Abstract
Purpose
This study examined the association of post-treatment changes in cognitive performance, APOE and smoking in breast cancer patients treated with adjuvant therapy.
Participants and Methods
Breast cancer patients treated with chemotherapy (N=55, age=51.9+/−7.1, education=15.7+/−2.6) were evaluated with a battery of neuropsychological tests prior to chemotherapy and at 1, 6, and 18 months post-chemotherapy. Matched groups of breast cancer patients not exposed to chemotherapy (N=68, age=56.8+/−8.3, education=14.8+/−2.2) and healthy controls (N=43, age=53.0+/−10.1, education=15.2+/−2.6) were evaluated at similar intervals. APOE epsilon 4 carrier status (APOE4+) and smoking history were also evaluated.
Results
The detrimental effect of APOE4+ genotype on post-treatment cognitive functioning was moderated by smoking history, i.e., patients without a smoking history had significantly lower performance on measures of processing speed and working memory compared to those with a smoking history and healthy controls. Exploratory analyses revealed that APOE4+ patients without a smoking history who were exposed to chemotherapy showed a decline in performance in processing speed, compared to patients with a smoking history. A similar, but less pronounced pattern was seen in the no chemotherapy group (primarily endocrine treatment). For working memory, the APOE4+ by smoking interaction was observed in the no chemotherapy group only.
Conclusions
The association between APOE status, breast cancer treatment, and cognitive functioning was moderated by smoking history suggesting that both chemotherapy and endocrine therapy interact with APOE status and smoking to influence cognition. A putative mechanism is that smoking corrects a deficit in nicotinic receptor functioning and dopamine levels in APOE4+ individuals.
Introduction
Increasing evidence suggests that breast cancer treatments (including chemotherapy and endocrine therapies) can cause long-term post-treatment cognitive changes as measured by performance on neuropsychological tests and imaging measures of brain structure and function [1-3]. Several specific mechanisms for cognitive change have been proposed including treatment-induced DNA damage, oxidative stress, and stimulation of neurotoxic cytokines [4]. However, other investigators have proposed a broader model implicating the interaction of cancer treatments and the biology of aging, resulting in an acceleration of the aging process [2-3]. Recent studies have suggested that older patients are more vulnerable to the cognitive decline associated with chemotherapy [5] and tamoxifen [6]. Further, age appears to interact with other risk factors like cognitive reserve to increase vulnerability to post-treatment cognitive decline (i.e., older patients with low pretreatment cognitive reserve, exposed to chemotherapy demonstrated reduced performance on measures of processing speed [5]).
The accelerated aging model leads to the hypothesis that cancer treatments should interact with genetic factors that influence cognitive aging. Apolipoprotein E (APOE) has been studied extensively in relation to cognitive functioning and the APOE4 allele has been associated with cognitive changes associated with normal aging as well as a variety of disorders with prominent cognitive dysfunction (e.g.,Alzheimer’s disease) [7]. ApoE is a complex glycolipoprotein that facilitates the uptake, transport, and distribution of lipids. It appears to play an important role in neuronal repair and plasticity after injury. A four exon gene codes for APOE on chromosome 19 in humans, with three major alleles: E2, E3, and E4. These alleles differ in amino acids at positions 112 and 158: E2 (cysteine/cysteine), E3 (cysteine/arginine), and E4 (arginine/arginine). The relationship of the APOE genotype to neuropsychological performance was examined in a cohort of long-term cancer survivors treated with standard dose chemotherapy. Survivors with at least one APOE4 allele scored significantly lower in the visual memory and spatial ability domains, with a trend to score lower in executive functioning, compared to survivors who did not carry an APOE4 allele [8].
Typically, smoking is associated with a variety of negative health outcomes, including accelerated cognitive aging [9]. However, research suggests that the detrimental effect of APOE4+ status on cognitive aging may be moderated by smoking history. In APOE4+ individuals with a smoking history, smoking has a less detrimental effect on cognitive aging as compared to APOE4− smokers [9-12]. A putative mechanism explaining the protective effect of smoking is that APOE4 carriers have fewer nicotinic receptor binding sites and lower activity of choline acetyltransferase (lower dopamine activity in frontal cortex) as compared to non-carriers, and smoking may correct this deficit [9-12].
Therefore, the associations among APOE status, smoking history, exposure to cancer treatments and post-treatment cognitive performance were evaluated within the context of a longitudinal study in which early stage breast cancer patients exposed to chemotherapy or not exposed to chemotherapy were evaluated with neuropsychological tests prior to the beginning of adjuvant therapy and at three follow-up assessments and compared to a matched healthy control group. The specific hypothesis evaluated whether breast cancer treatments interact with APOE4+ carrier status to cause a greater decrease in neuropsychological test performance compared to healthy controls and whether this effect is moderated by smoking history.
Participants and Methods
As described in our previous paper [5], consecutive newly diagnosed female breast cancer patients scheduled to receive adjuvant chemotherapy (N=60) or no chemotherapy as part of their adjuvant treatment (N=72) were recruited from the Breast Cancer Service of the Norris Cotton Cancer Center. Patients were eligible for participation if they were: 1) Diagnosed with noninvasive (Stage 0) or invasive (Stage 1, 2, or 3A) breast cancer; 2) Undergoing first treatment with systemic chemotherapy or surgery and/or local, non-CNS radiotherapy; 3) Between 18 and 70 years of age at time of diagnosis; 4) Fluent in English and able to read English. Patients were excluded based on the following criteria: 1) CNS disease; 2) Previous history of cancer (except basal cell carcinoma) or treatment with chemotherapy, CNS radiation, or intrathecal therapy; 3) Neurobehavioral risk factors including history of neurological disorder (e.g., Parkinson’s disease, seizure disorder, dementia), alcohol/substance abuse or moderate to severe head trauma (loss of consciousness >60 minutes or structural brain changes on imaging); 4) Axis I psychiatric disorder (DSM-IV) (e.g., schizophrenia, bipolar disorder).
Female healthy controls (N=45) who met the same inclusion (except for cancer diagnosis) and exclusion criteria were recruited through community advertisements. Healthy controls were frequency matched to patients on age and education. The methods and procedures were approved by the Committee for the Protection of Human Subjects of Dartmouth College and all participants provided written informed consent.
The pre-treatment assessment occurred following surgery, but prior to initiating adjuvant therapy. Follow-up assessments for patients treated with chemotherapy were conducted at 1, 6, and 18 months post-treatment. The test-retest interval for the first follow-up assessment for patients not exposed to chemotherapy and healthy control participants was frequency matched to the interval for the chemotherapy patients. Neuropsychological tests were grouped into domains to reduce the number of statistical comparisons based on expert opinion (AJS and BCM) guided by a factor analysis as described previously (13).
Neuropsychological Assessment Battery
Verbal Ability: Vocabulary (Wechsler Abbreviated Scale of Intelligence, WASI, 14), Verbal Fluency Test (Delis-Kaplan Executive Function System, D-KEFS, 15);
Verbal Memory California Verbal Learning Test-II [16], Logical Memory I and II (Wechsler Memory Scale-III, WMS-III, 17);
Visual Memory Faces I and II (WMS-III, 17);
Working Memory Paced Auditory Serial Addition Test (PASAT, 18);
Processing speed Digit Symbol-Coding (Wechsler Adult Intelligence Scale-III, WAIS-III, 17) Trail Making Test (D-KEFS, 15), Color-Word Interference Test (D-KEFS, 15), Grooved Pegboard [19];
Sorting Sorting Test (D-KEFS, 15);
Distractibility Continuous Performance Test (CPT, 20);
Reaction Time Continuous Performance Test (CPT, 20);
Block Design (WASI, 14)
Smoking status
Participants self-reported smoking history (current, former, or never smokers), Based on the response each participant was coded as ‘ever smoked’ or ‘never smoked’.
APOE Testing
Genotyping was performed in the Molecular Genetics Diagnostic Laboratory at the Dartmouth-Hitchcock Medical Center using standard techniques [21]. Briefly, genomic DNA was isolated from peripheral blood lymphocytes. APOE specific primers were used to amplify a fragment of the APOE gene by PCR, and the PCR product was digested using Hha1. The digested DNA was electrophoresed on an agarose gel and visualized under UV light using ethidium bromide. Alleles were determined based on the size of the fragments which was determined by each allele’s unique Hha1 restriction map.
Statistical Analysis
Standardized domain scores
To facilitate the interpretation of study findings across different neurocognitive domains listed above, the domain scores were standardized by the means and standard deviations of the non-cancer controls as norms within the same domain. Thus the standardized domain scores were mapped onto a comparable z-score scale. Overall, 89% of study participants completed all assessments.
Change scores since baseline
The baseline standardized domain scores were subtracted out of the standardized domain scores at post-treatment, 1, 6, and 18 months post-treatment to form the standardized change scores. Change scores since baseline have several practical advantages: 1) they yield clearly interpretable indications of the directions of individual change with precedence for their use in the literature [22]; and that 2) they help reduce noise due to individual variability at baseline.
Overall analytic approach
The overall analytic approach for this study followed that of our previous study [5]. Briefly, data visualizations such as plots of profiles of longitudinal neurocognitive performance over time were first carried out to summarize the data pattern. A mixed-effects model was then fitted. For each neurocognitive domain of interest, longitudinal change scores at 1, 6, and 18 months after treatment were fitted as the dependent variable (or the equivalent time periods in the yoked no chemotherapy and control groups). We began the analyses with the primary outcome of processing speed because previous work has showed that it was particularly sensitive in detecting treatment differences (2, 5). We first compared the patients against the controls and then further divided the patients into chemotherapy and no chemotherapy subgroups. This approach was taken because of increasing evidence showing that multiple aspects of breast cancer treatment contribute to post-treatment cognitive decline, including chemotherapy and endocrine therapy [2].
Covariates were entered as continuous fixed effects to adjust for baseline individual differences, including age, education, and baseline processing speed. The baseline processing speed was included to provide control over bias due to the common phenomena of ‘regression to the mean’ whenever longitudinal assessments are made (23). Also included as fixed categorical effects were time (as discrete assessment time points), group (patients vs. controls), APOE4 status (E4 allele absent or present), and history of smoking (a history of smoking vs. never smoked) by participant self-report. Pairwise two-way interaction terms were added between APOE4 status, group, and smoking history. Finally, a three-way interaction between these three factors completes the fixed effects. Random intercept terms allowed for participant-specific effects. As a precaution to control for potentially inflated p-values in slightly unbalanced group sizes and small samples in some sub-groups, the Kenward-Roger adjustment [24-25] was applied as part of the F-statistic for fixed effects.
Embedded in this full model are several research hypotheses testable by specific a priori statistical contrasts using the best linear unbiased estimates (BLUEs) for the fixed effects [26], including the main effect of APOE, the interaction of APOE and group, and the interaction of APOE, group and smoking history. The BLUEs are more parsimonious than the conventional approach of multiple post-hoc comparisons after a statistically significant Type-3 F statistic. The BLUEs are also more specific, for example, in addressing the extent to which APOE4 status conferred a detrimental influence in neurocognitive outcomes, more so among cancer patients as compared with controls (analogous to a two-way, APOE4 by group interaction in a conventional Type-3 test). Furthermore, a more detailed BLUE can drill down to examine, for example, whether or not the detrimental effects associated with an APOE4 positive status is more prominent for patients without a history of smoking as compared to controls. Despite its complexity, the full 3-way model affords the exploration of several important research questions. These BLUE estimates will be primary focus of the Results section. The mixed models were fitted with the PROC MIXED procedure in SAS (version 9.2; SAS Institute, Cary, NC).
Results
APOE genotyping was available for 55 (92%) patients treated with chemotherapy, 68 (94%) patients not treated with chemotherapy and 43 (96%) healthy controls. Examination of the demographic data (Table 1) demonstrated that the groups were well matched on education, and race/ethnicity; however, patients not treated with chemotherapy were significantly older (p=0.003). Smoking prevalence was similar across groups (>50%); however, the majority of participants were former smokers.
Table 1.
Baseline Information
| Chemotherapy (n=55) |
No Chemotherapy (n=68) |
Control (n=43) |
Overall p-value |
||||
|---|---|---|---|---|---|---|---|
| Age (mean, sd, range) | 51.9 (7.1, 31-66) | 56.8 (8.3, 37-69) | 53.0 (10.1, 30–68) | 0.004 | |||
| Education (mean, sd, range) | 15.7 (2.6, 11-25) | 14.8 (2.2, 9-20) | 15.2 (2.6, 12–20) | 0.113 | |||
| APOE4+ (n, %) | 14 (25%) | 18 (26%) | 7 (16%) | 0.428 | |||
| Race (n, %) | |||||||
| White | 53 (98%) | 67 (98%) | 42 (98%) | 0.484 | |||
| Asian | 1 (2%) | 0 (0%) | 0 (0%) | ||||
| Other | 0 (0%) | 1 (1%) | 0 (0%) | ||||
| Did not respond | 1 (2%) | 0 (0%) | 1 (2%) | ||||
| Ethnicity (n, %) | |||||||
| Hispanic | 1 (2%) | 0 (0%) | 1 (2%) | 0.476 | |||
| Not Hispanic | 51 (93%) | 67 (98%) | 41 (95%) | ||||
| Did not respond | 3 (5%) | 1 (1%) | 1 (2%) | ||||
| Smoking History | |||||||
| Never smoked | 24 (44%) | 25 (37%) | 17 (40%) | 0.741 | |||
| Ever smoked | 31 (56%) | 43 (63%) | 26 (60%) | ||||
|
|
|||||||
| (in ever smokers) a | former | current | former | current | Former | current | |
| 24 (77%) | 4 (13%) | 32 (74%) | 7 (16%) | 24 (92%) | 1 (4%) | ||
non-response to former/current smoker status was 3 in Chemo, 4 in No Chemo, and 1 in Control groups.
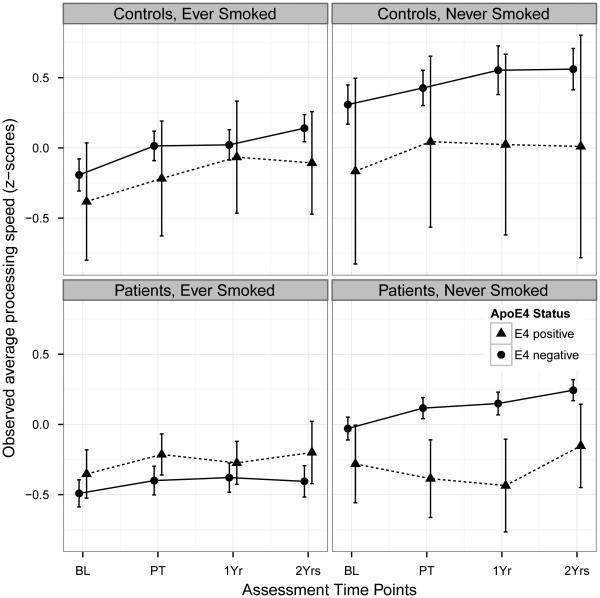
Figure 1 plots the profiles of observed longitudinal assessments of processing speed over time, stratified by group (patients vs. controls) and smoking history. The profiles are further divided by APOE4 status. Among the non-cancer controls (top two subplots), there appears to be no visible separation between the profiles of individuals who were APOE4-positive (‘APEO+’) and APOE4−negative (‘APOE4‐’), as can be seen in the overlapping error bars. All four profiles appear to follow the overall pattern of gradual improvement over time, with greatest improvements generally found between baseline and 1-month post treatment. Among the patients, the profiles appear more flat than those in the controls. Slight improvements are seen between baseline and 1-month post treatment, with the unique exception observed in APOE4+ patients who had never smoked. A decline is found in that group, up to 1-year post treatment, followed by a small recovery back to the baseline level at 2-years post treatment.
Figure 1.
Average domain scores for Processing Speed over assessment time points, stratified into four groups by smoking history and cancer.
Two lines are plotted for each sub panel, one for the ApoE4-positive status and the other for the ApoE4-negative status. The error bars are standard errors of the averages.
The visual observations in Figure 1 were supported by the mixed model BLUEs. There was no overall APOE4 effect (BLUE = −0.022, 95% CI: −0.142 – 0.096; p = 0.704), no APOE4 by group effect (BLUE = −0.155, 95% CI: −0.392 – 0.082; p = 0.198), and no APOE4 by smoking effect (BLUE = −0.096, 95% CI: −0.337 – 0.145; p = 0.433). We further examined the unique pattern in Figure 1, the declining processing speed in APOE4+ cancer patients who had never smoked. The 3-way contrast showed a worse outcome in APOE4+ patients without a smoking history as compared to controls (BLUE = −0.540, 95% CI: −1.015 – −0.064, p = 0.0263).
To facilitate the interpretation of these estimates, Figure 2 illustrates the observed post-treatment processing speed change scores (z-scores), averaged over the three followup time points, across the cohorts by contrasting the combined patient groups and controls (Figure 2(A)). The error bars represent 95% confidence intervals of the model-fitted BLUEs. In Figure 2(A), the lowest bar comes from APOE4+ patients who had never smoked; whereas all other bars show improved performance, a pattern consistent with what was observed in Figure 1. We found worse processing speed change among APOE4+ patients without a smoking history (contrast (a), BLUE=-0.366, 95% CI: −0.578 – −0.153, p = 0.0009). However, among individuals in the control cohort, we found no detrimental effect of APOE4+ and smoking history (p = 0.4247, (b)). A further comparison showed that the detrimental effect in APOE4+ individuals without a smoking history was more pronounced in patients as compared to controls (p = 0.0263, (c), BLUE = −0.540, 95% CI: −1.015 – −0.064, p = 0.0263).
Figure 2.
Impact of APOE and Smoking Status on Processing Speed for Patients vs. Controls (A) and by Treatment (B).
Bar plots of the observed average post-treatment processing speed outcomes for cancer patients and non-cancer controls (subplot (A)). The error bars represent the model-predicted 95% confidence intervals. Sample sizes of the subgroups are listed at the bottom of the graphs in parentheses. The interaction between APOE4 status and smoking history is represented with color, in the order as shown, with APOE4 present and a history of smoking represented by dark blue bars, APOE4 present but never smoked (light blue), APOE4 absent and a history of smoking (dark yellow), and APOE4 absent and never smoked (light yellow). Specific statistical contrasts were applied to test, in subplot (A): APOE4 and smoking history within patients (contrast (a)); APOE4 by smoking history interaction within non-cancer controls ((b)); and whether the pattern of the APOE4 by smoking history interaction is significantly different among the patients compared to non-cancer controls ((c)). In subplot (B), the two patient groups were further stratified by cancer treatment.
Figure 2 (B) further examines the difference between the two patient subgroups against the controls. The decline in processing speed was most distinct in patients treated with chemotherapy, at an observed average of −0.245 and a model-based confidence interval excluding the null. There was an APOE4 by smoking history interaction within the chemotherapy group (p = 0.006, (a)) and the no chemotherapy group (p = 0.050, (b)). By contrasting the BLUE effects in patient groups against that of the non-cancer controls, the detrimental effect in APOE positive, never smokers was more pronounced in chemotherapy-treated patients as compared to non-cancer controls (p = 0.023, (d)).
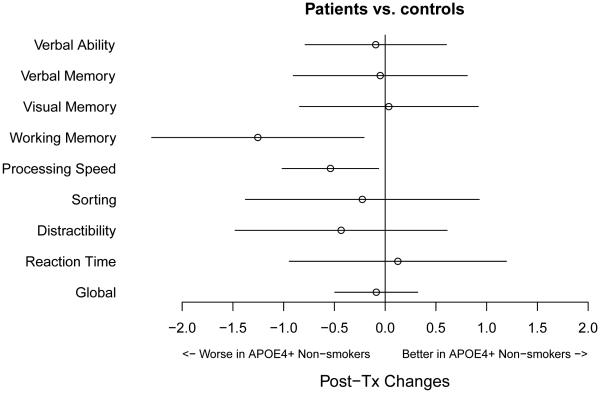
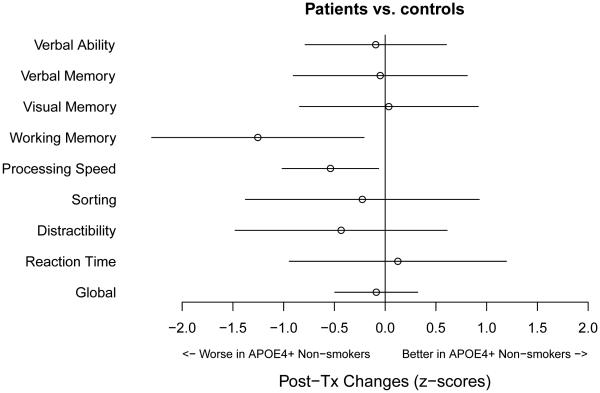
To further investigate whether or not similar result patterns were found in the other neurocognitive domains, we estimated the same effect in APOE4+ patients who had never smoked (chemotherapy and non-chemotherapy patients combined) as compared to controls. The results are summarized in Figure 3. The x-axis represents the magnitude of the standardized change scores (z-scores), with a negative value indicating that APOE4+, never smokers were worse off than ever smokers, and this discrepancy was greater in patients than in controls. This comparison was the same as, for example, the BLUE contrast (c) in Figure 2(A) for processing speed. In Figure 3, the points represent the BLUE estimates with corresponding error bars representing the 95% confidence intervals of the estimates. Figure 3 shows that statistically significant detrimental effects in APOE4+ patients who had never smoked were also found in working memory in addition to processing speed, shown by the error bars excluding the null. A similar interaction of APOE4 and smoking history was seen for working memory when comparing patients and controls (p=0.019).
Figure 3.
Estimates of the Detrimental Effects in APOE4+ patients who had Never Smoked Versus Controls Across Neurocognitive Domains.
The x-axis represents the magnitude of the standardized change scores, with a negative value indicating that APOE4 positive, never smokers were worse off than ever smokers, and this discrepancy is greater in patients than in controls. The horizontal error bars represent the 95% confidence intervals of the estimates. Statistically significant detrimental effects were found in processing speed and working memory, shown by the error bars excluding the null difference. The estimated effect in distractibility fell in the negative range, although the error bars did not exclude the null.
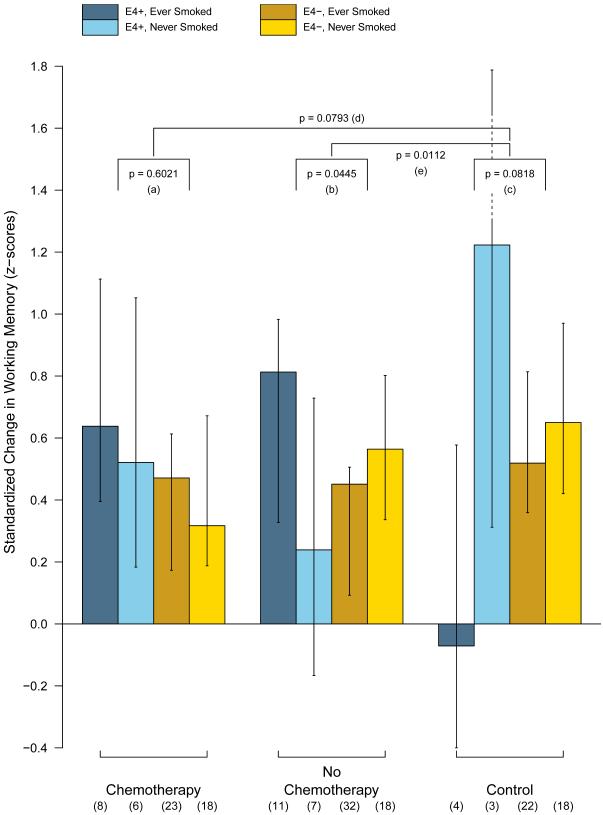
We further examined the working memory effect by plotting in Figure 4 the observed changes in working memory scores between the three groups to explore whether or not the detrimental influence of APOE4 on non-smokers was more prominent in chemotherapy-treated patients than non-chemotherapy-treated patients. Overall, the observed averages in Figure 4 are accompanied by wide error bars, the working memory domain had considerably higher variability than those in the processing speed domain. Specific statistical contrasts show that the APOE4 effect on non-smokers was more prominent in the no chemotherapy group (p = 0.0112, contrast (e)) but not in the chemotherapy-treated patients (p = 0.0793, (d)), as compared to controls.
Figure 4.
Impact of APOE and Smoking Status for Working Memory.
Bar plot of the observed average post-treatment working memory outcomes. The error bars represent the model-predicted 95% confidence intervals. Specific statistical contrasts were applied to test the APOE4 and smoking history interaction within (a) patients treated with chemotherapy; (b) patients not treated with chemotherapy; and (c) controls.
Discussion
There is consensus among investigators involved with the International Cognition and Cancer Task Force that only a subgroup of patients experience long-term post-treatment cognitive changes [1]. Therefore, the identification of risk factors that confer vulnerability to post-treatment cognitive change is critical to advancing the field. In our previous study, we identified age and cognitive reserve as important factors impacting post-treatment cognitive performance. In the current study, we examined APOE4 allele status as a risk factor for post-treatment cognitive decline. Since multiple aspects of cancer and treatment impact cognitive decline [1-2], we examined the influence of APOE status on post-treatment cognitive functioning in patients compared to healthy controls. No main effect for APOE or interactions of APOE and group were found. However, this analysis revealed that APOE4 was a risk factor for decline in the domains of processing speed and working memory, but only in patients without a smoking history. APOE4+ patients with a smoking history did not demonstrate the same pattern of decline, suggesting that smoking moderated the effect of APOE status.
The impact of treatment was then explored by dividing the patient group into chemotherapy exposed versus no chemotherapy groups. The interaction of APOE4 and smoking was more dramatic in the chemotherapy group for the processing speed domain, but remained to a lesser degree for the no chemotherapy group. However, for the working memory domain, the interaction was significant only for the no chemotherapy group. However, the precise pattern of results for treatment groups should be interpreted with caution since the cell sizes for certain groups are quite small.
The moderating effect of smoking in APOE4 carriers has been found in studies of cognitive changes associated with aging [9-12] and of risk for Alzheimer’s disease [27]. Although the mechanism for the moderating effects of smoking is not completely understood, one plausible explanation is that smoking corrects a deficit in nicotinic receptor binding sites and reduced dopaminergic activity [9-12, 27]. The role of dopamine in post-chemotherapy cognitive decline has been supported by results suggesting that breast cancer patients who were treated with chemotherapy and were carriers of the Val allele of catechol-o-methyltransferase (COMT), associated with lower levels of dopamine in prefrontal cortex, performed more poorly post-treatment compared to patients with the Met allele [28]. The role of frontal and prefrontal cortex in mediating the impact of chemotherapy-induced cognitive change has also been supported by imaging studies which have found changes in brain structure and function, primarily in frontal regions [29-30]. For patients not exposed to chemotherapy, the effect may be due to the impact of reduced estrogen levels in shaping dopamine-dependent cognitive functioning [31] since the majority of patients in this group were being treated with endocrine therapy.
It is noteworthy that the moderating effects of smoking were seen despite the fact that the majority of participants were not current smokers. Animal studies have demonstrated that even brief exposure to nicotine in adolescence can have a long-term impact on brain function [32], suggesting that there may be a critical period for smoking to have a moderating effect on the interaction of APOE and exposure to chemotherapy. The small number of current smokers may also account for the lack of a negative impact of smoking on APOE4− individuals, as has been found in previous studies (9-12).
Finally, these results, in combination with the prior COMT study [28], suggest that dopaminergic activity is one mechanism explaining post-treatment cognitive decline, and that interventions that stimulate nicotinic activity (e.g., nicotine patch) and/or enhance dopaminergic activity (e.g., certain antidepressants) may be protective for APOE4+ individuals without a smoking history. APOE status is not routinely assessed prior to treatment of breast cancer; however, as the field moves toward personalized medicine, APOE may be a candidate gene to consider adding to a pre-treatment gene array.
The above discussion is meant to provide a synthesis of findings from other areas which provide potential plausibility for the pattern of results found. However, we recognize that this discussion is highly speculative and should be interpreted with caution. On the other hand, it provides a context for understanding the current results and leads to testable hypotheses for future research.
The pattern of results from this study and our work examining age and cognitive reserve also provide a potential explanation for the inconsistencies seen in the results of recent longitudinal studies. The mixture of patient characteristics may impact on the degree of post-treatment cognitive change seen. For example, if the patient population is younger, more educated (higher cognitive reserve) and/or the prevalence of APOE4 and smoking varies, then there is less likelihood of finding a difference than if the sample consists of older, less educated (lower cognitive reserve) patients with a higher APOE4+ rate and lower rate of smoking history. Further, these results suggest the need to conduct purposeful recruitment based on patient characteristics so that there is sufficient power in the study sample to address specific hypotheses regarding treatment exposures and patient characteristics.
Additionally, as other studies have found, these results demonstrate that the majority of breast cancer patients show no clear evidence of deficits in cognitive performance, which is good news for patients making treatment decisions and survivors who are making the transition to their pre-diagnosis routines. However, those survivors who do experience post-treatment cognitive changes may feel that their cognitive problems are not “subtle” (as frequently described in the literature) as they struggle to return to work and school. Therefore, the extent of cognitive change may have been underestimated in the vulnerable subgroup of patients because the overall effect is attenuated by the majority of patients that improve.
The strengths of this study include the longitudinal assessment, including pre-treatment assessment, and the inclusion of breast cancer patients not exposed to chemotherapy and healthy women as comparison groups. The limitations include the small sample sizes in various subgroups and the lack of ethnic diversity in the sample. Further, due to sample size constraints, we were not able to compare current smokers with former smokers or interactions with age and cognitive reserve. Also, the domains identified in this study are different than the ones found in our previous study (visual memory and spatial ability). This discrepancy may be related to use of different measures or the impact of study design (longitudinal vs. cross-sectional) on the pattern of cognitive outcomes as discussed by Jim et al. [33]. Consequently, replication of these results is clearly necessary. However they provide interesting, hypothesis-generating data interactions of genetic, behavioral and treatment exposure factors that confer risk for post-treatment cognitive decline and point toward testable hypotheses regarding potential mechanisms and treatment / preventative strategies.
Acknowledgments
The authors thank Charlotte Furstenberg, Leigh Chesnut, Susan Horrigan, Carrie Kruck, Vivian Horovitch-Kelley, C. Harker Rhodes, and Becca Loeb for their assistance and all of the participants for their time and effort.
Supported by grants (R01 CA87845, R01 CA101318, R01 CA129769, and RO1 LM009012) from the National Institutes of Health, Bethesda, MD.
Footnotes
None of the authors have financial disclosures, conflicts of interest or acknowledgements
References
- 1.Wefel JS, Vardy J, Ahles TA, Schagen S. Internation Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in cancer patients. Lancet Oncology. 2011;12:703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 2.Ahles TA, Root JC, Ryan EL. Cancer and cancer treatment associated cogntive change: An update on the state of the science. Journal of Clinical Oncology. 2012;30:3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahles TA. Brain vulnerability to chemotherapy toxicities. Psycho-Onoclogy. 2012;21:1141–1148. doi: 10.1002/pon.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nature Reviews Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. Journal of Clinical Oncology. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schilder C, Seynaeve C, Beex LV, et al. Effects of tamoxifen and exemestane on cognitive function of postmenopausal patients with breast cancer: Results from the neuropsychological side study of the Tamoxifen and Exemestane Adjuvant Multinational Trial. Journal of Clinical Oncology. 2010;28:1294–1300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- 7.Bookheimer S, Burggren A. APOE-4 genotype and neurophysiological vulnerability to Alzheimer’s and cognitive aging. Annual Review of Clinical Psychology. 2009;5:343–362. doi: 10.1146/annurev.clinpsy.032408.153625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neurospsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psycho-Oncology. 2003;12:612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 9.Reitz C, Luchsinger J, Tang MX, Mayeux R. Effect of smoking and time on cognitive function in the elderly without dementia. Neurology. 2005;65:870–875. doi: 10.1212/01.wnl.0000176057.22827.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabia S, Kivimaki M, Kumari M, et al. Effect of apolipoprotein E 4 on the association between health behaviors and cognitive function in late midlife. Molecular Neurodegeneration. 2010;5:1–7. doi: 10.1186/1750-1326-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dufouil C, Tzourio C, Brayne C, et al. Influence of apolipoprotietn E genotype on the risk of cognitive deterioration in moderate drinkers and smokers. Epidemiology. 2000;11:280–284. doi: 10.1097/00001648-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Carmelli D, Swan GE, Reed T, et al. The effect of apolipoprotein E4 in the relationships of smoking and drinking to cognition. Neuroepidemiology. 1999;18:125–133. doi: 10.1159/000026204. [DOI] [PubMed] [Google Scholar]
- 13.Ahles TA, Saykin AJ, McDonald BC, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Research and Treatment. 2008;110:143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Psychological Corporation: Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 15.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- 16.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test-Second Edition: Adult Version Manual. The Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- 17.The Psychological Corporation . WAIS-III Wechsler Adult Intelligence Scale-Third Edition WMS-III Wechsler Memory Scale-Third Edition Updated Technical Manual. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 18.Fischer JS, Jak AJ, Kniker JE, et al. Multiple Sclerosis Functional Composite (MSFC) Administration and Scoring Manual. National Multiple Sclerosis Society; New York: 2001. [Google Scholar]
- 19.Lafayette Instrument: Grooved Pegboard: Instruction/Owner's Manual. Lafayette Instrument; Lafayette, IN: 2001. [Google Scholar]
- 20.Gordon M, McClure FD, Aylward GP. The Gordon Diagnostic System Instruction Manual and Interpretive Guide. Gordon Systems Inc.; Dewitt, NY: 1986. [Google Scholar]
- 21.Reymer W, Groenemeyer V, Van De Burg R, Kastelin J. Apolipoprotein E genotyping on agarose gels. Clinical Chemistry. 1995;41:1046–1047. [PubMed] [Google Scholar]
- 22.Flay BR, Miller TQ, Hedeker D, et al. The television, school, and family smoking prevention and cessation project. VIII. Student outcomes and mediating variables. Preventive Medicine. 1995;24:29–40. doi: 10.1006/pmed.1995.1005. [DOI] [PubMed] [Google Scholar]
- 23.Barnett AG, et al. Regression to the mean: what it is and how to deal with it. International Journal of Epidemiology. 2005;34:215–220. doi: 10.1093/ije/dyh299. [DOI] [PubMed] [Google Scholar]
- 24.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–97. [PubMed] [Google Scholar]
- 25.Brown H, Prescott R. Applied mixed models in medicine. John Wiley & Sons; New York: 2006. [Google Scholar]
- 26.Littell RC, Milliken GA, Stroup WW, et al. SAS system for mixed models. SAS Institute, Inc.; Cary, NC: 1996. [Google Scholar]
- 27.van Duijn CM, Havekes LM, Van Broidkhoven C, et al. Apolipoprotein E genotype and association between smoking and early onset Alzheimer’s disease. British Medical Journal. 1995;310:627–631. doi: 10.1136/bmj.310.6980.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Small BJ, Rawson KS, Walsh E, et al. Catechol-o-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. 2011;117:1369–1376. doi: 10.1002/cncr.25685. [DOI] [PubMed] [Google Scholar]
- 29.McDonald BC, Conroy SK, Ahles TA, et al. Gray matter reduction associated with systemic chemotherapy for breast cancer: A prospective MRI study Breast Cancer Research and Treatment. 2010;123:819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald BC, Conroy SK, Ahles TA, et al. Alterations in brain activation during working memory processing associated with breast cancer and treatment: A Prospective functional MRI study. Journal of Clinical Oncology. 2012;30:2500–2508. doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobs E, D’Esposito M. Estrogen shapes dopamine-dependent cognitive processes: Implications for women’s health. Journal of Neuroscience. 2011;31:5286–5293. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slotkin TA. Nicotine and the adolescent brain: Insights from an animal model. Neurotoxicology and Teratology. 2002;24:369–384. doi: 10.1016/s0892-0362(02)00199-x. [DOI] [PubMed] [Google Scholar]
- 33.Jim HSI, Phillips KM, Chait S, et al. Meta-Analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. Journal of Clinical Oncology. 2012;30:3578–3587. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]