Abstract
CD8+ cytotoxic T lymphocytes (CTL) are adept at killing virally infected cells and cancer cells and releasing cytokines (e.g. IFN-γ) to aid this response. However, during cancer and chronic viral infections, such as with Human Immunodeficiency Virus, this CTL response is progressively impaired due to a process called T-cell exhaustion. Previous work has shown that the glycoprotein, T-cell immunoglobulin and mucin domain-containing protein 3 (Tim-3) plays a functional role in establishing T-cell exhaustion. Tim-3 is highly upregulated on virus and tumor antigen-specific CD8+ T-cells and antagonizing Tim-3 helps restore function of CD8+ T cells. However, very little is known of how Tim-3 signals in CTLs. In this study, we assessed the role of Tim-3 at the immunological synapse as well as its interaction with proximal TCR signaling molecules in primary human CD8+ T cells. Tim-3 was found withinCD8+T cell lipid rafts at the immunological synapse. Blocking Tim-3 resulted in a significantly greater number of stable synapses being formed between Tim-3hi CD8+T cells and target cells, suggesting that Tim-3 plays a functional role in synapse formation. Further, we confirmed that Tim-3 interacts with Lck, but not the phospho-active form of Lck. Finally, Tim-3 colocalizes with receptor phosphatases CD45 and CD148, an interaction that is enhanced in the presence of the Tim-3 ligand, galectin-9. Thus, Tim-3 interacts with multiple signaling molecules at the immunological synapse and characterizing these interactions could aid in the development of therapeutics to restore Tim-3-mediated immune dysfunction.
Introduction
The immune system has developed multiple mechanisms to limit T-cell responses to self-proteins to prevent autoimmunity. However, these mechanisms also limit T-cell responses to cancer antigens and chronic infection [1, 2]. During chronic infections, such as Human Immunodeficiency Virus Type 1 (HIV) and Hepatitis C Virus (HCV), CD8+T cells become exhausted causing CD8+ T cells to lose their effector function. Loss of production of IL-2 and TNF-α characterizes early exhaustion, whereas the production of IFN-γ is usually maintained until late-stage exhaustion [3–5]. Increased expression of multiple negative, co-inhibitory checkpoints, including Programmed death receptor 1(PD-1), has been associated with the exhausted phenotype. In addition, antagonizing these receptors with antibodies or soluble fusion proteins results in partial rescue of effector function [6]. We have previously shown that T-cell immunoglobulin and mucin domain-containing protein 3 (Tim-3) is highly expressed on exhausted HIV-specific CD8+T cells [4]. Similar to the other co-inhibitory receptors, Tim-3 blockade partially rescues the proliferation, cytokine production and cytotoxicity of virus-specific CD8+T cells suggesting that Tim-3 plays a functional role in T-cell exhaustion [4, 7]. However, unlike PD-1, Tim-3 is relatively uncharacterized in terms of how it manipulates the cell to dampen T-cell responses.
Human Tim-3 is a type I transmembrane protein with extracellular Ig V-like and mucin domains with two N- and one O-linked glycosylation sites [8]. Tim-3 is expressed at low levels on naïveCD8+T cells, Th1 and Th17 cells and regulatory CD4 T cells (Tregs), increased on activated CD8+T cells, and constitutively expressed on NK cells, dendritic cells (DCs), monocytes, and macrophages [4, 9–15]. Known ligands for murine Tim-3 include phosphatidylserine and galectin-9 [16, 17]. The interaction between galectin-9 and Tim-3 is carbohydrate dependent [17], and as such, galectin-9’scarbohydrate binding, lectin properties suggest that it may also interact or co-interact with other surface glycoproteins including CD44 [18] and integrins [19], allowing association with Tim-3.
Currently, the galectin-9 induced Tim-3 signaling cascade is unknown. We and others have shown that exhausted Tim-3hi CD8+T cells respond more efficiently to TCR stimulation when the Tim-3 pathway is blocked, suggesting that Tim-3 engagement antagonizes TCR signaling pathways [4, 5, 7, 20–23]. Previous studies have investigated Tim-3 signaling in artificial systems such as cell lines and transfection systems. Tyrosine phosphorylation of the Tim-3 cytoplasmic tail and enhancement of this phosphorylation with addition of galectin-9 has been reported in epithelial cell lines [24, 25]. In addition, Tim-3 was shown to bind to Fyn, p85 (the PI3K adaptor), and Lck [26, 27], further suggesting a role for Tim-3 in TCR proximal signaling. Finally, Tim-3 expression was shown to suppress NFAT dephosphorylation and AP-1 transcription [28]. However, these reports did not study Tim-3 in the context of intact TCR signaling with concurrent galectin-9 engagement. In addition, the pathway still remains to be studied in primary human CD8+T cells. Here we characterized the interaction between Tim-3 on primary human CD8+ T cells and early signaling events, which may lead to the exhausted phenotype of CD8+ T cells found in association with chronic viral infection or cancer.
Materials and Methods
Study Participants
Healthy HIV–seronegative human volunteers were recruited for blood specimens obtained via Leukophoresis. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Pacque PLUS (GE Healthcare Bio-Sciences, Uppsala, Sweden). Informed consent was obtained in accordance with the guidelines for conduction of clinical research at the University of Toronto and St. Michael’s Hospital institutional ethics boards.
T cell isolation, culture and activation
CD8+ T cells were isolated using EasySep™ Human CD8+ T Cell Negative Enrichment Kit (StemCell Technologies, Vancouver, BC, Canada). Cells achieved a purity of at least 95%, assessed via flow cytometry. Isolated CD8+ T cells were cultured in R-10 medium (RPMI 1640, 10% FBS, 1 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine; Wisent, Saint-Bruno, QC, Canada) and stimulated with1 μg/mL LEAF-purified anti-CD3 monoclonal antibody (mAb) (Clone OKT3; BioLegend, San Diego, CA) and 1 μg/mL LEAF-purified anti-CD28 mAb (Clone 28.8; BioLegend) with 50U/mL of recombinant IL-2 (National Institutes of Health, Bethesda, MD) for 5–7 days to upregulate Tim-3 [29]. Cells were then washed three times in R-10 and rested overnight in R-10 with no additional stimulation. For Tim-3 blocking experiments, cells were washed in R-10 medium and treated with 10μg/mL LEAF-purified anti-Tim-3 antagonistic mAb (Clone 2E2; BioLegend) or 10μg/mL LEAF-purified isotype control antibody (BioLegend) overnight without additional stimulation. EBV-transformed patient B-cells were cultured in R-10 for up to 20 passages.
Lipid Raft Isolation
5×106 of rested Tim-3hi CD8+T cells were harvested and resuspended in 20μl of TBS. The cells were lysed in 250μl of 0.5% Brij-58 TKM Lysis Buffer (50mM Tris-HCl, pH 7.4, 25mM KCl, 5mM MgCl2, 1mM EDTA, 1mM NaVO4, 5mM NaF with 1X Roche Complete Mini EDTA-free Protease Inhibitor) for 30 minutes at 4°C and mixed with equal parts of 80% sucrose in TKM Buffer. 36% sucrose in TKM Buffer (4.3mL) was layered on top of the 40% sucrose cell lysate solution and subsequently layered with 5% sucrose in TKM Buffer (0.2mL). The samples were ultracentrifuged at 50,000rpm for 16 hours at 4°C in a SW Ti-55 rotor (Beckman Coulter, Brea, CA) to isolate lipid rafts, which float to the top of the gradient (fraction 1), while non-lipid raft membrane should remain at the bottom of the gradient (fraction 10). 0.5mL fractions were collected from each 5mL gradient. To assess the presence of lipid rafts, 100μl of each fraction was blotted onto nitrocellulose using a dot blot apparatus and probed with a 1:3000 dilution of Cholera toxin-HRP (Sigma-Aldrich, Germany) to detect GM-1, a lipid raft resident ganglioside.
Soluble Tim-3 Staining and Flow Cytometry
Soluble Tim-3 (sTim-3) was produced and purified as previously described [4] and biotinylated using the EZ-Link Sulfo-NHS-LC-Biotinylation Kit (Thermo Scientific, Rockford, IL). B-cells were washed twice in 2% FBS/PBS and incubated with 1μg of biotinylated sTim-3 per 3×105 cells for 30 minutes at 4°C with or without α-lactose followed by staining with streptavidin-APC (Invitrogen, Carlsbad, CA). B-cells were also stained with Galectin-9-PE (BioLegend). Data were acquired on a BD FACS Calibur and analyzed on FlowJo software.
Conjugate formation and immunofluorescent staining
B-cell: T-cell conjugates were prepared as described in [30] with some modifications. Briefly, B cells were labeled with Cell Tracker Blue CMAC (Invitrogen) and incubated with 2μg/mL Staphylococcal enterotoxin B (SEB; Sigma-Aldrich) for 30 minutes at 37°C. Rested or Tim-3 blocked Tim-3hi CD8+T cells were mixed 2:1 with the B-cells and spun at 200X g for 5 minutes, gently resuspended and further incubated for 10 minutes at 37°C. The cells were placed onto poly-L-lysine coated coverslips and allowed to adhere for 15 minutes. The coverslips were gently rinsed with PBS and fixed with 4% methanol-free formaldehyde for 15 min at 37°C. Cells were washed with PBS and then permeabilized using 0.2% Triton-X-100 for 5 minutes at room temperature then washed with PBS. Blocking was performed using 10% BSA for 1 hour at room temperature followed by primary antibody incubation overnight at 4°C using combinations of anti-Tim-3 (R&D Systems, Minneapolis, MN; Cat#AF2365), anti-CD3 (Abcam, Cambridge, UK; Cat#ab5690), anti-phospho-ERK1/2 (R&D Systems; Cat#MAB1018), phospho-Lck Y394 (R&D Systems; Cat#MAB7500), anti-CD45 (Abcam; Cat#ab33533), and anti-CD148 (Abcam; Cat#ab140236) antibodies. Following subsequent washes in PBS, the coverslips were then incubated with appropriate secondary antibody mixes for 45 minutes at 37°C. Coverslips were mounted on slides using N-propyl gallate mounting buffer and sealed.
Microscopy and synapse counting
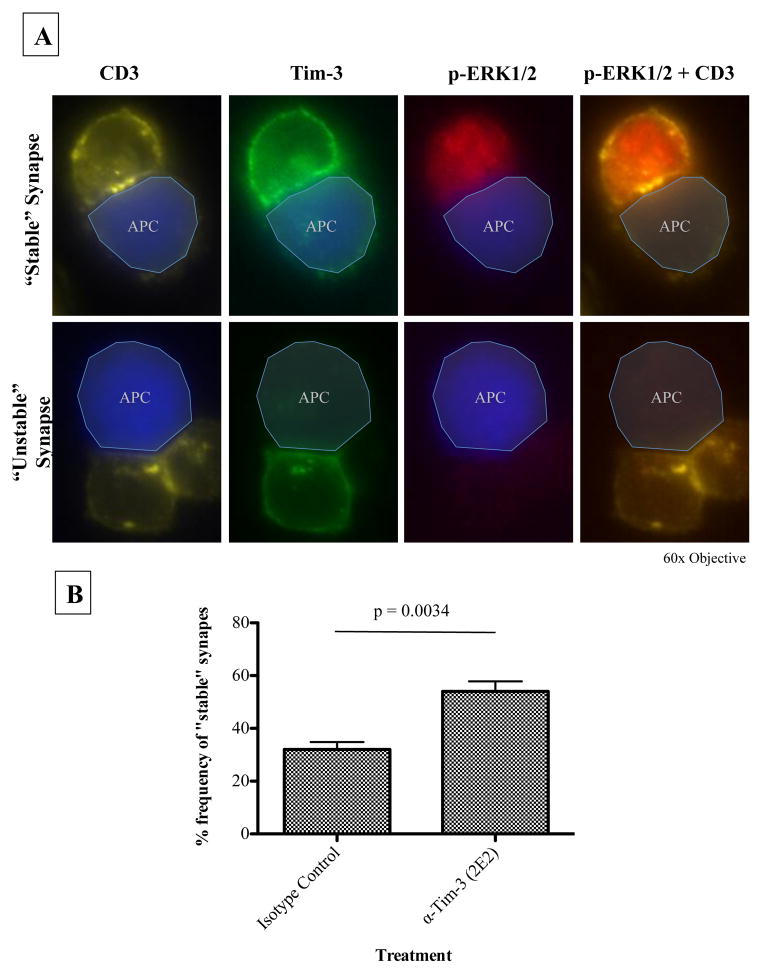
The slides were analyzed using a Zeiss LSM700 Confocal Microscope with a 63x oil immersion objective. Images of T cells, which formed an immunological synapse, were analyzed using LSM Zen LE 2011 Acquisition Software. For semi-quantification of stable synapses, slides were analyzed using an epifluorescent microscope with 60x oil immersion objective. Representative pictures were taken and analysis was performed using ImageJ software. Pictures were taken of multiple fields of view and 25 pairs of T cell: B-cell conjugates were counted per slide. “Stable” synapses were defined as a T-cell: B-cell conjugate with concentrated CD3 at the interface between the two cells and the T-cell was phospho-ERK+. “Unstable” synapses were defined as a T-cell-B-cell conjugate in which CD3 was not concentrated at the interface between the two cells or the T-cell was not phospho-ERK+. Only conjugates with a Tim-3+ T-cell were included in the counts. The number of “stable” synapses was blindly counted per 25 pairs of T cell: B-cell conjugates.
Immunoprecipitations and western blot analysis
1.5×107 rested Tim-3hi CD8+T cells were washed in PBS and resuspended in 1mL serum-free media then incubated at 37°C for 10 minutes. Cells were treated with or without 1mM pervanadate for 5 minutes at room temperature and then lysed in 0.5mL NP-40 Lysis Buffer (Invitrogen) at 4°C for 30 minutes. Cleared lysate was incubated with isotype or Tim-3 antibody (R&D Systems, Cat#AF2365) coupled Protein-G Dynabeads (Invitrogen) for 3 hours at 4°C. Beads were washed 5 times with 1mL of ice cold lysis buffer and bound protein was eluted in LDS sample buffer with 50mM DTT (Invitrogen). For anti-CD3 immunoprecipitations, 2×106 rested Tim-3hi CD8+T cells were pre-treated with anti-CD3 antibody-coupled Dynabeads (Invitrogen) beads for 30 minutes followed by addition of buffer or in-house produced recombinant galectin-9 for 1 hour. The cells were lysed in 0.5% Brij-58 TKM lysis buffer and anti-CD3 beads were collected. CD3-immunoprecipitated protein was eluted in LDS sample buffer with 50mM DTT (Invitrogen). Samples were prepared in LDS sample buffer and 50mM DTT as per manufacturer’s instructions and analyzed via 4–12% Bis-Tris SDS-PAGE with subsequent western blotting. Membranes were probed using anti-phosphotyrosine (EMD Millipore, Billerica, MA; Cat#05-321), anti-Tim-3 (R&D Systems; Cat#AF2365), anti-Lck (Abcam; Cat#ab3885), anti-CD45 (Abcam; Cat#ab33533), anti-CD148 (R&D Systems; Cat#AF1934) and anti-CD3 (Abcam; Cat#ab5690) antibodies followed by the appropriate light-chain-specific secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Detection was performed using chemiluminescence (Thermo Scientific).
Production and purification of human galectin-9
The medium isoform of human galectin-9 (UNIPROT ID: O00182-2) with a C-terminal 3XFLAG tag was codon optimized and subcloned into a pET15b vector. Following expression in ArcticExpress (DE3) competent cells (Agilent Technologies, Palo Alto, CA), the cells were induced with 0.5mM isopropyl b-D-thiogalactoside and incubated at 12°C for 24 hours. Cells were harvested and lysed using B-PER protein extraction reagent (Thermo Scientific) as per the manufacturer’s instructions. The lysate was cleared via centrifugation at 25,000X g for 30 min at 4°C. Functional galectin-9 was isolated using α-lactose agarose (Sigma-Aldrich) and eluted using 200mM α-lactose. Polishing purification was performed using a Superdex 75 (GE Healthcare, Little Chalfont, Buckinghamshire, UK) via AKTA HPLC. A purity >90% was obtained (assessed via SDS-PAGE and Coomassie and silver staining analysis), and endotoxin was <1 EU/mg protein. Identity of the protein was confirmed via galectin-9 western blot (R&D Systems; Cat#AF2045).
Surface Plasmon Resonance
The binding of recombinant human galectin-9 to recombinant human sTim-3 was tested using a Biacore X instrument (GE Healthcare, Little Chalfont, UK). A CM5 chip was coupled with 1.82ng of sTim-3 at pH 4.5 (equivalent to 1820 response units). A twofold serial dilution of recombinant human galectin-9, from 563nM to 18nM, was injected over the sTim-3 surface and the change in response units was measured.
Galectin-9 affinity precipitation and mass spectrometry analysis
10μg of in-house produced recombinant galectin-9 was immobilized onto magnetic anti-FLAG beads (Sigma-Aldrich) and incubated with RIPA (Cell Signaling, Beverly, MA) lysate prepared from 5×106 rested Tim-3hi CD8+T cells for 3 hours at 4°C. Following collection of the flow through and subsequent washing with lysis buffer, bound proteins were first eluted with 200mM α-lactose in lysis buffer followed by a subsequent denaturing elution in LDS sample buffer with 50mM DTT. Lysate, flow-throughs and elutions were analyzed via SDS-PAGE and western blotting or in-gel silver staining (Sigma-Aldrich). Silver stained bands were excised from the gel and sent for in-gel tryptic digestion and identification via LC/MS/MS. Analysis of peptide hits was performed using Scaffold 4 software.
Results
Tim-3 is a lipid raft resident protein and is found at the immunological synapse
TCR signaling predominantly occurs within distinct membrane microdomains called lipid rafts [31, 32]. Productive TCR signaling requires shuttling of essential proximal signaling molecules in and out of the rafts during initial interaction with the target cell [33]. We tested whether Tim-3 could be found within lipid rafts of CD8+ T cells. To do this, primary human CD8+ T cells were obtained from peripheral blood of normal human volunteers, and stimulated in culture for 5–7 days with α-CD3, α-CD28 and IL-2 to upregulate Tim-3 as previously described in Mujib et al [29]. Following in vitro stimulation, the cells were washed three times to remove residual antibodies and IL-2 and the cells were rested overnight in plain medium to dampen any residual proximal signaling. Assessment of Tim-3’s membrane location in these primary rested Tim-3hi CD8+T cells using sucrose density gradient purification revealed the presence of a large amount of Tim-3 in lipid rafts (Figure 1a). Approximately 40% of total cellular Tim-3 was found in the first fraction collected from the sucrose gradient, which contained approximately 87% of GM-1, a lipid raft ganglioside. Lipid raft clustering is required for immunological synapse assembly [34], which provided us with a rationale to further investigate the role of Tim-3 at the immunological synapse.
Figure 1. Tim-3 is found in lipid rafts and is recruited to the immunological synapse.
(A) Rested Tim-3hi CD8+ T-cells were lysed and subjected to sucrose gradient centrifugation followed by fraction collection to isolate lipid rafts. Fractions were subjected to dot-blot analysis using cholera-toxin-HRP to detect lipid raft resident protein GM-1, or Tim-3 western blot. Fraction 1 contains the lipid rafts. Densitometry analysis using ImageJ was used to determine the fraction of total Tim-3 and GM-1 in fractions 1–3 and 8–10. (B) Rested Tim-3hi CD8+ T-cells were used to form conjugates with Cell-Tracker Blue stained, SEB-loaded B-cells followed by indirect immunofluorescent staining for CD3 (magenta – marker of the cSMAC) with Tim-3 (green) and analyzed via confocal microscopy. White indicates co-localization. The following are representative of 5 experiments
Formation of an immunological synapse requires a T-cell in conjugate with a target cell to provide a TCR stimulus and surface ligands/receptors on both cells to coordinate multiple co-stimulatory/inhibitory and immune accessory molecules [35, 36]. In order to assess for immunological synapse formation we utilized and modified a previous system [30] in which effector CD8+T cells are co-incubated with B cell lines as antigen presenting cells, that are pulsed with SEB as the antigen. To confirm the presence of endogenous Tim-3 ligand expressed on antigen presenting cells, we employed a biotinylated soluble recombinant Tim-3 (sTim-3) with labeled streptavidin secondary to stain our target cells (human B-cell line). The B-cells bound labeled sTim-3 indicating the presence of the Tim-3 ligand on their surface. In addition, the cells stained for an antibody to galectin-9, a Tim-3 ligand (Supplementary Figure 1a and b), indicating that surface associated galectin-9 was present on these cells. To confirm that galectin-9 was acting as the ligand, we treated the cells with sTim-3 in the presence of α-lactose, a galectin-9 substrate. This significantly inhibited the binding of the sTim-3 (Supplementary Figure 1b), suggesting that galectin-9 was a major ligand for Tim-3 on these cells. However, α-lactose treatment of B cells did not completely abrogate sTim-3 binding, indicating that either there were non-carbohydrate dependent ligands for Tim-3 on these B cells, as previously described [37], or the affinity of galectin-9 for α-lactose was less than that for Tim-3. Thus, we have confirmed that in the CTL/B-cell line system that we will be using to model TCR stimulation, the requisite receptors and ligands are present to allow for simultaneous Tim-3 engagement.
Following conjugate formation with rested Tim-3hi CD8+T cells and the target, i.e., SEB-loaded B-cells, the cells were stained for CD3 (as a marker for the central supramolecular activation cluster (cSMAC)) and Tim-3, and then analyzed via confocal microscopy. As shown in Figure 1b, Tim-3 was recruited to the immunological synapse, suggesting a role for Tim-3 in proximal TCR signaling. Similarly, PD-1 has also been found at the immunological synapse and negatively effects synapse stability, as defined by concentrated and centered CD3at the T-cell synapse [38]. Using our conjugate forming assay, we assessed whether blocking Tim-3 had an effect on synapse stability. Using epifluorescnce microscopy, we defined stable synapses as conjugates with concentrated CD3 at the interface between the T-cell and APC. In addition, to increase the stringency of our approach, we included phospho-ERK signaling, which results from an integration of signals from both the TCR and activated LFA-1[39], as an additional parameter to define a “stable” synapse, which is capable of eliciting downstream signaling. Representative pictures of a “stable” and “unstable” synapse are shown in Figure 2a. Blocking Tim-3 overnight on resting Tim-3hi CD8+ T-cells using the antagonistic Tim-3 mAb [10] significantly increased the number of stable synapses formed between T cells and their SEB pulsed targets to 54% (+/− 3.8%) compared to 32% (+/−2.8%) for the isotype control (Figure 2b). This suggests that Tim-3 signaling plays a role in stable synapse formation.
Figure 2. Blocking Tim-3 enhances synapse formation.
(A) Tim-3hi CD8+ T-cells were rested overnight with either an isotype control or the antagonistic Tim-3 mAb, 2E2, and subjected to conjugate formation and subsequent immunofluorescence staining with Cell-Tracker Blue stained, SEB-loaded B-cells. Depicted are a T-cell: B-cell conjugate with a “stable” synapse (top) and a T-cell: B-cell conjugate with an “unstable” synapse (bottom). “Stable” synapses were defined as a T-cell: B-cell conjugate with concentrated CD3 at the interface between the two cells and the T-cell was phospho-ERK+. “Unstable” synapses were defined as a T-cell: B-cell conjugate in which CD3 was not concentrated at the interface between the two cells or the T-cell was not phospho-ERK+. Only conjugates with a Tim-3+ T-cell were included in the counts. (B) Bar graph showing the difference in frequency of “stable” synapse formation between cells treated with the isotype control or antagonistic antibody, 2E2. For semi-quantification of “stable” synapses, pictures were taken of multiple fields of view and 25 pairs of T cell: B-cell conjugates were blindly counted per slide. Statistical analysis was performed using a two-tailed paired student’s t-test from 4 independent experiments.
Tim-3 can be phosphorylated and associates with Lck
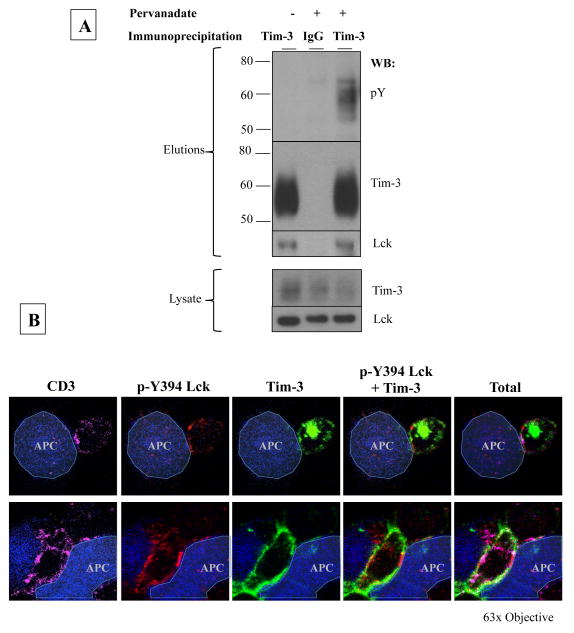
Given that Tim-3 is localized in lipid rafts, which are crucial to immunological synapse formation, and that Tim-3 is located at the immunological synapse, we postulated that Tim-3 plays a role in proximal signaling events. Proximal signaling is regulated, in part, by tyrosine phosphorylation. While previous studies have shown that Tim-3 can be phosphorylated in epithelial cell lines and is enhanced by addition of galectin-9[24, 25], it is still undetermined if this is the case in primary CD8+T cells. While attempts were made to determine Tim-3’s phosphorylation status with ligand treatment, we found that the addition of galectin-9 rendered Tim-3 insoluble even in harsh RIPA buffer, which hindered subsequent Tim-3 immunoprecipitation (data not shown). In addition, we could not detect Tim-3 phosphorylation with TCR stimulation (data not shown). However, it is possible that only a very small fraction of Tim-3 is being phosphorylated during TCR stimulation. To assess the potential for Tim-3 phosphorylation, we immunoprecipitated Tim-3 from rested Tim-3hi CD8+ T cells treated with or without pervanadate, a potent phosphatase inhibitor, and analyzed the elutions via western blot. Under these conditions, Tim-3 was found to contain phosphorylated tyrosine motifs (Figure 3a), further suggesting Tim-3’s involvement in proximal signaling. Probing for additional proximal signaling molecules revealed that Tim-3 co-immunoprecipitated with Lck, confirming previous findings [27], but this binding however was not dependent upon Tim-3 phosphorylation (see lane 1, Fig 3A). In addition, assessment of conjugates between Tim-3hi CD8+ T-cells and SEB-loaded B-cells revealed that Tim-3 co-localized minimally, if not at all, with the active form of Lck (p-Y394) at the immunological synapse (Figure 3b – top panel). Interestingly, large, concentrated areas of active phospho-Lck appeared to exclude Tim-3 (Figure 3b – bottom panel). This suggests the possibility that Tim-3 may be recruiting a phosphatase to dampen Lck phosphorylation and subsequent activity.
Figure 3. Tim-3 interacts with Lck.
(A) Rested Tim-3hi CD8+ T-cells were treated with or without pervanadate and Tim-3 was immunoprecipitated following lysis of the cells. Elutions and lysate were analyzed via SDS-PAGE and western blotting for phospho-tyrosine (pY), stripped and reprobed for Tim-3, followed by Lck. (B)Rested Tim-3hi CD8+ T-cells were used to form conjugates with Cell-Tracker Blue stained, SEB-loaded B-cells followed by indirect immunofluorescent staining for CD3 (magenta), phospho-Y394 Lck (p-Y394 Lck; red), and Tim-3 (green) and analyzed via confocal microscopy. Yellow indicates co-localization of p-Y394 Lck and Tim-3 while white indicates co-localization of CD3 and Tim-3. The following is representative picture of 3 experiments
Galectin-9 serves as the canonical human ligand for Tim-3 and receptor phosphatases in T cells
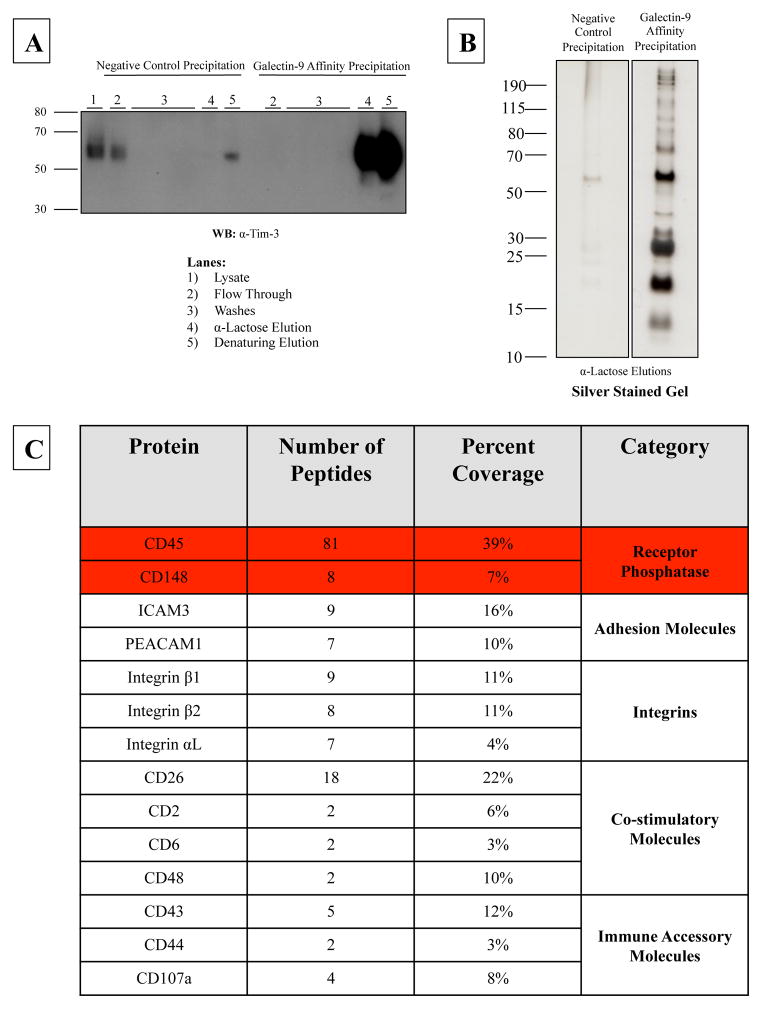
A previous study has demonstrated that mouse Tim-3 binds to mouse galectin-9 [17], however the data is unresolved on this interaction in humans. To assess the binding of human Tim-3 to human galectin-9, we first produced and purified a FLAG-tagged recombinant human galectin-9 (Supplemental Figure 2a). Next, we performed two independent surface plasmon resonance experiments to show that immobilized recombinant human Tim-3 was able to bind to recombinant human galectin-9 (Supplemental Figure 2b). Results were comparable using our in-house produced recombinant human galectin-9 and human soluble Tim-3 and recombinant proteins obtained from R&D Systems (data not shown). However, since the interaction between galectin-9 and Tim-3 is carbohydrate dependent, endogenous, surface expressed Tim-3 might have a different glycosylation state, which may prevent it from binding to galectin-9. We performed a galectin-9 affinity precipitation to determine if our recombinant human galectin-9 was able to precipitate endogenous Tim-3 from Tim-3hi CD8+T cells. Galectin-9 bound to endogenous Tim-3 in a carbohydrate dependent manner, as shown by the presence of Tim-3 in the α-lactose elution (Figure 4a – lane 4). Following the α-lactose elution, any remaining proteins were removed via boiling/denaturing elution (Figure 4a – lane 5). A large portion of Tim-3 was found in the denaturing elution, indicating that either the 200mM α-lactose elution could not completely disrupt the interaction between Tim-3 and galectin-9 or that Tim-3 is also able to bind to galectin-9 in a non-carbohydrate dependent manner. In order to determine other binding partners of galectin-9, besides, Tim-3, the α-lactose elutions were analyzed via SDS-PAGE and silver staining. As shown in Figure 4b, multiple proteins bound to galectin-9 in a carbohydrate-specific manner. Mass spectrometry analysis identified most of these proteins as immune accessory molecules including the phosphatases CD45 and CD148 (Figure 4c). This suggests that while galectin-9 is the ligand for Tim-3, it is able to coordinate multiple surface receptors, including receptor phosphatases.
Figure 4. Galectin-9 binds to multiple immune accessory molecules including receptor phosphatases CD45 and CD148.
(A) Bead immobilized recombinant galectin-9 or beads alone (negative control) were incubated with lysate from rested Tim-3hi CD8+ T-cells. Bound proteins were eluted with 200mM α-lactose followed by a subsequent boiling/denaturing elution in reducing sample buffer. Lysate, flow throughs and elutions were analyzed via SDS-PAGE and western blotting for Tim-3. (B) In addition, α-lactose elutions were analyzed via SDS-PAGE and subsequent silver staining. Differential bands were excised from the galectin-9 affinity precipitation α-lactose elution and sent for LC/MS/MS identification. (C) Analysis of the mass spectrometry results using Scaffold 4 software yielded identification of multiple immune accessory molecules and receptor phosphatases.
Galectin-9 enhances the interaction between Tim-3 and CD45
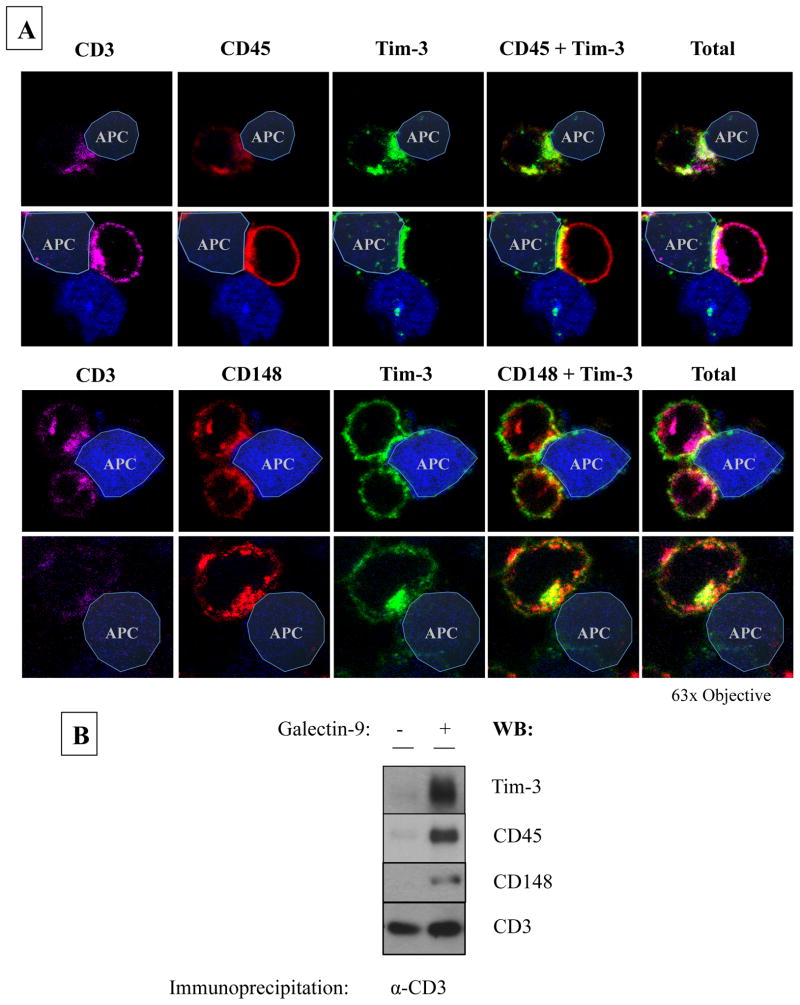
Since galectin-9 has two carbohydrate recognition domains (CRDs) joined by a flexible linker, it is possible that galectin-9 may co-localize Tim-3 with CD45 or CD148, allowing Tim-3 to associate with a phosphatase in a ligand-dependent manner. Assessment of conjugates between Tim-3hi CD8+ T-cells and SEB-loaded B-cells showed thatTim-3 co-localized with CD45 and CD148 at the immunological synapse formed between Tim-3hiCD8+T cells and Tim-3-ligand expressing APCs (Figure 5a). Interestingly, addition of recombinant galectin-9 to Tim-3hi CD8+T cells during CD3-engagement resulted in accumulation of Tim-3, CD45 and CD148 within CD3 signaling complexes, as shown by CD3 immunoprecipitation and subsequent western blotting (Figure 5b). These data suggest that Tim-3 may mediate its inhibitory function through the recruitment of the receptor phosphatases to the synapse via its ligand, galectin-9.
Figure 5. Tim-3 co-localizes with receptor phosphatases, an interaction that is enhanced in the presence of galectin-9.
(A) Rested Tim-3hi CD8+ T-cells were used to form conjugates with Cell-Tracker Blue stained, SEB-loaded B-cells followed by indirect immunofluorescent staining for CD3 (magenta), Tim-3 (green) and either CD45 (red – top panel) or CD148 (red – bottom panel) and analyzed via confocal microscopy. Yellow indicates co-localization. The following are two representative pictures of 4 experiments. (B) Rested Tim-3hi CD8+ T-cells were pre-treated with anti-CD3 beads followed by addition of buffer or recombinant galectin-9. The cells were lysed and anti-CD3 beads were collected. CD3-immunoprecipitated protein was eluted using reducing sample buffer and analyzed via SDS-PAGE and western blot for Tim-3. The blot was stripped and reprobed for CD45, CD148 and CD3. The following is representative of 3 experiments
Discussion
Our study provides novel insight into the Tim-3 receptor coordination during immunological synapse formation and involvement in proximal TCR signaling. This is the first study to show that human Tim-3 is found in lipid rafts and is recruited to the immunological synapse. Further, Tim-3 may be involved in the process of “stable” synapse formation, suggesting a role for Tim-3 in proximal signaling. Tim-3 was found to have the potential for tyrosine phosphorylation in primary CD8+T cells and bind to Lck, further confirming the finding by Rangachari et al that Tim-3 associates with this essential proximal signaling molecule [27]. However, Tim-3 was not found to associate with the active form of Lck, phospho-Y394, at the synapse, suggesting that Tim-3 may be recruiting a phosphatase. Interestingly, in addition to confirming galectin-9 as the Tim-3 ligand, we described novel interactions between Galectin-9 and receptor phosphatases CD45 and CD148. Finally, Galectin-9 was able to enhance the interaction between these phosphatases and Tim-3 within CD3 signaling complexes. These data suggest the possibility that Tim-3 may regulate Lck function via interaction with CD45 or CD148 at the synapse, an interaction mediated by its ligand galectin-9.
Proximal TCR signaling is a complex process regulated not only by post-translational modifications, including tyrosine phosphorylation, but also by lipid raft dynamics and compartmentalization of proteins. For T cells to initiate signaling, first the peptide-loaded MHC interacts with the TCR. This results in recruitment to the lipid rafts and exclusion of CD45[33]. Fyn and/or non-CD8 bound Lck become associated with the TCR-CD3 signaling complex, resulting in phosphorylation of CD3 ITAMs and recruitment of ZAP70. Following ZAP70 phosphorylation, the SH2 domain of CD8-bound Lck binds to phospho-ZAP70, recruiting CD8 to the MHC-TCR complex. CD8 binds to the MHC, increasing the stability of the MHC-TCR complex [40], sustaining proximal signaling and initiation of downstream signaling.
The finding that more than 40% of Tim-3 resides in lipid rafts and is recruited to the immunological synapse provides evidence that Tim-3 is involved in proximal signaling. While this study assessed membrane location of Tim-3 on resting cells, further work is warranted to determine if Tim-3 accumulates within or is excluded from lipid rafts during TCR signaling, similar to Lck and CD45, respectively. However, since TCR triggering results in the accumulation of lipid rafts at the synapse [34], it is likely that Tim-3 would accumulate. In addition, we found that blocking Tim-3 using the antagonistic antibody, 2E2, resulted in significantly more conjugates that had “stable” synapses. Initial proximal TCR signaling, such as Lck phosphorylation and subsequent downstream signaling to LAT, is required for stable synapse formation [41], further providing evidence that Tim-3 is involved in TCR proximal signaling. While the exact mechanism of how Tim-3 effects proximal signaling is still under investigation, our work provides preliminary evidence that Tim-3 may be involved in Lck regulation.
Our finding that Tim-3 binds to Lck is not novel [27], however, we did show that this interaction occurred in primary human CD8+T cells. In addition, we showed that Tim-3 did not localize with the active form of Lck, phospho-Y394. Lck activity is regulated via phosphorylation of the positive regulatory tyrosine Y394 in the kinase domain and the negative regulatory tyrosine Y505 in the C-terminal end [33]. When Y505 is phosphorylated, it forms a bond with its own SH2 domain, preventing substrate access to the kinase domain. However, when Y394 is phosphorylated, the protein achieves full kinase activity. It would be prudent to assess Tim-3’s location in relation the inactive form of Lck (pY505) versus the active form of Lck (pY394), however, the inactive form pY505 is bound to its own SH2 domain, preventing access to the phosphorylated motif by any antibody used for immunofluorescence. Thus we are unable to assess, given currently available reagents, whether Tim-3 preferentially binds pY505Lck. Phosphorylation of Y505 and Y394 are achieved by the kinase Csk and via trans/autophosphorylation, respectively [33]. Dephosphorylation of either tyrosine is mediated by the receptor phosphatase CD45. Interestingly, CD45 plays a dual role in positively and negatively regulating Lck, depending on the expression level of CD45 [42]. Low to intermediate concentrations of CD45 result in dephosphorylation of Lck at Y505, resulting in an increase in Lck activity. However, high levels of CD45 result in dephosphorylation of Y394, effectively decreasing Lck kinase activity.
Despite a recent study that claimed human Tim-3 was not the receptor for human galectin-9 [43], we confirmed that galectin-9 was the physiological ligand for Tim-3 using two different biochemical assays. In addition, we discovered that galectin-9 bound to receptor phosphatases CD45 and CD148, suggesting a possible mechanism of how Tim-3 could regulate Lck activity. Inducing high local concentrations of CD45 in proximity to Tim-3 and associated Lck could result in dephosphorylation of Lck Y394 and Y505. In addition, we showed that galectin-9 was able to increase levels of Tim-3, CD45 and CD148 within CD3 signaling rafts. Thus, while CD45 and CD148 are normally excluded from the synapse [33, 44], concurrent ligation of these receptor phosphatases with Tim-3 via galectin-9 could enhance the presence of these phosphatases at the synapse, resulting in negative regulation of Lck as well as dampening of TCR signaling.
Importantly, all of this work was performed using primary CD8+T cells with minimal manipulation of the T cells or target cells. While Tim-3 expression was induced via in vitro stimulation, no proteins were ever overexpressed which can lead to artificial results and false positives, such as chaperones binding to overexpressed proteins [45]. In addition, this work studied Tim-3 under near physiological conditions. Use of primary CD8+T cells provides an intact TCR signaling platform, including presence of proximal signaling molecules, regulation via lipid raft dynamics and presence of receptor phosphatases that are essential for the initiation and final dampening of TCR signaling [44, 46, 47]. This is absent in many of the cell lines used to study Tim-3 thus far [24, 25, 27]. In addition, our system uses SEB loaded on target cells to provide a TCR stimulus, which does not bypass proximal signaling, unlike the phorbol myristate acetate (PMA)/Ionomycin stimulus used to study Tim-3 in Jurkat cells [26, 28]. Finally, we provide a target cell, which expresses the physiological Tim-3 ligand as shown by sTim-3 binding to the B-cell in a carbohydrate-specific manner. This is essential since previous studies have engaged Tim-3 using putative agonistic antibodies [13, 27]. Galectin-9 is a soluble protein, not a transmembrane protein, like PD-L1 and PD-L2. It can associate with the membrane via interaction with other glycans, however, its behaviour during T-cell engagement and synapse formation is currently unknown, stressing the importance to understand how galectin-9 effects Tim-3 before using antibodies to engage Tim-3. Indeed, using a galectin-9 affinity precipitation, we did show that galectin-9 was able to bind to various immune accessory molecules. Thus, the Tim-3-galectin-9 interaction is not a closed system but a complex interplay with various other surface glycosylated molecules. While tempted to use agonistic antibodies to study the effects of Tim-3, the reality is that when Tim-3 is engaged under physiological conditions, so are other receptors, including receptor phosphatases like CD45.
Using the data we have collected, we wish to propose a working model. In the case of Tim-3 expressed on the T-cell and galectin-9 expressed on the surface of the APC, both CD45 and Tim-3 get recruited to the TCR signaling clusters at the immunological synapse. This results in CD45-mediated dephosphorylation of the active tyrosine (Y394) of Lck, and accumulation of inactive Lck within TCR signaling clusters. This increases the ratio of inactive Lck to active Lck and eventual dampening TCR signaling. These results, thus far, are observational and future functional investigations focusing on the Tim-3 intracellular pathway and galectin-9-mediated effects using our physiological T-cell: APC system are warranted.
Efforts need to be made to understand the signaling pathways associated with co-inhibitory receptors to allow for better development of therapeutic interventions. The signaling pathways for co-inhibitory receptors CTLA-4 and PD-1 are currently known. In addition, these proteins have antagonistic antibodies currently used in clinic or in Phase III trials, respectively, for cancer treatment [48]. Co-inhibitory molecules are not redundant as shown by the synergistic effects of dual blockade. This stresses the importance of understanding what specific effects each inhibitory receptor has on T-cell function to better formulate blockades that are most effective against different diseases such as cancer and chronic viral infections.
Supplementary Material
Acknowledgments
Grant Support
This work was supported by a grant from the Canadian Institutes of Health Research. K.L.C and M.O gratefully acknowledge salary support from the Ontario HIV Treatment Network
We thank Dr. Michael Julius for his help and expertise with the lipid raft isolation, Dr. Andrew Wilde for the use of his microscope equipment, Dr. Walid Houry for his help and expertise with the protein purifications and use of his equipment. We would like to thank Dr. R. Brad Jones for his helpful comments.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 3.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vali B, Jones RB, Sakhdari A, Sheth PM, Clayton K, Yue FY, et al. HCV-specific T cells in HCV/HIV co-infection show elevated frequencies of dual Tim-3/PD-1 expression that correlate with liver disease progression. European Journal of Immunology. 2010;40:2493–2505. doi: 10.1002/eji.201040340. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakhdari A, Mujib S, Vali B, Yue FY, Macparland S, Clayton K, et al. Tim-3 Negatively Regulates Cytotoxicity in Exhausted CD8(+) T Cells in HIV Infection. Plos One. 2012;7:e40146. doi: 10.1371/journal.pone.0040146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 10.Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, et al. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. European Journal of Immunology. 2009;39:2492–2501. doi: 10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seki M, Oomizu S, Sakata KM, Sakata A, Arikawa T, Watanabe K, et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clinical Immunology. 2008;127:78–88. doi: 10.1016/j.clim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao D, et al. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J Hepatol. 2010;52:322–329. doi: 10.1016/j.jhep.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 14.Baitsch L, Legat A, Barba L, Fuertes Marraco SA, Rivals JP, Baumgaertner P, et al. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. Plos One. 2012;7:e30852. doi: 10.1371/journal.pone.0030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, et al. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119:3734–3743. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakayama M, Akiba H, Takeda K, Kojima Y, Hashiguchi M, Azuma M, et al. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 2009;113:3821–3830. doi: 10.1182/blood-2008-10-185884. [DOI] [PubMed] [Google Scholar]
- 17.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 18.Katoh S, Ishii N, Nobumoto A, Takeshita K, Dai SY, Shinonaga R, et al. Galectin-9 inhibits CD44-hyaluronan interaction and suppresses a murine model of allergic asthma. Am J Respir Crit Care Med. 2007;176:27–35. doi: 10.1164/rccm.200608-1243OC. [DOI] [PubMed] [Google Scholar]
- 19.Bi S, Hong PW, Lee B, Baum LG. Galectin-9 binding to cell surface protein disulfide isomerase regulates the redox environment to enhance T-cell migration and HIV entry. Proc Natl Acad Sci U S A. 2011;108:10650–10655. doi: 10.1073/pnas.1017954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu W, Shi Y, Li S, Zhang Y, Liu Y, Wu Y, et al. Blockade of Tim-3 signaling restores the virus-specific CD8(+) T-cell response in patients with chronic hepatitis B. European Journal of Immunology. 2012;42:1180–1191. doi: 10.1002/eji.201141852. [DOI] [PubMed] [Google Scholar]
- 21.McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, et al. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120:4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van de Weyer PS, Muehlfeit M, Klose C, Bonventre JV, Walz G, Kuehn EW. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem Biophys Res Commun. 2006;351:571–576. doi: 10.1016/j.bbrc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 25.Vega-Carrascal I, Reeves EP, Niki T, Arikawa T, McNally P, O’Neill SJ, et al. Dysregulation of TIM-3-galectin-9 pathway in the cystic fibrosis airways. Journal of Immunology. 2011;186:2897–2909. doi: 10.4049/jimmunol.1003187. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Su EW, Zhu C, Hainline S, Phuah J, Moroco JA, et al. Phosphotyrosine-dependent coupling of Tim-3 to T-cell receptor signaling pathways. Mol Cell Biol. 2011;31:3963–3974. doi: 10.1128/MCB.05297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rangachari M, Zhu C, Sakuishi K, Xiao S, Karman J, Chen A, et al. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion. Nat Med. 2012 doi: 10.1038/nm.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee MJ, Woo MY, Chwae YJ, Kwon MH, Kim K, Park S. Down-regulation of interleukin-2 production by CD4(+) T cells expressing TIM-3 through suppression of NFAT dephosphorylation and AP-1 transcription. Immunobiology. 2012;217:986–995. doi: 10.1016/j.imbio.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Mujib S, Jones RB, Lo C, Aidarus N, Clayton K, Sakhdari A, et al. Antigen-independent induction of Tim-3 expression on human T cells by the common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 is associated with proliferation and is dependent on the phosphoinositide 3-kinase pathway. Journal of Immunology. 2012;188:3745–3756. doi: 10.4049/jimmunol.1102609. [DOI] [PubMed] [Google Scholar]
- 30.Ramsay AG, Johnson AJ, Lee AM, Gorgun G, Le Dieu R, Blum W, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118:2427–2437. doi: 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kabouridis PS. Lipid rafts in T cell receptor signalling. Mol Membr Biol. 2006;23:49–57. doi: 10.1080/09687860500453673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otahal P, Angelisova P, Hrdinka M, Brdicka T, Novak P, Drbal K, et al. A new type of membrane raft-like microdomains and their possible involvement in TCR signaling. Journal of Immunology. 2010;184:3689–3696. doi: 10.4049/jimmunol.0902075. [DOI] [PubMed] [Google Scholar]
- 33.Filipp D, Ballek O, Manning J. Lck, Membrane Microdomains, and TCR Triggering Machinery: Defining the New Rules of Engagement. Front Immunol. 2012;3:155. doi: 10.3389/fimmu.2012.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dupre L, Aiuti A, Trifari S, Martino S, Saracco P, Bordignon C, et al. Wiskott-Aldrich syndrome protein regulates lipid raft dynamics during immunological synapse formation. Immunity. 2002;17:157–166. doi: 10.1016/s1074-7613(02)00360-6. [DOI] [PubMed] [Google Scholar]
- 35.Saito T, Yokosuka T. Immunological synapse and microclusters: the site for recognition and activation of T cells. Curr Opin Immunol. 2006;18:305–313. doi: 10.1016/j.coi.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao E, Zang X, Ramagopal UA, Mukhopadhaya A, Fedorov A, Fedorov E, et al. T cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand-binding surface. Immunity. 2007;26:311–321. doi: 10.1016/j.immuni.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li D, Molldrem JJ, Ma Q. LFA-1 regulates CD8+ T cell activation via T cell receptor-mediated and LFA-1-mediated Erk1/2 signal pathways. J Biol Chem. 2009;284:21001–21010. doi: 10.1074/jbc.M109.002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gascoigne NR, Casas J, Brzostek J, Rybakin V. Initiation of TCR phosphorylation and signal transduction. Front Immunol. 2011;2:72. doi: 10.3389/fimmu.2011.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ou-Yang CW, Zhu M, Fuller DM, Sullivan SA, Chuck MI, Ogden S, et al. Role of LAT in the granule-mediated cytotoxicity of CD8 T cells. Mol Cell Biol. 2012;32:2674–2684. doi: 10.1128/MCB.00356-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNeill L, Salmond RJ, Cooper JC, Carret CK, Cassady-Cain RL, Roche-Molina M, et al. The differential regulation of Lck kinase phosphorylation sites by CD45 is critical for T cell receptor signaling responses. Immunity. 2007;27:425–437. doi: 10.1016/j.immuni.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 43.Leitner J, Rieger A, Pickl WF, Zlabinger G, Grabmeier-Pfistershammer K, Steinberger P. TIM-3 does not act as a receptor for galectin-9. PLoS Pathog. 2013;9:e1003253. doi: 10.1371/journal.ppat.1003253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin J, Weiss A. The tyrosine phosphatase CD148 is excluded from the immunologic synapse and down-regulates prolonged T cell signaling. J Cell Biol. 2003;162:673–682. doi: 10.1083/jcb.200303040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gingras AC, Gstaiger M, Raught B, Aebersold R. Analysis of protein complexes using mass spectrometry. Nat Rev Mol Cell Biol. 2007;8:645–654. doi: 10.1038/nrm2208. [DOI] [PubMed] [Google Scholar]
- 46.Zikherman J, Jenne C, Watson S, Doan K, Raschke W, Goodnow CC, et al. CD45-Csk phosphatase-kinase titration uncouples basal and inducible T cell receptor signaling during thymic development. Immunity. 2010;32:342–354. doi: 10.1016/j.immuni.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hermiston ML, Zikherman J, Zhu JW. CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells. Immunol Rev. 2009;228:288–311. doi: 10.1111/j.1600-065X.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nirschl CJ, Drake CG. Molecular Pathways: Co-Expression of Immune Checkpoint Molecules: Signaling Pathways and Implications for Cancer Immunotherapy. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.