Abstract
Purpose
Allergen-specific immunotherapy is the only currently available treatment to modify the natural history of allergic rhinitis (AR). If patients are polysensitized, it is difficult to identify the allergen causing the allergic symptoms. We evaluated the effectiveness of immunotherapy against house dust mites (HDMs) in AR patients polysensitized to both HDMs and seasonal allergens.
Methods
Thirty AR patients polysensitized to both HDMs and seasonal allergens (group A) and 30 patients sensitized to HDMs only (group B) were enrolled in this study. All subjects who received immunotherapy against HDMs for more than 2 years were evaluated by the multiple allergen simultaneous test (MAST) to determine the specific IgE level in luminescence units, total eosinophil counts in peripheral blood, serum total IgE, total nasal symptom scores, and the rhinoconjunctivitis quality of life questionnaire (RQLQ) before and after immunotherapy.
Results
There were no statistical differences in levels of total and specific IgE, or total eosinophil count between the two groups. The total nasal symptom scores, RQLQ and medication scores significantly decreased after immunotherapy in both groups, however no significant differences were noted between the two groups.
Conclusions
We determined that the primary causative allergen of AR in Seoul, Korea is perennial allergens, such as HDMs, rather than seasonal allergens. This study provides a reference for the selection of allergens to use in immunotherapy for polysensitized AR patients living in an urban environment.
Keywords: Immunotherapy, allergic rhinitis, house dust mite, polysensitization
INTRODUCTION
Allergic rhinitis (AR) is the most common disease mediated by IgE. The prevalence of AR has increased gradually, especially in industrialized countries where pollution is more severe. Treatments for AR include avoidance, pharmacologic therapy and allergen immunotherapy. Immunotherapy has been verified in many reports since Noon and Freeman first applied it to treat pollinosis in 1911 and the therapeutic goal of immunotherapy is to induce immunological tolerance to allergens.1
It is important to identify the causative allergen before applying immunotherapy. When immunotherapy includes allergens causing a positive reaction on allergy skin test, its effect decreases, because the concentration of the actual causative allergen decreases. Among the allergens showing high serum specific IgE levels, the most likely causative allergen can be selected based on the patient's clinical and allergen exposure history. A previous study evaluating the causative allergens in AR patients reported that house dust mite (HDM) is the most prevalent allergen in Korea, representing 65.4% of patients sensitized to perennial allergens, 15.2% sensitized to seasonal allergens, and 19.3% polysensitized to both perennial and seasonal allergens.2 Furthermore, Jeong et al. confirmed the importance of HDM an inhalant allergen in Korea, and the level of exposure to HDM was clinically significant.3 Allergens such as HDMs (98.9%), pollens (72.8%), and animal dander (23.9%) have been used for immunotherapy by allergy specialists in Korea.4
The causative allergens of AR are variable and different according to area and degree of urbanization. It remains difficult to identify the causative allergen in patients who are polysensitized to both perennial and seasonal allergens. In this study, we evaluated the effects of allergen immunotherapy with HDMs in AR patients who are polysensitized to both HDMs and seasonal allergens compared to those sensitized to HDMs only.
MATERIALS AND METHODS
Subjects and study design
Sixty patients receiving subcutaneous immunotherapy (SCIT) for AR at the AR clinic of Kyung Hee University Hospital from January 2009 to December 2011 were enrolled in the study. Among the AR patients receiving immunotherapy for perennial allergens for more than 2 years, 30 who were sensitized to both HDMs and seasonal allergens were included in group A and 30 who were sensitized to HDMs only were included in group B. All patients had positive skin prick tests and a positive serum test for HDMs and seasonal allergens. The 2 groups were treated with immunotherapy against HDMs only, which was administered by soluble vaccine (Hollister-Stier Laboratories, Spokane, WA, USA) injection according to the schedule. Doses were increased every week for more than 6 months until an efficacious dose was reached. The injection interval was then decreased to semimonthly and then monthly injections. After the induction phase, the maintenance dose was administered once a month, once every 2 months, and then once every 3 months. Upon each visit, the patient's clinical condition and suitability for injection was assessed by the clinical investigator. The patients were observed for 30 minutes after each injection. Modification of the dose was made if systemic or local side effects occurred, with either no increment or a reduction in the dose depending on the severity of the side effects. The patients with accompanying chronic rhinosinusitis, nasal polyposis, or asthma were excluded from the study. The study protocol was approved by the Institutional Review Board of the Medical Research Institute, Kyung Hee University Medical Center. Written informed consent was obtained from all subjects.
Allergic test
Skin prick tests with 8 common allergens (Dermatophagoides farinae, Dermatophagoides pteronyssinus, cockroach, tree mix, grass mix, weed mix, Aspergillus, and Alternaria) were performed on the forearm of patients using aqueous extracts (Allergopharma, Reinbeck, Germany); histamine was used as a positive control and saline as a negative control. A positive response to an allergen was defined as a wheal diameter of more than 3 +(greater than the diameter produced by histamine). Using a multiple allergen simultaneous test-chemiluminescent assay (MAST-CLA, Hitachi Chemical Diagnostics Inc., CA, USA), the levels of specific IgE against 35 kinds of allergens were determined based on a semi-quantitative scoring system; class 2 (66-142 unit) and higher were considered positive. Before and after immunotherapy, MAST-CLA (Hitachi Chemical Diagnostics Inc. was performed to examine changes in allergen sensitization and the presence of new allergen sensitization between the two groups.
Objective clinical indicators
Blood taken before and after treatment was anticoagulated using EDTA, and peripheral blood cells were analyzed using a Coulter automated hematology analyzer (Hialeah FL, Miami, FL, USA) to measure total eosinophils. Serum was separated from peripheral blood collected before and after treatment by centrifugation at 1,200 rpm and then stored at -20℃ until measurement of total IgE. Total IgE level was measured by ImmunoCAP system (Thermo Scientific, Uppsala, Sweden) and the results for total serum IgE levels and eosinophil count measurements were expressed in international unit(s) per milliliter (IU/mL) and cells per mm3 (cells/mm3), respectively.
Assessment of symptoms and medication scores
Nasal symptom scores and quality of life were measured using questionnaires as described before.5 A visual analogue scale ranging from 0 to 4 was used for nasal symptoms (sneezing, runny nose, congestion, itchy nose), and a rhinoconjunctivitis quality of life questionnaire (RQLQ) modified by our clinic was used to assess quality of life.5 Symptoms before immunotherapy were recorded in the questionnaire and then again after receiving immunotherapy for more than 2 years. The RQLQ was designed to measure the health status of patients with allergic rhinoconjunctivitis according to the following seven domains: activities, sleep, non-nose/eye symptoms, practical problems, nasal symptoms, eye symptoms, and emotional well-being. Each question was scored using an impairment rating scale of 0-6, with higher scores indicative of more severe impairment of quality of life. Amelioration of seasonal allergy symptoms was also evaluated.
The following drugs were allowed during the study: antihistamine (5 mg levocetirizine tablets), intranasal corticosteroid (mometasone) and a 4 mg tablet of methylprednisolone. The patients recorded the use of medications, and a specific weekly score was assigned (1 point: used only nasal corticosteroids at a minimum of 1 per day every week; 2 points: the previous therapy plus one tablet of levocetirizine at a minimum of 1 per day; 3 points: the previous therapies plus one 4 mg tablet of methylprednisolone at a minimum of 1 per day).
Statistics
Statistical analyses were performed using SPSS version 12.0. Baseline data were analyzed using the chi-square test or Fisher's exact test. Symptom scores and serum measurements before and after immunotherapy were compared between groups using the paired t-test. A P value less than 0.05 was considered statistically significant.
RESULTS
Clinical characteristics and sensitization to allergens
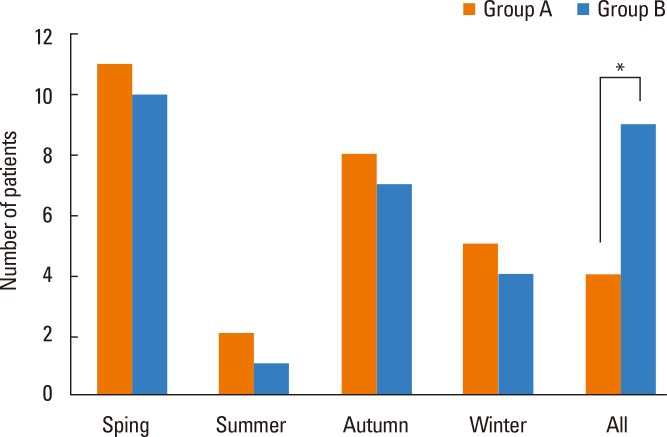
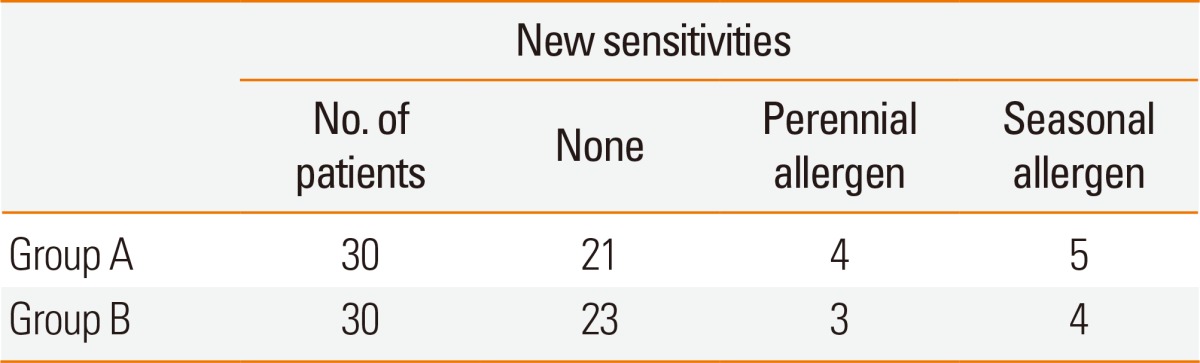
The median age of patients in group A was 26 (range 15-42) years, and the average period of immunotherapy treatment was 31±9.2 months. The median age of patients in group B was 23 (range 17-39) years, and the average treatment period was 29±9.1 months. There were no significant differences in the baseline data, number of years with rhinitis symptoms, and periods of immunotherapy between the 2 groups (Table 1). The distribution of sensitized patients to specific allergens in each group is shown in Table 2. In group A, 4 patients were sensitized to 2 allergens, 5 to three allergens, 14 to 4 allergens, and 8 to 5 allergens. In the questionnaire on amelioration of seasonal nasal symptoms, group A showed a greater tendency for deterioration of symptoms than did group B, but there was no statistical significance. In addition, group B consisted of more patients whose symptoms continued regardless of the season (Fig. 1). MAST values (luminescence units) tended to increase after immunotherapy; however, statistical analyses could not be performed due to the small sample size of the target group (Table 2). After immunotherapy, 30% of patients in group A and 23% in group B showed new sensitization. In groups A and B, 4 and 3 patients were sensitized to new perennial allergens, respectively, and 5 and 4 were sensitized to new seasonal allergens, respectively; however, there was no significant difference between the 2 groups (Table 3).
Table 1.
Clinical characteristics of groups A and B
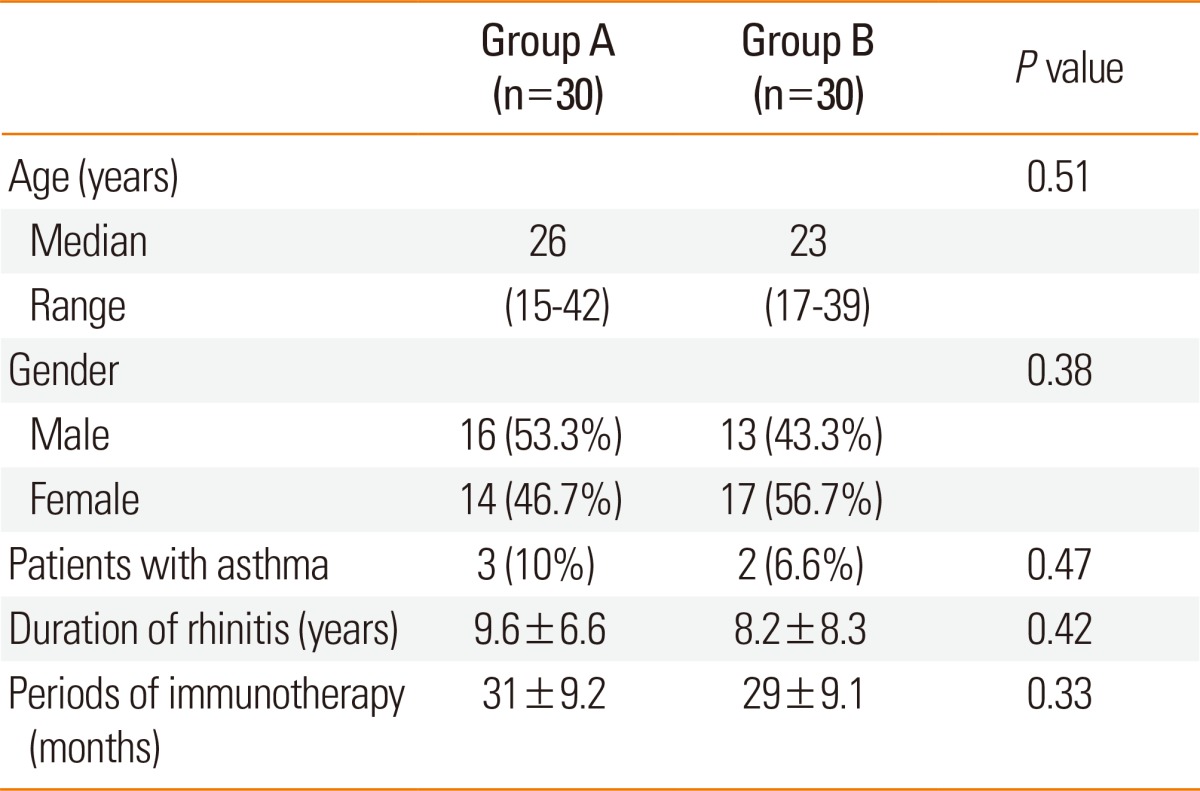
Table 2.
Comparison of pre- and post-treatment allergen specific IgE levels (luminescence unit) measured by MAST between groups A and B
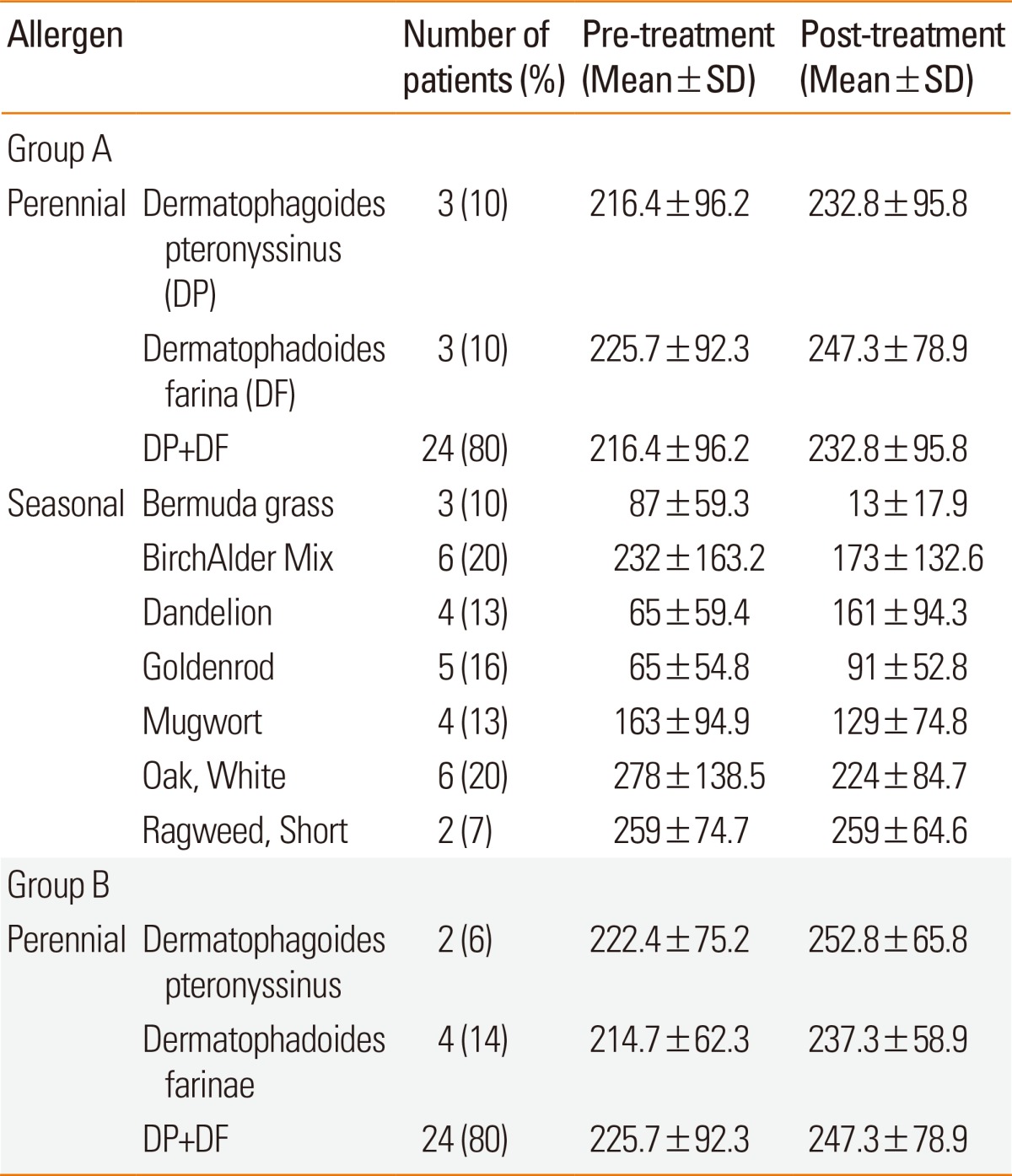
DP, Dermatophagoides pteronyssinus; DF, Dermatophagoides farinae.
Fig. 1.
Number of patients with severe allergic rhinitis symptoms during the season (*P<0.05).
Table 3.
Comparison of the development of new sensitization between Groups A and B
Total eosinophils and total IgE level
Before immunotherapy, the average number of total eosinophils was 387.5/mm3 in group A and 353.6/mm3 in group B. After immunotherapy, the average was 339/mm3 and 323.5/mm3, respectively, revealing no difference between the 2 groups. Before immunotherapy, the mean total IgE level was 476.3 IU/mL in group A and 458.7 IU/mL in group B. After immunotherapy, the mean levele was 418.2 IU/mL and 412.9 IU/mL, respectively, with no difference between the 2 groups.
Symptom and medication scores
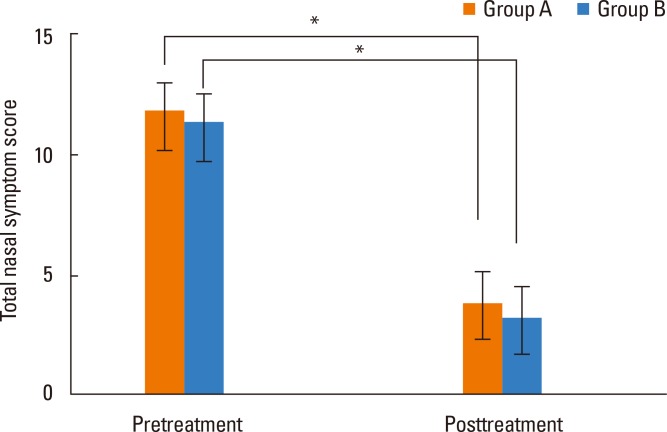
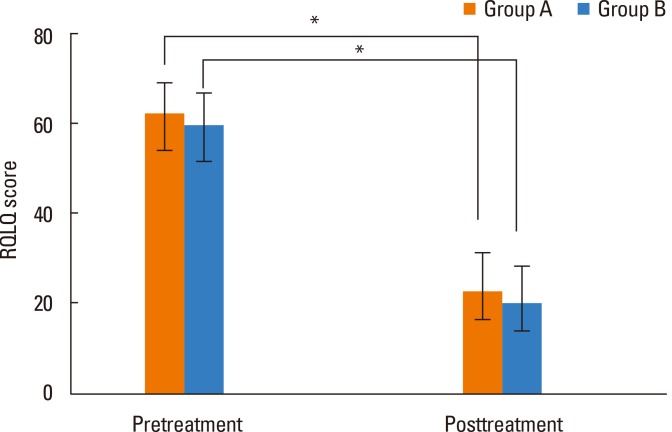
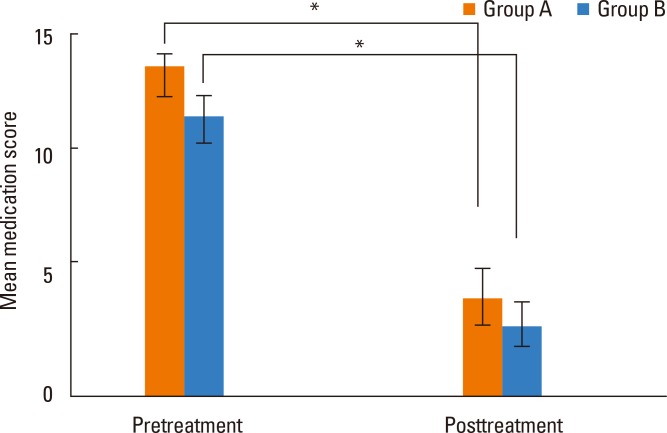
Total nasal symptom scores were 11.8 in group A and 11.3 in group B, with no difference between the 2 groups. After immunotherapy, total scores were 3.8 and 3.2, respectively, with a significant decrease in both groups after immunotherapy but no difference between the 2 groups (Fig. 2). The average life quality score was 62.4 in group A and 59.7 in group B, showing no significant difference between the 2 groups. After immunotherapy, life quality scores were 22.8 and 20.3, respectively, which were significantly decreased in both groups relative to before immunotherapy, but there was no difference between the 2 groups (Fig. 3). Mean medication scores also showed a significant decrease in both groups after immunotherapy but no difference between the 2 groups (Fig. 4).
Fig. 2.
Comparison of total nasal symptom scores pre- and post-treatment between Groups A and B (*P<0.05).
Fig. 3.
Comparison of RQLQ scores pre- and post-treatment between Groups A and B (*P<0.05).
Fig. 4.
Comparison of mean medication scores pre- and post-treatment between groups A and B (*P<0.05).
DISCUSSION
The appropriate use of immunotherapy for polysensitized AR patients remains unclear.6,7 With respect to SCIT, one double-blind trial using a multi-allergen mix in children with perennial asthma and multiple allergies showed no difference compared with the placebo group.8 Frew et al. examined participants with seasonal hay fever whose symptoms were largely confined to during the grass pollen season.9 In this previous study, grass pollen immunotherapy using an alum-based single species grass vaccine was equally effective in subjects with multiple positive skin tests compared with those monosensitized to grass pollen. In addition, Ciprandi et al. showed that immunotherapy using a single allergen extract significantly improves the severity grade of AR and quality of life in polysensitized patients.10 Taken together, this evidence demonstrates that single allergen immunotherapy may be effective against the relevant allergen in polysensitized patients. Of course, a single allergen is likely to play a critical role in the development of nasal symptoms. This study observed a curative effect of immunotherapy against HDMs in AR patients living in Seoul, Korea, who were polysensitized to HDM as well as perennial and seasonal allergens. As a result, there were no differences in serological tests between groups before and after treatment. However, AR symptoms and quality of life showed improvements similar to those of patients treated with immunotherapy after monosensitization to HDM. Therefore, this study demonstrates that HDM allergens, rather than seasonal allergens, play a critical role in the development of allergic symptoms in Seoul, Korea.
Treatment for AR includes avoidance, medical therapy, and immunotherapy. Among these, immunotherapy is commonly used and is the only treatment for AR that is potentially curative.11 Immunotherapy for AR shows better results for seasonal than for perennial allergens due to smaller amounts of allergen, lower age, and less severe symptoms before treatment.12 Specifically, a considerable number of AR patients have asthma; therefore, immunotherapy is required in patients diagnosed with airway hyperreactivity.13
It is necessary to confirm the results of skin reaction, serum tests or nasal challenge tests before targeting a specific allergen for immunotherapy.14 However, immunotherapy cannot be performed for every allergen that shows a positive reaction. For immunotherapy to be most effective, it is important to determine the specific allergen that will be used for treatment. Among allergens positive to specific IgE antibody, the first one selected for immunotherapy should be common in the surrounding environment, and contact with the allergen is based on the patient's history. When immunotherapy is performed for every allergen that is positive to specific IgE antibody, the concentration of actual causative allergen used is reduced, diminishing the effectiveness of the treatment. Because immunotherapy is less effective with a large number of allergens, it is better to minimize the number used for treatment. For polysensitized patients affected by many allergens, the number of allergens can be reduced by considering cross-allergenicity. When performing immunotherapy against more than 2 allergens using allergen compounds, cross-reaction between allergens, the amount of each allergen within the compound, and the potential for allergen cleavage by proteases should be considered.15 Yu et al. confirmed that the Dermatophagoides pteronyssinus, the most important perennial allergen, has the highest positivity rate of 78.4%.2 Dermatophagoides farinae, pollens, animal furs, and cockroaches show the next highest positivity rates, respectively. Serman reported that many patients with perennial AR are also sensitized to pollens, and 77.7% are polysensitized to more than 2 allergens.16 Boulet performed skin tests for asthma and AR and reported 66.4% polysensitization to both perennial and seasonal allergens.17 The present study divided patients into 2 groups: those who were sensitized to HDMs only and those who were sensitized to HDMs as well as seasonal and perennial allergens. Some patients in groups A and group B were sensitized to new perennial allergens, such as cockroaches and Aspergillus, as well as new seasonal allergens, such as mugwort, oak and ragweed, but there was no significant difference between the 2 groups.
Evaluation of immunotherapy is based on the symptoms, administration score, induction reaction test, and immunological changes in cell markers and cytokines. Regular re-evaluation of skin prick tests or MAST to assess the success of immunotherapy is not effective, because these tests do not reflect improvement of symptoms after immunotherapy.17 IgE is the most important immunoglobulin in immunopathology, and specific IgE and IgG play important roles in immune responses to allergens. Immunotherapy has beneficial clinical effects by modulating immunoglobulin levels.18 Once immunotherapy is applied, IgE increases in the early phase and then slowly decreases in the maintenance phase; however, the period between the decrease in IgE antibody and improvement of symptoms was shown to be irrelevant.19 When pollens are used for immunotherapy, there is little change in blood IgE levels, and increases in IgE are slow during the season that pollens pervade.19 A previous report showed no correlation between the success of immunotherapy or improved clinical symptoms and a decrease in specific IgE.20 This study showed no significant difference between MAST, total eosinophils and total IgE before or after therapy between the 2 groups. Assessment of a patient's reaction to treatment is generally performed by objectively evaluating the improvement in quality of life of the AR patient. This study included an ophthalmic symptoms section of Juniper's questionnaire to evaluate quality of life.21 The modified questionnaire, which was tailored to the surrounding environment of Seoul, Korea, was comprised of 6 sections, including sleeping disorder, nasal symptoms, everyday life, activity restriction, entire body symptoms, and emotional status, to evaluate the condition of patients with AR.5 In the present study, immunotherapy using perennial allergens showed improvements in symptoms and quality of life in both the monosensitized and polysensitized groups, with no significant difference.
This study showed that improvement in polysensitized patients was similar to that in the monosensitized group. However, if there was no response to immunotherapy (especially in polysensitized AR patients), additional immunotherapy should be considered. Therefore, immunotherapy is an effective treatment for AR, and the main causative allergen in polysensitized patients is the HDM.
CONCLUSIONS
In this study, we determined that the primary causative allergen of AR in Seoul, Korea is perennial allergens, such as HDM, rather than seasonal allergens. Therefore, this study provides a reference for the selection of allergens to use in immunotherapy for polysensitized AR patients living in an urban environment.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Finegold I. Immunotherapy historical perspective. Ann Allergy Asthma Immunol. 2001;87:3–4. doi: 10.1016/s1081-1206(10)62187-4. [DOI] [PubMed] [Google Scholar]
- 2.Yu YI, Cho JS, Lee KH, Kim KH, Hong SM, Kim SW. Clinical statistical study on offending allergens of patients with allergic rhinitis: prevalence of multiple sensitization. Korean J Otolaryngol-Head Neck Surg. 2003;46:48–53. [Google Scholar]
- 3.Jeong KY, Park JW, Hong CS. House dust mite allergy in Korea: the most important inhalant allergen in current and future. Allergy Asthma Immunol Res. 2012;4:313–325. doi: 10.4168/aair.2012.4.6.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hur GY, Kim TB, Han MY, Nahm DH, Park JW Allergen and Immunotherapy Work Group of the Korean Academy of Asthma, Allergy and Clinical Immunology (KAAACI) A survey of the prescription patterns of allergen immunotherapy in Korea. Allergy Asthma Immunol Res. 2013;5:277–282. doi: 10.4168/aair.2013.5.5.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park KH, Cho JS, Lee KH, Shin SY, Moon JH, Cha CI. Rhinoconjunctivitis quality of life questionnaire (RQLQ) as an evaluator of perennial allergic rhinitis patients-the first report. Korean J Otolaryngol-Head Neck Surg. 2002;45:254–262. [Google Scholar]
- 6.Calderón MA, Cox L, Casale TB, Moingeon P, Demoly P. Multiple-allergen and single-allergen immunotherapy strategies in polysensitized patients: looking at the published evidence. J Allergy Clin Immunol. 2012;129:929–934. doi: 10.1016/j.jaci.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Bahceciler NN, Galip N, Cobanoglu N. Multiallergen-specific immunotherapy in polysensitized patients: where are we? Immunotherapy. 2013;5:183–190. doi: 10.2217/imt.12.161. [DOI] [PubMed] [Google Scholar]
- 8.Adkinson NF, Jr, Eggleston PA, Eney D, Goldstein EO, Schuberth KC, Bacon JR, Hamilton RG, Weiss ME, Arshad H, Meinert CL, Tonascia J, Wheeler B. A controlled trial of immunotherapy for asthma in allergic children. N Engl J Med. 1997;336:324–331. doi: 10.1056/NEJM199701303360502. [DOI] [PubMed] [Google Scholar]
- 9.Frew AJ, Powell RJ, Corrigan CJ, Durham SR UK Immunotherapy Study Group. Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment-resistant seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:319–325. doi: 10.1016/j.jaci.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Ciprandi G, Incorvaia C, Puccinelli P, Soffia S, Scurati S, Frati F. Polysensitization as a challenge for the allergist: the suggestions provided by the Polysensitization Impact on Allergen Immunotherapy studies. Expert Opin Biol Ther. 2011;11:715–722. doi: 10.1517/14712598.2011.576246. [DOI] [PubMed] [Google Scholar]
- 11.Veling MC, Trevino RJ. The treatment of allergic rhinitis with immunotherapy: a review of 1,000 cases. Ear Nose Throat J. 2001;80:542–543. [PubMed] [Google Scholar]
- 12.Finegold I. Allergen immunotherapy: present and future. Allergy Asthma Proc. 2007;28:44–49. doi: 10.2500/aap.2007.28.2971. [DOI] [PubMed] [Google Scholar]
- 13.Portnoy JM. Immunotherapy for inhalant allergies. Guidelines for why, when, and how to use this treatment. Postgrad Med. 2001;109:89–90. 93–94, 99–100. doi: 10.3810/pgm.2001.05.929. [DOI] [PubMed] [Google Scholar]
- 14.Malling HJ. Allergen-specific immunotherapy in allergic rhinitis. Curr Opin Allergy Clin Immunol. 2001;1:43–46. doi: 10.1097/01.all.0000010983.53424.26. [DOI] [PubMed] [Google Scholar]
- 15.Joint Task Force on Practice Parameters. Allergen immunotherapy: a practice parameter. American Academy of Allergy, Asthma and Immunology. American College of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 2003;90:1–40. [PubMed] [Google Scholar]
- 16.Sherman WB. Hypersensitivity: mechanisms and management. Philadelphia (PA): Saunders; 1968. [Google Scholar]
- 17.Boulet LP, Turcotte H, Laprise C, Lavertu C, Bédard PM, Lavoie A, Hébert J. Comparative degree and type of sensitization to common indoor and outdoor allergens in subjects with allergic rhinitis and/or asthma. Clin Exp Allergy. 1997;27:52–59. [PubMed] [Google Scholar]
- 18.Ortolani C, Pastorello E, Moss RB, Hsu YP, Restuccia M, Joppolo G, Miadonna A, Cornelli U, Halpern G, Zanussi C. Grass pollen immunotherapy: a single year double-blind, placebo-controlled study in patients with grass pollen-induced asthma and rhinitis. J Allergy Clin Immunol. 1984;73:283–290. doi: 10.1016/s0091-6749(84)80021-4. [DOI] [PubMed] [Google Scholar]
- 19.Gehlhar K, Schlaak M, Becker W, Bufe A. Monitoring allergen immunotherapy of pollen-allergic patients: the ratio of allergen-specific IgG4 to IgG1 correlates with clinical outcome. Clin Exp Allergy. 1999;29:497–506. doi: 10.1046/j.1365-2222.1999.00525.x. [DOI] [PubMed] [Google Scholar]
- 20.McHugh SM, Lavelle B, Kemeny DM, Patel S, Ewan PW. A placebo-controlled trial of immunotherapy with two extracts of Dermatophagoides pteronyssinus in allergic rhinitis, comparing clinical outcome with changes in antigen-specific IgE, IgG, and IgG subclasses. J Allergy Clin Immunol. 1990;86:521–531. doi: 10.1016/s0091-6749(05)80208-8. [DOI] [PubMed] [Google Scholar]
- 21.Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Validation of the standardized version of the Rhinoconjunctivitis Quality of Life Questionnaire. J Allergy Clin Immunol. 1999;104:364–369. doi: 10.1016/s0091-6749(99)70380-5. [DOI] [PubMed] [Google Scholar]