear to be modulated by SFA. At both FTO and APOA2 loci, the presence of variant alleles in individuals consuming high amounts of SFAs was associated with greater BMI compared to individuals without those alleles.7,8,12 As previously reported,7 we also obtained similar results for the interaction terms with MUFA intake, probably because of the strong correlation that exists between MUFA and SFA consumption in North American populations, where the major source of MUFA is animal fat (meat and dairy products). However, instead of using a single locus, we combined over 50 well-established obesity variants to calculate an obesity GRS. Although the use of a GRS may be less informative at a biological level, it is emerging as a preferred method in analyses of gene-environment interactions.31
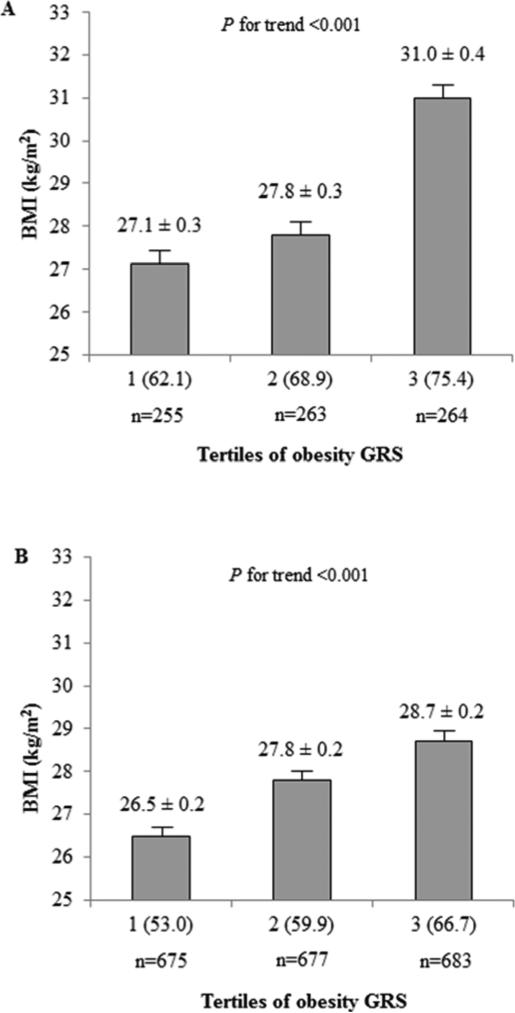
Obesity GRS evaluated by tertiles for BMI
With the GRS evaluated by tertiles for BMI, participants with a higher GRS had a higher BMI (Figure 2; P for trend <0.001 in both populations). We next examined differences in anthropometric, biochemical, lifestyle and dietary measurements in the lowest and highest GRS tertiles and by obesity status (Table 4). With low genetic obesity predisposition (Tertile 1), non-obese participants were younger than obese in GOLDN, while age did not differ by obesity status in MESA. Furthermore, GOLDN obese participants showed similar total energy intake compared to non-obese, suggesting that obese individuals may be modifying their diets to limit intakes. Similar results were observed in MESA when we age-matched MESA participants (<60 years) to resemble GOLDN. In the highest GRS tertile, all types of fats were lower in GOLDN and MESA in non-obese compared to obese participants.
Figure 2.
Relationship between the obesity GRS and BMI. (A) BMI by tertiles of obesity GRS in GOLDN. Ranges (minimum-maximum) for tertiles 1 through 3 are 44.8-66.3, 66.4-71.5 and 71.6-85.7, and values in parentheses are means of tertiles of obesity GRS. P for trend was calculated by the generalized estimating equation approach with exchangeable correlation structure implemented in the SAS GENMOD procedure to adjust for familial relationships, gender, age and center. (B) BMI by tertiles of obesity GRS in MESA. Ranges (minimum-maximum) for tertiles 1 through 3 are 37.6-56.3, 56.4-62.2 and 62.3-83.1, and values in parentheses are means of tertiles of obesity GRS. P for trend was calculated by using a general linear regression model adjusted by population structure, gender, age and center. BMI, body mass index; GRS, genetic risk score; SFA, saturated fatty acid.
Table 4.
Anthropometric, biochemical, lifestyle and dietary characteristics in the population with the lowest (tertile 1) and highest tertile (tertile 3) of the obesity GRS according to obesity statusa
|
GOLDN study
|
MESA study
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Tertile 1 of the obesity GRS
|
P-valueb |
Tertile 3 of the obesity GRS
|
P-valueb |
Tertile 1 of the obesity GRS
|
P-valuec |
Tertile 3 of the obesity GRS
|
P-valuec | |||||
|
Non-obese (BMI<30 kg/m2) |
Obese (BMI≥30 kg/m2) |
Non-obese (BMI<30 kg/m2) |
Obese (BMI>30 kg/m2) |
Non-obese (BMI<30 kg/m2) |
Obese (BMI≥30 kg/m2) |
Non-obese (BMI<30 kg/m2) |
Obese (BMI≥30 kg/m2) |
|||||
| mean ± SE | mean ± SE | mean ± SE | mean ± SE | mean ± SE | mean ± SE | mean ± SE | mean ± SE | |||||
| N (men/women) | 195 (86/109) | 60 (24/36) | 125 (65/60) | 139 (68/71) | 545 (289/256) | 130 (67/63) | 435 (216/219) | 248 (118/130) | ||||
| Age, y | 46.4 ± 1.0 | 53.9 ± 2.0 | 0.001 | 45.5 ± 1.4 | 52.6 ± 1.5 | 0.001 | 63.6 ± 0.5 | 63.0 ± 0.9 | 0.601 | 63.4 ± 0.6 | 60.4 ± 0.7 | <0.001 |
| Weight, kg | 74.7 ± 0.8 | 96.1 ± 1.3 | <0.001 | 78.3 ± 0.9 | 102.5 ± 1.4 | <0.001 | 72.0 ± 0.5 | 94.5 ± 0.9 | <0.001 | 74.5 ± 0.6 | 99.6 ± 0.8 | <0.001 |
| Waist, cm | 88.8 ± 0.8 | 108.3 ± 1.2 | <0.001 | 91.4 ± 1.0 | 113.8 ± 1.5 | <0.001 | 91.1 ± 0.5 | 112.3 ± 0.9 | <0.001 | 93.4 ± 0.6 | 115.6 ± 0.8 | <0.001 |
| Waist-to-hip ratio | 0.87 ± 0.01 | 0.93 ± 0.01 | <0.001 | 0.89 ± 0.01 | 0.95 ± 0.01 | <0.001 | 0.91 ± 0.01 | 0.97 ± 0.01 | <0.001 | 0.91 ± 0.01 | 0.98 ± 0.01 | <0.001 |
| BMI, kg/m2 | 25.2 ± 0.2 | 33.2 ± 0.4 | <0.001 | 26.1 ± 0.2 | 35.1 ± 0.4 | <0.001 | 25.0 ± 0.1 | 33.3 ± 0.3 | <0.001 | 25.6 ± 0.2 | 34.6 ± 0.2 | <0.001 |
| Systolic blood pressure, mmHg | 111.8 ± 1.0 | 117.1 ± 1.9 | 0.026 | 113.6 ± 1.5 | 123.2 ± 1.5 | <0.001 | 121.6 ± 0.8 | 126.9 ± 1.6 | 0.002 | 121.9 ± 1.0 | 129.3 ± 1.4 | <0.001 |
| Diastolic blood pressure, mmHg | 66.6 ± 0.6 | 69.9 ± 1.1 | 0.016 | 68.1 ± 0.7 | 70.9 ± 0.9 | 0.008 | 69.4 ± 0.4 | 71.2 ± 0.8 | 0.042 | 70.7 ± 0.5 | 72.2 ± 0.7 | 0.052 |
| Cholesterol, mg/dLd | 189.2 ± 2.7 | 199.7 ± 4.6 | 0.079 | 193.9 ± 3.9 | 195.0 ± 3.9 | 0.837 | 195.0 ± 1.5 | 200.9 ± 3.1 | 0.094 | 193.9 ± 1.9 | 194.7 ± 2.7 | 0.837 |
| LDL-cholesterol, mg/dLd | 120.0 ± 2.3 | 132.7 ± 4.3 | 0.018 | 122.7 ± 3.1 | 129.3 ± 3.1 | 0.083 | 117.3 ± 1.5 | 120.7 ± 2.7 | 0.226 | 115.3 ± 1.5 | 116.5 ± 2.3 | 0.602 |
| HDL-cholesterol, mg/dLd | 48.4 ± 1.2 | 42.2 ± 1.2 | 0.001 | 48.0 ± 1.2 | 42.2 ± 0.8 | 0.001 | 54.6 ± 0.8 | 48.8 ± 1.2 | <0.001 | 53.4 ± 0.8 | 46.1 ± 0.8 | <0.001 |
| Triglycerides, mg/dLe | 124.9 ± 6.2 | 166.6 ± 13.3 | 0.010 | 137.3 ± 9.7 | 157.7 ± 12.4 | 0.192 | 117.8 ± 3.5 | 150.6 ± 6.2 | <0.001 | 126.7 ± 7.1 | 171.9 ± 8.9 | <0.001 |
| Fasting glucose, mg/dLf | 98.1 ± 1.1 | 104.9 ± 1.6 | 0.001 | 100.1 ± 1.3 | 105.3 ± 1.8 | 0.019 | 87.7 ± 0.7 | 93.6 ± 1.3 | <0.001 | 89.1 ± 1.4 | 101.2 ± 2.0 | <0.001 |
| Total energy intake (kcal/d) | 2086.3 ± 57.0 | 2055.4 ± 89.5 | 0.740 | 2017.6 ± 61.9 | 2151.0 ± 64.9 | 0.125 | 1497.2 ± 28.2 | 1667.9 ± 53.5 | 0.003 | 1544.9 ± 40.7 | 1805.6 ± 52.4 | <0.001 |
| Total fats (%)g | 35.6 ± 0.5 | 35.7 ± 0.9 | 0.905 | 34.4 ± 0.6 | 36.5 ± 0.7 | 0.035 | 31.4 ± 0.4 | 32.8 ± 0.7 | 0.058 | 31.9 ± 0.4 | 33.0 ± 0.5 | 0.006 |
| SFAs (%)g | 12.0 ± 0.2 | 11.8 ± 0.3 | 0.695 | 11.2 ± 0.3 | 12.2 ± 0.3 | 0.024 | 10.5 ± 0.2 | 11.1 ± 0.3 | 0.063 | 10.5 ± 0.2 | 11.3 ± 0.2 | 0.002 |
| MUFAs (%)g | 13.3 ± 0.2 | 13.5 ± 0.4 | 0.774 | 13.0 ± 0.3 | 13.7 ± 0.3 | 0.041 | 12.0 ± 0.2 | 12.4 ± 0.3 | 0.188 | 12.0 ± 0.2 | 12.7 ± 0.2 | 0.004 |
| PUFAs (%)g | 7.7 ± 0.1 | 7.7 ± 0.3 | 0.809 | 7.6 ± 0.2 | 7.8 ± 0.2 | 0.374 | 6.2 ± 0.1 | 6.4 ± 0.2 | 0.190 | 6.3 ± 0.1 | 6.4 ±0.1 | 0.887 |
| Proteins (%)g | 15.8 ± 0.2 | 16.3 ± 0.3 | 0.215 | 15.9 ± 0.2 | 16.2 ± 0.3 | 0.352 | 16.0 ± 0.2 | 16.0 ± 0.3 | 0.992 | 15.5 ± 0.2 | 15.6 ± 0.2 | 0.706 |
| Carbohydrates (%)g | 48.6 ± 0.6 | 48.8 ± 1.1 | 0.876 | 50.2 ± 0.8 | 47.9 ± 0.7 | 0.010 | 51.2 ± 0.4 | 50.5 ± 0.8 | 0.420 | 51.2 ± 0.5 | 50.0± 0.6 | 0.093 |
| Screen timeh | 2.6 ± 0.1 | 3.0 ± 0.2 | 0.154 | 2.6 ± 0.1 | 2.9 ± 0.1 | 0.097 | 739.9 ± 27.8 | 955.7 ± 52.6 | <0.001 | 784.8 ± 32.9 | 986.7 ± 42.4 | <0.001 |
| Physical activityi | 34.0 ± 0.5 | 32.6 ± 0.7 | 0.084 | 33.9 ± 0.6 | 34.8 ± 0.6 | 0.325 | 2050.8 ± 124.1 | 1329.9 ± 235.3 | 0.004 | 1856.2 ± 123.9 | 1226.6 ±159.6 | <0.001 |
| Obesity GRS | 62.1 ± 0.3 | 63.3 ± 0.4 | 0.031 | 74.7 ± 0.3 | 75.8 ± 0.3 | 0.008 | 53.0 ± 0.2 | 53.6 ± 0.3 | 0.054 | 66.5 ± 0.2 | 66.8 ± 0.2 | 0.183 |
Abbreviations: BMI, body mass index; LDL-cholesterol, low-density lipoprotein cholesterol; HDL-cholesterol, high-density lipoprotein cholesterol; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; GRS, genetic risk score.
Values are expressed as mean ± SE or n (%).
P-value in GOLDN study was calculated by using the generalized estimating equation approach with exchangeable correlation structure implemented in the SAS GENMOD procedure adjusted by familial relationships, gender, age and center.
P-value in MESA study was calculated by using a general linear regression model adjusted by population structure, gender, age and center.
To convert mg/dL cholesterol to mmol/L, multiply mg/dL by 0.026. To convert mmol/L cholesterol to mg/dL, multiply mmol/L by 38.7.
To convert mg/dL triglyceride to mmol/L, multiply mg/dL by 0.0113. To convert mmol/L triglyceride to mg/dL, multiply mmol/L by 88.6.
To convert mg/dL glucose to mmol/L, multiply mg/dL by 0.0555. To convert mmol/L glucose to mg/dL, multiply mmol/L by 18.0.
%“ refers to total daily energy intake.
”Screen time" refers to the number of hours/day spent viewing television or using a computer in GOLDN study and light leisure TV (MET-minutes/week) in MESA study.
“Physical activity” was measured by a physical activity score in the GOLDN study and self-reported intentional physical activity (MET-minutes/week) in MESA study (as described in Methods).
Physical inactivity as reflected by screen time may affect obesity in both studies. In MESA, physical inactivity was greater in obese compared to non-obese participants, and a similar but non-significant difference was observed in GOLDN, with its lower statistical power. In contrast in GOLDN, when the genetic obesity predisposition was high, no differences in physical inactivity were observed by obesity status. Hence, non-obese status may be attributed to a lower intake of total fat and SFAs rather than by a lower physical inactivity or other behaviors that affect energy balance as indicated by comparing non-obese to obese participants.
The finding that a high SFA intake amplified the genetic predisposition to obesity suggests that individuals with a high obesity GRS may be more SFA-sensitive and may derive the most benefit from dietary manipulation of this macronutrient. Reducing SFA intake may be more effective in preventing obesity, especially among individuals with a high genetic predisposition to obesity. Therefore, dietary recommendations to reduce BMI in populations with a high obesity GRS could include reducing total fat intake mainly by limiting SFAs.
Postulated mechanisms
Although the association between SFA intake and obesity risk is controversial,32,33 high SFA intake may contribute to obesity through several potential mechanisms. Rodent studies have reported that SFA intake activates hypothalamic toll-like receptor signaling and promotes resistance to anorexigenic signals thought to contribute to obesity via increased energy intake.34 Increasing SFA intake in humans is known to increase obesity risk.35,36 Experimental evidence suggests a lower satiating37 and thermogenic effect38 of SFAs than unsaturated fatty acids. Moreover, long-chain SFAs were observed to be the least oxidized after examining different fatty acid oxidation rates compared to short-chain SFA and other types of fatty acids, including MUFA and PUFA.38 Nevertheless, it is unclear whether these mechanisms linking SFA intake to obesity also mediate SFA modulation of genetic susceptibility as reflected by gene-diet interaction studies. Since we cannot establish causal mechanisms using the current study design, additional laboratory-based studies are needed to clarify possible mechanisms of the interaction between SFA intake and genetic predisposition to obesity.
Strengths and limitations
Strengths of this study include use of an obesity GRS weighted by its relative effect size for each SNP in each population, a greater proportion of the variation in BMI is explained by GRS than by single SNPs, comprehensive coverage of established BMI associated genetic variants, the use of well-validated dietary questionnaires, and replication of the interaction in a different population. Principal limitations of the current study derive from the cross-sectional study design, which prevents us from establishing causality. Moreover, different methodologies used to assess dietary and lifestyle variables between populations may introduce measurement imprecision that may reduce statistical power, as well as our ability to achieve complete replication.
CONCLUSIONS
Our data provide novel findings that dietary fat intake interacts with an obesity genetic risk score in determining BMI in two US populations. SFAs were particularly important in modulating the relationship between genetic risk and BMI. Total fat and especially SFA intake may be more relevant in preventing weight gain in susceptible individuals. These results are preliminary, and we cannot refute the possibility that SFAs may represent a marker for poor dietary quality or other substandard lifestyle habits, either of which can increase obesity risk. The use of an obesity GRS may be a practical application to establish personalized nutritional recommendations, especially for those individuals with high genetic predisposition to obesity.
ACKNOWLEDGEMENTS
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. The USDA is an equal opportunity provider and employer. We also acknowledge the contributing investigators of the Multi-Ethnic Study of Atherosclerosis. The data of the Multi-Ethnic Study of Atherosclerosis were obtained from dbGaP.
Supported by National Institutes of Health (1R21AR055228-01A1, HL54776, 5R21HL114238-02, U01 HL72524), the National Institute of Diabetes and Digestive and Kidney Diseases (DK075030) and the US Department of Agriculture Research Service (53-K06-5-10 and 58–1950-9-001), K08 HL112845-01, SHARE genotyping was provided by NHLBI contract NO2-HL-64278, the provision of genotyping data was supported in part by the National Center for Advancing Translational Sciences, CTSI grant UL1TR000124, and the National Institute of Diabetes and Digestive and Kidney Disease Diabetes Research Center (DRC) grant DK063491 to the Southern California Diabetes Endocrinology Research Center; and this research has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. PIOF-GA-2010-272581.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors’ responsibilities were as follows—PC-A: hypothesis generation, analysis of data and writing of the manuscript; DKA, JIR: design of the experiment; CES, C-QL, LDP, MA, YDI and LFW: provision of significant advice; C-QL, SSR, IBB and Y-CL: collection and analysis of data; JMO: design of the experiment, critical reading of the manuscript, and provision of significant advice; JMO and PC-A had primary responsibility for final content. All the authors read and approved the final manuscript.
There were no potential conflicts of interest.
Contributor Information
Patricia Casas-Agustench, Nutrition and Genomic Laboratory, Jean Mayer United States Department of Agriculture Human Nutrition Research Center on Aging at Tufts University 711 Washington Street, Boston, MA 02111-1524, USA.; Instituto Madrileño de Estudios Avanzados (IMDEA) Alimentación, CEI UAM+CSIC C/ Faraday, 7, 1ª planta D1.11, Ciudad Universitaria de Cantoblanco, Ctra. de Colmenar Km.15, Madrid, 28049, Spain.
Donna K. Arnett, Department of Epidemiology, School of Public Health, and Clinical Nutrition Research Center, University of Alabama at Birmingham, AL, USA 1665 University Blvd, Birmingham, AL 35294, USA Telephone number: +1- 205-934-7066 arnett@uab.edu.
Caren E. Smith, Nutrition and Genomic Laboratory, Jean Mayer United States Department of Agriculture Human Nutrition Research Center on Aging at Tufts University 711 Washington Street, Boston, MA 02111-1524, USA. Telephone number: +1-617-630-1739 Fax number: +1-617-556-3211 caren.smith@tufts.edu.
Chao-Qiang Lai, Nutrition and Genomic Laboratory, Jean Mayer United States Department of Agriculture Human Nutrition Research Center on Aging at Tufts University 711 Washington Street, Boston, MA 02111-1524, USA. Telephone number: +1-617-556-3206 Fax number: +1-617-556-3211 ChaoQiang.Lai@ARS.USDA.GOV.
Laurence D. Parnell, Nutrition and Genomic Laboratory, Jean Mayer United States Department of Agriculture Human Nutrition Research Center on Aging at Tufts University 711 Washington Street, Boston, MA 02111-1524, USA. Telephone number: +1-617-556-3089 Fax number: +1-617-556-3211 Laurence.Parnell@ARS.USDA.GOV.
Ingrid B. Borecki, Division of Statistical Genomics in the Center for Genome Sciences, Washington University School of Medicine 660 S. Euclid Ave. St. Louis, MO 63110-1093 Telephone number: +1-314-362-3365 ingrid@dsgmail.wustl.edu.
Alexis C. Frazier-Wood, Division of Epidemiology, Human Genetics and Environmental Sciences. The University of Texas School of Public Health 1200 Herman Pressler, Rm E-517, Houston, TX, 77030, USA. Telephone number: +1-713-500-9814 Fax number: +1-713-500-0900 LekkiWood@Gmail.com.
Matthew Allison, Department of Family and Preventive Medicine University of California San Diego 9500 Gilman Drive, Mailcode 0965, La Jolla, CA 92093-0965, USA. Telephone number: +1-858-822-7671 Fax number: +1-858-822-7662 mallison@ucsd.edu.
Yii-Der Ida Chen, Laboratory for Biochemistry, Microarray, and Molecular Phenotyping, Institute for Translational Genomics and Population Sciences, Los Angeles Biomedical Research Institute and Department of Pediatrics at Harbor-UCLA Medical 1124 West Carson Street, Building E-5, Torrance, CA 90502,, USA. Telephone number: +1-310-974-9338 Fax number: +1-310-533-8519 ichen@labiomed.org.
Kent D. Taylor, Laboratory for High Throughput Genotyping and Bioinformatics, Institute for Translational Genomics and Population Sciences, Los Angeles Biomedical Research Institute and Department of Pediatrics at Harbor-UCLA Medical 1124 West Carson Street, Building E-5, Torrance, CA 90502, USA. Telephone number: +1-310-974-9323 Fax number: +1-310-533-8519 ktaylor@labiomed.org.
Stephen S. Rich, Center for Public Health Genomics, School of Medicine, University of Virginia Health System West Complex Room 3231, Charlottesville, USA Telephone number: +1-434-243-7356 Fax number: +1-434-924-8437 ssr4n@eservices.virginia.edu.
Jerome I. Rotter, Institute for Translational Genomics and Population Sciences, Los Angeles Biomedical Research Institute and Department of Pediatrics at Harbor-UCLA Medical Center 1124 West Carson Street, Building E-5, Torrance, CA 90502, USA. Telephone number: +1- 310-974-9501 Fax number: +1-310-533-8519 jrotter@labiomed.org.
Yu-Chi Lee, Nutrition and Genomic Laboratory, Jean Mayer United States Department of Agriculture Human Nutrition Research Center on Aging at Tufts University 711 Washington Street, Boston, MA 02111-1524, USA. Telephone number: +1-617-818-3236 Fax number: +1-617-556-3211 Yu-Chi.Lee@tufts.edu.
José M. Ordovás, Nutrition and Genomic Laboratory, Jean Mayer United States Department of Agriculture Human Nutrition Research Center on Aging at Tufts University Nutrition and Genomics Laboratory, Jean Mayer USDA HNRCA at Tufts University, 711 Washington Street, Boston, MA 02111-1524, USA.; Instituto Madrileño de Estudios Avanzados (IMDEA) Alimentación, CEI UAM+CSIC C/ Faraday, 7, 1ª planta D1.11, Ciudad Universitaria de Cantoblanco, Ctra. de Colmenar Km.15, Madrid, 28049, Spain. Department of Cardiovascular Epidemiology and Population Genetics, Centro Nacional de Investigaciones Cardiovasculares (CNIC) C/ Melchor Fernández Almagro, 3, Madrid, 28029, Spain Telephone number: +1-617-556-3102 Fax number: +1-617-556-3344 jose.ordovas@tufts.edu
LITERATURE CITED
- 1.Day FR, Loos RJ. Developments in obesity genetics in the era of genome-wide association studies. J Nutrigenet Nutrigenomics. 2011;4(4):222–238. doi: 10.1159/000332158. [DOI] [PubMed] [Google Scholar]
- 2.Poppitt SD, Prentice AM. Energy density and its role in the control of food intake: evidence from metabolic and community studies. Appetite. 1996;26(2):153–174. doi: 10.1006/appe.1996.0013. [DOI] [PubMed] [Google Scholar]
- 3.Jéquier E, Tappy L. Regulation of body weight in humans. Physiol Rev. 1999;79(2):451–480. doi: 10.1152/physrev.1999.79.2.451. [DOI] [PubMed] [Google Scholar]
- 4.Choquet H, Meyre D. Molecular basis of obesity: current status and future prospects. Curr Genomics. 2011;12(3):154–168. doi: 10.2174/138920211795677921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YC, Lai CQ, Ordovas JM, Parnell LD. A Database of Gene-Environment Interactions Pertaining to Blood Lipid Traits, Cardiovascular Disease and Type 2 Diabetes. J Data Mining Genomics Proteomics. 2011;2(1):pii:106. doi: 10.4172/2153-0602.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonestedt E, Roos C, Gullberg B, Ericson U, Wirfält E, Orho-Melander M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. Am J Clin Nutr. 2009;90(5):1418–1425. doi: 10.3945/ajcn.2009.27958. [DOI] [PubMed] [Google Scholar]
- 7.Corella D, Arnett DK, Tucker KL, et al. A high intake of saturated fatty acids strengthens the association between the fat mass and obesity-associated gene and BMI. J Nutr. 2011;141(12):2219–2225. doi: 10.3945/jn.111.143826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moleres A, Ochoa MC, Rendo-Urteaga T, et al. Dietary fatty acid distribution modifies obesity risk linked to the rs9939609 polymorphism of the fat mass and obesity-associated gene in a Spanish case-control study of children. Br J Nutr. 2012;107(4):533–538. doi: 10.1017/S0007114511003424. [DOI] [PubMed] [Google Scholar]
- 9.Lamri A, Abi Khalil C, Jaziri R, et al. Dietary fat intake and polymorphisms at the PPARG locus modulate BMI and type 2 diabetes risk in the D.E.S.I.R. prospective study. Int J Obes (Lond) 2012;36(2):218–224. doi: 10.1038/ijo.2011.91. [DOI] [PubMed] [Google Scholar]
- 10.Memisoglu A, Hu FB, Hankinson SE, et al. Interaction between a peroxisome proliferator-activated receptor gamma gene polymorphism and dietary fat intake in relation to body mass. Hum Mol Genet. 2003;12(22):2923–2929. doi: 10.1093/hmg/ddg318. [DOI] [PubMed] [Google Scholar]
- 11.Garaulet M, Smith CE, Hernández-González T, Lee YC, Ordovás JM. PPARγ Pro12Ala interacts with fat intake for obesity and weight loss in a behavioural treatment based on the Mediterranean diet. Mol Nutr Food Res. 2011;55(12):1771–1779. doi: 10.1002/mnfr.201100437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corella D, Peloso G, Arnett DK, et al. APOA2, dietary fat, and body mass index: replication of a gene-diet interaction in 3 independent populations. Arch Intern Med. 2009;169(20):1897–1906. doi: 10.1001/archinternmed.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corella D, Tai ES, Sorlí JV, et al. Association between the APOA2 promoter polymorphism and body weight in Mediterranean and Asian populations: replication of a gene-saturated fat interaction. Int J Obes (Lond) 2011;35(5):666–675. doi: 10.1038/ijo.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi Q, Chu AY, Kang JH, et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med. 2012;367(15):1387–1396. doi: 10.1056/NEJMoa1203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rukh G, Sonestedt E, Melander O, et al. Genetic susceptibility to obesity and diet intakes: association and interaction analyses in the Malmö Diet and Cancer Study. Genes Nutr. 2013;8(6):535–547. doi: 10.1007/s12263-013-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corella D, Arnett DK, Tsai MY, et al. The -256T>C polymorphism in the apolipoprotein A-II gene promoter is associated with body mass index and food intake in the genetics of lipid lowering drugs and diet network study. Clin Chem. 2007;53(6):1144–1152. doi: 10.1373/clinchem.2006.084863. [DOI] [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 18.MESA Coordinating Center. Multi-Ethnic Study of Atherosclerosis Field Center Manual of Operations. University of Washington; Seattle, WA: 2001. [Google Scholar]
- 19.Thompson FE, Subar AF, Brown CC, et al. Cognitive research enhances accuracy of food frequency questionnaire reports: results of an experimental validation study. J Am Diet Assoc. 2002;102(2):212–225. doi: 10.1016/s0002-8223(02)90050-7. [DOI] [PubMed] [Google Scholar]
- 20.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 21.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43(12):1327–1335. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 22.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, et al. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol. 1999;9(5):314–324. doi: 10.1016/s1047-2797(98)00070-2. [DOI] [PubMed] [Google Scholar]
- 23.Nettleton JA, Steffen LM, Mayer-Davis EJ, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2006;83(6):1369–1379. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999;8(6):805–813. doi: 10.1089/152460999319129. [DOI] [PubMed] [Google Scholar]
- 25.Smith CE, Arnett DK, Tsai MY, et al. Physical inactivity interacts with an endothelial lipase polymorphism to modulate high density lipoprotein cholesterol in the GOLDN study. Atherosclerosis. 2009;206(2):500–504. doi: 10.1016/j.atherosclerosis.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox CS, Heard-Costa N, Cupples LA, Dupuis J, Vasan RS, Atwood LD. Genome-wide association to body mass index and waist circumference: the Framingham Heart Study 100K project. BMC Med Genet. 2007;8:S1–18. doi: 10.1186/1471-2350-8-S1-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garaulet M, Lee YC, Shen J, et al. CLOCK genetic variation and metabolic syndrome risk: modulation by monounsaturated fatty acids. Am J Clin Nutr. 2009;90(6):1466–1475. doi: 10.3945/ajcn.2009.27536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O'Donnell CJ, de Bakker PI. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24(24):2938–2939. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aslibekyan S, An P, Frazier-Wood AC, et al. Preliminary evidence of genetic determinants of adiponectin response to fenofibrate in the Genetics of Lipid Lowering Drugs and Diet Network. Nutr Metab Cardiovasc Dis. 2013;23(10):987–994. doi: 10.1016/j.numecd.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shetty PB, Qin H, Namkung J, Elston RC, Zhu X. Estimating heritability using family and unrelated individuals data. BMC Proc. 2011;5(9):S34. doi: 10.1186/1753-6561-5-S9-S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Zhao JH, Luan J, et al. Physical activity attenuates the genetic predisposition to obesity in 20,000 men and women from EPIC-Norfolk prospective population study. PLoS Med. 2010;7(8) doi: 10.1371/journal.pmed.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moussavi N, Gavino V, Receveur O. Could the quality of dietary fat, and not just its quantity, be related to risk of obesity? Obesity (Silver Spring) 2008;16(1):7–15. doi: 10.1038/oby.2007.14. [DOI] [PubMed] [Google Scholar]
- 33.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297(9):969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 34.Milanski M, Degasperi G, Coope A, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29(2):359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunner EJ, Wunsch H, Marmot MG. What is an optimal diet? Relationship of macronutrient intake to obesity, glucose tolerance, lipoprotein cholesterol levels and the metabolic syndrome in the Whitehall II study. Int J Obes Relat Metab Disord. 2001;25(1):45–53. doi: 10.1038/sj.ijo.0801543. [DOI] [PubMed] [Google Scholar]
- 36.Doucet E, Alméras N, White MD, Després JP, Bouchard C, Tremblay A. Dietary fat composition and human adiposity. Eur J Clin Nutr. 1998;52(1):2–6. doi: 10.1038/sj.ejcn.1600500. [DOI] [PubMed] [Google Scholar]
- 37.Alfenas RC, Mattes RD. Effect of fat sources on satiety. Obes Res. 2003;11(2):183–187. doi: 10.1038/oby.2003.29. [DOI] [PubMed] [Google Scholar]
- 38.Casas-Agustench P, López-Uriarte P, Bulló M, Ros E, Gómez-Flores A, Salas-Salvadó J. Acute effects of three high-fat meals with different fat saturations on energy expenditure, substrate oxidation and satiety. Clin Nutr. 2009;28(1):39–45. doi: 10.1016/j.clnu.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 39.DeLany JP, Windhauser MM, Champagne CM, Bray GA. Differential oxidation of individual dietary fatty acids in humans. Am J Clin Nutr. 2000;72(4):905–911. doi: 10.1093/ajcn/72.4.905. [DOI] [PubMed] [Google Scholar]