Abstract
The ability to control desires, whether for food, sex, or drugs, enables people to successfully function within society. Yet, in tempting situations, strong impulses often result in self-control failure. Although many triggers of self-control failure have been identified, the question remains as to why some individuals are more likely to give in to temptation than others. Here, we combined functional neuroimaging and experience sampling to determine if there are brain markers that predict whether people act upon their food desires in daily life. To that end, we examined food cue-related activity in the nucleus accumbens (NAcc), as well as activity associated with response inhibition in the inferior frontal gyrus (IFG). NAcc activity was associated with greater likelihood of self-control failures, whereas IFG activity supported successful resistance to temptations. These findings demonstrate an important role for the neural mechanisms underlying desire and self-control in people’s real-world experiences of temptations.
Keywords: Self-control, Neuroimaging, Individual Differences
The inability to curb desires and control impulses has far-reaching implications and costs for individuals and society at large (Baumeister, Heatherton, & Tice, 1994; Schroeder, 2007). Indeed, it has been estimated that up to 40% of deaths in the United States every year are attributable to self-control failures (Mokdad, Marks, Stroup, & Gerberding, 2004; Schroeder, 2007). Many models portray self-control as the outcome of a balance between the strength of impulses (e.g., desires and cravings) and the exertion of self-control (Hare, Camerer, & Rangel, 2009; Heatherton & Wagner, 2011; Hofmann, Friese, & Strack, 2009; Metcalfe & Mischel, 1999). These models also predict that whenever this balance is tipped in favor of impulses, a person is especially prone to self-control failure (Heatherton & Wagner, 2011). Although previous research has identified some predictors of self-control failure, such as negative affect or resource depletion (see Wagner & Heatherton, In Press, for review), it is still unclear why certain people generally succeed at regulating their impulses and behaviors while others consistently fail. Indeed, identifying those individuals who are most likely to give in to temptation in the short term may help health practitioners develop programs that prevent this behavioral tendency from turning into a chronic, unhealthy lifestyle in the long run (e.g., overeating and obesity in the food domain).
To address this question, we set out to model how successful (and unsuccessful) people are in controlling their desires to eat on a daily basis. While merely asking people to report their past eating behaviors might adequately capture differences in self-control, there are several reasons why retrospective self-report can be problematic. For instance, people’s recall of past behaviors may be subject to memory biases (Gorin & Stone, 2001; Schwarz, 1999) and could lead them to misreport how often they have succumbed to a temptation. People also tend to grossly underestimate the number of eating decisions they make on a daily basis (Wansink, 2007). Together, this can make it difficult to test hypotheses about why some are better at self-control than others using retrospective measures that rely on participants having perfect recall of past events. Because of this, it can be difficult to tease apart competing accounts of diet failure, such as those that posit that excessive appetite and desire strength are critical factors (Hofmann & Dillen, 2012) compared to others that propose that compromised willpower is to blame (Mischel, Cantor, & Feldman, 1996).
One way to get around the inherent response biases in self-reports is to assess underlying neural correlates. We took this approach in the present study by pairing functional neuroimaging with subsequent smartphone experience sampling technology to test whether reward activity in response to viewing appetizing food cues (Demos, Heatherton, & Kelley, 2012; Kelley, 2004; Wagner, Boswell, Kelley, & Heatherton, 2012) predicts the strength of everyday food desires, and whether activity in brain regions previously identified in studies of response inhibition (Berkman, Falk, & Lieberman, 2011; Menon, Adleman, White, Glover, & Reiss, 2001) predicts an individual’s successful resistance of those desires. To elicit food cue specific reward activity, we used a cue-reactivity paradigm adapted from previous work in our lab (e.g., see Demos et al., 2012) and to examine the role of regions related to self-control, we administered a go/no-go response inhibition task (Casey et al., 1997).
We then determined whether brain activity in response to food cues or evoked during a self-control task (i.e., go/nogo) predicted participants’ daily eating behaviors, as assessed via experience sampling. Specifically, we tested whether activity in the ventral striatum (specifically the nucleus accumbens, NAcc) predicted desire for food, tendency to give in to desire, and amount of food consumed. We also tested whether activity in self-control regions (e.g., the inferior frontal gyrus; IFG) predicted successful resistance to these desires and thereby decreased likelihood of eating. Overall, we aimed to determine whether these brain markers would be able to predict daily eating behaviors and affect self-regulatory outcomes—above and beyond self-report.
Methods
Thirty-one female participants completed an initial fMRI scanning session consisting of the abovementioned cue reactivity and response inhibition tasks. At the end of the scanning session we also collected trait-level measures of interest, such as dieting status (Herman & Polivy, 1980) and sensitivity to external food cues (van Strien, Peter Herman, & Anschutz, 2012). The fMRI session was followed by experience sampling of participants’ food desires and eating behaviors for one week, in accordance with Hofmann and colleagues’ procedure (Hofmann, Baumeister, Förster, & Vohs, 2012). Specifically, all participants were provided with Blackberry smartphones and each day were signaled with seven short surveys (randomly administered across seven 2-hour intervals) that prompted them to report on recent desire episodes that might have occurred within the last half hour. Whenever participants indicated a food desire they were asked to report on the following variables: desire strength, resistance to that desire, enactment (whether or not they gave in to the desire and already ate), and if so, amount eaten.
Participants
We recruited thirty-one females (mean age = 21.1 years, range = 18-28) from the Dartmouth College community to participate in the study. Sample size was determined based on previous studies conducted using the brain-as-predictor approach (see Berkman et al., 2011), and based on sample sizes of studies conducted using our cue reactivity paradigm (Wagner, Altman, Boswell, Kelley, & Heatherton, 2013). Accordingly, we sought to enroll 30 participants in the study with the stop rule being that eligible participants had to be able to complete all phases of the study by the end of the relevant term of participation. We ran only female participants to be consistent with previous cue-reactivity studies that sampled from the same population (Demos et al., 2012), as well as to avoid the confound of gender effects on eating behaviors (Holm-Denoma, Joiner, Vohs, & Heatherton, 2008). All participants were right-handed, had normal or corrected-to-normal vision, and reported no history of psychiatric or neurological disorders. Because we were interested in capturing variability in eating behaviors in the general population, we did not recruit participants based on dieting status per se, but in order to account for differences in dietary restraint, all participants completed the Restraint Scale (Heatherton, Peter, Polivy, King, & McGree, 1988; Herman & Polivy, 1980). At the beginning of the study, we informed participants that the study was about cognition and emotion in everyday life. Upon successful completion of the fMRI and experience sampling portions of the study, participants were debriefed on the general goals of the study. All participants gave their informed consent based on guidelines set by Dartmouth’s Committee for the Protection of Human Subjects.
Stimuli
All images in the food cue reactivity task were adapted from previous work in our lab (Demos et al., 2012; Demos, Kelley, & Heatherton, 2011). In total, there were 90 high-caloric foods, including 30 dessert items, 30 fast food meals, and 30 snacks. Stimuli in the go/no-go task consisted of different images types (e.g., social scenes, food images). In the present analyses images were collapsed across all go and no-go conditions to test whether domain-general response inhibition would encompass and be predictive of resistance to food impulses.
Imaging Apparatus
All neuroimaging data were collected with a 3T Philips Intera Achieva scanner (Philips Medical Systems, Bothell, WA) equipped with a SENSEitivity Encoding head coil. Stimuli were presented using SuperLab 4.0 (Cedrus Corporation) and projected to an Epson ELP-7000 LCD screen positioned at the end of the scanner bore. Participants were able to view the screen via a mirror mounted on the head coil. Participants made all responses with button-presses on a Lumina LU-400 fMRI response pad.
Imaging procedure
In the fMRI scanning session, all participants completed the cue reactivity task first, followed by the go/no-go task. In the food cue reactivity task, we instructed participants to make simple perceptual judgments as to whether each image they viewed depicted an indoor or outdoor scene. All judgments were made with a button press. Since the task incorporated multiple image types, including images of people and nature scenes, participants were naïve to the true purpose of the task. In the go/no-go task, we asked participants to respond to certain image types (go condition) by making a button press and to withhold responding for other image types (no-go condition) by refraining from pressing the button. All go/no-go conditions were counterbalanced.
Both tasks implemented a rapid event-related design. In the cue reactivity task, each trial consisted of an image (a food item, people, or a nature scene) displayed for 2.5 seconds. We randomized the order of trial types and inter-stimulus interval (ISI). During the ISI, a white fixation cross was displayed on a black background to create jittered intervals of variable fixation (0-12.5 seconds) for more accurate estimation of task effects. In the go/no-go task, a stimulus was presented on a black background for 500 milliseconds, followed by a jittered ISI ranging from two to 9.5 seconds. Across both runs of the task, there were in total 108 go trials and 36 no go trials.
For each task, data were collected in two functional runs. Each run of cue reactivity task consisted of 250 whole-brain volumes and each run of the go/no-go task consisted of 266 volumes, with the same acquisition parameters for both tasks (36 axial slices per whole-brain volume, 3.5-mm thickness, 0.5-mm gap; 3 × 3 mm in-plane resolution).
Image Preprocessing and Analysis
The fMRI data were analyzed using Statistical Parametric Mapping software (SPM8, Wellcome Department of Cognitive Neurology, London, UK) in conjunction with a suite of tools for preprocessing and analysis (available at http://github.com/ddwagner/SPM8w). For each functional run, data were preprocessed to remove sources of noise and artifact, and corrected for differences in slice-timing. Functional data were realigned within and across runs to correct for head movement and were unwarped to reduce residual movement-related image distortions that realignment may have failed to correct. Functional data were normalized into a standard stereotaxic space (3-mm isotropic voxels) based on the SPM8 EPI template that follows the ICBM 152 brain template space (Montreal Neurological Institute). To spatially smooth the normalized images, a Gaussian kernel was applied (6-mm full width at half maximum). For the go/no-go task, three participants’ data were not included in subsequent analyses (one participant failed to complete the task and two participants showed extreme motion-related artifact).
For each participant and for both tasks, we ran a general linear model (GLM) that included task effects and covariates of no interest (instruction trials, error trials for the go/no-go task, a linear trend and six motion parameters derived from realignment corrections). GLMs were convolved with a canonical hemodynamic response function and used to compute parameter estimates for comparisons at each voxel. For the cue reactivity task, contrast images comparing food versus all other stimuli (people and nature scenes) were entered into a second-level random effects analysis, with the participant treated as the random effect.
For the go/no-go task, both go and no-go trials were modeled separately. Since we were interested in interrogating brain activity associated with response inhibition, for each subject we generated contrast images comparing no-go versus go trials. These images were subsequently subjected to a second-level random effects analysis, with the participant again treated as the random effect. Importantly, any trials in which participants made errors, whether omission or commission errors, were excluded from all analyses. This allowed for easier interpretation of response inhibition related activity in the no-go > go contrast.
To localize our nucleus accumbens region of interest (ROI) from the cue reactivity task, we applied a functionally defined, spherical mask (4 mm) to the right nucleus accumbens (MNI coordinates: 12, 9, −3) based on previous work in our lab (Demos et al., 2011). In the present study, this ROI showed significant food cue specific activation, t(30) = 2.87, p = .007. We extracted the mean beta values from this region to be used in subsequent multilevel regression models predicting intensity and enactment of food desires, and amount eaten.
For the go/no-go task, we first ran Monte Carlo simulations using AFNI’s AlphaSim to calculate the minimum cluster size at an uncorrected threshold of p < .005 (required for a whole brain correction of p < .05). We performed simulations (1,000 iterations) on the volume of the study-wide whole-brain mask. These simulations estimated a minimum cluster size of 180 voxels. Based on this estimation, we selected a spherical ROI (6 mm) in the left inferior frontal gyrus centered on peak voxels (MNI coordinates: −36, 30, −3). Again, we extracted beta values from this ROI to be used in our multilevel models.
Experience sampling procedure
Following the fMRI scan, all participants underwent a short training session in which they received oral and written instructions on how to use the Blackberry smartphones. The experience sampling protocol was administered on the smartphones via a customized Java ME software application that determined the assessment schedule, questionnaire administration, and logging of data.
Participants carried the smartphones on their person during the one-week experience sampling period. Each day, seven signals were distributed across a 14-hour time window, with each signal occurring randomly within a 2-hour time block, as per Hektner and colleagues’ recommendation (Hektner, Schmidt, & Csikszentmihalyi, 2007). Any two signals were constrained to be at least 30 minutes apart. If the smartphone was turned off during the time of a signal, the program postponed the signal until later in the time block, or, if the time block passed, the response would be logged as missing. If the smartphone was turned on but participants did not respond within 15 minutes of the signal’s onset, the program would turn off and would categorize the response as missing. To ensure enough data collection points per participant, the experience sampling period was extended an additional day if participants responded to fewer than five signals on any given day. If that occurred, a pop-up message would occur on the screen, asking participants to carry the device an additional day. For each signal, participants reported the following: (1) desire strength: the experienced strength of the food desire on a scale from 0 (none at all) to 6 (irresistible); (2) resistance: how much they attempted to resist these desires on a scale from 0 (not at all) to 6 (very much); (3) enactment: whether or not they gave in to the desire and already ate (yes/no); and, if so (4) amount eaten: how much they had eaten on a scale from 1 (a tiny bit) to 6 (much more than a regular portion/stuffed).
None of the participants reported any difficulty or disruption associated with responding to the questionnaires, since the average response time for each signal was only 2.65 minutes. And overall there was high compliance, as participants completed an average of 83% of all signals during the experience sampling period and reported having food desires more than half of the time (54.4%).
Multi-level analysis procedure
All multi-level regression models were run using the software package Hierarchical Linear Modeling (HLM) (Raudenbush, 2004). Across all models, dependent variables were not transformed but since enactment of food desires is a dichotomous variable, logistic multilevel regression was applied by specifying the Bernoulli model in HLM (Raudenbush, 2004). All Level 1 predictors, such as the measurements of desire and resistance during the experience sampling period, were person-mean centered, whereas Level 2 predictors, such as personality measures, were grand-mean centered. To incorporate neural data from the cue reactivity and response inhibition tasks into the models, we treated beta values from the ROIs described above as Level 2 continuous predictors (grand-mean centered). For all models, we accounted for individual differences in dietary restraint by including scores from the Restraint Scale (Heatherton et al., 1988; Herman & Polivy, 1980) and scores from the external eating subscale of the Dutch Eating Behavior Questionnaire (van Strien et al., 2012) as level 2 predictors.
To address our main questions, we ran several hierarchical linear regression models with situational variables (e.g., desire strength, resistance) at Level 1 and person-based variables (e.g., NAcc activity, personality measures) at Level 2 to accommodate the nested structure of the experience sampling data (i.e., observations within persons). All models incorporated Level 1 random intercepts, and models 2 and 3 included Level 1 random slopes for the relationship between resistance and the given outcome variable—as determined by variance components tests (all p’s < .005) (Hox, 2010).
Results
In the first model, we regressed desire strength on two brain predictors used in all subsequent, reported models. The brain predictors included signal change values from (1) a region of the NAcc that showed significant activation for appetitive food images during the cue reactivity task, and (2) an area in interior frontal gyrus associated with successful response inhibition from the go/no-go task (see Methods for ROI selection procedures). Individuals with higher NAcc activity in response to appetitive food images during fMRI scanning experienced more intense food desires than individuals with lower NAcc activity, B = 0.27, p = .003 (Fig. 1). Additionally, participants who reported being more sensitive to external food cues (van Strien et al., 2012), tended to have stronger desires, B = 0.05, p = .023. No other effects reached significance (see Table 1 for all estimated model parameters).
Figure 1.
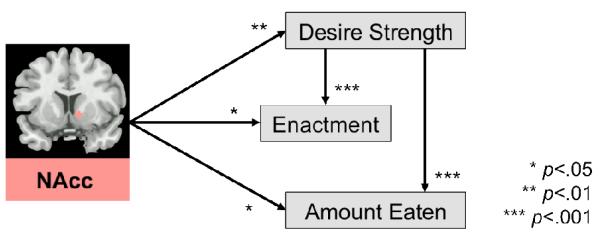
Summary of effects of nucleus accumbens’ (MNI Coordinates: 12, 9, −3) activity on multiple behavioral outcomes in the experience sampling period (from all multilevel regression models). Desire strength refers to responses to the question “How strong is your desire to eat the food?” Enactment is operationalized as positively answering the question “Did you already consume some of the food you desire?” Amount eaten refers to responses to the question “How much did you eat?” See Tables 1 and 2 for all other predictors and effects.
Table 1.
Multilevel regression of desire strength on Level 2 trait/brain predictors. Level 2 (person) predictors include the NAcc region-of-interest (ROI) from the cue reactivity task and the inferior frontal gyrus (IFG) ROI from the go/no-go task, while controlling for external eating and dietary restraint (see above).
| Predictor | B | SE | p |
|---|---|---|---|
| Base predictors (Level 1) | |||
| Intercept | 4.20 | 0.09 | <.001 |
| Trait/brain predictors (Level 2) | |||
| NAcc | 0.27 | 0.08 | .003 |
| IFG | −0.13 | 0.21 | .547 |
| Dietary restraint | −0.02 | 0.02 | .200 |
| External eating | 0.05 | 0.02 | .023 |
In the second model, our outcome variable was whether or not people gave in to their temptations to eat (i.e., enactment; see Table 2 for complete results from the enactment model). We replicated previous work (Hofmann et al., 2012) by showing that situational variation in food desire strength and resistance affected the likelihood of enactment, with desire strength predicting more frequent enactment, Blog = 0.32, p <.001, and higher resistance to food desires predicting less frequent enactment, Blog = −0.30, p = .004. In addition to these effects, there was a main effect of NAcc activity on enactment, such that those participants who showed higher NAcc activity in the cue reactivity task were more likely to give in to their temptations to eat, Blog = 0.38, p = .014. (Fig. 1). Greater sensitivity to external food cues (van Strien et al., 2012) also predicted enactment, Blog = 0.05, p = .022.
Table 2.
Multilevel logistic regression of enactment on desire, resistance, and Level 2 trait/brain predictors. Level 1 predictors include desire strength (i.e., “How strong is your desire to eat the food?”) and resistance (i.e., “How much did you try to resist the desire to eat this food?”) participants reported throughout the experience sampling period.
| Predictor | B log | SE | p |
|---|---|---|---|
| Base predictors (Level 1) | |||
| Intercept | −0.39 | 0.68 | .010 |
| Desire strength | 0.32 | 0.09 | < .001 |
| Resistance | −0.30 | 0.09 | .004 |
| Trait/brain predictors (Level 2) | |||
| NAcc | 0.38 | 0.14 | .014 |
| IFG | 0.14 | 0.23 | .555 |
| Dietary restraint | −0.03 | 0.02 | .160 |
| External eating | 0.05 | 0.02 | .022 |
| Interactions with Desire Strength (DS) | |||
| NAcc ×DS | 0.19 | 0.10 | .069 |
| IFG ×DS | −0.30 | 0.10 | .003 |
| Dietary restraint ×DS | −0.03 | 0.02 | .178 |
| External eating ×DS | <.001 | 0.01 | .979 |
| Interactions with Resistance (RST) | |||
| NAcc ×RST | −0.23 | 0.07 | .004 |
| IFG ×RST | −0.42 | 0.17 | .019 |
| Dietary restraint ×RST | −0.03 | 0.02 | .276 |
| External eating ×RST | −0.02 | 0.02 | .398 |
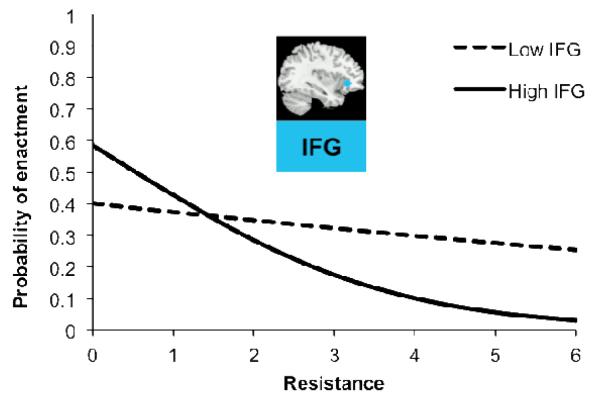
In this model we also observed moderating effects of IFG activity associated with successful response inhibition from the go/no-go task. On average, individuals with higher IFG activity during the go/no-go task less frequently acted upon their desires, as indicated by greater IFG activity weakening the relationship between desire strength and enactment, Blog = −0.30, p = .003, as well as enhancing the inhibitory link between resistance and enactment, Blog = −0.42, p = .019. An interaction plot (Fig. 2) shows that in particularly tempting situations (i.e., those characterized active resistance to those desires) high IFG individuals (+1 SD) were considerably more successful at regulating their food consumption compared to low IFG individuals (-1 SD). For instance, at high levels of resistance, low IFG individuals were an estimated 8.2 times more likely to give in to a food desire than those high in IFG activity (Fig. 2).
Figure 2.
Interaction plot depicting reported resistance on the x-axis, probability of giving in to temptation to eat on the y-axis, and the moderating influence of left IFG (MNI Coordinates: −36, 30, −3) recruitment during successful response inhibition in the go/no-go task (lines represent +/ 1SD above and below mean IFG activity). Predicted log-odds have been transformed to probabilities.
The last model we ran included the same predictor variables as the desire and enactment models, but with amount of food eaten as the outcome variable. We found a similar pattern of effects, including main effects of NAcc activity (B = 0.23, p = .025) and food cue sensitivity (B = 0.05, p = .002) on amount eaten. IFG activity weakened the relationship between desire strength and amount eaten, B = −0.19, p = .012, such that those individuals with higher IFG activity ate less when faced with temptations to eat (i.e., an experienced desire). See Table 3 for results from the third model.
Table 3.
Multilevel regression of amount eaten on desire, resistance, and Level 2 trait/brain predictors.
| Predictor | B | SE | p |
|---|---|---|---|
| Base predictors (Level 1) | |||
| Intercept | 1.17 | 0.11 | <.001 |
| Desire strength | 0.23 | 0.06 | < .001 |
| Resistance | −0.21 | 0.06 | .004 |
| Trait/brain predictors (Level 2) | |||
| NAcc | 0.23 | 0.10 | .025 |
| IFG | 0.08 | 0.19 | .667 |
| Dietary restraint | <0.01 | 0.02 | .879 |
| External eating | 0.05 | 0.01 | .002 |
| Interactions with Desire Strength (DS) | |||
| NAcc ×DS | 0.20 | 0.06 | .001 |
| IFG ×DS | −0.19 | 0.08 | .012 |
| Dietary restraint ×DS | −0.02 | 0.01 | .187 |
| External eating ×DS | 0.02 | 0.01 | .071 |
| Interactions with Resistance (RST) | |||
| NAcc ×RST | −0.10 | 0.06 | .089 |
| IFG × RST | −0.14 | 0.11 | .224 |
| Dietary restraint ×RST | −0.01 | 0.02 | .459 |
| External eating ×RST | −0.01 | 0.01 | .562 |
Discussion
Taken together, the results from the present study provide initial evidence for neural markers of everyday eating behaviors that can identify individuals who are more likely to give in to temptations to eat. Food cue reactivity in the NAcc, a part of the mesolimbic dopamine system associated with reward processing (Schultz, 2006), played a significant role predicting strength of food desires, enactment of those desires, and even amount eaten. Additionally, the moderating effects of IFG activity suggest that the IFG is a critical brain region that can influence self-regulatory outcomes, especially when people are faced with strong temptations and self-control is required (Fig. 2). Those individuals who recruited the IFG more during the response inhibition task tended to be less likely to succumb to temptations and also ate less.
The present findings also demonstrate the importance of individual differences in how people experience and respond to temptation in their day-to-day lives. These differences appear to arise from not only how temptation is experienced in the moment (as measured by desire strength and desire resistance), but also from neural mechanisms associated with both reward processing (NAcc) and response inhibition (IFG). Indeed, the models we report here indicate that variation in these brain regions’ activity predicts how well (or poorly) individuals exert self-control when confronted with temptations to eat. We observed these effects in models that accounted for variance captured by self-report, suggesting that neuroimaging can provide an independent means to validate different accounts of why certain people are prone to self-regulation failure. And rather than supporting one account exclusively over another, our findings support multiple accounts of self-control failure. For example, cue exposure is a well-known threat to self-regulation (Heatherton & Wagner, 2011), and our study’s NAcc effects demonstrate that higher reward-related activity during cue exposure is associated with greater likelihood of failure to resist temptations to eat. Other theories propose that self-regulatory failure is more likely whenever executive functions, supported by various regions of prefrontal cortex (e.g., IFG, Aron, Robbins, & Poldrack, 2004), are not engaged to modulate or dampen the reward value of a tempting stimulus (Heatherton & Wagner, 2011). In accordance with this account, we observed that individuals who showed lower IFG activity associated with response inhibition were prone to give in to their temptations, while those with higher IFG activity were more successful in resisting desires to eat.
To conclude, the brain-behavior relationships in the present study support and extend previous research on everyday desires (Hofmann et al., 2012) and test theories of self-control behaviors by incorporating neural markers of these behaviors. Related work has linked reward activity in the NAcc to long-term weight change (e.g., Demos et al., 2012), but the present study applied a brain-as-predictor approach (Berkman & Falk, 2013) to shed light on neural mechanisms of more proximal, short-term eating behaviors, which, over time, may give rise to chronic patterns of overeating and possibly weight gain. Future investigations should explore the extent to which brain systems associated with reward (e.g., NAcc) and self-control (e.g., IFG) can serve as neural markers of other appetitive and addictive behaviors—from binge drinking, to compulsive gambling, to risky sexual behaviors.
Acknowledgments
This work was supported by the National Institute on Drug Abuse (R01 DA022582), the National Heart Lung and Blood Institute (1R21HL114092-01), the National Cancer Institute (1F31CA177203-01), and Grant HO 4175/4-1 from the German Science Foundation.
References
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. doi:10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Heatherton TF, Tice DM. Losing Control: How and Why People Fail at Self-Regulation. Vol. xi. Academic Press; San Diego, CA, US: 1994. [Google Scholar]
- Berkman ET, Falk EB. Beyond brain mapping: Using neural measures to predict real-world outcomes. Current Directions in Psychological Science. 2013;22(1):45–50. doi: 10.1177/0963721412469394. doi:10.1177/0963721412469394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ET, Falk EB, Lieberman MD. In the trenches of real-world self-control: Neural correlates of breaking the link between craving and smoking. Psychological Science. 2011;22(4):498–506. doi: 10.1177/0956797611400918. doi:10.1177/0956797611400918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997;9(6):835–847. doi: 10.1162/jocn.1997.9.6.835. doi:10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. The Journal of Neuroscience. 2012;32(16):5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. doi:10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos KE, Kelley WM, Heatherton TF. Dietary restraint violations influence reward responses in nucleus accumbens and amygdala. Journal of Cognitive Neuroscience. 2011;23(8):1952–1963. doi: 10.1162/jocn.2010.21568. doi:10.1162/jocn.2010.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin A, Stone A. Recall biases and cognitive errors in retrospective self-reports: A call for momentary assessments. In: Baum A, Revenson T, Singer J, editors. Handbook of Health Psychology. Lawrence Erlbaum Associates Inc.; Mahwah, NJ: 2001. pp. 405–13. [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–648. doi: 10.1126/science.1168450. doi:10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Peter C, Polivy J, King GA, McGree ST. The (mis)measurement of restraint: An analysis of conceptual and psychometric issues. Journal of Abnormal Psychology. 1988;97(1):19–28. doi: 10.1037//0021-843x.97.1.19. doi:10.1037/0021-843X.97.1.19. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences. 2011;15(3):132–139. doi: 10.1016/j.tics.2010.12.005. doi:10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hektner JM, Schmidt JA, Csikszentmihalyi M. Experience Sampling Method: Measuring the Quality of Everyday Life. SAGE; 2007. [Google Scholar]
- Herman C, Polivy J. Obesity. Saunders; Philadelphia: 1980. Restrained eating. [Google Scholar]
- Hofmann W, Baumeister RF, Förster G, Vohs KD. Everyday temptations: An experience sampling study of desire, conflict, and self-control. Journal of Personality and Social Psychology. 2012;102(6):1318–1335. doi: 10.1037/a0026545. doi:10.1037/a0026545. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Dillen LV. Desire: The new hot spot in self-control research. Current Directions in Psychological Science. 2012;21(5):317–322. doi:10.1177/0963721412453587. [Google Scholar]
- Hofmann W, Friese M, Strack F. Impulse and self-control from a dual-systems perspective. Perspectives on Psychological Science. 2009;4(2):162–176. doi: 10.1111/j.1745-6924.2009.01116.x. doi:10.1111/j.1745-6924.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- Holm-Denoma JM, Joiner TE, Vohs KD, Heatherton TF. The “freshman fifteen” (the “freshman five” actually): Predictors and possible explanations. Health Psychology. 2008;27(1, Suppl):S3–S9. doi: 10.1037/0278-6133.27.1.S3. doi:10.1037/0278-6133.27.1.S3. [DOI] [PubMed] [Google Scholar]
- Hox J. Multilevel Analysis: Techniques and Applications. Second Edition Routledge; 2010. [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neuroscience & Biobehavioral Reviews. 2004;27(8):765–776. doi: 10.1016/j.neubiorev.2003.11.015. doi:10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a go/no-go response inhibition task. Human Brain Mapping. 2001;12(3):131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. doi:10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: Dynamics of willpower. Psychological Review. 1999;106(1):3–19. doi: 10.1037/0033-295x.106.1.3. doi:10.1037/0033-295X.106.1.3. [DOI] [PubMed] [Google Scholar]
- Mischel W, Cantor N, Feldman S. Principles of self-regulation: The nature of willpower and self-control. In: Higgins ET, Kruglanski AW, editors. Social psychology: Handbook of basic principles. Guilford Press; New York, NY, US: 1996. pp. 329–360. [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the united states. JAMA. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. doi:10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW. HLM 6: Hierarchical Linear and Nonlinear Modeling. Scientific Software International; 2004. [Google Scholar]
- Schroeder SA. We can do better — Improving the health of the American people. New England Journal of Medicine. 2007;357(12):1221–1228. doi: 10.1056/NEJMsa073350. doi:10.1056/NEJMsa073350. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annual Review of Psychology. 2006;57(1):87–115. doi: 10.1146/annurev.psych.56.091103.070229. doi:10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Schwarz N. Self-reports: How the questions shape the answers. American Psychologist. 1999;54(2):93–105. doi:10.1037/0003-066X.54.2.93. [Google Scholar]
- Van Strien T, Peter Herman C, Anschutz D. The predictive validity of the DEBQ-external eating scale for eating in response to food commercials while watching television. International Journal of Eating Disorders. 2012;45(2):257–262. doi: 10.1002/eat.20940. doi:10.1002/eat.20940. [DOI] [PubMed] [Google Scholar]
- Wagner DD, Altman M, Boswell RG, Kelley WM, Heatherton TF. Self-regulatory depletion enhances neural responses to rewards and impairs top-down control. Psychological Science. 2013;24(11):2262–2271. doi: 10.1177/0956797613492985. doi:10.1177/0956797613492985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Boswell RG, Kelley WM, Heatherton TF. Inducing negative affect increases the reward value of appetizing foods in dieters. Journal of Cognitive Neuroscience. 2012;24(7):1625–1633. doi: 10.1162/jocn_a_00238. doi:10.1162/jocn_a_00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Heatherton TF. Self-Regulation and its Failure: Seven Deadly Threats to Self-Regulation. In: Borgida E, Bargh J, editors. APA Handbook of Personality and Social Psychology. Vol. 1 - Attitudes and Social Cognition. American Psychological Association; (In Press) [Google Scholar]
- Wansink B. Mindless Eating: Why We Eat More Than We Think. Random House LLC; New York: 2007. [Google Scholar]