Abstract
Background
Evidence for a role of leptin in depression is limited and conflicting. Inconclusive findings may be explained by the complex effect of obesity on leptin signaling. In particular, both hyperleptinemia due to leptin resistance in obese persons as well as low leptin in lean persons can imply that low leptin biological signaling is associated with an increased risk of significant depressive symptoms. We tested whether the relationship between leptin and depressive symptoms is modulated by abdominal adiposity in two population-based studies.
Methods
Data were from 851 participants (65–94 years) of the InCHIANTI Study and 1,064 (26–93 years) of the Baltimore Longitudinal Study of Aging (BLSA). Plasma concentrations of leptin, waist circumference and depressive symptoms via the Center for Epidemiological Studies-Depression scale (CES-D) were assessed. In longitudinal InCHIANTI analyses onset of depressed mood (CES-D≥20) was evaluated over a 9-year follow-up.
Results
In pooled cross-sectional analyses the interaction between leptin and waist circumference was significantly associated with CES-D scores ((log)leptin-by-waist interaction p=0.01). Also in longitudinal analyses, the (log)leptin-by-waist interaction term significantly (p=0.04) predicted depressed mood onset over time; depressed mood risk was especially increased for high levels of both leptin and waist circumference.
Conclusions
The present findings suggest that low leptin signaling rather than low leptin concentration is a risk factor for depression. Future studies should develop proxy measures of leptin signaling by combining information on abdominal adiposity and leptin level to be used for clinical and research applications.
Keywords: leptin, depression, abdominal adiposity, obesity, aging
INTRODUCTION
Increasing evidence indicates that depression is associated with obesity-related (especially abdominal adiposity) metabolic alterations(Penninx et al., 2013; Xu et al., 2011 ). In this context a role in depression has been proposed for leptin(Lu, 2007; Zupancic and Mahajan, 2011), the peptide hormone secreted by white adipose tissue that exerts a primary homeostatic function by suppressing nutritional intake and allowing energy expenditure. Leptin receptors are expressed in limbic substrates related to mood regulation (e.g. hippocampus and amygdala), and in animal models leptin has shown to improve learning and memory and to exert antidepressant behavioral effects(Krishnan and Nestler, 2010; Paz-Filho et al., 2010).
However, preliminary small clinical studies in humans have had conflicting results, showing both increased(Antonijevic et al., 1998; Zeman et al., 2009; Rubin et al., 2002) and decreased(Jow et al., 2006; Kraus et al., 2001; Atmaca et al., 2008; Lawson et al., 2012) leptin levels in depressed patients, whereas another study showed no association(Deuschle et al., 1996). These conflicting findings may be explained by the complexity of leptin response as a function of obesity, which is often associated with high levels of leptin. The failure of high levels of leptin to suppress food intake and decrease body weight/adiposity in obese persons is thought to be caused by a mechanism of leptin resistance (similar to the one that links type 2 diabetes and insulin resistance) that blunts leptin central action despite increasing concentrations(Munzberg and Myers, 2005). Based on these observations, it has been hypothesized that is not the absolute leptin concentration but rather reduced leptin signaling to the central nervous system that affects mood(Zupancic and Mahajan, 2011; Lu, 2007). Therefore, hyperleptinemia due to functional resistance in obese persons may represent a phenotype risk for depression similar to leptin insufficiency in lean patients.
In a recent study(Milaneschi et al., 2012) conducted in 2,502 community-dwelling older persons, we showed that leptin and visceral fat had an interactive effect in predicting the onset of depressive symptoms over a 5-year follow-up in men, with risk especially high for persons with high levels of both leptin and visceral fat. Another recent cross-sectional study(Morris et al., 2012) in 537 adults reported a borderline significant association between leptin and the interaction of depressive symptoms with body mass index. However, when testing interactive effects the sample size may represent a critical issue.
In the present study we used data from two well-characterized population-based studies, the InCHIANTI Study and the Baltimore Longitudinal Study of Aging. We tested whether the relationship between leptin and depressive symptoms is modulated by abdominal adiposity. We hypothesized that the interaction between leptin and waist circumference would be significantly associated with depressive symptoms cross-sectionally (pooling together data from both studies) and would predict the development of clinically relevant depressive symptoms over a 9-year follow-up (InCHIANTI).
METHODS AND MATERIALS
Study Population – InCHIANTI
The InCHIANTI Study is a prospective population-based study of older persons (65 years and older) in Tuscany (Italy) designed to investigate factors contributing to decline in mobility in later life(Ferrucci et al., 2000). Briefly, in 1998–2000 the sample was randomly selected from two sites using a multistage stratified sampling method. Data collection included a home interview, a medical examination and blood drawing. Participants were evaluated again at three-year (2001–2003), six-year (2004–2006) and nine-year (2007–2009) follow-up visits. All respondents signed an informed consent and the Italian National Institute of Research and Care on Aging Ethical Committee approved the study protocol. We selected 876 participants with available data on leptin, waist circumference and depressive symptoms at baseline. We then excluded 15 participants with dementia and another 10 with values above 3 standard deviations for leptin (≥71.1 ng/mL). This left 851 participants (65–94 years of age) as the primary sample. The majority of the sample (51.1%) had depressive symptoms measured at all three follow-ups and 69.7% of participants had at least 2 follow-up assessments. In longitudinal analyses, among the 675 participants free of depressed mood (Center for Epidemiological Studies- Depression Scale score <20) at baseline, 596 had at least one measure of depressive symptoms over the follow-up period (median=8.8 years; range=2.3–9.8 years). Those lost at follow-up, as compared to those available, were older (79.7 vs. 72.8 years, p<.0001) and had lower leptin (10.4 vs. 12.3 ng/mL; p=0.03) but did not differ in terms of sex, waist circumference or depressive symptoms.
Study Population –BLSA
The BLSA is an ongoing longitudinal study of community-dwelling volunteers living primarily in the Baltimore-Washington area (USA), who have been continually recruited since 1958(Ferrucci, 2008). Participants are healthy at study entry and undergo extensive evaluations including medical evaluations and physiological, functional, and neuropsychological testing at each clinic visit (at regular intervals of approximately 2 years). All participants provided informed consent and the study was approved by the MedStar Research Institute Institutional Review Board. Using all available BLSA data at the time this project was undertaken, we identified 1,114 participants with complete data on leptin (assay introduced since 2001), waist circumference and depressive symptoms at the same visit (2001–2010). We then excluded 12 participants already diagnosed with dementia and 19 for whom the onset of dementia was identified before our analytical visit and were diagnosed at a following visit (the method for determination of dementia status has been detailed elsewhere(Kawas et al., 2000)). After the additional exclusion of 19 participants above 3 standard deviations for leptin (≥109.5 ng/mL) the remaining 1,064 (26–93 years of age) represented the analytical sample. Data from subsequent visits after our analytical baseline were not available for 24.5% of the participants and 51.9% had only 1 follow-up CES-D assessment beyond baseline, therefore appropriate longitudinal analyses could not be performed at the current stage.
Leptin
Morning fasting plasma samples were used to assay leptin using an enzyme-linked immunosorbent assay (ELISA) (Human Leptin ELISA Kit; Linco Research, Inc, St. Charles, MO, USA) at the Laboratory of Immunology (InCHIANTI) and at the Laboratory of Clinical Investigation (BLSA) of the National Institute on Aging (Baltimore, MD, USA). Intra-assay and inter-assay variations were, respectively, 2.6–4.6% and 2.6–6.2% for InCHIANTI, and 1.1–5.0% and 3.9–5.3% for BLSA.
Abdominal and general adiposity
The same measurements for abdominal/general adiposity were available in both studies. Waist circumference (cm), defined as the minimal abdominal circumference between the lower edge of the rib cage and the iliac crests, was measured by trained clinical staff according to a standardized procedure as index of abdominal adiposity. Height and weight were measured to calculate body mass index (BMI) in kg/m2 as index of general adiposity.
Depressive symptoms
In both studies depressive symptoms were assessed using the Center for Epidemiological Studies-Depression scale (CES-D)(Radloff, 1977). The CES-D is a 20-item self report scale assessing the frequency of a variety of depressive symptoms within the previous week. Items are rated on a four-point scale from 0 (rarely) to 3 (most or all of the time) and total CES-D scores ranges from 0 to 60.
In analyses based on longitudinal data from InCHIANTI a cut-off score ≥20 at either the 3-year, 6-year or 9-year follow-up was used to define clinically relevant “depressed mood”. While a cut-off of 16 is generally considered to represent relevant depression, we selected a cut-off of 20 that has been shown to avoid overestimation of depressed mood in older subjects(Beekman et al., 1997).
Covariates
Putative confounders were a priori selected on the basis of previously reported associations with leptin and depression and of availability in both studies. Sociodemographic characteristics included age, sex, race (only in BLSA: white vs. others) and years of education. Smoking status was coded as never/former/current. Diabetes was defined as self-report of physician diagnosis, hypoglycemic (oral agents and insulin) medication use or clinical findings according to the 2011 American Diabetes Association criteria(American Diabetes Association, 2013) (plasma fasting glucose ≥126 mg/dL in InCHIANTI and BLSA, or 2-h plasma glucose ≥200 mg/dl during an oral glucose tolerance test in BLSA). Use of antidepressants was coded according to Anatomical Therapeutic Chemical (ATC) classification system(WHO, 2007). In longitudinal analyses focusing only on InCHIANTI additional covariates available in this cohort were selected to take into account the health profile of this older sample. Total number of chronic diseases (heart failure, coronary heart disease including angina and myocardial infarction, stroke, chronic obstructive lung disease, hypertension, cancer, Parkinson’s disease and hip arthritis) was calculated as a global marker of poor physical health; diseases were ascertained according to standardized, pre-established criteria and algorithms based upon those used in the Women’s Health and Aging Study(Guralnik et al., 1995) using information on self-reported history, pharmacological treatments, medical exam data and hospital discharge records. Presence of activities of daily livings (ADL) disabilities was defined as self-report of inability or needing personal help in performing any basic activities of daily living(Katz and Akpom, 1976). The Mini Mental State Examination (MMSE)(Folstein et al., 1975) was used to determine the global cognitive status.
Statistical analyses
Variables were reported as percentages or means ± standard deviation as appropriate. Leptin concentrations were presented as median [interquartile range] and were log-transformed for analyses due to their skewed distribution (Figure S1). In descriptive analyses, differences in leptin levels across groups were calculated using analysis of variance (leptin was presented back-transformed to facilitate interpretation) and the correlation with continuous variables was expressed by Pearson’s coefficient. In cross-sectional analyses, data from the two cohorts were pooled together using a multilevel analysis approach with subjects clustered within studies and a random intercept at the study level to take into account the correlation between measurements within the same study. In a linear model with (log)leptin (a (log)leptin2 term was tested and found to be non-significant (p=0.72) and therefore not further included in analyses) and waist circumference predicting CES-D scores, a (log)leptin-by-waist term was introduced. To examine whether the interactive effect of leptin and waist circumference differed across gender or race, in distinct analyses a (log)leptin-by-waist-by-sex and a (log)leptin-by-waist-by-race interaction term (along with the other interaction terms nested within) were entered in the multilevel model. Next, another analytical approach was undertaken to confirm results: the same linear regression model including the (log)leptin-by-waist term was tested separately in each study, and the pooled effect for the interaction term was estimated using a fixed-effects meta-analysis. In longitudinal analyses in InCHIANTI, the risk of developing depressed mood over the follow-up among participants free of depressed mood at baseline was estimated using a Cox proportional hazards model with (log)leptin, waist and (log)leptin-by-waist as predictors. For participants who developed depressed mood time-to-event was calculated as the time between baseline and the follow-up in which the outcome appeared for the first time; those who did not develop depressed mood were censored at the date of their last follow-up. The proportional hazards model was checked by including a time-to-event-by-(log)leptin interaction term and was met in the analysis. Since in Cox regression the coefficient of the interaction term estimates departure from multiplicativity, we additionally tested departure from additivity using the Relative Excess Risk due to Interaction (RERI) measure proposed by Knol et al. (2007). The outcome from Cox regression was used to calculate the RERI and the 95% Confidence Intervals (95%CI) were estimated using bootstrapping techniques (10,000 bootstrap samples); the RERI was deemed significant if the 95%CI did not include zero. All analyses were adjusted for relevant covariates. Multilevel analyses were performed with MLwiN (v. 2.22, Centre for Multilevel Modeling, University of Bristol) and meta-analysis with “rmeta” package (v. 2.16 ) available in R (v. 3.0.1, R Project for Statistical Computing). The “bootstrap” package (v. 2012.04-1) in R was used to estimate 95%CI for the RERI. All other analyses were performed with SAS (v. 9.2, SAS Institute, Inc., Cary, NC). Significance level was set at p<0.05, two-tailed.
RESULTS
Descriptives
Table 1 shows the main characteristics of participants from InCHIANTI and BLSA study. The InCHIANTI sample was older as expected by design, while the BLSA cohort included highly educated participants and 34.2% of participants were not white (25.2% African-Americans). In InCHIANTI and BLSA mean CES-D scores were, respectively, 12.7±8.8 and 5.7±5.9 and median leptin was 8.6[4.1–8.6] and 17.7[8.3–35.1] ng/mL.
Table 1.
Characteristics of the study populations in InCHIANTI and BLSA
| Characteristics | InCHIANTI (n=851) |
BLSA (n=1,064) |
|
|---|---|---|---|
| Age (years) (mean±SD) | 74.0 ± 6.7 | 65.1 ± 12.6 | |
| Sex(%) | |||
| Males | 44.0 | 50.9 | |
| Females | 56.0 | 49.1 | |
| Race (Caucasian) (%) | 100 | 65.8 | |
| Education (years) (mean±SD) | 5.5 ± 3.3 | 16.9 ± 2.4 | |
| Smoking status (%) | |||
| non smoker | 58.5 | 51.7 | |
| former smoker | 28.0 | 43.8 | |
| current smoker | 13.5 | 4.4 | |
| Diabetes (%) | 10.8 | 14.1 | |
| BMI (kg/m2)(mean±SD) | 27.5 ± 4.0 | 27.2 ± 4.7 | |
| Waist (cm)(mean±SD) | 92.6 ± 10.1 | 91.9 ± 12.6 | |
| CES-D (mean±SD) | 12.7 ± 8.8 | 5.7 ± 5.9 | |
| Antidepressant use (%) | 4.4 | 7.3 | |
| Leptin (ng/mL)(median[IQR]) | 8.6 [4.1–8.6] | 17.7 [8.3–35.1] | |
Abbreviations: BMI, body mass index; CES-D, Center for Epidemiological Studies-Depression scale
Leptin was higher for women and for higher years of education in both studies and was lower for smokers in InCHIANTI and white participants in BLSA (all univariate association between leptin and study characteristics are reported in supplemental Table S1). Furthermore, (log)leptin was significantly and positively associated with waist circumference (InCHIANTI: r= 0.36, p<.0001; BLSA: r= 0.22, p<.0001) and BMI (InCHIANTI: r= 0.54, p<.0001; BLSA: r= 0.47, p<.0001). In addition, (log)leptin was significantly associated with CES-D scores in InCHIANTI (r= 0.16, p<.0001) but not in BLSA (r= −0.03, p=0.31); however, in both studies participants on antidepressant medications had higher (log)leptin as compared to non-users (InCHIANTI: 16.2[6.2–22.8] vs 8.4[4.0–15.9] ng/mL, p=0.01; BLSA: 23.7[10.9–54.1] vs 17.5[8.3–35.1] ng/mL, p=0.07).
Cross-sectional analyses
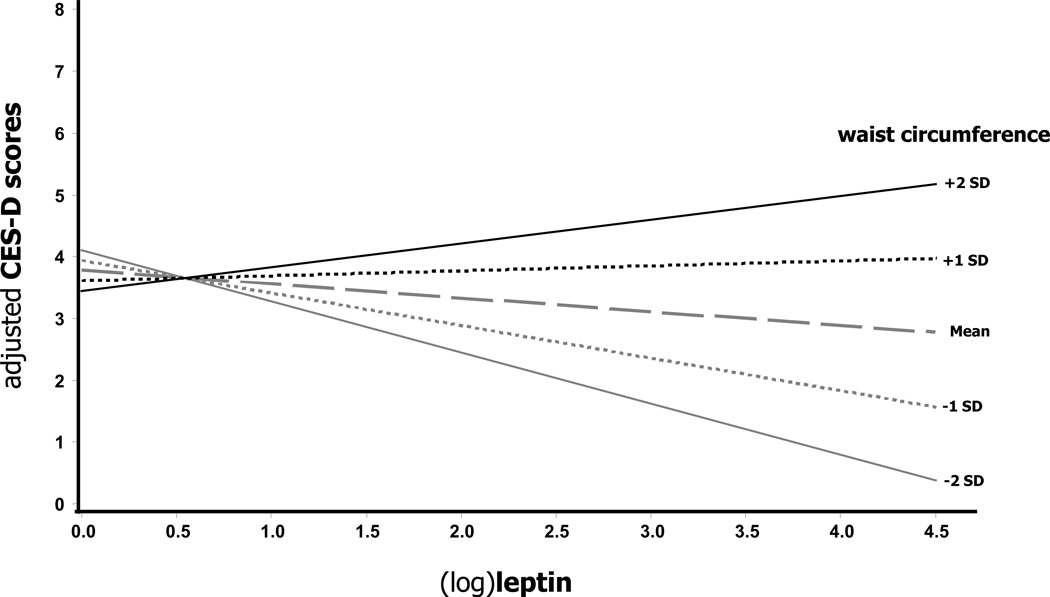
The results of multilevel analyses measuring the association of leptin, waist circumference and their interaction with CES-D scores pooling together data from both cohorts are reported in Table 2. In analyses adjusted for age, age2 (recent findings from BLSA showed that depressive symptoms follow a U-shaped pattern across adulthood(Sutin et al., 2013)), sex, race, education, smoking, diabetes and antidepressant use, (log)leptin did not predict CES-D scores. However, CES-D scores were predicted by waist circumference (β=0.06, SE=0.02, p=0.003). The log(leptin)-by-waist interaction term introduced in the same models was found to be significant (p=0.01). From the previous model we plotted the relationship between (log)leptin and CES-D scores across different waist circumferences (Figure 1): higher mean CES-D scores were evident for both higher leptin and waist circumference levels. In subsequent separate analyses a non-significant interaction was found for the (log)leptin-by-waist-by-sex term (p=0.79) and for the (log)leptin-by-waist-by-race term (p=0.49) included in fully adjusted models, suggesting that the interactive effect of leptin with waist circumference did not differ across gender or race. A significant (log)leptin-by-waist interaction was confirmed also using a fixed-effects (Woolf’s test for heterogeneity p=0.75) meta-analysis pooling together the separate results (supplemental Table S2) from fully adjusted linear regressions in the two cohort ((log)leptin-by-waist: summary effect 0.02, 95%CI 0.0005–0.04, p=0.045).
Table 2.
Adjusted cross-sectional association between leptin and waist circumference with depressive symptoms in pooled data from InCHIANTI and BLSA
| CES-D scores |
||||
|---|---|---|---|---|
| Model 1 |
Model 2 |
|||
| β (SE) | p) | β (SE) | p | |
| (log)leptin | 0.06 (0.02) | 0.35 | −0.02 (0.18) | 0.91 |
| (log)leptin | −0.31 (0.21) | 0.13 | −0.36 (0.21) | 0.09 |
| Waist | 0.06 (0.02) | 0.001 | 0.06 (0.02) | 0.003 |
| (log)leptin | −2.98 (1.11) | 0.01 | −2.62 (1.13) | 0.02 |
| waist | −0.02 (0.04) | 0.55 | −0.01 (0.04) | 0.72 |
| (log)leptin-by-waist | 0.03 (0.01) | 0.01 | 0.03 (0.01) | 0.01 |
Abbreviations: SE, standard error; CES-D, Center for Epidemiological Studies-Depression scale
Model 1: adjusted for age, age2 and sex
Model 2: additionally adjusted for race, education, smoking, diabetes and antidepressant use
Figure 1.
Adjusted cross-sectional association of leptin with depressive symptoms according to waist circumference in pooled data from InCHIANTI and BLSA
Abbreviations: CES-D, Center for Epidemiological Studies-Depression scale; SD, Standard Deviations
Adjusted for age, age2, sex, race, education, smoking, diabetes and antidepressant use. Waist circumference: mean=92.2 cm; −2 SD=69.0; −1 SD=80.6 cm; +1 SD=103.9 cm; +2 SD=115.3 cm
We repeated the multilevel analyses replacing waist circumference with BMI. In the fully adjusted model BMI was not associated with CES-D scores (β=0.07, SE=0.05, p=0.16) and the (log)leptin-by-BMI interaction term was non-significant (p=0.33).
Longitudinal analyses (InCHIANTI)
In the InCHIANTI cohort, of the 596 participants who were free of depressed mood at baseline (CES-D <20) and with available longitudinal data, 183 (30.7%) developed depressed mood over the course of follow-up (incidence rate: 44.9 cases per 1,000 person-years). In Cox regression models (Table 3) the risk of developing depressed mood over follow-up was not associated with (log)leptin or waist circumference. Again, the (log)leptin-by-waist interaction term subsequently entered in the fully adjusted model was significant (p=0.04), suggesting that the relationship between leptin and risk of depressed mood varied based on waist circumference levels. Results were substantially consistent using different CES-D cut-offs (from 20 to 16) to define depressed mood (Figure S2): however for the lower cut-off a higher number of participants classified as already depressed at baseline were removed from the analyses substantially reducing the sample size (N=512), while the proportion of cases with depressed mood onset was largely overestimated (46.7%). Furthermore, the (log)leptin-by-waist-by-sex interaction term subsequently included in the same model was non-significant (p=0.95). Moreover, after replacing waist circumference with BMI the (log)leptin-by-BMI interaction term was found to be non-significant (p=0.16). Finally the RERI for interaction between (log)leptin and waist calculated from a Cox model adjusted for age, sex and baseline CES-D (continuous) scores was significant (RERI=0.02, 95%CI=0.001–0.05), suggesting departure from additivity.
Table 3.
Adjusted risks of depressed mood onset over a 9-year follow-up according to baseline leptin levels and waist circumference in the InCHIANTI Study.
| Risk of Depressed Mood Onset | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N=596 | Model 1 | Model 2 | Model 3 | |||||||
| H.R. | 95% C.I. | p | H.R. | 95% C.I. | p | H.R. | 95% C.I. | p | ||
| (log)leptin | 1.17 | (0.98 –1.40) | 0.08 | 1.16 | (0.97 –1.39) | 0.10 | 1.14 | (0.96 –1.37) | 0.14 | |
| (log)leptin | 1.12 | (0.92 –1.36) | 0.27 | 1.11 | (0.91 –1.35) | 0.31 | 1.10 | (0.90 –1.34) | 0.38 | |
| waist | 1.01 | (0.99 –1.03) | 0.27 | 1.01 | (0.99 –1.03) | 0.32 | 1.01 | (0.99 –1.03) | 0.35 | |
| (log)leptin | 0.30 | (0.09 –1.01) | 0.05 | 0.28 | (0.08 –0.96) | 0.04 | 0.30 | (0.09 –1.06) | 0.06 | |
| waist | 0.98 | (0.94 –1.01) | 0.18 | 0.97 | (0.94 –1.01) | 0.15 | 0.98 | (0.94 –1.01) | 0.19 | |
| (log)leptin-by-waist | 0.03 | 0.03 | 0.04 | |||||||
Abbreviations: HR, Hazard Ratios; 95%CI, 95% Confidence Intervals
Depressed Mood: Center for Epidemiological Studies-Depression (CES-D) score ≥ 20 at any follow-up assessments in participants with CES-D < 20 at baseline
Model 1: adjusted for age and sex
Model 2: additionally adjusted for CES-D (continuous) scores at baseline
Model 3: additionally adjusted for education, smoking, diabetes, antidepressant use, education, smoking, diabetes, use of antidepressants, number of chronic diseases, presence of ADL disability and MMSE
In order to further explore the shape of the associations under study, we categorized participants in tertiles of leptin distribution and then divided each leptin-tertile in two sub-groups based on the group sex-specific waist circumference median; Figure 2 shows that, as compared to participants with lower waist circumference in the middle leptin-tertile, the fully adjusted risk of developing depressed mood over the 9-year follow-up was significantly higher for those with both high leptin and high waist circumference (HR=1.90, 95%CI=1.15–3.13, p=0.01); the second highest risk, although non-significant (HR=1.45, 95%CI=0.84–2.50, p=0.18), was found for those with both low leptin and low waist circumference.
Figure 2.
Adjusted risks of depressed mood onset over a 9-year follow-up according to baseline leptin levels and waist circumference in the InCHIANTI Study
Depressed Mood: Center for Epidemiological Studies-Depression (CES-D) score ≥ 20 at any follow-up assessments in participants with CES-D < 20 at baseline
Adjusted for age, sex, education, smoking, diabetes, use of antidepressants, number of chronic diseases, presence of ADL disability, MMSE and CES-D (continuous) scores at baseline
Leptin: low (tertile 1) < 5.6 ng/mL; normal (tertile 2) 5.6 – 13.2 ng/mL; high (tertile 3) > 13.2 ng/mL
−/+ waist: low/high waist within leptin groups based on group sex-specific median of waist circumference; 91.0 cm (low leptin), 99.0 cm (normal leptin) and 101.0 cm (high leptin) for men; 82.5 cm (low leptin), 88.0 cm (normal leptin) and 94.0 cm (high leptin) for women
Adjusted risks. Low leptin: - waist HR=1.45, 95%CI=0.84–2.50, p=0.18; + waist HR=0.75, 95%CI=0.40–1.40, p=0.36. Normal leptin: - waist Reference group; + waist HR=1.04, 95%CI=0.59–1.81, p=0.90. High leptin: - waist HR=1.38, 95%CI=0.82–2.30, p=0.23; + waist HR=1.90, 95%CI=1.15–3.13, p=0.01
DISCUSSION
Using data from two large population-based studies, we showed that the relationship between leptin blood concentrations and depressive symptoms is modified by abdominal adiposity. We found that leptin and waist circumference significantly interacted in predicting depressive symptoms both in cross-sectional and longitudinal analyses. In the latter, among participants free of depressed mood at baseline, those with high levels of both leptin and waist circumference had the highest risk of developing depressed mood over a 9-year follow-up. These results only partially replicate those of our previous study(Milaneschi et al., 2012) showing an interactive effect of leptin and visceral fat in predicting the development of relevant depressive symptoms over a 5-year follow-up in older men. However, in the current study we did not find evidence for a gender-specific interactive effect of leptin and waist on depressive symptoms. Differences in key study characteristics may be considered in order to explain this discrepant result. First, while the previous study was based on a sample of very old persons (75 years and older), in the current analyses younger participants were also included. Moreover, while in the present study waist circumference was used as a general measure of abdominal obesity, in the previous study abdominal visceral (distinguished from subcutaneous) fat was specifically assessed via computerized tomography. It has been consistently shown that different fat indicators are differentially distributed across genders, with a higher visceral component in men and a higher subcutaneous component in women(Koster et al., 2010). Finally, as compared to our previous study the sample size for the current analyses (despite pooling together data from two cohorts) was smaller and, therefore, possibly underpowered to adequately study multiple interactions between leptin, waist circumference and gender.
Nevertheless, the result of an interactive effect of leptin and waist circumference in predicting depressive symptoms is consistent with the hypothesis that it is not the absolute concentration of leptin that is correlated with mood but rather its ability to induce an effect at the receptor/post-receptor level(Lu, 2007; Zupancic and Mahajan, 2011). In this context, reduced leptin signaling to central nervous systems may be due to both leptin insufficiency in lean subjects or functional resistance-related hyperleptinemia in obese persons. Moreover, we showed that the interactive effect of leptin with adiposity was specific only for abdominal obesity. This is consistent with increasing evidence confirming that abdominal obesity, as compared to overall obesity, is specifically linked with the risk of depression(Penninx et al., 2013; Xu et al., 2011). White adipose tissue, especially in the abdominal area, is an active endocrine organ and a major contributor to pathogenic immuno-metabolic responses linked to metabolic diseases and depression(Osborn and Olefsky, 2012). In contrast, other types of fat (e.g. subcutaneous fat) have been shown to have less detrimental immuno-metabolic profiles and health consequences(Canepa et al., 2012; Koster et al., 2010). The immuno-metabolic alterations linked to abdominal obesity may have a role in the development of central leptin resistance, which is due to impaired leptin transport across the blood–brain barrier, reduced function of the leptin receptor and defects in leptin signal transduction(Munzberg and Myers, 2005). Obesity-associated inflammation can disrupt leptin hypothalamic action through IKKβ/NFkβ (inhibitor of kB kinase-β/nuclear factor-kβ) regulation of SOCS-3 (suppressor of cytokine signaling-3), a key inhibitor of leptin signaling(Zhang et al., 2008). Moreover, in animal models it has been shown that C-reactive protein, the acute phase protein produced by hepatocytes in response to pro-inflammatory cytokines stimulation, may directly bind to leptin and attenuate its physiological functions and this effect has been postulated to rise in proportion to the severity of obesity(Chen et al., 2006). Finally, in rodents the injection in the cerebral ventricles of interleukin-1 receptor antagonist (that in humans has been found to increase with obesity(Meier et al., 2002) and to be associated with depression(Milaneschi et al., 2009; Howren et al., 2009) inhibited the leptin-induced reduction in food intake(Luheshi et al., 1999).
Leptin may influence depressive symptoms via different biological mechanisms: leptin receptors (in particular the isoform LRb that activates intracellular transduction pathways) are expressed in the hippocampus and amygdala, suggesting a potential neuroactive function(Krishnan and Nestler, 2010a; Lu, 2007). The systemic administration of leptin in rodents has been shown to exert antidepressant behavioral effects and to improve learning and memory through long-term potentiation of hippocampal neurons(Lu et al., 2006; Oomura et al., 2006). Leptin has also been shown to affect hippocampal and cortical structure through its actions on neurogenesis, axon growth, synaptogensis and dendritic morphology regulation(Garza et al., 2008; Bouret, 2010). Moreover, accumulating evidence shows that leptin modulates hypothalamus-pituitary-adrenal (HPA) axis activity, which has been implicated in depression and obesity(Bornstein et al., 2006). Chronic administration of leptin can reverse hypercortisolemia even prior to weight loss in mice(Stephens et al., 1995), and there is an inverse relationship between plasma leptin, glucocorticoids and circadian rhythm activity(Licinio et al., 1997).
Alternative mechanisms may be also considered in order to explain the relationship between leptin and depressive symptoms, especially in conditions at the extremes of the weight spectrum that can be associated with high/low leptin concentrations. Abdominal obesity is associated with chronic diseases such as diabetes and cardiovascular disorders that, in turn, have been shown to be risk factors for depression(Penninx et al., 2013). Similarly, an upregulated inflammatory response have been extensively reported both in obesity and depression(Osborn and Olefsky, 2012; Meier et al., 2002; Milaneschi et al., 2009; Howren et al., 2009; Penninx et al., 2013). Interestingly, it has been shown that leptin may partially mediate the pathway from expanded adipose tissue to pro-inflammatory biomarkers increase(Miller et al., 2013). Furthermore, obesity may be associated with other lifestyle factors, such as unhealthy dietary patterns, lower level of physical activity, alcohol consumption and smoking habit, considered bio-behavioral risk factors for depression(Luppino et al., 2010). Finally, being overweight and the perception of overweight may increase psychological distress and depressive symptoms(Luppino et al., 2010). At the opposite extreme of body weight, low leptin has been associated with clinical conditions such as anorexia nervosa, in which depressive symptoms are common(Lawson et al., 2012).
A major strength of the present study was the possibility of examining data from two well-characterized population-based studies including participants from different countries. Moreover, the interaction of leptin and waist circumference was confirmed as departure from both multiplicativity and additivity, which has been proposed to better reflect biological synergism(Knol et al., 2007). Limitations should be also considered. First, depression was measured only through symptom questionnaires instead of assessing psychiatric diagnoses by means of clinical interviews. However, depressive disorders are not highly prevalent among elderly persons in the community, while chronic subsyndromal symptoms are more common with prevalences of 8–16% in the general older population and 17–35% among older persons in long-term care(Alexopoulos, 2005). Furthermore, although BLSA included also younger participants, the very low CES-D scores suggested that the presence of depressive disorders in this cohort was unlikely. Another limitation is that longitudinal data for the variables of interest were available only in one of the cohorts. Furthermore, the sample for longitudinal analyses was not adequately sized to test for the interactive effect of leptin and waist circumference on persistent depressed mood. Moreover, participants lost at follow-up in longitudinal analyses had lower leptin levels at baseline. Censoring of these subjects may have led to an underestimation of the relationship between low leptin and depressed mood onset. Finally, since the age-range of the cohorts included in the analyses was skewed towards older ages, the results may not be generalizable to younger populations. Similarly, since four fifth of the participants were white results cannot be generalized to other ethnic groups. Further studies in larger and relatively younger samples, including participants of different ethnic groups and well characterized in terms of psychiatric diagnoses are needed.
The present findings have important research and clinical implications. Our findings suggest that in planning future studies assessing the relationship between leptin and depression the major effect of adiposity, especially abdominal obesity, on this association needs to be taken into account. Not considering this issue may lead to contradictory or inconsistent conclusions. From a clinical point of view, these data sustain the idea that the key to understand the pathophysiology of leptin may lie in its function and its impaired central action and not merely in its circulating level. Therefore, in obese depressed patients therapeutic interventions on leptin downstream pathways should target leptin central resistance rather than leptin itself(Lu, 2007; Zupancic and Mahajan, 2011). A recent study(Yamada et al., 2011) showed that leptin homeostatic and antidepressant effect were impaired in the hippocampus of diet-induced obese mice, resulting in a severe depressive state despite hyperleptinemia; diet substitution and weight loss in the obese mice normalized leptin levels and restored the antidepressant effect of leptin.
In conclusion, our findings suggest that low leptin signaling rather than low leptin concentration is a risk factor for depression. The present study suggests a potential new mechanism by which two of the main contributors of disease and disability in Western countries, obesity and depression, may be linked. Future studies should develop proxy measures of leptin signaling by combining information on abdominal adiposity and leptin level to be used for clinical and research applications.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- Antonijevic IA, Murck H, Frieboes RM, Horn R, Brabant G, Steiger A. Elevated nocturnal profiles of serum leptin in patients with depression. J.Psychiatr.Res. 1998;32:403–410. doi: 10.1016/s0022-3956(98)00032-6. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Kuloglu M, Tezcan E, Ustundag B. Serum leptin and cholesterol values in violent and non-violent suicide attempters. Psychiatry Res. 2008;158:87–91. doi: 10.1016/j.psychres.2003.05.002. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Deeg DJ, Van LJ, Braam AW, De Vries MZ, Van TW. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol.Med. 1997;27:231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Schuppenies A, Wong ML, Licinio J. Approaching the shared biology of obesity and depression: the stress axis as the locus of gene-environment interactions. Mol.Psychiatry. 2006;11:892–902. doi: 10.1038/sj.mp.4001873. [DOI] [PubMed] [Google Scholar]
- Bouret SG. Neurodevelopmental actions of leptin. Brain Res. 2010;1350:2–9. doi: 10.1016/j.brainres.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canepa M, Strait JB, Abramov D, Milaneschi Y, AlGhatrif M, Moni M, Ferrucci L. Contribution of central adiposity to left ventricular diastolic function (from the Baltimore Longitudinal Study of Aging) Am.J.Cardiol. 2012;109:1171–1178. doi: 10.1016/j.amjcard.2011.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Li F, Li J, Cai H, Strom S, Bisello A, Zhao AZ. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat.Med. 2006;12:425–432. doi: 10.1038/nm1372. [DOI] [PubMed] [Google Scholar]
- Deuschle M, Blum WF, Englaro P, Schweiger U, Weber B, Pflaum CD, Heuser I. Plasma leptin in depressed patients and healthy controls. Horm.Metab Res. 1996;28:714–717. doi: 10.1055/s-2007-979885. [DOI] [PubMed] [Google Scholar]
- Ferrucci L. The Baltimore Longitudinal Study of Aging (BLSA): a 50-year-long journey and plans for the future. J.Gerontol.A Biol.Sci.Med.Sci. 2008;63:1416–1419. doi: 10.1093/gerona/63.12.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J.Am.Geriatr.Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J.Psychiatr.Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Garza JC, Guo M, Zhang W, Lu XY. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J.Biol.Chem. 2008;283:18238–18247. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME. The Women's Health and Aging Study: Health and Social Characteristics of Older Women With Disability. Bethesda, Md: NIH publication; 1995. pp. 95–400. [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom.Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Jow GM, Yang TT, Chen CL. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J.Affect.Disord. 2006;90:21–27. doi: 10.1016/j.jad.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Katz S, Akpom CA. A measure of primary sociobiological functions. Int.J.Health Serv. 1976;6:493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- Knol MJ, van der Tweel I, Grobbee DE, Numans ME, Geerlings MI. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int.J.Epidemiol. 2007;36:1111–1118. doi: 10.1093/ije/dym157. [DOI] [PubMed] [Google Scholar]
- Koster A, Stenholm S, Alley DE, Kim LJ, Simonsick EM, Kanaya AM, Harris TB. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity.(Silver.Spring) 2010;18:2354–2361. doi: 10.1038/oby.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus T, Haack M, Schuld A, Hinze-Selch D, Pollmacher T. Low leptin levels but normal body mass indices in patients with depression or schizophrenia. Neuroendocrinology. 2001;73:243–247. doi: 10.1159/000054641. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am.J.Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson EA, Miller KK, Blum JI, Meenaghan E, Misra M, Eddy KT, Klibanski A. Leptin levels are associated with decreased depressive symptoms in women across the weight spectrum, independent of body fat. Clin.Endocrinol.(Oxf) 2012;76:520–525. doi: 10.1111/j.1365-2265.2011.04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licinio J, Mantzoros C, Negrao AB, Cizza G, Wong ML, Bongiorno PB, Gold PW. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat.Med. 1997;3:575–579. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- Lu XY. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr.Opin.Pharmacol. 2007;7:648–652. doi: 10.1016/j.coph.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc.Natl.Acad.Sci.U.S.A. 2006;103:1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luheshi GN, Gardner JD, Rushforth DA, Loudon AS, Rothwell NJ. Leptin actions on food intake and body temperature are mediated by IL-1. Proc.Natl.Acad.Sci.U.S.A. 1999;96:7047–7052. doi: 10.1073/pnas.96.12.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch.Gen.Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- Meier CA, Bobbioni E, Gabay C, Assimacopoulos-Jeannet F, Golay A, Dayer JM. IL-1 receptor antagonist serum levels are increased in human obesity: a possible link to the resistance to leptin? J.Clin.Endocrinol.Metab. 2002;87:1184–1188. doi: 10.1210/jcem.87.3.8351. [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Corsi AM, Penninx BW, Bandinelli S, Guralnik JM, Ferrucci L. Interleukin-1 receptor antagonist and incident depressive symptoms over 6 years in older persons: the InCHIANTI study. Biol.Psychiatry. 2009;65:973–978. doi: 10.1016/j.biopsych.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaneschi Y, Simonsick EM, Vogelzangs N, Strotmeyer ES, Yaffe K, Harris TB, Penninx BW. Leptin, abdominal obesity, and onset of depression in older men and women. J.Clin.Psychiatry. 2012;73:1205–1211. doi: 10.4088/JCP.11m07552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain.Behav.Immun. 2003;17:276–285. doi: 10.1016/s0889-1591(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Morris AA, Ahmed Y, Stoyanova N, Hooper WC, De SC, Gibbons G, Vaccarino V. The association between depression and leptin is mediated by adiposity. Psychosom.Med. 2012;74:483–488. doi: 10.1097/PSY.0b013e31824f5de0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzberg H, Myers MG., Jr Molecular and anatomical determinants of central leptin resistance. Nat.Neurosci. 2005;8:566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, Sasaki K. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27:2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat.Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- Paz-Filho G, Wong ML, Licinio J. The procognitive effects of leptin in the brain and their clinical implications. Int.J.Clin.Pract. 2010;64:1808–1812. doi: 10.1111/j.1742-1241.2010.02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 2013;11:129. doi: 10.1186/1741-7015-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl.Psychol.Measure. 1977;1:385–401. [Google Scholar]
- Rubin RT, Rhodes ME, Czambel RK. Sexual diergism of baseline plasma leptin and leptin suppression by arginine vasopressin in major depressives and matched controls. Psychiatry Res. 2002;113:255–268. doi: 10.1016/s0165-1781(02)00263-9. [DOI] [PubMed] [Google Scholar]
- Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Kriauciunas A. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377:530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- Sutin AR, Terracciano A, Milaneschi Y, An Y, Ferrucci L, Zonderman AB. The Trajectory of Depressive Symptoms Across the Adult Life Span. JAMA Psychiatry. 2013;70:803–811. doi: 10.1001/jamapsychiatry.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology. Anatomical Therapeutic Chemical Classification. Geneva, Switzerland: WHO; 2007. [Google Scholar]
- Xu Q, Anderson D, Lurie-Beck J. The relationship between abdominal obesity and depression in the general population: A systematic review and meta-analysis. Obes.Res.Clin.Practice. 2011;5:e267–e278. doi: 10.1016/j.orcp.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Yamada N, Katsuura G, Ochi Y, Ebihara K, Kusakabe T, Hosoda K, Nakao K. Impaired CNS leptin action is implicated in depression associated with obesity. Endocrinology. 2011;152:2634–2643. doi: 10.1210/en.2011-0004. [DOI] [PubMed] [Google Scholar]
- Zeman M, Jirak R, Jachymova M, Vecka M, Tvrzicka E, Zak A. Leptin, adiponectin, leptin to adiponectin ratio and insulin resistance in depressive women. Neuro.Endocrinol.Lett. 2009;30:387–395. [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NFkappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupancic ML, Mahajan A. Leptin as a neuroactive agent. Psychosom.Med. 2011;73:407–414. doi: 10.1097/PSY.0b013e31821a196f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.