Abstract
Rationale
Monoamine reuptake inhibitors can stimulate expression of brain-derived neurotrophic factor (BDNF) and alter long-term potentiation (LTP), a widely used model for the synaptic mechanisms that underlie memory formation. BDNF expression is up-regulated during LTP, and BDNF in turn positively modulates LTP. Previously, we found that treatment with venlafaxine, a serotonin and norepinephrine reuptake inhibitor (SNRI), but not citalopram, a selective serotonin reuptake inhibitor (SSRI) reduced LTP in hippocampal area CA1 without changing hippocampal BDNF protein expression.
Objectives
We tested the hypothesis that combined serotonin and norepinephrine reuptake inhibition is necessary for LTP impairment, and we reexamined the potential role of BNDF by testing for region-specific changes in areas CA1, CA3 and dentate gyrus. We also tested whether early events in the LTP signaling pathway were altered to impair LTP.
Methods
Animals were treated for 21 days with venlafaxine, imipramine, fluoxetine, or maprotiline. In vitro hippocampal slices were used for electrophysiological measurements. Protein expression was measured by enzyme-linked immunosorbent assay (ELISA) and western blotting.
Results
LTP was impaired only following treatment with combined serotonin and norepinephrine reuptake inhibitors (venlafaxine, imipramine) but not with selective serotonin (fluoxetine) or norepinephrine (maprotiline) reuptake inhibitors. BDNF protein expression was not altered by venlafaxine or imipramine treatment, nor were postsynaptic depolarization during LTP inducing stimulation or synaptic membrane NMDA receptor subunit expression affected.
Conclusions
LTP is impaired by chronic treatment with antidepressant that inhibit both serotonin and norepinephrine reuptake; this impairment results from changes that are downstream of postsynaptic depolarization and calcium-influx.
Keywords: Hippocampus, antidepressant, long-term potentiation, brain-derived neurotrophic factor (BDNF), tropomyosin-related kinase B (TrkB), N-methyl-D-aspartate (NMDA) receptor.
Introduction
Antidepressants have been used for over five decades, but the fundamental mechanisms of their therapeutic action are still unknown. The original theory of antidepressant action was based on the ability of the tricyclics and monoamine oxidase inhibitors to elevate norepinephrine and serotonin concentrations (Schildkraut 1965). While monoamine levels are altered quickly by antidepressants, a delay of 2-3 weeks precedes detectable clinical response, indicating that the therapeutic response is mediated by additional processes (Tardito et al 2006). Nibuya et al (1995) reported that chronic antidepressant treatment increases expression of BDNF mRNA. This finding stimulated formation of the neurotrophin hypothesis, which explains the delay between treatment and relief of symptoms by the time required for altered neurotrophin expression and consequent synaptic plasticity (Castrén et al 2007; Duman and Monteggia 2006; Calabrese et al 2009). The neurotrophin hypothesis is supported by animal studies showing elevated BDNF mRNA following antidepressant treatment (Nibuya et al 1996; Russo-Neustadt et al 2000; DeFoubert et al 2004). Further supporting evidence comes from human studies reporting decreased serum BDNF in depressed patients (Karege et al 2002; Shimizu et al 2003), and elevated serum BDNF after antidepressant treatment (Shimizu et al 2003; Aydemir et al 2005; Gonul et al 2005).
Neurotrophins are released in an activity-dependent manner (Greenberg et al 2009). During LTP, an activity-dependent form of synaptic plasticity (Blundon and Zakharenko 2008), BDNF mRNA is up-regulated (Patterson et al 1992; Castrén et al 1993). In addition, BNDF modulates LTP induction and persistence (Figurov et al 1996; Patterson et al 1996; Pang et al 2004). These findings predict that elevated BDNF levels following antidepressant treatment should affect LTP. In a previous study, we found that chronic treatment with venlafaxine, a serotonin and norepinephrine reuptake inhibitor, impaired LTP in the hippocampus without affecting hippocampal BDNF protein levels, whereas a selective serotonin reuptake inhibitor, citalopram, did not impair LTP (Cooke et al 2009). Our previous findings suggest that antidepressant effects on LTP might be limited to drugs that inhibit both serotonin and norepinephrine reuptake, and that effects on LTP are not mediated by BDNF. In this study, we tested selective serotonin, selective norepinephrine, and combined reuptake inhibitors for effects on LTP. We also examined BDNF levels in the CA1, CA3, and dentate gyrus regions of the hippocampus, to determine if our previous failure to find changes in BDNF protein may have been due to our use of whole hippocampus homogenates. Finally, we investigated several potential mechanisms for the inhibition of LTP by antidepressants.
Methods
Animals
A total of 134 male Sprague-Dawley rats (350-450g, Hilltop) were used. Animals were housed in pairs, on a 12 h light/dark schedule under controlled temperature and relative humidity, with food and water available ad libitum. Antidepressant drugs were administered orally as described previously (Cooke et al 2009) in sweetened condensed milk. Animals received vehicle solution for 3-5 days before assignment to a drug or control treatment. Animals in a drug treatment group received one of four drugs (all at 10 mg/kg in 10 ml vehicle, once per day): venlafaxine (an SNRI), imipramine, a tricyclic which inhibits reuptake of serotonin and norepinephrine, maprotiline, a norepinephrine reuptake inhibitor (NRI), or fluoxetine (an SSRI). Drug treated animals were run in small groups of 2-4, with an equal number of control animals (10 ml vehicle only). Treatment duration was 21 days, after which the animals were humanely euthanized by inhalation of a CO2/air mixture and decapitated.
After euthanasia, the brain was quickly removed and immersed in chilled artificial cerebrospinal fluid (ACSF), composed of 124 mM NaCl, 26 mM NaHCO3, 3.4 mM KCl, 1.2 mM NaH2PO4, 2.0 mM CaCl2, 2.0 mM MgSO4, 10 mM glucose, pH 7.35, equilibrated with 95% O2, 5% CO2. Coronal slices (400 μm) were cut using a vibrating microtome (Campden Instruments). Slices containing the hippocampus in a transverse orientation were dissected to isolate the hippocampus from surrounding brain tissue, yielding 10-14 individual hippocampal slices. Half of the slices were stored at room temperature in an interface holding chamber for later electrophysiological recordings. The remaining slices were micro-dissected into areas CA1, CA3, and dentate gyrus. Micro-dissected slices were rapidly frozen, and stored at −80°C for later protein analysis.
Electrophysiology
Our electrophysiological methods have been described previously (Cooke et al 2009; Grover et al 2009). Slices were transferred to an interface recording chamber (35°C) for extracellular field potential or whole cell patch clamp recording, and perfused continuously at 1-1.6 ml/min with ACSF.
Extracellular recordings
A glass micropipette recording electrode (3-5 MΩ) filled with ACSF was placed in stratum radiatum of area CA1. Field potentials were amplified (gain 100-1000), filtered (0.1 Hz - 10 kHz), digitized (10-100 kHz; National Instruments), and stored on a personal computer using WinWCP software (Strathclyde Electrophysiology Software, John Dempster, University of Strathclyde). A bipolar stimulating electrode was placed in stratum radiatum, about 500 m from the recording electrode. Biphasic constant voltage stimuli (0.1 ms duration) were delivered at 0.067 Hz. Stimulation voltage was varied from 1 to 10 V to create an input/output curve plotting field excitatory postsynaptic potential (EPSP) slope vs stimulus intensity. The population spike threshold intensity was determined and this intensity was used for the remainder of the recording.
Test stimulation was given for at least 15 min to establish a stable baseline response, after which we attempted to induce LTP with high frequency stimulation (HFS; 100 Hz, 1 s) or theta burst stimulation (TBS; 20 bursts of stimuli repeated at 5 Hz, each burst consisting of 4 stimuli at 100 Hz). Test stimulation was resumed approximately 15 s following attempted LTP induction, and continued for at least 1 h. EPSP slopes were normalized relative to the mean slope during the last 5 min of the baseline recording period, and expressed as percentage change from baseline. Normalized EPSP slopes at 55-60 min post-tetanus were compared between drug treated and control groups. In one set of recordings, slices received HFS in the presence of bicuculline, a GABAA receptor antagonist.
Whole cell recordings
Patch electrodes (3-5 MΩ) were filled with 140 mM potassium gluconate, 10 mM sodium HEPES, and 3 mM MgCl2, adjusted to 285-290 mOsm, pH 7.2. Recordings were made in current-clamp mode using an Axoclamp 2B (Axon Instruments). Membrane potentials were amplified (gain 10), low pass filtered (3 kHz), digitized (10-100 kHz; National Instruments), and recorded on a personal computer using WinWCP or WinEDR (Strathclyde Electrophysiology Software, John Dempster, University of Strathclyde) programs. Membrane potentials were not corrected for the liquid junction potential. Input resistance was estimated from the steady-state change in membrane potential in response to -100 to -400 pA current stimulation. Responses to single stimuli and HFS were evoked as described above. Responses were first recorded in ACSF, then with GABAA receptors blocked by picrotoxin (100 μM).
Protein Analysis
Protein samples were prepared as described (Cooke et al 2009) from frozen, micro-dissected hippocampal slices. Tissue was homogenized in ice cold lysis buffer (150 mM NaCl, 0.5% sodium deoxycholate, 1% NP-40, 50 mM Tris pH 8.0, 0.1% sodium dodecyl sulfate) containing phosphatase inhibitors (Sigma Phosphatase Inhibitor Cocktail 1 and 2) and protease inhibitors (Roche Complete Mini), using an ultrasonic cell disruptor (Misonix). Homogenates were centrifuged (12,000g, 4°C), and the supernatant was collected. Protein concentrations were determined using the Bradford assay (BioRad) with bovine serum albumin (Sigma) as the standard. BDNF protein concentrations were assessed using an ELISA kit (Promega BDNF Emax® ImmunoAssay System) following the manufacturer's instructions. Samples were diluted 1:10 prior to assay.
Synaptosomal membranes were prepared by Dounce homogenization as described (Kim et al 2010). Samples were homogenized in sucrose buffer (320 mM sucrose , 10 mM Tris-HCl, pH 7.4) with phosphatase and protease inhibitors, then centrifuged for 10 min at 1,000g, yielding a pellet (P1), and supernatant (S1). The S1 fraction was decanted and spun at 10,000g for 20 min to obtain crude synaptosomal membranes (P2). The P2 pellet was resuspended and lysed in water with protease and phosphatase inhibitors (30 min). The lysed P2 fraction was centrifuged at 25,000g for 20 min, and the pellet (LP1, synaptosomal membrane fraction) was resuspended in lysis buffer and inhibitors. Pellets were washed with sucrose buffer after each centrifugation.
Western blots were performed on proteins from tissue homogenates or synaptosomal membranes. Proteins (15-20 μg ) were separated by electrophoresis on gradient gels (4-15%, BioRad), transferred to nitrocellulose membranes, and probed with antibodies against the NMDA receptor subunits NR2A, NR2B, and NR1 (Chemicon/Millipore, #AB1555P, 1:5000; BD Biosciences, #610416, 1:5000; BD Biosciences, #556308, 1:5000); PSD-95 (Millipore, #MAB1598, 1:10,000) and TrkB (clone 80E3, Cell Signaling, cat # 4603); -actin (Sigma, #A2228, 1:10,000) was used as a loading control. After exposure to primary antibody, membranes were washed (tris-buffered saline containing 0.05% Tween 20) then incubated with HRP conjugated secondary antibodies (Sigma, 1:20,000 - 1:50,000), and washed again. Blots were then incubated with ECL Advance (GE Healthcare) solution and exposed to film. Densitometry was performed using ImageJ software (Wayne Rasband, NIH). For NMDA receptor subunits, blots were initially probed for NR1, NR2A or NR2B, then stripped using Restore™ buffer (Thermo Scientific) and reprobed for remaining NMDA receptor subunits and PSD-95; all blots were stripped and reprobed for ® -actin.
Statistics
Comparisons were made between drug-treated and control using unpaired student's t-tests (two-tailed). A p-value 0.05 was considered significant. Statistical analysis was performed using Gnumeric (http://www.gnome.org/projects/gnumeric/) and Origin software (Microcal). Values are reported as mean ± 1 SEM.
Results
LTP was impaired by serotonin and norepinephrine reuptake inhibitors
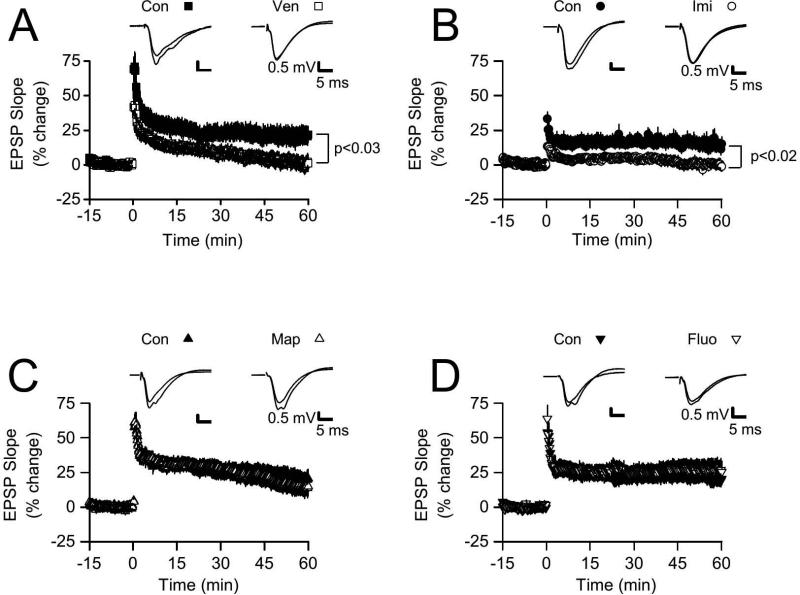
We examined LTP in slices from animals that were treated with venlafaxine for 3 weeks. As we reported previously (Cooke et al 2009), LTP induced by HFS was significantly reduced by chronic venlafaxine treatment (Fig.1A). Although HFS initially increased the EPSP slope in both groups, potentiation was maintained only in slices prepared from control animals. In control slices (n = 12), EPSP slopes at 55-60 min following HFS were increased by 20.7 ± 6.5%, while slices from the venlafaxine treatment group (n = 16) had a mean change in EPSP slope of only 1.1 ± 5.6% (p < 0.03 vs control). We next examined slices from imipramine-treated animals. While slices from control animals (n = 18) showed reliable LTP (see Fig.1B), with a mean increase in EPSP slope at 55-60 min post tetanus of 14.3 ± 4.3%, LTP again failed in slices from imipramine-treated animals (n = 16), which showed no change in EPSP slope at 55-60 min post-tetanus (0.3 ± 3.3%, p < 0.02 vs control).
Figure 1. LTP was significantly reduced in slices from venlafaxine (Ven) and imipramine (Imi) treated animals, but not in slices from maprotiline (Map) and fluoxetine (Fluo) treated animals.
Graphs shows normalized extracellular field EPSP slopes evoked every 15 s, with HFS was delivered at time 0. A. At 55-60 min post-HFS, normalized EPSP slopes for slices in the Ven group were significantly reduced (p < 0.03) compared to the Con group. Insets at top show EPSPs from representative slices from the Con and Ven groups; for each example, EPSPs were averaged over the final 5 min of baseline recording period, and are shown superimposed with averaged EPSPs from the final 5 min of the post-HFS recording period. B. At 55-60 min post-HFS, normalized EPSP slopes for slices in the Imi group were significantly reduced (p < 0.02) compared to the Con group. Insets (top) show superimposed, averaged EPSPs (final 5 min of baseline and final 5 min of post-HFS recording) from representative slices from the Con and Imi groups. C. Normalized EPSP slopes at 55-60 min post-HFS were not significantly different between the Map and Con groups (p > 0.65). Insets show superimposed, averaged EPSPs (baseline, final 5 min) from representative slices from the Con and Map groups. D. Normalized EPSP slopes at 55-60 min post-HFS were not significantly different between the Fluo and Con groups (p > 0.40). Insets show superimposed, averaged EPSPs (baseline, final 5 min) from representative slices from the Con and Map groups.
These data indicate that chronic inhibition of both serotonin and norepinephrine reuptake is sufficient to impair LTP. To determine if inhibition of reuptake for both neurotransmitters is necessary, we examined drugs which selectively inhibit reuptake of only one neurotransmitter: first, maprotiline, an NRI, and second, fluoxetine, an SSRI. In contrast to results obtained with venlafaxine and imipramine, three weeks of treatment with maprotiline or fluoxetine had no significant effects on LTP (see Figs. 1C, D). Slices from maprotiline-treated animals (n = 19) demonstrated a mean increase in EPSP slope of 15.1 ± 6.9% at 55-60 min post-tetanus, which did not differ from control slices (n = 24), 19.4 ± 6.7% (p > 0.65). Similarly, LTP in slices from fluoxetine-treated animals did not differ from LTP in slices from control animals. At 55-60 min post-tetanus, EPSP slopes in slices in the fluoxetine group (n = 14) were increased by 26.7 ± 7.6%, while control slices (n = 12) averaged 19.5 ± 3.9% (p > 0.40). These data indicate that chronic inhibition of both serotonin and norepinephrine reuptake is required for LTP impairment, and inhibition of either serotonin or norepinephrine reuptake by itself is not sufficient to impair LTP. Since antidepressants have previously been shown to alter BDNF expression, and since BDNF is implicated in LTP, we examined hippocampal tissue for changes in BDNF and TrkB expression.
BDNF and TrkB protein levels in hippocampus were unaffected by drug treatment
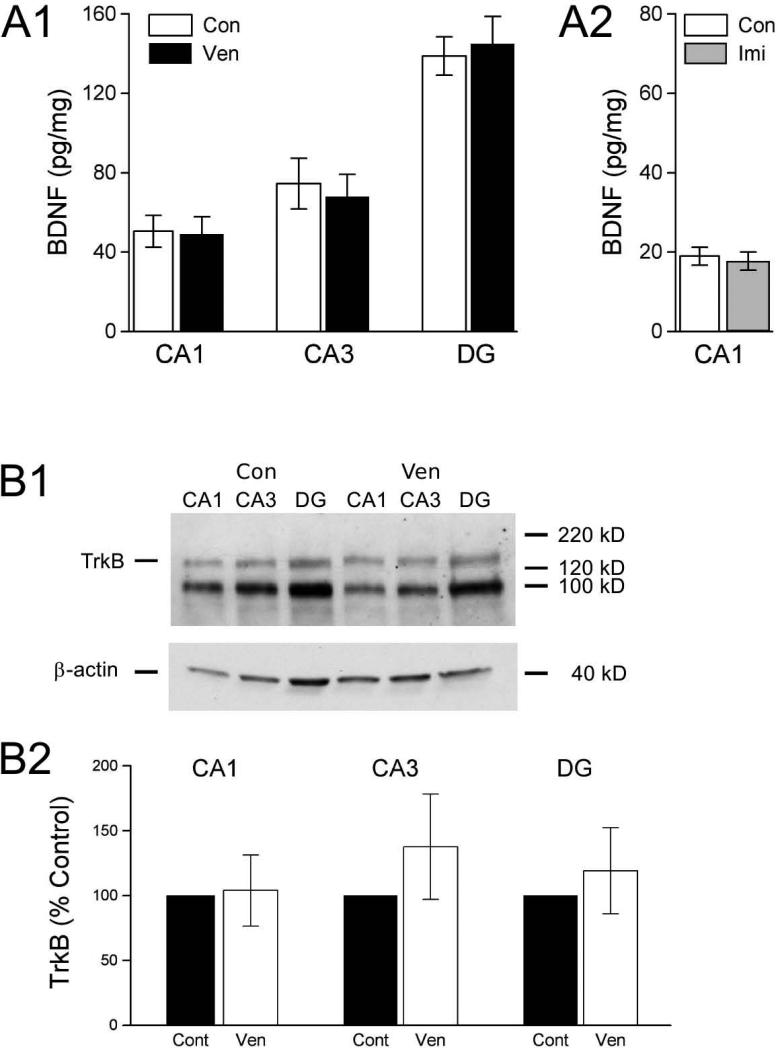
Previously, we found no effect of venlafaxine treatment on BDNF protein (Cooke et al 2009). However, in that study we examined BDNF expression in homogenates of whole hippocampus, and region-specific changes may have been obscured. To test for region-specific differences, we micro-dissected hippocampal slices into CA1, CA3, and dentate gyrus, and analyzed BDNF separately in each of these areas. We examined all three areas in tissue from venlafaxine-treated animals, and area CA1 in hippocampus from imipramine-treated animals (Fig.2A). In hippocampus from the venlafaxine-treated group (n = 6), area CA1 contained 50.6 ± 8.1 pg BDNF/mg protein, which was virtually identical to the value obtained area CA1 from control animals (n = 7), 49.1 ± 8.7 pg BDNF/mg protein). For area CA3, the venlafaxine treatment condition had 68.0 ± 11.3 pg BDNF/mg protein, while controls had 74.5 ± 12.8 pg BDNF/mg protein. A similar pattern was obtained in dentate gyrus, with 145.0 ± 13.8 pg BDNF/mg protein in the venlafaxine condition, and 138.8 ± 9.7 pg BDNF/mg protein in controls. None of these differences were significant (all p-values > 0.70). We also examined BDNF expression in area CA1 -- the area in which we conducted our electrophysiological experiments -- following imipramine treatment. As with venlafaxine, imipramine treatment failed to alter BDNF expression. Area CA1 from imipramine-treated animals (n = 9) contained 17.8 ± 2.3 pg BDNF/mg protein, a value that did not differ (p > 0.70) from controls (n = 9; 19.0 ± 2.3 pg BDNF/mg protein). Because neither venlafaxine nor imipramine affected BDNF protein expression, their effects on LTP cannot be explained by changes in BDNF abundance, but BDNF signaling could have been affected by changes in TrkB expression. However, treatment with venlafaxine failed to alter TrkB expression (Fig.2B) in any of the three major subdivisions of the hippocampus (p-values > 0.35 for areas CA1, CA3, and dentate gyrus).
Figure 2. BDNF and TrkB protein expression in micro-dissected areas of hippocampus were not affected by treatment with venlafaxine (Ven) or imipramine (Imi).
A1. BDNF protein levels in CA1, CA3, and dentate gyrus (DG) did not differ between Ven and control (Con) groups (all p-values > 0.70). A2. No difference in BDNF protein levels in area CA1 between Imi and Con groups (p > 0.70). B1. Representative TrkB western blot from one replication. Blot with proteins from micro-dissected CA1, CA3 and DG regions from one Ven and one Con animal were probed with TrkB antibody (top), then stripped and reprobed with -actin antibody (bottom) for loading control. Positions of molecular weight markers are shown at right, expected positions of full-length TrkB (145 kD) and -actin proteins are shown at left. The lower molecular weight (approximately 95 kD) protein recognized by the TrkB antibody is most likely the truncated TrkB receptor lacking the kinase domain (Klein et al 1990; Middlemas et al 1991). B2. For each protein sample, TrkB expression was normalized to the β-actin loading control, then all samples on the same blot were normalized relative to control, and results were averaged across blots. There were no significant differences (p-values > 0.35; n = 7 for both Con and Ven groups).
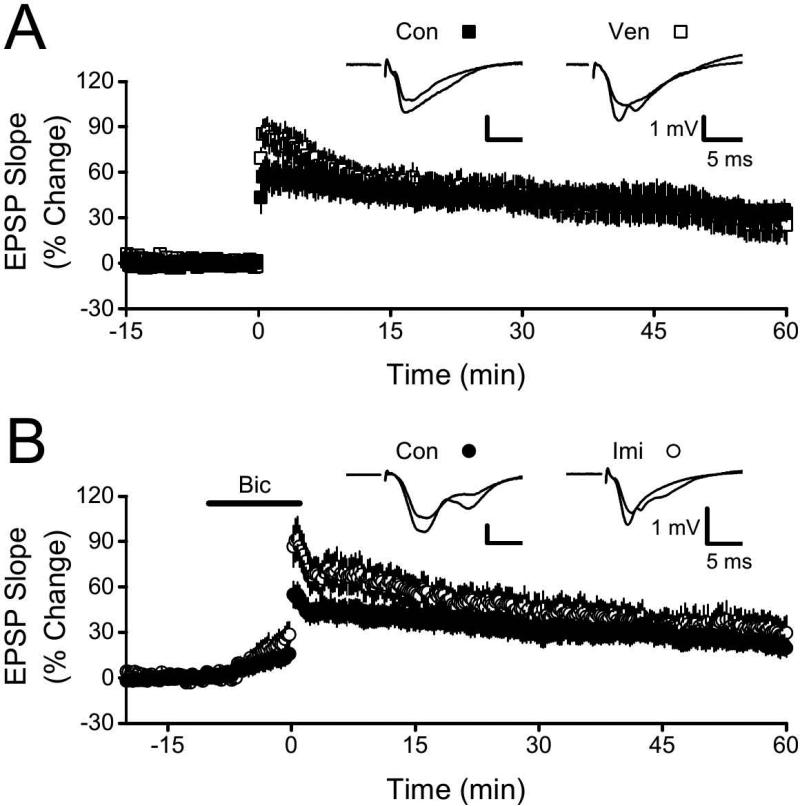
Normal LTP observed with TBS or bicuculline treatment
The HFS protocol that we used above to test effects of antidepressants produces a relatively modest level of potentiation. Inhibition of LTP by venlafaxine and imipramine might be overcome by a stronger LTP induction protocol. We tested this possibility using two additional LTP induction protocols that yield more robust potentiation: theta burst stimulation (TBS; Larson et al. 1986; Grover et al. 2009) and HFS given during block of GABAA receptors (Wigström and Gustafsson 1983; Grover and Chen 1999). In control slices (n = 12), TBS produced a large amplitude LTP with a mean EPSP slope increase of 33.1 ± 6.2% at 55-60 min following LTP induction (Fig.3A). In contrast to earlier results (compare Figs 3A and 1A), slices from the venlafaxine treatment group (n = 10) showed persistent potentiation following TBS; at 55-60 min post, EPSP slopes were increased by 26.3 ± 11.5%, which was not different from control (p > 0.55). Similarly, when we delivered HFS in the presence of bicuculline (GABAA receptor antagonist), LTP in control slices (n = 12; increase in EPSP slope of 22.8 ± 6.6% at 55-60 min post) did not differ from LTP in slices (n = 9) from the imipramine treatment group (EPSP slope increase of 31.2 ± 10.7%, p > 0.45 vs control). Together, these two experiments indicate that a sufficiently strong stimulation protocol can overcome the LTP impairment. .
Figure 3. LTP impairment in slices from venlafaxine (Ven) and imipramine (Imi) treated animals could be overcome.
A. Normalized EPSP slopes at 55-60 min following theta burst stimulation were not significantly different between the Ven and control (Con) groups (p > 0.55). Insets show superimposed, averaged EPSPs (baseline, final 5 min) from representative slices from the Con and Ven groups. B. Normalized EPSP slopes at 55-60 min following HFS in the presence of bicuculline (Bic; application period indicated by labeled horizontal bar) were not significantly different between the Imi and Con groups (p > 0.45). Insets show superimposed, averaged EPSPs (baseline, final 5 min) from representative slices from the Con and Imi groups.
Effects of antidepressant treatment on synaptic responses
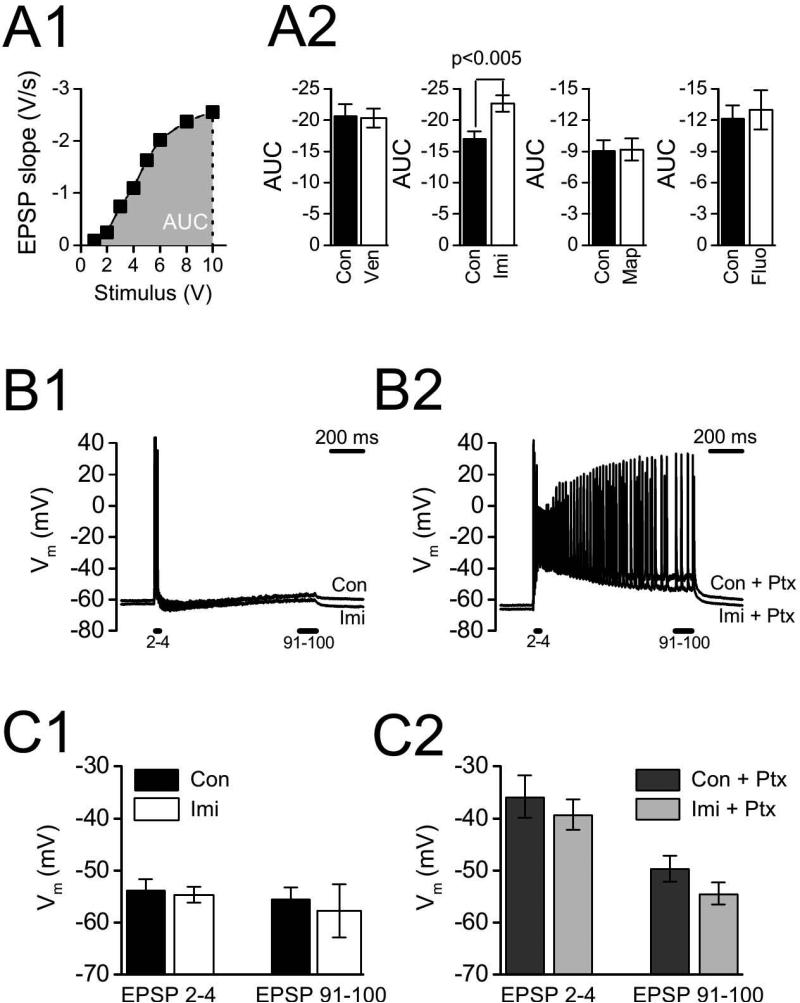
Chronic antidepressant drug treatment might itself have caused potentiation, so that subsequent in vitro testing for additional potentiation through LTP failed due to a ceiling effect. If this were the case, then basal excitatory synaptic transmission should be enhanced by antidepressant drug treatments that inhibit LTP. To test this possibility, we compared input/output curves between slices from drug-treated and control animals (Fig.4A). We calculated the area under the curve (AUC) for each slice, and compared slices in each drug treatment condition with slices from their corresponding control animals. Although imipramine treatment significantly increased basal synaptic transmission (increased AUC, p < 0.005), neither venlafaxine (p > 0.75), maprotiline (p > 0.95), nor fluoxetine (p > 0.70) significantly affected AUC. Because venlafaxine and imipramine both inhibited LTP, but venlafaxine did not alter basal excitatory synaptic transmission, a ceiling effect cannot account for the inhibition of LTP. This conclusion is supported by the strong LTP we obtained in slices from the imipramine treatment group when GABAA receptors were blocked (Fig.3B).
Figure 4. Antidepressant effects on synaptic transmission.
A. Imipramine (Imi), but not other antidepressants, increased the input-output function. A1. Example input-output curve from control (Con) slice. Field EPSPs were evoked by stimuli of varying intensity (1-10 V), and EPSP slopes were plotted against stimulus intensity to generate input-output curve. Area under the curve (AUC) was calculated then averaged across all slices from the same treatment condition. A2. AUC for slices from antidepressant treated and control animals. AUC was significantly greater for Imi group compared to Con (p < 0.005), but not for venlafaxine (Ven), maprotiline (Map), or fluoxetine (Fluo) groups relative to their controls (all p-values > 0.75). B, C. No effect of imipramine treatment on postsynaptic membrane potential during HFS. B1,2. Whole cell current clamp recordings of membrane potentials in representative CA1 pyramidal cells from imipamine-treated and control animals. Recordings were made first in normal ACSF (B1) and then following application of 100 μM picrotoxin (Ptx; B2); HFS was 100 Hz for 1 s. For each cell, membrane potentials during HFS were compared over 2 time periods: early during HFS (mean membrane potential measured immediately before EPSPs 2-4), and at the end of HFS (mean membrane potential measured immediately before EPSPs 91-100). C1. Membrane potentials during HFS in normal ACSF. C2. Membrane potentials during HFS after blocking GABAA receptors with Ptx. There were no significant differences in C1 or C2 between the Imi and Con groups (all p-values > 0.12).
LTP in hippocampal area CA1 is induced by postsynaptic, voltage-dependent calcium influx, and antidepressant drug treatment could interfere with LTP by reducing postsynaptic depolarization during induction. Reduced postsynaptic depolarization could result from decreased glutamate receptor function or enhanced GABA receptor function. To test these possibilities, we made whole cell current clamp recordings from CA1 pyramidal neurons. There were no differences in resting membrane potential between cells from control animals (−60.7 ± 2.1 mV, n = 8) and cells from imipramine-treated animals (−62.8 ± 1.6 mV, n = 9; p > 0.25), nor were there differences in input resistance (control:46.9 ± 6.2 MΩ, n = 4; imipramine-treated: 41.7 ± 2.8 M , n = 5; p > 0.40) To assess possible changes in glutamate and GABA receptor function, we first recorded membrane potentials during HFS in normal ACSF, where membrane potential is affected by both glutamate and GABA synaptic transmission, and then recorded membrane potentials with GABAA receptors bocked by picrotoxin (see Fig.4B, C), where postsynaptic membrane potential reflects glutamate receptor stimulation only. Postsynaptic membrane potential during HFS was quantified by measuring the membrane potential immediately prior to each stimulus during HFS; this measurement reflects the summed EPSPs from previous stimuli, with 〈-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptors primarily contributing to membrane depolarization early during HFS, and both AMPA and NMDA receptors contributing later during HFS (Herron et al 1986). For each cell, membrane potential measurements were averaged for EPSPs 2 - 4 (AMPA receptor mediated) and for the final 10 evoked EPSPs (AMPA + NMDA receptor mediated). For EPSPs 2 - 4 during HFS in ACSF, membrane potentials averaged −54.7 ± 1.5 mV for cells (n = 9) from the imipramine-treated group and −54.7 ± 1.5 mV for control cells (n = 8; not significantly different, p > 0.55). For the same cells, membrane potentials averaged −57.7 ± 1.8 mV (imipramine) and −55.4 ± 2.1 mV (control) over the final 10 EPSPs (also not significantly different, p > 0.20). Membrane potentials at the same points during HFS with GABAA receptors blocked also were not significantly different: for EPSPs 2 - 4, −39.2 ± 2.9 mV (imipramine, n = 7) and −35.8 ± 4.0 mV (control, n = 7; p > 0.60), and for EPSPs 91-100, −54.4 ± 2.1 mV (imipramine) and −49 ± 2.5 mV (control; p > 0.12). Because postsynaptic membrane potentials during HFS were not different between imipramine and control treatment groups, we conclude that impairment of LTP by antidepressants is not explained by altered postsynaptic depolarization
Synaptic membrane NMDA receptor subunits were not altered with AD treatment
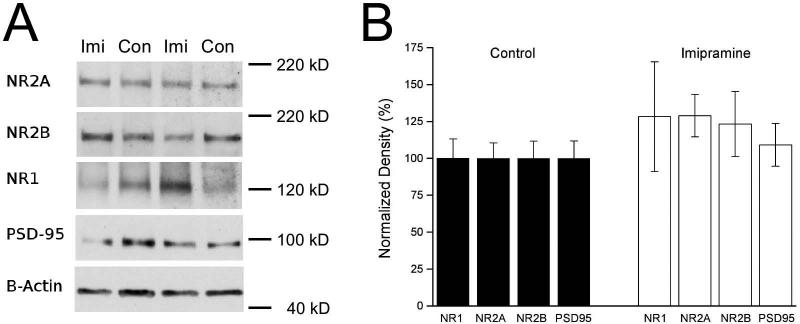
We examined expression of NR1, NR2A and NR2B NMDA receptor subunits in synaptic membranes prepared from area CA1 of control (n = 6) and imipramine-treated (n = 8) animals (Fig.5). Although NMDA receptor subunit expression appeared to be increased after imipramine treatment, with NR1 at 129 ± 37.2 % of control, NR2A at 128.9 ± 14.4 % of control, and NR2B at 123.2 ± 22.0 % of control, none of these differences were significant (all p-values > 0.15). We examined PSD-95 expression, but it, too, was unaffected by imipramine treatment (109.1 ± 14.5 % of control, p > 0.60).
Figure 5. No effect of imipramine (Imi) treatment on NMDA receptor subunit proteins in synaptic membranes.
A. Representative blot showing synaptic membrane proteins from two Imi and two control (Con) animals; proteins were probed for NMDA receptor subunits (NR1, NR2A, NR2B), PSD-95, and β-actin (loading control). B. For each protein, expression was normalized to β-actin, and then all values on the same blot were normalized relative to the mean control value. Results were averaged across blots. There were no significant differences in NMDA receptor subunit expression (all p-values > 0.15) or PSD-95 (p > 0.60).
Discussion
Antidepressant impairment of LTP requires both serotonin and norepinephrine reuptake inhibition
Our major finding is that chronic treatment with drugs (venlafaxine, imipramine) that inhibit reuptake of both serotonin and norepinephrine impairs LTP in hippocampal area CA1, but treatment with drugs that selectively inhibit either serotonin (fluoxetine in this study, citalopram in Cooke et al 2009) or norepinephrine (maprotiline) do not impair LTP. Because our experiments were done in vitro after completion of drug treatment, the LTP impairment we observed reflects a change in hippocampal synaptic function that outlasts immediate drug effects on monoamine reuptake, and any direct drug effects on signaling mechanisms required for LTP.
Several previous studies have examined effects of antidepressants on LTP, with mixed results probably reflecting variation in methods, including in vivo vs in vitro recording, acute vs chronic treatment, and brain region examined. Acute (single dose) studies, with LTP assessed in vivo, have reported the following findings: no effect of fluvoxamine (SSRI) on LTP in the hippocampal-prefrontal cortex pathway (Ohashi et al 2002) or the prefrontal cortex-basolateral amygdala pathway (Ohashi et al 2003); inhibition of LTP in area CA1 by milnacipran (SNRI) and fluvoxamine or citalopram (both SSRIs) (Kojima et al 2003; Tachibana et al 2004; Mnie-Filali et al 2006; Tachibana et al 2006); enhancement of LTP in dentate gyrus by tianeptine (serotonin uptake enhancer). Chronic antidepressant treatment with LTP assessed in vivo has also yielded variable results. Stewart and Reid (2000) treated animals for 15 days with fluoxetine, and found reduced LTP in the dentate gyrus in anesthetized animals. In contrast, Ohashi et al (2002) reported that 21 days of treatment with fluvoxamine enhanced LTP in the hippocampal-prefrontal cortex pathway, but similar treatment did not affect LTP in the prefrontal cortex-basolateral amygdala pathway (Ohashi et al, 2003). Interpretation of in vivo studies is complicated since effects of antidepressant treatment may reflect ongoing changes in monoamine neurotransmission, consequences of prior changes in monoamine neurotransmission, or direct effects of antidepressant drugs on one or more components of the LTP signaling pathway.
In vitro studies may offer more direct insight into the mechanisms through which antidepressants affect LTP. Watanabe et al (1993) applied tricyclic antidepressants (imipramine, desipramine, amitriptyline) directly to slices while recording; these investigators reported inhibition of LTP, but this could reflect a direct drug effect, since the drugs were present during LTP recordings. Massicotte et al (1993) found reduced LTP in area CA1 of hippocampal slices from animals treated for 7-9 days with trimipramine, a tricyclic antidepressant with atypical properties including weak inhibition of serotonin and norepinephrine reuptake (Randrup and Braestrup 1977). Wang et al (2008) treated animals with fluoxetine and found reduced LTP in the dentate gyrus in vitro after 5 days of treatment, but increased LTP after 25 days of treatment. A similar enhancement of LTP in the mouse dentate gyrus after 28 days of fluoxetine treatment was reported by Bath et al (2012), who also reported the loss of this enhancement in BDNF Val66Met knock-in animals. In contrast, in our earlier study (Cooke et al 2009), we found no effect on LTP after 7 or 21 days of treatment with citalopram, but inhibition of LTP following 7 or 21 days of venlafaxine treatment, whereas Rubio et al (2013) reported inhibition of both LTP and LTD at Schaffer Collateral-CA1 synapses after 28 days of fluoxetine treatment. However, Rubio et al used TBS to induce LTP, while we used HFS to examine effects of fluoxetine treatment. It is difficult to see a unifying pattern among all of these studies, and it is quite likely that many of these apparently anomalous findings result from key methodological differences including brain region examined (e.g., CA1 vs dentate gyrus) and LTP induction protocol (e.g., TBS vs HFS). In addition, our findings (this study and Cooke et al 2009) indicate that the reuptake inhibition profile of the antidepressant drug used is a critical factor determining effects on LTP.
Possible mechanisms of antidepressant impairment of LTP
We examined several mechanisms that might influence LTP induction: changes in glutamate receptor function and expression, GABA receptor function, and BNDF/TrkB expression. Our experiments show that changes in these factors do not explain the inhibition of LTP that we observed after chronic treatment with antidepressant drugs that inhibit reuptake of both serotonin and norepinephrine.
Glutamate Receptor Function
. NMDA receptor activation has a critical role in LTP induction in the Schaffer collateral-commissural pathway in the hippocampus CA1 field (Bliss and Collingridge 1993; Malenka and Bear 2004). Antidepressants can inhibit NMDA receptor function (Reynolds and Miller 1988; Sernagor et al 1989; Watanabe et al 1993), and this might explain impaired LTP in some previous studies where antidepressants were present during LTP recordings. However, this cannot explain the lack of LTP in our study, since antidepressants were not present during our in vitro recordings. Still, it is possible that chronic antidepressant treatment might persistently down-regulate NMDA receptors. Our findings argue against down-regulation of NMDA receptors: first, postsynaptic depolarization during HFS, which is partially NMDA receptor mediated (Herron et al 1986), was unaffected, and second, NMDA receptor subunit abundance in synaptic membranes was not reduced. However, it is possible that our methods may not have been sufficiently sensitive to detect a subtle change in NMDA receptor function or expression.
Altered AMPA receptor function might also impair LTP, through a ceiling effect, if antidepressant treatment potentiated AMPA receptor function, or by reduced postsynaptic depolarization during HFS, if antidepressant treatment reduced AMPA receptor function. Our data argue against both possibilities. Input/output curves reflect the strength of basal AMPA receptor-mediated excitatory transmission. Although the input/output relationship was significantly increased by imipramine, it was not altered by venlafaxine, yet both impaired LTP. Moreover, impairment of LTP by imipramine was overcome by delivering HFS in the presence of a GABAA antagonist. In addition, we found no effect of prior antidepressant treatment on postsynaptic depolarization during HFS, arguing against insufficient postsynaptic depolarization.
Inhibitory Function
Inhibition of LTP could result from enhanced GABA receptor function. Benzodiazepines, which enhance GABAA activation, impair LTP (del Cerro et al 1992; Higashima et al 1998), and GABAA receptor antagonists enhance LTP (Grover and Chen 1999; Wigstrom and Gustafsson 1983). Any increase in inhibitory function that might impair LTP should have reduced postsynaptic depolarization during HFS. However, our whole cell recordings showed equivalent membrane potential changes during HFS in cells from control and antidepressant-treated animals.
BDNF and TrkB
BDNF is important in LTP (Poo 2001; Minichiello 2009), and previous studies reported increased BDNF expression after antidepressant treatment (Castrén and Rantamäki 2010; Duman and Monteggia 2006). To investigate possible contribution of altered BDNF levels, we measured BDNF protein after venlafaxine and imipramine treatment. We failed to find any changes in BDNF protein in areas CA1, CA3, and dentate gyrus. This finding is consistent with previous studies by us and others (Altar et al 2003; Balu et al 2008; Cooke et al 2009). Since BDNF primarily exerts its actions through TrkB receptor binding, we measured TrkB protein expression by western blotting, but failed to find any effect of antidepressant treatment. Our results argue against the contribution of a sustained change in BDNF or TrkB expression in the impairment of LTP by antidepressant drugs. We cannot exclude a short-lived change in BDNF signaling that did not persist through the entire 21 day treatment period, but that nonetheless may have had a critical role in initiating the changes that lead to impaired LTP. Also, we cannot exclude an increase in TrkB signaling which could occur even in the absence of any detectable change in BDNF or TrkB protein. This could occur, for example, if increased BDNF release was accompanied by a matching increase in BDNF production. An increase in TrkB signaling might be detected by changes in TrkB phosphorylational status (Reichardt 2006); however, the absence of such changes at the 21 day time point could not exclude earlier, non-sustained but potentially critical changes in TrkB signaling.
Other Explanations
We were unable to implicate early signaling events (postsynaptic depolarization during HFS, altered NMDA receptor expression) suggesting that the impairment of LTP by prior antidepressant treatment involves downstream events in LTP induction. Based on their known importance for LTP (Silva et al 1992; Deisseroth et al 1996; Lu et al 1999; Kasahara et al 2001), likely candidates are Ca2+-calmodulin dependent protein kinase (CaMK) II or IV, and cAMP response element binding protein (CREB). Prior work has established that CaMKs (Tiraboschi et al 2004a,b; Barbiero et al 2007) and CREB (Schwaninger et al 1995; Nibuya et al 1996; Thome et al 2000) are affected by antidepressant treatment.
Variability in LTP Magnitude and BDNF Protein
We observed unexpected variability in LTP magnitude, with weaker potentiation in slices from both imipramine-treated animals and their controls compared to other antidepressant treatments and their respective controls (compare results in panel B of Fig. 1 with panels A, C and D). Because we ran animals in small groups (2-4 treated and equal number control animals at one time) with treatment duration of 3 weeks, in order to achieve the necessary number of replications, each experimental treatment required running multiple groups and therefore several months. As a consequence, our experiments were run over the course of approximately 2 years. We do not have an explanation for the variability in LTP magnitude, but it might reflect a seasonal effect on LTP (Grey and Burrell 2011; Walton et al 2011). We not believe this variability affected the outcome of our study, however, because all of our comparisons were made between control and treated animals that were run at the same time. In support, we note that the magnitude of effect of venlafaxine and imipramine (the difference between control and treated) was nearly identical, despite the differences in control LTP magnitude.
We also observed unexpected variability in BDNF protein levels for area CA1, with great BDNF protein in the the venlafaxine-treatment group and its control (approximately 50 pg/mg protein) compared to the imipramine-treatment group and its control (approximately 20 pg/mg protein). Again, because our experiments required running several small groups of animals over a long period of time, it is possible that seasonal effects might account for this variability, but we do not believe that this variablity can explain the lack of antidepressant effect on BDNF protein, since all of our comparisons between control and antidepressant treatments were made on animals that were run at the same time, and any effect of venlafaxine or imipramine on BDNF protein would have been detected by comparison against the relevant controls.
Relevance to Antidepressant Drug therapy
Does the effect of antidepressant drugs on LTP have relevance for antidepressant drug therapy? In addition to the impairment of LTP by monoamine reuptake inhibitors that we and others have reported, electroconvulsive shock both relieves depression (Linington and Harris 1988; Eitan and Lerer 2006) and inhibits LTP (Anwyl et al 1987; Trepel and Racine 1999; Stewart and Reid 2000). Similarly, sleep deprivation has long been recognized for beneficial effects in some depressed patients (Roy and Bhanji 1976) and more recently has been show to impair LTP (Campbell et al 2002; Davis et al 2003; Kim et al 2005, 2010). Finally, treatment with the NMDA receptor antagonist, ketamine, which like other NMDA receptor blockers can prevent LTP (Desmond et al 1991; Maren et al 1991), rapidly improves symptoms of depression (Skolnick et al 2009; Hashimoto 2011). We suggest that impairment of LTP or an LTP-like process may contribute to the effectiveness of antidepressant therapies.
Conclusions
We demonstrated that LTP is impaired by chronic treatment with antidepressant that inhibit both serotonin and norepinephrine reuptake. Our data indicate that reduced postsynaptic depolarization and NMDA receptor function do not underlie the impairment of LTP; this impairment may result from changes in one or more of the calcium-regulated components of the LTP signaling pathway. Our data indicate that a sustained alteration in BDNF availability is not required for antidepressant effects on LTP.
Acknowledgements
This work was supported by NIH grants P20RR016477 and P20GM103434 to the West Virginia INBRE. None of the authors declare a conflict of interest. All animal procedures were approved by the Marshall University Institutional Animal Care and Use Committee, and comply with the US Public Health Service policy on humane care and use of laboratory animals.
References
- Altar CA, Whitehead RE, Chen R, et al. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry. 2003;54:703–709. doi: 10.1016/s0006-3223(03)00073-8. [DOI] [PubMed] [Google Scholar]
- Anwyl R, Walshe J, Rowan M. Electroconvulsive treatment reduces long-term potentiation in rat hippocampus. Brain Res. 1987;435:377–379. doi: 10.1016/0006-8993(87)91629-5. [DOI] [PubMed] [Google Scholar]
- Aydemir O, Deveci A, Taneli F. The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: a preliminary study. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:261–265. doi: 10.1016/j.pnpbp.2004.11.009. doi: 10.1016/j.pnpbp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Balu DT, Hoshaw BA, Malberg JE, et al. Differential regulation of central BDNF protein levels by antidepressant and non-antidepressant drug treatments. Brain Res. 2008;1211:37–43. doi: 10.1016/j.brainres.2008.03.023. doi: 10.1016/j.brainres.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbiero VS, Giambelli R, Musazzi L, et al. Chronic antidepressants induce redistribution and differential activation of alphaCaM kinase II between presynaptic compartments. Neuropsychopharmacology. 2007;32:2511–2519. doi: 10.1038/sj.npp.1301378. doi: 10.1038/sj.npp.1301378. [DOI] [PubMed] [Google Scholar]
- Bath KG, Jing DQ, Dincheva I, et al. BDNF Val66Met impairs fluoxetine-induced enhancement of adult hippocampus plasticity. Neuropsychopharmacology. 2012;37:1297–1304. doi: 10.1038/npp.2011.318. doi: 10.1038/npp.2011.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Blundon JA, Zakharenko SS. Dissecting the components of long-term potentiation. Neuroscientist. 2008;14:598–608. doi: 10.1177/1073858408320643. doi: 10.1177/1073858408320643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Racagni G, Riva MA. Neuronal plasticity: a link between stress and mood disorders. Psychoneuroendocrinology 34 Suppl. 2009;1:S208–216. doi: 10.1016/j.psyneuen.2009.05.014. doi: 10.1016/j.psyneuen.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol. 2002;88:1073–1076. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- Castrén E, Pitkänen M, Sirviö J, et al. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport. 1993;4:895–898. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- Castrén E, Rantamäki T. Role of brain-derived neurotrophic factor in the aetiology of depression: implications for pharmacological treatment. CNS Drugs. 2010;24:1–7. doi: 10.2165/11530010-000000000-00000. doi: 10.2165/11530010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Castrén E, Võikar V, Rantamäki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Del Cerro S, Jung M, Lynch G. Benzodiazepines block long-term potentiation in slices of hippocampus and piriform cortex. Neuroscience. 1992;49:1–6. doi: 10.1016/0306-4522(92)90071-9. [DOI] [PubMed] [Google Scholar]
- Cooke JD, Grover LM, Spangler PR. Venlafaxine treatment stimulates expression of brain-derived neurotrophic factor protein in frontal cortex and inhibits long-term potentiation in hippocampus. Neuroscience. 2009;162:1411–1419. doi: 10.1016/j.neuroscience.2009.05.037. doi: 10.1016/j.neuroscience.2009.05.037. [DOI] [PubMed] [Google Scholar]
- Davis CJ, Harding JW, Wright JW. REM sleep deprivation-induced deficits in the latency-to-peak induction and maintenance of long-term potentiation within the CA1 region of the hippocampus. Brain Res. 2003;973:293–297. doi: 10.1016/s0006-8993(03)02508-3. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- Desmond NL, Colbert CM, Zhang DX, Levy WB. NMDA receptor antagonists block the induction of long-term depression in the hippocampal dentate gyrus of the anesthetized rat. Brain Res. 1991;552:93–98. doi: 10.1016/0006-8993(91)90664-h. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Eitan R, Lerer B. Nonpharmacological, somatic treatments of depression: electroconvulsive therapy and novel brain stimulation modalities. Dialogues Clin Neurosci. 2006;8:241–258. doi: 10.31887/DCNS.2006.8.2/reitan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, et al. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- De Foubert G, Carney SL, Robinson CS, et al. Fluoxetine-induced change in rat brain expression of brain-derived neurotrophic factor varies depending on length of treatment. Neuroscience. 2004;128:597–604. doi: 10.1016/j.neuroscience.2004.06.054. doi: 10.1016/j.neuroscience.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Gonul AS, Akdeniz F, Taneli F, et al. Effect of treatment on serum brain-derived neurotrophic factor levels in depressed patients. Eur Arch Psychiatry Clin Neurosci. 2005;255:381–386. doi: 10.1007/s00406-005-0578-6. doi: 10.1007/s00406-005-0578-6. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey KB, Burrell BD. Seasonal variation of long-term potentiation at a central synapse in the medicinal leech. J. Exp. Biol. 2011;214:2534–2539. doi: 10.1242/jeb.057224. doi: 10.1242/jeb.057224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover LM, Kim E, Cooke JD, Holmes WR. LTP in hippocampal area CA1 is induced by burst stimulation over a broad frequency range centered around delta. Learn Mem. 2009;16:69–81. doi: 10.1101/lm.1179109. doi: 10.1101/lm.1179109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover LM, Yan C. Blockade of GABAA receptors facilitates induction of NMDA receptor-independent long-term potentiation. J Neurophysiol. 1999;81:2814–2822. doi: 10.1152/jn.1999.81.6.2814. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. The role of glutamate on the action of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1558–1568. doi: 10.1016/j.pnpbp.2010.06.013. doi: 10.1016/j.pnpbp.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Herron CE, Lester RA, Coan EJ, Collingridge GL. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature. 1986;322:265–268. doi: 10.1038/322265a0. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- Higashima M, Kinoshita H, Koshino Y. Differences in the effects of zolpidem and diazepam on recurrent inhibition and long-term potentiation in rat hippocampal slices. Neurosci Lett. 1998;245:77–80. doi: 10.1016/s0304-3940(98)00178-5. [DOI] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, et al. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Kasahara J, Fukunaga K, Miyamoto E. Activation of calcium/calmodulin-dependent protein kinase IV in long term potentiation in the rat hippocampal CA1 region. J Biol Chem. 2001;276:24044–24050. doi: 10.1074/jbc.M100247200. doi: 10.1074/jbc.M100247200. [DOI] [PubMed] [Google Scholar]
- Kim E, Grover LM, Bertolotti D, Green TL. Growth hormone rescues hippocampal synaptic function after sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1588–1596. doi: 10.1152/ajpregu.00580.2009. doi: 10.1152/ajpregu.00580.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Mahmoud GS, Grover LM. REM sleep deprivation inhibits LTP in vivo in area CA1 of rat hippocampus. Neurosci Lett. 2005;388:163–167. doi: 10.1016/j.neulet.2005.06.057. doi: 10.1016/j.neulet.2005.06.057. [DOI] [PubMed] [Google Scholar]
- Klein R, Conway D, Parada LF, Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990;61:647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- Kojima T, Matsumoto M, Togashi H, et al. Fluvoxamine suppresses the long-term potentiation in the hippocampal CA1 field of anesthetized rats: an effect mediated via 5-HT1A receptors. Brain Res. 2003;959:165–168. doi: 10.1016/s0006-8993(02)03756-3. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Research. 1986;368:347–50. doi: 10.1016/0006-8993(86)90579-2. doi: 3697730. [DOI] [PubMed] [Google Scholar]
- Linington A, Harris B. Fifty years of electroconvulsive therapy. BMJ. 1988;297:1354–1355. doi: 10.1136/bmj.297.6660.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YF, Kandel ER, Hawkins RD. Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. J Neurosci. 1999;19:10250–10261. doi: 10.1523/JNEUROSCI.19-23-10250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Maren S, Baudry M, Thompson RF. Differential effects of ketamine and MK-801 on the induction of long-term potentiation. Neuroreport. 1991;2:239–242. doi: 10.1097/00001756-199105000-00006. [DOI] [PubMed] [Google Scholar]
- Massicotte G, Bernard J, Ohayon M. Chronic effects of trimipramine, an antidepressant, on hippocampal synaptic plasticity. Behav Neural Biol. 1993;59:100–106. doi: 10.1016/0163-1047(93)90808-u. [DOI] [PubMed] [Google Scholar]
- Middlemas DS, Lindberg RA, Hunter T. trkB, a neural receptor protein-tyrosine kinase: evidence for a full-length and two truncated receptors. Mol Cell Biol. 1991;11:143–153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850–860. doi: 10.1038/nrn2738. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- Mnie-Filali O, El Mansari M, Espana A, et al. Allosteric modulation of the effects of the 5-HT reuptake inhibitor escitalopram on the rat hippocampal synaptic plasticity. Neurosci Lett. 2006;395:23–27. doi: 10.1016/j.neulet.2005.10.044. doi: 10.1016/j.neulet.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi S, Matsumoto M, Otani H, et al. Changes in synaptic plasticity in the rat hippocampomedial prefrontal cortex pathway induced by repeated treatments with fluvoxamine. Brain Res. 2002;949:131–138. doi: 10.1016/s0006-8993(02)02973-6. [DOI] [PubMed] [Google Scholar]
- Ohashi S, Togashi H, Matsumoto M, et al. Changes in synaptic properties in cortical-limbic communications induced by repeated treatments with fluvoxamine in rats. J Pharmacol Sci. 2003;92:100–107. doi: 10.1254/jphs.92.100. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, et al. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Randrup A, Braestrup C. Uptake inhibition of biogenic amines by newer antidepressant drugs: relevance to the dopamine hypothesis of depression. Psychopharmacology (Berl) 1977;53:309–314. doi: 10.1007/BF00492370. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds IJ, Miller RJ. Tricyclic antidepressants block N-methyl-D-aspartate receptors: similarities to the action of zinc. Br J Pharmacol. 1988;95:95–102. doi: 10.1111/j.1476-5381.1988.tb16552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Bhanji S. Sleep deprivation treatment in depression: a review. Postgrad Med J. 1976;52:50–52. doi: 10.1136/pgmj.52.603.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio FJ, Ampuero E, Sandoval R, et al. Long-term fluoxetine treatment induces input-specific LTP and LTD impairment and structural plasticity in the CA1 hippocampal subfield. Front Cell Neurosci. 2013;7:66. doi: 10.3389/fncel.2013.00066. doi: 10.3389/fncel.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101:305–312. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Schwaninger M, Schöfl C, Blume R, et al. Inhibition by antidepressant drugs of cyclic AMP response element-binding protein/cyclic AMP response element-directed gene transcription. Mol Pharmacol. 1995;47:1112–1118. [PubMed] [Google Scholar]
- Sernagor E, Kuhn D, Vyklicky L, Jr, Mayer ML. Open channel block of NMDA receptor responses evoked by tricyclic antidepressants. Neuron. 1989;2:1221–1227. doi: 10.1016/0896-6273(89)90306-1. [DOI] [PubMed] [Google Scholar]
- Shakesby AC, Anwyl R, Rowan MJ. Overcoming the effects of stress on synaptic plasticity in the intact hippocampus: rapid actions of serotonergic and antidepressant agents. J Neurosci. 2002;22:3638–3644. doi: 10.1523/JNEUROSCI.22-09-03638.2002. doi: 20026326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Okamura N, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Stewart CA, Reid IC. Repeated ECS and fluoxetine administration have equivalent effects on hippocampal synaptic plasticity. Psychopharmacology (Berl) 2000;148:217–223. doi: 10.1007/s002130050045. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Matsumoto M, Koseki H, et al. Electrophysiological and neurochemical characterization of the effect of repeated treatment with milnacipran on the rat serotonergic and noradrenergic systems. J Psychopharmacol (Oxford) 2006;20:562–569. doi: 10.1177/0269881106059694. doi: 10.1177/0269881106059694. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Matsumoto M, Togashi H, et al. Milnacipran, a serotonin and noradrenaline reuptake inhibitor, suppresses long-term potentiation in the rat hippocampal CA1 field via 5-HT1A receptors and alpha 1-adrenoceptors. Neurosci Lett. 2004;357:91–94. doi: 10.1016/j.neulet.2003.11.016. doi: 10.1016/j.neulet.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Tardito D, Perez J, Tiraboschi E, et al. Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: a critical overview. Pharmacol Rev. 2006;58:115–134. doi: 10.1124/pr.58.1.7. doi: 10.1124/pr.58.1.7. [DOI] [PubMed] [Google Scholar]
- Thome J, Sakai N, Shin K, et al. cAMP response element-mediated gene transcription is upregulated by chronic antidepressant treatment. J Neurosci. 2000;20:4030–4036. doi: 10.1523/JNEUROSCI.20-11-04030.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraboschi E, Giambelli R, D'Urso G, et al. Antidepressants activate CaMKII in neuron cell body by Thr286 phosphorylation. Neuroreport. 2004a;15:2393–2396. doi: 10.1097/00001756-200410250-00018. [DOI] [PubMed] [Google Scholar]
- Tiraboschi E, Tardito D, Kasahara J, et al. Selective phosphorylation of nuclear CREB by fluoxetine is linked to activation of CaM kinase IV and MAP kinase cascades. Neuropsychopharmacology. 2004b;29:1831–1840. doi: 10.1038/sj.npp.1300488. doi: 10.1038/sj.npp.1300488. [DOI] [PubMed] [Google Scholar]
- Trepel C, Racine RJ. Blockade and disruption of neocortical long-term potentiation following electroconvulsive shock in the adult, freely moving rat. Cereb Cortex. 1999;9:300–305. doi: 10.1093/cercor/9.3.300. [DOI] [PubMed] [Google Scholar]
- Walton JC, Chen Z, Weil ZM, Pyter LM, Travers JB, Nelson RJ. Photoperiod-mediated impairment of long-term potention and learning and memory in male white-footed mice. Neurosci. 2011;175:127–132. doi: 10.1016/j.neuroscience.2010.12.004. doi: 10.1016/j.neuroscience.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J- W, David DJ, Monckton JE, et al. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Saito H, Abe K. Tricyclic antidepressants block NMDA receptor-mediated synaptic responses and induction of long-term potentiation in rat hippocampal slices. Neuropharmacology. 1993;32:479–486. doi: 10.1016/0028-3908(93)90173-z. [DOI] [PubMed] [Google Scholar]
- Wigström H, Gustafsson B. Facilitated induction of hippocampal long-lasting potentiation during blockade of inhibition. Nature. 1983;301:603–604. doi: 10.1038/301603a0. [DOI] [PubMed] [Google Scholar]