Abstract
Background/Aims
Several recent studies have reported that the early use of infliximab (IFX) improves the prognosis of Crohn's disease (CD). However, no data are available from Asian populations, as the forementioned studies have all been conducted in Western countries. The aim of the current study was to evaluate the impact of early use of IFX on the prognosis of Korean patients with CD.
Methods
Patients with a diagnosis of CD established between July 1987 and January 2012 were investigated in 12 university hospitals in Korea. Because insurance coverage for IFX treatment began in August 2005, patients were assigned to either of 2 groups based on diagnosis date. The first group included patients diagnosed from July 1987 to December 2005, and the second from January 2006 to January 2012. We compared the cumulative probabilities of operation and reoperation between the two groups using the Kaplan-Meier method and a log-rank test.
Results
Of the 721 patients investigated, 443 (61.4%) comprized the second group. Although the cumulative probabilities of immunosuppressant (P<0.001) and IFX use (P<0.001) after diagnosis were significantly higher in the second group, there were no significant differences in cumulative probabilities of operation (P=0.905) or reoperation (P=0.418) between two groups.
Conclusions
The early use of IFX did not reduce CD-related surgery requirements in Korean patients with CD. These study results suggest that the early use of IFX may have little impact on the clinical outcome of CD in Korean patients in the setting of a conventional step-up algorithm.
Keywords: Crohn disease, Infliximab, Prognosis
INTRODUCTION
Crohn's disease (CD) is a chronic and relapsing inflammatory bowel disorder that can affect the entire gastrointestinal tract from the mouth to the anus. Major symptoms include abdominal pain, diarrhea, and weight loss. Additionally, CD can induce complications in the bowel such as stenosis, perforation, abscess, and fistula. Such complications may require repetitive operative procedures in some cases. However, the pathogenic mechanism of CD has not been completely elucidated. CD has been shown to be more common in developed countries and relatively rare in Korea, however, the prevalence of CD has shown recent and continuous increases in Asia. As a result, the socioeconomic burden of CD is gradually increasing.1
Step-up treatment has been the preferred method for managing CD and involves selecting medications according to severity of CD for the induction and maintenance of remission before initiating treatment with stronger medications. Thus, 5-aminosalicylic acid (5-ASA), antibiotics, and steroids are used as primary drugs. Immunosuppressive therapy with azathioprine (AZA) or 6-mercaptopurine (6-MP) is administered to patients with steroid dependency and refractoriness, followed by treatment with the biological agent infliximab (IFX) in cases that show no response to AZA or 6-MP treatment.
Several studies have reported that the early use of tumor necrosis factor-alpha (TNF-α) blockers, including IFX, decreased the need for hospitalization and surgery by inducing intestinal mucosal healing which can prevent or delay the occurrence of complications such as intestinal perforation, fistula, abscess and stenosis.2,3 Thus, a new concept of top-down treatment has emerged based on improvements in the intractable progression and prognosis of CD based on the early use of IFX. Previous studies that assessed the impact of early use of IFX on patients with CD were performed exclusively in Western countries and included relatively short follow-up periods. To date, there have been no studies that have investigated the impact of early use of IFX on Asian or Korean patients. Therefore, the aim of the current study was to identify the effects of early use of IFX on the natural progress and prognosis of CD in Korean patients through a long-term follow-up investigation. The current study hypothesized that the early administration of IFX increased dramatically after 2006, as insurance began to cover IFX treatment in patients with CD as of August 2005. Based on this assertion, the current study analyzed the impact of early use of IFX on the prognosis of Korean patients with CD. Therefore, the current study was designed to examine the effects of early administration of IFX on the prognosis of Korean patients with CD by comparing the prognosis (cumulative probabilities of operation and reoperation) between patient groups diagnosed with CD before and after 2006.
METHODS
In this multi-center study, the medical records of 721 patients diagnosed with CD in 12 university hospitals from July 1987 through January 2012 were reviewed retrospectively. The authors retrieved data on follow-up duration, the classification of behavior and location according to the Montreal Classification, hospitalization and surgical history related to CD, the number of operations, and the use of drugs including steroids, immunosuppressants and IFX. CD was diagnosed according to international standards and interpreted comprehensively based on previous clinical characteristics, endoscopic findings, radiological findings, and pathologic findings.1 Upper gastrointestinal invasion was defined in patients with indications of CD based on endoscopic lesions (non-specific lesions such as esophageal or gastric ulcer) in the upper gastrointestinal tract including the esophagus, stomach and duodenum, and a bamboo joint-like appearance of the stomach characterized by swollen longitudinal folds transversed by erosive fissures or linear furrows. Perianal lesions were defined as complications including anal ulcer, fistula, and fissure caused by CD in the perianal region. Perianal lesion-related surgeries such as anal fistulectomy were excluded from CD-related bowel resection. Hospitalization history included only CD-related admissions and a family history of IBD limited to immediate family members. Smoking status was categorized into current, former, and never smokers.
Variables were presented as percentage (%) and mean±SD. Continuous variables were analyzed using the Student t-test, and categorical variables were compared using Chi-square and Fisher's exact tests. Kaplan-Meier analysis and log rank tests were used to compare the cumulative probabilities of operation and reoperation between groups diagnosed with CD before and after 2006. Statistical analyses were performed using SPSS PASW Statistics 18.0 (IBM, Armonk, NY, USA) and P-values <0.05 were considered statistically significant. Institutional review board approval for the current study was obtained from the Kangbuk Samsung Hospital.
RESULTS
A total of 721 patients with CD were enrolled and divided into 2 groups diagnosed with CD before 2006 (First group, n=278) and after 2006 (Second group, n=443). The number of male patients was greater in both groups, 194 (69.8%) and 322 (72.7%), respectively. Mean ages at diagnosis were 30.0 years (±12.8 years) and 28.3 years (±12.6 years), respectively, in the 2 groups. According to the Montreal classification at diagnosis, most of the patients were affected by CD in the ileocecal region. The numbers of patient cases with invasion in the perianal region were 76 (27.3%) and 129 (29.1%), respectively, in each group. Furthermore, the numbers of patient cases with invasion in the upper gastrointestinal tract were 21 (7.6%) and 65 (14.7%), respectively, in each group and were significantly higher in the second group (P=0.004). There were 129 (46.4%) and 163 (36.8%) patients, respectively, with stricturing or penetrating CD in each group. The percentage of patients with stricturing or penetrating CD was significantly lower in the second group (P=0.011) (Table 1).
Table 1.
Baseline Characteristics of Patients With CD
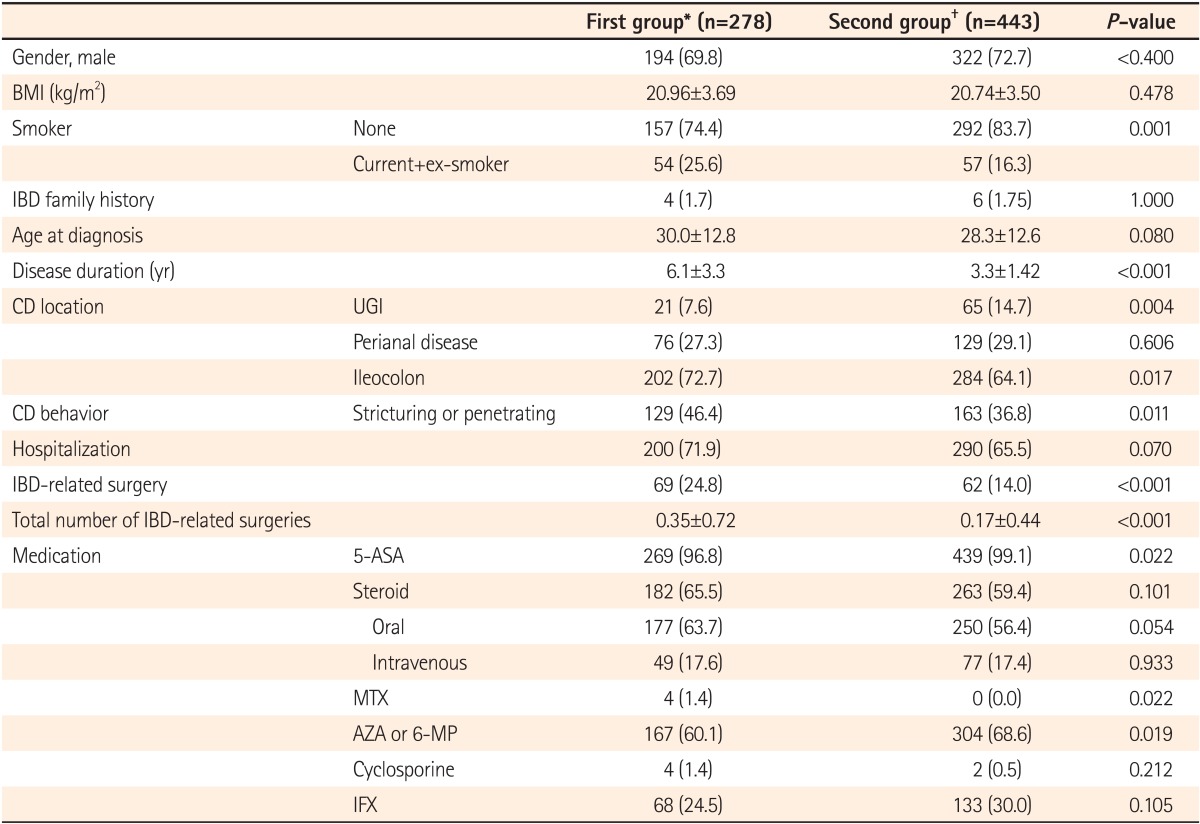
Values are presented as mean&SD or n (%).
*Diagnosed with CD before December 31, 2005.
†Diagnosed with CD after January 1, 2006.
UGI, Upper gastrointestinal; 5-ASA, 5-aminosalicylic acid; MTX, methotrexate; AZA, azathioprine; 6-MP, 6-mercaptopurine; IFX, Infliximab.
Disease duration (average follow-up after diagnosis) was longer in the first group (6.1±3.3, 3.3±1.4, P<0.001). The proportion of patients who underwent CD-related operations (69 [24.8%] vs. 62 [14.0%], P<0.001) and the total number of operations (0.35±0.72 vs. 0.17±0.44, P<0.001) were significantly higher in the first group. Intestinal stenosis (23.9%, 25.4%) accounted for the largest percentage of all CD complications requiring surgical intervention, followed by fistula, and abscess. There was no difference in CD complications between the 2 groups. The numbers of patients receiving immunosuppressive therapy with AZA or 6-MP were 167 (60.1%) in the first group and 304 (68.6%) in the second group, and were significantly higher in the second group. However, no significant differences were found in the rates of steroid (182 cases [65.5%] vs. 263 cases [59.4%], P=0.101) and IFX use (68 cases [24.5%] vs. 133 cases [30.3%], P=0.105) between the 2 groups (Table 1).
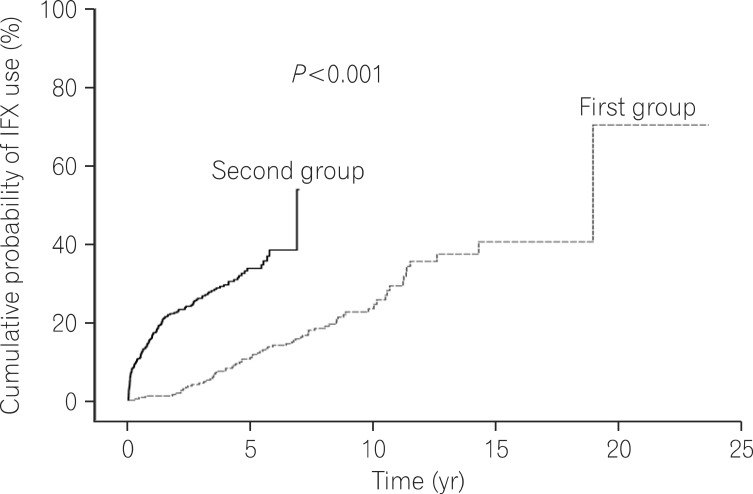
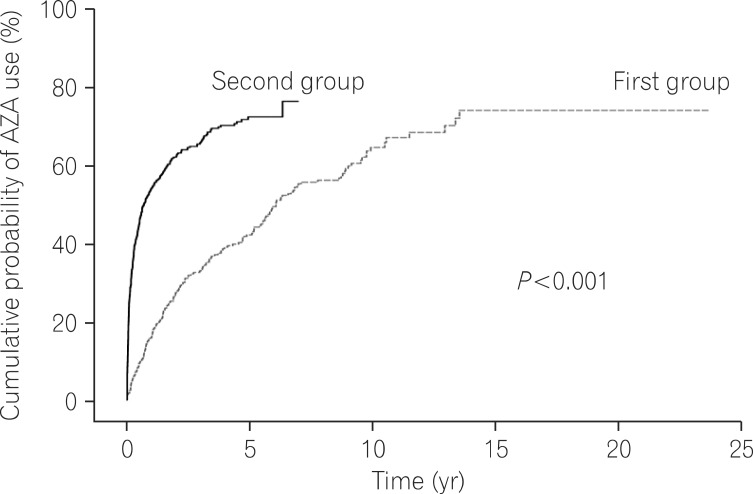
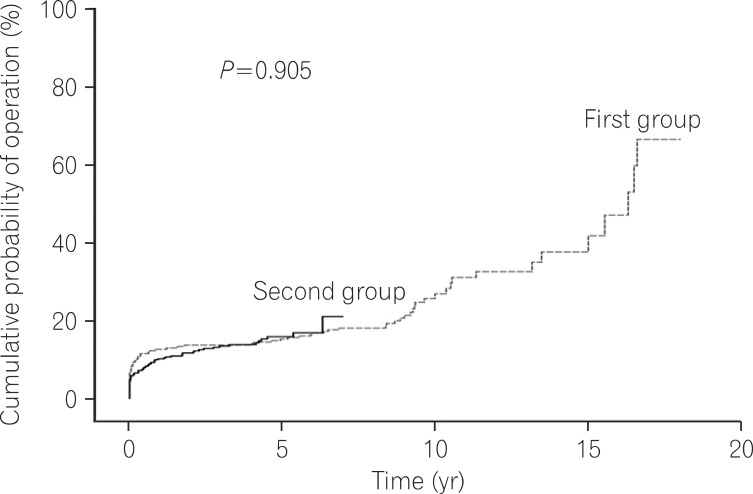
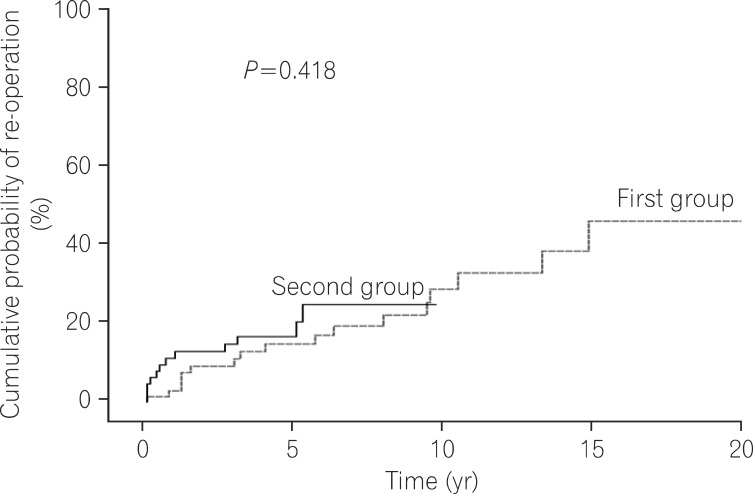
Log rank tests and Kaplan-Meier analyses were conducted in order to compare the cumulative probabilities of IFX use between groups. The cumulative probabilities of IFX use were 1.1% vs. 16.8%, 4.4% vs. 26.0%, and 11.3% vs. 33.6% between the first and second groups at 1, 3, and 5 years after diagnosis. The cumulative probabilities were significantly higher in the second group (P<0.001) (Fig. 1). The cumulative probabilities of AZA use were 16.6% vs. 54.2%, 33.4% vs. 66.0%, and 42.2% vs. 73.0% between the 2 groups at 1, 3, and 5 years after diagnosis. The cumulative probabilities of immunosuppressive drugs were significantly higher in the second group (P<0.001) (Fig. 2). Conversely, the cumulative probabilities of operation were 12.6% vs. 10.2%, 13.7% vs. 13.4%, and 16.0% vs. 15.8% between the 2 groups at 1, 3, and 5 years after diagnosis. No statistically significant difference was found in the cumulative probabilities of operation between the 2 groups (P=0.905) (Fig. 3). Moreover, the cumulative probabilities of reoperation were 7.6% vs. 11.2%, 13.0% vs. 16.8%, and 38.7% vs. 25.0% between the 2 groups at 1, 3, and 5 years after the first surgery, and no statistically significant difference was found in the cumulative probabilities of reoperation between the 2 groups (P=0.418) (Fig. 4). No statistically significant difference was detected in the cumulative probabilities of operation and reoperation in a Cox-proportional hazard regression analysis conducted after adjusting for potentially confounding variables such as gender, smoking status, disease duration, the location and behavior of CD, and medication use.
Fig. 1.
Cumulative probability of infliximab (IFX) use during follow-up.
Fig. 2.
Cumulative probability of azathioprine (AZA) use during follow-up.
Fig. 3.
Cumulative probability of operation during follow-up.
Fig. 4.
Cumulative probability of re-operation during follow-up.
DISCUSSION
In treating CD, IFX prevents TNF-α binding to the cell surface of inflammatory cells in the injured intestinal mucosa. Several multi-center, large-scale, prospective studies have proven the effectiveness of IFX in inducing and maintaining remission in patients with intestinal or fistula CD refractory to standard therapy.4,5,6,7,8 In recent years, the use of TNF-α blockers has become an important therapeutic strategy to induce and maintain remission in patients intolerant to conventional therapies. However, several recent studies have reported that the initiation of immunosuppressive or biological agents in patients with recurrent CD refractory to conventional therapeutic algorithms can result in the early development of severe CD-related complications.9,10 Therefore, an aggressive treatment approach has emerged as a therapeutic method aimed at improving the natural progression of CD by inducing rapid and consistent healing of the intestinal mucosa and preventing or delaying the occurrence of complications such as intestinal perforation, fistula, abscess, and stenosis. A number of studies have shown the effectiveness of this form of top-down treatment starting with the use of TNF-α blockers in the early period of disease to limit complications by preventing disease progression through prompt management of mucosal inflammation and minimizing the use of steroids.
A comparative study (Step-up/Top-down study) reported the usefulness of early administration of biological agents by dividing patients newly diagnosed with CD into 2 groups; those treated with conventional management and those treated with early combined use of immunosuppressive drugs (IFX, AZA).11 In the Crohn's Disease Clinical Study Evaluating Infliximab in a New Long-Term Treatment Regimen I (ACCENT I)4 and Crohn's Trial of the Fully Human Antibody Adalimumab for Remission Maintenance (CHARM) trials,12 the early administration of biological agents decreased hospital admission and operation rates attributable to the complications of CD by inducing clinical remission and mucosal healing in patients with CD.13,14
In conclusion, top-down treatment with TNF-α blockers, immunosuppressant medications, or their combination has been shown to improve the prognosis of CD. However, these previous studies were limited in their ability to sufficiently identify a long-term prognosis due to a relatively short follow-up period. Moreover, the aforementioned studies were mostly comprised of Western patients with CD such that no studies have yet analyzed Asian or Korean patients with CD.
The current study aimed to investigate changes in the long-term prognosis of patients with CD due to an increase in the early use of IFX in Korean patients with CD. This study was predicated on the hypothesis that the early administration of IFX increased dramatically after 2006 because health insurance began to cover IFX treatment in Korea as of August 2005. This long-term follow-up study compared prognosis between 2 groups newly diagnosed with CD before and after 2006. Patients diagnosed with CD after 2006 were administered IFX or AZA therapy earlier than those diagnosed with CD before 2006. However, surgical intervention for CD was neither delayed nor prevented. Consequently, we concluded that the early use of IFX had no influence in improving the prognosis of CD. Similarly, comparable results were shown in a Spanish study which determined that increased early use of IFX did not improve the prognosis of CD.15
Regarding possible explanations for the results of the current study, health insurance coverage of IFX in Korea is limited essentially to those who have experienced adverse effects or displayed refractoriness to treatment with AZA or 6-MP. To be more exact, IFX could not have been selected as the first drug to be administered to patients diagnosed with CD after 2006, and therefore these patients would have been limited to a step-up treatment algorithm. This would seem to be the primary cause of a lack of impact from the early use of IFX on the prognosis of CD. Additionally, this research was conducted as a multi-center retrospective study. An electronic medical record system was first introduced in Korean university hospitals during the years 2005-2008. Therefore, missing data on drug use, complications, operations, re-operations, and other information recorded before 2006 may have introduced biases that could have influenced the results. Furthermore, the diagnosis rate of IBD and its complications has increased with the widespread use of colonoscopy, elevated awareness of CD, and advances in radiological technology including CT enterography. Subsequently, operation and re-operation rates for complications may have increased spontaneously in patients diagnosed with CD after 2006 as compared with those diagnosed with CD before 2006. Lastly, it is already known that there are differences in the clinical and epidemiologic characteristics and genetic factors between Westerners and Asians.16 Although racial differences are anticipated to influence the effect of IFX on CD, additional studies will be required to provide further clarification.
There are some limitations associated with the current study. The prognosis of CD was based only on the cumulative probabilities of operation and reoperation. The prognostic factors may have been analyzed insufficiently, as cumulative admission rate, cumulative rates of intestinal complication incidence including stenosis, fistula, and perforation, and cumulative rate of steroid use were not examined. Furthermore, we were unable to investigate the degree of improvement in endoscopic mucosal activity and patient quality of life. Lastly, we had insufficient data on the periods of administration of other medications (ASA, steroids, and AZA/6-MP) used concurrently or prior to IFX, or the duration, dose, and efficacy of IFX use.
In conclusion, the current study demonstrated that an increase in the early use of IFX did not reduce the cumulative probabilities of operation and reoperation in Korean patients with CD. The study findings imply that the early use of IFX may not change or improve the long-term clinical outcomes of Korean patients with CD within the conventional framework of a step-up treatment algorithm. In order to further clarify the long-term effects of early use of IFX in Korean patients with CD, multi-center, large-scale, and prospective studies with long-term follow-up will need to be conducted.
Footnotes
Financial support: None.
Conflict of interest: None.
References
- 1.Ye BD, Yang SK, Shin SJ, et al. Guidelines for the management of Crohn's disease. Intest Res. 2012;10:26–66. [Google Scholar]
- 2.Armuzzi A, De Pascalis B, Fedeli P, De Vincentis F, Gasbarrini A. Infliximab in Crohn's disease: early and long-term treatment. Dig Liver Dis. 2008;40(Suppl 2):S271–S279. doi: 10.1016/S1590-8658(08)60537-X. [DOI] [PubMed] [Google Scholar]
- 3.Fasci Spurio F, Aratari A, Margagnoni G, Doddato MT, Papi C. Early treatment in Crohn's disease: do we have enough evidence to reverse the therapeutic pyramid? J Gastrointestin Liver Dis. 2012;21:67–73. [PubMed] [Google Scholar]
- 4.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 5.Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398–1405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 6.Targan SR, Hanauer SB, van Deventer SJ, et al. Crohn's Disease cA2 Study Group. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 7.Richter JA, Bickston SJ. Infliximab use in luminal Crohn's disease. Gastroenterol Clin North Am. 2006;35:775–793. doi: 10.1016/j.gtc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Sands BE, Blank MA, Diamond RH, Barrett JP, Van Deventer SJ. Maintenance infliximab does not result in increased abscess development in fistulizing Crohns disease: results from the ACCENT II study. Aliment Pharmacol Ther. 2006;23:1127–1136. doi: 10.1111/j.1365-2036.2006.02878.x. [DOI] [PubMed] [Google Scholar]
- 9.Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–782. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 11.D'Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet. 2008;371:660–667. doi: 10.1016/S0140-6736(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 12.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 13.Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology. 2004;126:402–413. doi: 10.1053/j.gastro.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Rutgeerts P, Diamond RH, Bala M, et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn's disease. Gastrointest Endosc. 2006;63:433–442. doi: 10.1016/j.gie.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Domenech E, Zabana Y, Garcia-Planella E, et al. Clinical outcome of newly diagnosed Crohn's disease: a comparative, retrospective study before and after infliximab availability. Aliment Pharmacol Ther. 2010;31:233–239. doi: 10.1111/j.1365-2036.2009.04170.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim ES, Kim WH. Inflammatory bowel disease in Korea: epidemiological, genomic, clinical, and therapeutic characteristics. Gut Liver. 2010;4:1–14. doi: 10.5009/gnl.2010.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]