Abstract
Background/Aims
Only moderate to severe Crohn's Disease (CD) patients without a satisfactory conventional therapy effect are eligible to get reimbursement from the National Health Insurance of Taiwan for using adalimumab. These are more stringent criteria than in many Western countries and Japan and Korea. We aim to explore the efficacy of using adalimumab in CD patients under such stringent criteria.
Methods
A retrospective analysis was conducted in nine medical centers in Taiwan and we collected the results of CD patients receiving adalimumab from Sep 2009 to Mar 2014. The clinical characteristics, response measured by CDAI (Crohn's Disease Activity Index), adverse events and survival status were recorded and analyzed. CR-70, CR-100, and CR-150 were defined as attaining a CDAI decrease of 70, 100 or 150 points compared with baseline.
Results
A total of 103 CD patient records were used in this study. Sixty percent of these patients received combination therapy of adalimumab together with immunomodulators. CR-70 was 68.7%, 74.5% and 88.4% after week 4, 8 and 12 of treatment, respectively. The steroid-free rate, complications and survival were 47.6%, 9.7% and 99% of patients, respectively. In considering the mucosal healing, only 25% patients achieve mucosal healing after treatment for 6 to 12 months. Surgery was still needed in 16.5% of patients. Combination treatment of adalimumab with immunomodulators further decreased the level of CDAI at week 8 when compared with the monotherapy.
Conclusions
Even under the stringent criteria for using adalimumab, the response rate was comparable to those without stringent criteria.
Keywords: Crohn disease, Adalimumab, Efficacy, Safety
INTRODUCTION
The incidence and prevalence of IBD, which includes CD and UC, are lower in Asian countries than in Western countries. However, this has been recently changing.1 CD causes intestinal transmural inflammation, presenting with ulceration and can involve the entire gastrointestinal tract. Prolonged ulceration with inflammation and fibrosis without healing can lead to stricture or other complications.2,3 Therefore, mucosal healing is one of the important goals in treating CD. Treating the inflamed mucosa is associated with a decreased hospitalization risk and surgical resection. Current medications such as 5-aminosalicylic acid and other immunomodulators can relieve the symptoms, but usually do not achieve deep mucosal remission. On the other hand, tumor necrosis factor-alpha antibodies (Anti-TNF-α) have been found to be effective in treating both the symptoms and mucosal inflammations of CD.4
Adalimumab (ADA), one of the available anti-TNF-α antibodies, is an effective therapy for inducing and maintaining remission of moderate to severe CD.5 In one retrospective analysis of 149 patients treated with ADA at a mean duration of 20 months, the results showed 45% anti-TNF-naïve patients and 32% anti-TNF-exposed patients achieved clinical remission. Fistulas healed in 19% and an improvement was seen in the additional 19% of the patients. Adverse events were also seen in 38% of patients, infection being the most common (20%). Mortality in CD patient due to ADA is rare (<1%).6
In Taiwan, dating back to 2008 and till 2014, ADA is the only one available biologics that could be used for CD patients. The criteria of ADA usage is more stringent than most of the other countries, such as Japan and Korea. So far, there was no published data about using ADA in moderate to severe CD patients in Asia. This study aims to evaluate the efficacy and adverse effects of using ADA for CD patients in Taiwan.
METHODS
1. Patients
Collaborating with the Taiwan Society of Inflammatory Bowel Disease (TSIBD), we conducted a retrospective analysis of 9 medical centers in Taiwan from Sep 2009 to Mar 2014. Moderate to severe CD patients who were treated with ADA were included and analyzed. Diagnosis of CD was based on clinical, radiological, and histological features. The clinical response and remission was measured by the standardized CDAI and clinical characteristics were collected and analyzed.
2. Inclusion Criteria
According to the guidelines from the National Health Insurance of Taiwan, reimbursement for ADA therapy depends on the following criteria: First of all, the diagnosis of CD has to be validated and the patient must be registered as having a catastrophic illness. Secondly, patients have received conventional therapies including 5-aminosalicylic acid, immunomodulators or steroids without having satisfactory results. Thirdly, the following medical criteria must be fulfilled: (1) Moderate to severe degree of CD (CDAI >250) despite conventional treatments; (2) Persistent abdominal fistula or anal fistula with symptoms despite medical or surgical treatments with CDAI >100.
3. Definitions of the Efficacy and Safety
The response of ADA was recorded using the following definitions: (1) Steroid-free: taper and then discontinue of steroid use after introducing the ADA. (2) Mucosal healing: Crohn's disease endoscopic index of severity (CDEIS) <6, or no ulceration in the endoscopic finding. (3) CR-70: a decrease in CDAI of at least 70 points. CR-100: a decrease in CDAI of at least 100 points. CR-150: a decrease in CDAI of at least 150 points. Clinical remission was defined as CDAI <150 or healing of fistula. (4) Primary non-response 70 (PNR-70) was defined as not attaining a CDAI decrease of 70 points compared with baseline, primary non-response 100 (PNR-100) was defined as not attaining a CDAI decrease of 100 points compared with baseline, and primary non-response 150 (PNR-150) was defined as not attaining a CDAI decrease of 150 points compared with baseline at week 12 respectively. (5) Adverse events: an undesired harmful effect during ADA treatment.
Extracted and analyzed data included demographics, remission and response rates with ADA, and the safety and efficacy of ADA.
4. Statistical Analysis
Continuous data was expressed as mean±SD and categorical data as percentages. Student's t-test was used for continuous variables and chi-square test for categorical variables. A value of P <0.05 was considered statistically significant. Regressions with a generalized linear model were also performed to assess the relationship between the level of CDAI-decrease and other parameters. The β-value and 95% CI were used to examine the potential relationships of the CDAI-decreased level and other parameters. All statistical analyses were conducted using SAS software, version 9.2 (SAS institute Inc., Cary, North Carolina, USA).
RESULTS
A total of 103 patients were enrolled from 9 medical centers. As shown in Table 1, about two thirds of the patients were male (66.9%). Their ages ranged from 16 to 86 years-old, with a mean±SD of 37.9±15.7 years-old. The duration of treatment ranged from 1 to 253 weeks, with the mean±SD of 74.6±59.1 weeks. Sixty percent of the patients received a combo therapy with ADA combined with azathioprine or methotrexate. The survival rate was 99% after the ADA treatment. For the Montreal classification, most patients were between 17 and 40 years-old (A2) (71.8%) with bowel stricturing (B2) (38.8%) located in the ileocolonic area (L3) (44.7%) (Table 1).
Table 1.
Demographics of the Study Patients
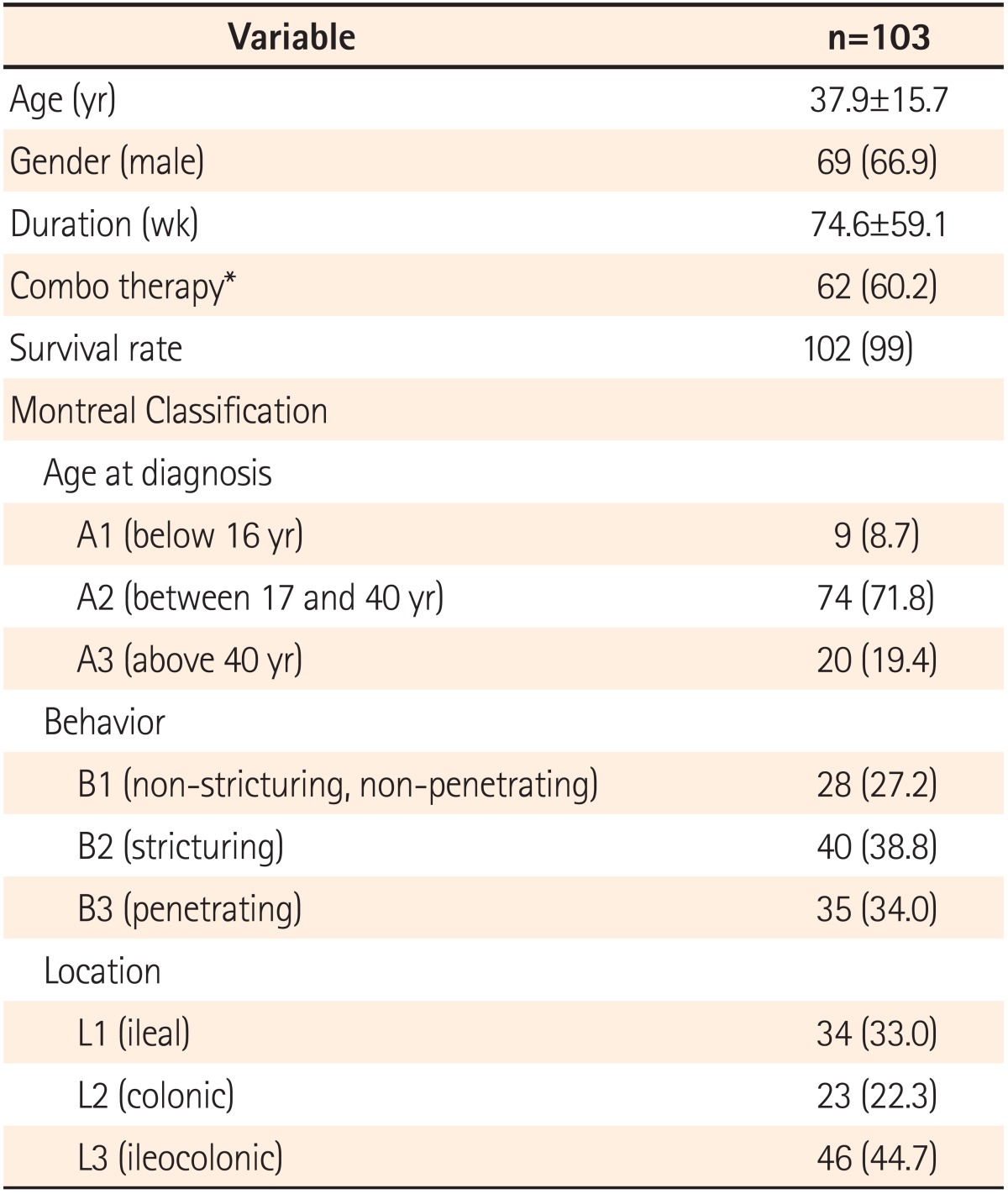
Values are presented as mean±SD or n (%).
*Combination with azathioprine or methotrexate.
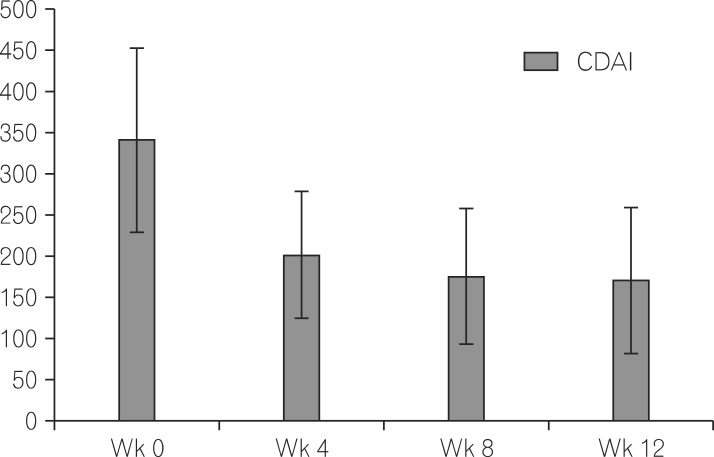
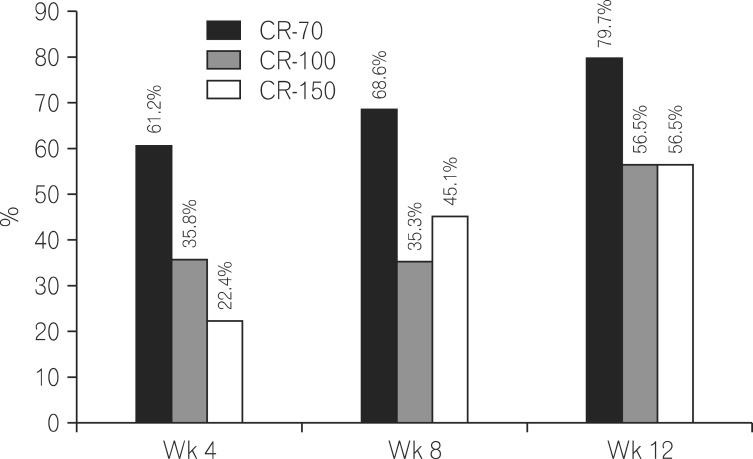
As shown in Fig. 1, the clinical response and remission after ADA treatment, was a CDAI (mean±SD) of 342.2±113.5, 201.3±77.2, 175.3±80.9 and 170.4±89.0 at weeks 0, 4, 8 and 12, respectively. The rates of CR-70 were 68.7%, 74.5% and 88.4% at weeks 4, 8 and 12, respectively. The rates of CR-100 were 61.2%, 68.6% and 79.7% at weeks 4, 8 and 12, respectively. In addition, the rates of CR-150 were 35.8%, 35.3% and 56.5% at weeks 4, 8 and 12, respectively (Fig. 2). After 12 weeks of treatment, 56.5% patients achieved clinical remission with the definition of CDAI <150 (Table 2).
Fig. 1.
Levels of CDAI at week 0, week 4, week 8 and week 12 of CD patients receiving adalimumab treatment.
Fig. 2.
The percentages of CR-70, CR-100 and CR-150 at week 4, week 8 and week 12. CR-70, decrease in CDAI of at least 70 points; CR-100, decrease in CDAI of at least 100 points; CR-150, decrease in CDAI of at least 150 points.
Table 2.
The Decrease of CDAI Compared With Baseline at Week 4, 8 and 12
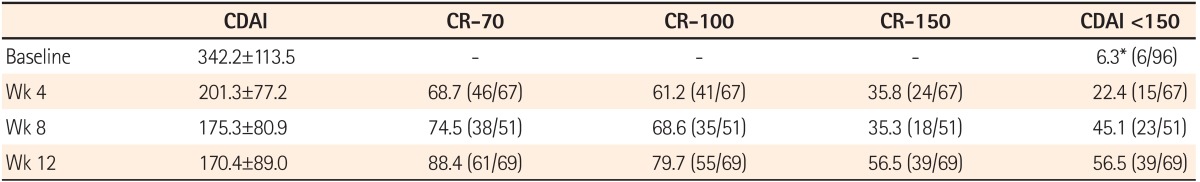
Valuses are presented as mean±SD or % (n).
*There were 6 patients who received adalimumab due to persistent abdominal fistula and the baseline CDAI <150.
CR-70, decrease in CDAI of at least 70 points; CR-100, decrease in CDAI of at least 100 points; CR-150, decrease in CDAI of at least 150 points.
47.6% of the patients receiving ADA became steroid-free as far as treatment. Sixty eight patients underwent endoscopic evaluation for mucosal healing from 6 to 12 months after ADA treatment. Of these patients, 25% (17/68) patients had CDEIS <6 or no mucosal ulceration and 27.9% (19/68) patients had improved endoscopic findings but still had ulcers (partial healing). When we used the CR-70 at 12 weeks after treatment as a cut-off point for primary response, the PNR-70 was only 11.6%. However, if we used the CR-100 at 12 weeks after treatment, the PNR-100 increased to 20.3%. When we used the CR-150 at 12 weeks after treatment, the PNR-150 was as high as 43.5%. There were still 16.5% patients who underwent ADA treatment that needed surgical intervention, mostly due to the stenosis or perforation complications (Table 3).
Table 3.
Efficacy of Adalimumab (n=103)
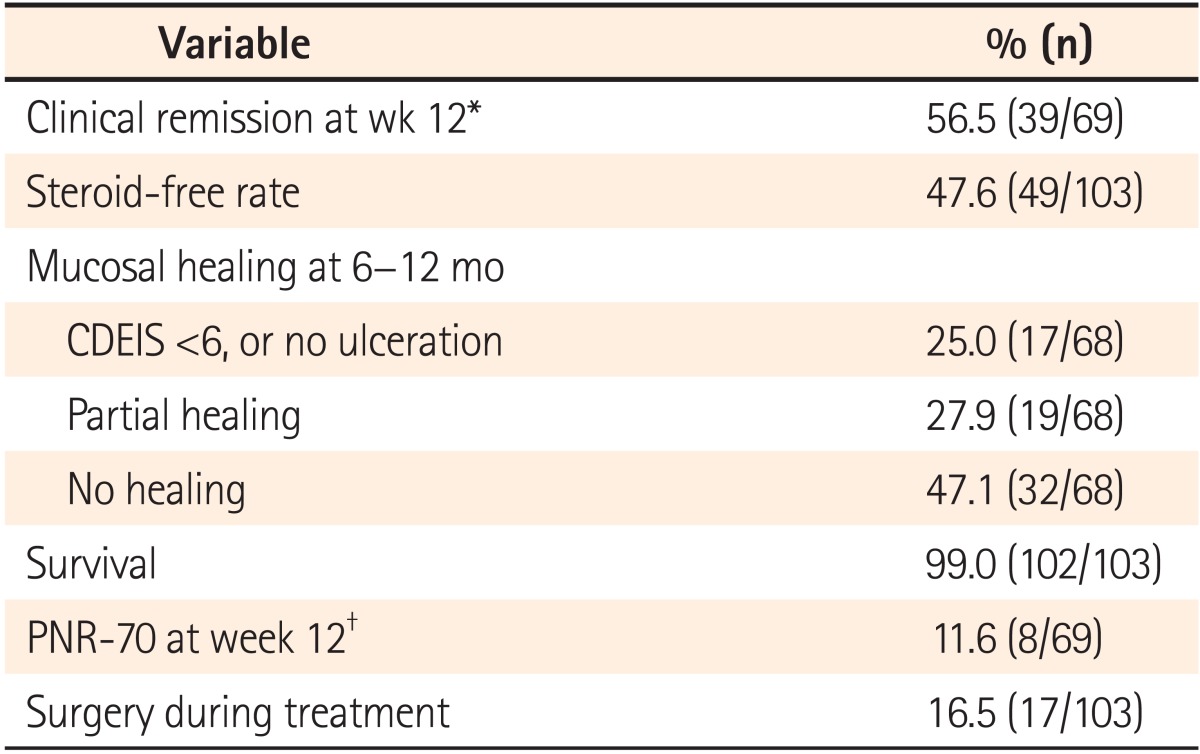
*The definition of clinical remission is CDAI <150 points.
†PNR-70 (primary non-response): did not attain a CDAI decrease of 70 points compared with baseline.
CDEIS, Crohn's disease endoscopic index of severity.
About 60% of the patients received the ADA and an immunomodulator as combo therapy. By using generalized linear regression models, we found that there was a significant difference in the CDAI-decreased level at week 8 when comparing the combo and monotherapy (using ADA alone) with the CDAI decreasing for the combo group by 158.5±114.2 and 101.0±75.6 for the monotherapy group (P =0.02) (Table 4).
Table 4.
The Relationship Between CDAI-Decrease Levels (Week 4, 8 and 12) and Parameters*
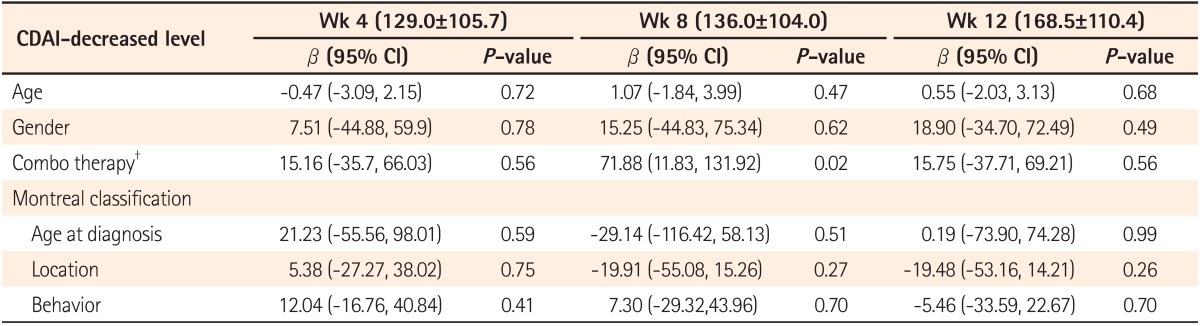
*Data was analyzed with generalized linear regression models.
†Combination of azathioprine/methotrexate and adalimumab.
Adverse events were observed in 9.7% of the patients receiving ADA treatment. Six patients (6/103, 5.8%) had infections and one patient died of sepsis. Other associated adverse events such as intracranial hemorrhage (1/103), myofascial pain (1/103), palpitation (1/103) and a new onset right parotid pleomorphic adenoma (1/103) were also noted. No malignancies were found, so far, in these patients (Table 5). There was also no statistical difference between monotherapy and combo therapy (P =0.18, chi-square test) in adverse events.
Table 5.
Adverse Events During Adalimumab Treatment (n=103)
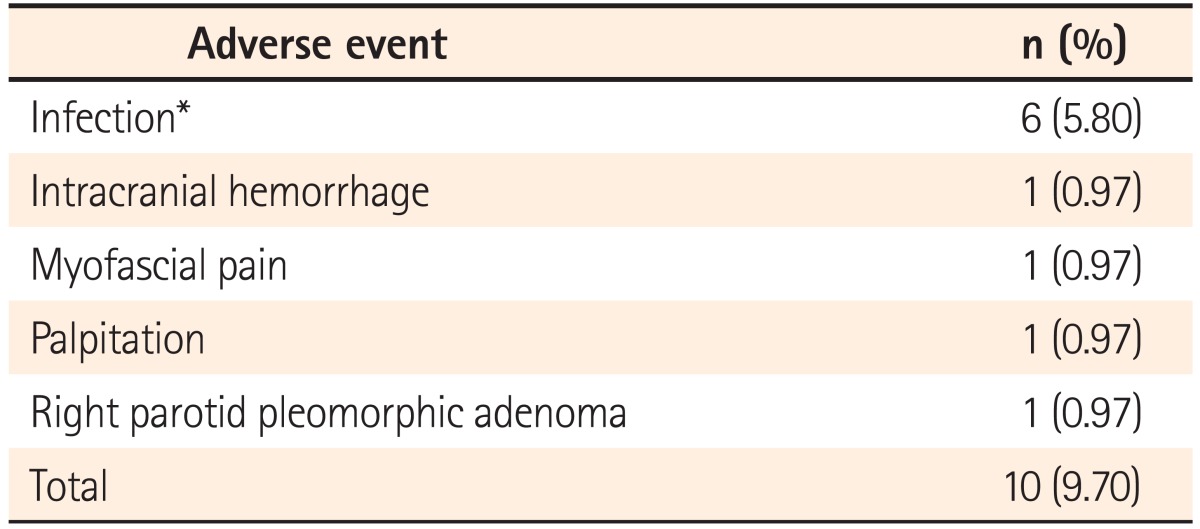
*Two bacterial infections, 2 tuberculosis and 2 herpes zoster infections. In addition, one patient died due to sepsis.
DISCUSSION
The safety and efficacy of ADA for CD have been extensively tested in Western countries, but much less so in Asian countries.3,6,7,8,9,10,11 In Japan, ADA was approved for CD treatment since 2010. Anti-TNF-α agents, such as infliximab (IFX) or ADA, are suggested for refractory cases, remission maintenance, prevention of postoperative relapse and anal lesions. Refractory cases are defined as steroid-resistant, steroid-dependent and cases that surgical treatment is a consideration.12 In Taiwan, as of September 2014, ADA is the only available anti-TNF-α antibody for CD patients. According to the guidelines from the National Health Insurance of Taiwan, the criteria for using ADA in CD are more stringent and limited to the moderate to severe CD with CDAI more than 250 and not responded to conventional therapy. Historically, IBD has been rare in the Far East, however, the incidence and prevalence of IBD are increasing now.13 This phenomenon was also found to be true in Taiwan.14 In Asia, Japan and Korea, so far, have the highest incidence and prevalence for IBD and they approved the use of anti-TNF antibodies similar to its use in western countries. The governments of these countries also support the treatment when IBD patients register and are in need of the antibody treatment. However, most other Asian countries do not provide such governmental support for IBD patients or only providing limited support. Therefore, balancing the cost and benefit of such a treatment is an important issue. Therefore, we set out to examine whether there is an added benefit of antibody treatment using the very stringent criteria used in Taiwan, compared with those countries using the anti-TNF antibody treatment without such stringent criteria.
CDAI is a validated score for CD monitoring with a range from 0 to approximately 600. CDAI below 150 points is considered a reasonable non-active disease status. A CDAI score of 150-219 is defined as a mildly active disease and scores of 220-450 as moderately active disease. Finally, a severe degree is defined as a cut-off value of more than 450 points.15 In our study, the criteria of "moderate to severe degree" is even more stringent, which the CDAI was more than 250 points in order to get the reimbursement of ADA usage. In the Clinical Assessment of ADA Safety and Efficacy Studied as Induction Therapy in Crohn's Disease I (CLASS I) and the Crohn's Trial of the Fully Human Antibody ADA for Remission Maintenance (CHARM) studies, the CR-70 was 54-59% and 64.1% at week 4, respectively.16,17 Our result showed similar findings with 68.7% of the patients attaining CR-70 at week 4, and a better CR-70 at week 12 (88.4%) compared with the Extend the Safety and Efficacy of ADA Through Endoscopic Healing (EXTEND) trial (65-66%).4 As for the clinical remission rate, previous studies also had different results. In the EXTEND trial, the rates of remission were 52% and 28% at week 12 and week 52, respectively. In the CHARM study, the remission rates were 40% in the every other week ADA treatment group, and 47% in the group of weekly ADA treatment, respectively, at week 26.17 Our results showed that 56.5% patients had clinical remission at week 12. For comparing the combo or monotherapy, we found that ADA with concomitant azathioprine was significantly more effective for decreasing the CDAI level after treatment, which was consistent to the previous report.18 In considering the side effects, there's no statistically difference between the combo and monotherapy group (12.9%, 4.9%, respectively; P =0.18).
Becoming steroid-free is another aim in treating CD patients. According to our results, 47.6% patients achieved steroid-free disease management. A previous study showed similar findings with a 44.4% steroid-free rate during anti-TNF-α (IFX) treatment.19 Mucosal healing is the current goal of treating CD patients to reduce the need for hospitalizations and surgery.7 In our study, the mucosal healing rate was 25% after 6-12 months treatment, which is similar to the EXTEND trial of 27% at week 12.4 In a previous report, when using anti-TNF treatment (ADA 74, IFX 109) in CD patients with the median treatment duration as 23 months, 43% patient case had achieved deep mucosal healing.20 Our results showed that most of our patients did not achieve deep mucosal healing after 6-12 months treatment, it implies that longer treatment is needed to help improve the mucosal healing rate, especially when treating the moderate to severe CD patients.
Adverse events of ADA are another important issue for treating CD patients. From the EXTEND trial, adverse events and serious adverse events were noted in 42.2% and 6.3% of the patients.7 Serious infections developed during IFX treatment for CD was noted in 4.9% of the patients.19 Our study also showed that the adverse events rate is 9.7%, which were mostly infections (5.8%). In CHARM trial, there were 3 cases (0.2%) with active tuberculosis (TB) during treatment.21 Two cases of TB (1.9%) were also noted during treatment in our study. One was de novo TB and the other as latent TB, both received anti-TB treatment consequently.
There were limitations of our study. First, this is a retrospective study with missing data. Therefore, how was the proportion of patients with perianal lesions, how many patients were under steroid before they used the ADA were not able to be known from this study. Second, since there was no universal protocol of endoscopic evaluation for mucosal healing, we could only have the endoscopic results as a range of 6 to 12 months for evaluating the mucosa healing rate.
In summary, our results have demonstrated that even using more stringent criteria for choosing CD patients to treat with anti-TNF-α, the efficacy is comparable to those not under such stringent criteria. We also observed similar rates of adverse effects. However, more than one year of anti-TNF treatment might be needed to increase the deep mucosal healing rate for CD patients.
Footnotes
Financial support: None.
Conflict of interest: None.
References
- 1.Sung JJ, Kamm MA, Marteau P. Asian perspectives in the management of inflammatory bowel disease: findings from a recent survey. J Gastroenterol Hepatol. 2010;25:183–193. doi: 10.1111/j.1440-1746.2009.06024.x. [DOI] [PubMed] [Google Scholar]
- 2.Rieder F, Fiocchi C. Intestinal fibrosis in inflammatory bowel disease - current knowledge and future perspectives. J Crohns Colitis. 2008;2:279–290. doi: 10.1016/j.crohns.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Tursi A, Elisei W, Picchio M, et al. Effectiveness and safety of infliximab and adalimumab for ambulatory Crohn's disease patients in primary gastroenterology centres. Eur J Intern Med. 2014;25:485–490. doi: 10.1016/j.ejim.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology. 2012;142:1102–1111. doi: 10.1053/j.gastro.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 5.Peters CP, Eshuis EJ, Toxopeus FM, et al. Adalimumab for Crohn's disease: long-term sustained benefit in a population-based cohort of 438 patients. J Crohns Colitis. 2014;8:866–875. doi: 10.1016/j.crohns.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Teriaky A, Gregor J, Yan B, Ponich T, Chande N, Mosli M. The safety and efficacy of adalimumab in patients with Crohn's disease: the experience of a single Canadian tertiary care centre. Scand J Gastroenterol. 2014;49:280–286. doi: 10.3109/00365521.2013.865785. [DOI] [PubMed] [Google Scholar]
- 7.Asgharpour A, Cheng J, Bickston SJ. Adalimumab treatment in Crohn's disease: an overview of long-term efficacy and safety in light of the EXTEND trial. Clin Exp Gastroenterol. 2013;6:153–160. doi: 10.2147/CEG.S35163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zorzi F, Zuzzi S, Onali S, et al. Efficacy and safety of infliximab and adalimumab in Crohn's disease: a single centre study. Aliment Pharmacol Ther. 2012;35:1397–1407. doi: 10.1111/j.1365-2036.2012.05100.x. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenstein GR, Panaccione R, Mallarkey G. Efficacy and safety of adalimumab in Crohn's disease. Therap Adv Gastroenterol. 2008;1:43–50. doi: 10.1177/1756283X08092548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seiderer J, Brand S, Dambacher J, et al. Adalimumab in patients with Crohn's disease--safety and efficacy in an open-label single centre study. Aliment Pharmacol Ther. 2007;25:787–796. doi: 10.1111/j.1365-2036.2007.03253.x. [DOI] [PubMed] [Google Scholar]
- 11.Hinojosa J, Gomollon F, Garcia S, et al. Efficacy and safety of short-term adalimumab treatment in patients with active Crohn's disease who lost response or showed intolerance to infliximab: a prospective, open-label, multicentre trial. Aliment Pharmacol Ther. 2007;25:409–418. doi: 10.1111/j.1365-2036.2006.03232.x. [DOI] [PubMed] [Google Scholar]
- 12.Ueno F, Matsui T, Matsumoto T, Matsuoka K, Watanabe M, Hibi T. Evidence-based clinical practice guidelines for Crohn's disease, integrated with formal consensus of experts in Japan. J Gastroenterol. 2013;48:31–72. doi: 10.1007/s00535-012-0673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thia KT, Loftus EV, Jr, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103:3167–3182. doi: 10.1111/j.1572-0241.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 14.Wei SC, Lin MH, Tung CC, et al. A nationwide population-based study of the inflammatory bowel diseases between 1998 and 2008 in Taiwan. BMC Gastroenterol. 2013;13:166. doi: 10.1186/1471-230X-13-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sostegni R, Daperno M, Scaglione N, Lavagna A, Rocca R, Pera A. Review article: Crohn's disease: monitoring disease activity. Aliment Pharmacol Ther. 2003;17(Suppl 2):11–17. doi: 10.1046/j.1365-2036.17.s2.17.x. [DOI] [PubMed] [Google Scholar]
- 16.Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 17.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 18.Ishida K, Inoue T, Fujiwara K, et al. Clinical effects of adalimumab treatment with concomitant azathioprine in Japanese Crohn's disease patients. World J Gastroenterol. 2013;19:2676–2682. doi: 10.3748/wjg.v19.i17.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 20.Molander P, Sipponen T, Kemppainen H, et al. Achievement of deep remission during scheduled maintenance therapy with TNFα-blocking agents in IBD. J Crohns Colitis. 2013;7:730–735. doi: 10.1016/j.crohns.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Panaccione R, Colombel JF, Sandborn WJ, et al. Adalimumab maintains remission of Crohn's disease after up to 4 years of treatment: data from CHARM and ADHERE. Aliment Pharmacol Ther. 2013;38:1236–1247. doi: 10.1111/apt.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]