Abstract
Purpose
Based on the close relationship between histamine and interleukin 6 (IL-6), we hypothesized that histamine may regulate the production of cytokines, such as IL-6, during allergic inflammation. Here, we examined the role of histamine in IL-6 production and histamine receptor activity in nasal fibroblasts, along with the mechanisms underlying these effects.
Methods
Experiments were performed using nasal fibroblasts from 8 normal patients. RT-PCR was used to identify the major histamine receptors expressed in nasal fibroblasts. Fibroblasts were then treated with histamine with or without histamine-receptor antagonists, and monitored for IL-6 production using an ELISA. Four potential downstream signaling molecules, p38, extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and NF-κB, were evaluated by Western blot, and a luciferase reporter assay.
Results
Elevated expression was seen for all histamine receptors, with IL-6 protein levels increasing significantly following histamine stimulation. Among the histamine-receptor specific antagonists, only the H1R antagonist significantly decreased IL-6 production in histamine-stimulated nasal fibroblasts. Histamine increased the expression level of phosphorylated p38 (pp38), pERK, and pJNK, as well as NF-κB induction. The H1R antagonist actively suppressed pp38 and NF-κB expression in histamine-induced nasal fibroblasts, but not pERK and pJNK. The p38 inhibitor strongly attenuated IL-6 production in histamine-stimulated nasal fibroblasts.
Conclusions
The data presented here suggest that antihistamines may be involved in the regulation of cytokines, such as IL-6, due to the role of histamine as an inflammatory mediator in nasal fibroblasts.
Keywords: Nose, fibroblast, histamine, IL-6, allergic rhinitis
INTRODUCTION
Histamine is one of the major inflammatory mediators of allergy, and is released from mast cells in the immediate-phase and basophils in the late-phase response.1 Nasal histamine production induces the majority of symptoms associated with allergic rhinitis, including itching, sneezing, rhinorrhea, and nasal obstruction.2 As histamine-1 receptor (H1R) antagonists control symptoms related to histamine expression, H1R is generally accepted as the primary receptor of histamine activity. An immunohistochemical study demonstrated intense immunoreactivity for H1R in nasal epithelial cells and vascular endothelial cells.3 Furthermore, it has been shown that histamine affects vascular permeability, allowing for leukocyte infiltration and edema formation via H1R.4 However, if we instead consider allergic symptoms as the result of a complex series of reactions orchestrated by several mediators, including cytokines, chemokines, neuropeptides, adhesion molecules, and cells, it becomes important to understand what effects of histamine might have on those processes.
Interleukin 6 (IL-6) is a pleiotropic cytokine that plays a central role in host defense.5 Though mononuclear phagocytic cells are the primary source of IL-6, it is also produced by a number of other cell types, including lymphocytes, granulocytes, endothelial cells, keratinocytes, mast cells, and fibroblasts.6 IL-6 is traditionally considered an activator of acute phase responses and a lymphocyte stimulatory factor.7 Recent evidence has shown that IL-6 secreted by cells of the innate immune system can induce expansion of Th2 effector cell populations.8 Moreover, as a proinflammatory cytokine, IL-6 is thought to amplify the allergic inflammatory response.9
There is a close relationship between histamine and IL-6. Histamine has been shown to induce exocytosis and IL-6 production in human lung macrophages through its interaction with H1Rs,10 as well as inducing IL-6 production in human endothelial cells.11 Microenvironmental controls of inflammatory processes have been shown to regulate the initiation and perpetuation of allergic inflammation through their effects on cell differentiation and cell structure.12 Taken together, these data suggest that histamine may play a role in the production of cytokines, such as IL-6, in nasal fibroblasts. In this report, we examined the role of histamine on IL-6 production and histamine receptor activity in nasal fibroblasts, along with the mechanisms underlying these effects.
MATERIALS AND METHODS
Reagents
Histamine, fexofenadine (H1R antagonist), ranitidine (H2R antagonist), clobenpropit (H3R antagonist), and JNJ7777120 (H4R antagonist) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ranitidine and clobenpropit were dissolved in distilled water. Fexofenadine and JNJ7777120 were dissolved in dimethyl sulfoxide (DMSO). The maximum final concentration of DMSO was <0.1%.
Nasal fibroblast culture
Inferior turbinate specimens of 8 patients were obtained during corrective septorhinoplasty at the Department of Otorhinolaryngology, Korea University Medical Center, Korea. Informed consents were obtained from each patient, and the study was approved by the Korea University Medical Center Institutional Review Board (KUGH-12200). None of the patients had a history of allergy, asthma, or aspirin sensitivity. Nasal fibroblasts were isolated from surgical tissues by enzymatic digestion with collagenase (500 U/mL, Sigma), hyaluronidase (30 U/mL, Sigma), and DNase (10 U/mL, Sigma). Cells were cultured in Dulbecco's Modified Eagle's Medium containing 10% (v/v) heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA, USA), 1% (v/v), 10,000 units/mL penicillin, and 10,000 µg/mL streptomycin (Invitrogen). The medium was changed every 4 days until confluence. Cells were used after being passaged 4 times.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was denatured at 65℃ for 5 minutes. After cooling on ice, the following components were added to the samples: 5X RT-buffer, 0.1 M dithiothreitol, RNase OUT, and 2.5 mM dNTP of Moloney murine leukemia virus RT. After 60 minutes at 37℃, the RT was inactivated by heating the mixture at 95℃ for 5 minutes. PCR was performed using the following primer combinations: H1R (sense sequence 5'-GTC TAA CAC AGG CCT GGA TT-3', anti-sense sequence 5'-GGA TGA AGG CTG CCA TGA TA-3'); histamine 2 receptor (H2R, sense sequence 5'-ATT AGC TCC TGG AAG GCAGC-3', anti-sense sequence 5'-CTG GAG CTT CAG GGG TTT CT-3'); histamine 3 receptor (H3R, sense sequence 5'-TCG TGC TCA TCA GCT ACG AC-3', anti-sense sequence 5'-AAG CCG TGA TGA GGA AGT AC-3'); histamine 4 receptor (H4R, sense sequence 5'-GGC TCA CTA CTG ACT ATC TG-3', anti-sense sequence 5'-CCT TCA TCC TTC CAA GAC TC-3'); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, sense sequence 5'-GTG GAT ATT GTT GCC ATC AAT GAC C-3', anti-sense sequence 5'-GCC CCA GCC TTC TTC ATG GTG GT-3').
IL-6 measurements
IL-6 was assayed using a commercial ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol. In brief, nasal fibroblasts were incubated in 2.5% FBS, and the culture media were collected at the end of the incubation. Each well was blocked with blocking buffer for a minimum of 2 hours at room temperature, and washes were performed by filling each well with wash buffer. For detection, IL-6 antibody was added and incubated for 2 hours. A substrate solution was introduced to each well and was incubated for 20 minutes in the dark. Stop solution was added to each well and incubated for 30 minutes in the dark. If the subsequent color change did not appear uniform, the plate was gently tapped to ensure thorough mixing. The optical density of each well was determined within 30 minutes using a microplate reader (F2000; Hitachi Ltd., Tokyo, Japan) set to 450 nm.
Western blot analysis
Nasal fibroblasts were lysed in PRO-PREP protein extraction solution (iNtRON Biotechnology, Seongnam, Korea). Cell lysates were subjected to SDS-PAGE and transferred to PVDF membranes (Millipore Inc., Billerica, MA, USA). The membranes were then blocked for 1 hour in 3% non-fat dry milk, and incubated at 4℃ overnight with anti-mouse monoclonal pJNK, GAPDH, anti-rabbit polyclonal pERK (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and p-p38, p38, JNK, ERK antibodies (Cell Signaling, Boston, MA, USA). After incubation, membranes were washed 3 times for 5 minutes, and then treated with a peroxidase-conjugated anti-mouse or anti-rabbit antibody (Vector Laboratories, Burlingame, CA, USA) for 1 hour. After washing the membranes, substrate was added using an enhanced chemiluminescence reagent kit (Du Pont, Boston, MA, USA).
Luciferase reporter assay
Nasal fibroblasts were cotransfected with pGL4.32 (luc2P/NF-κB-RE/Hygro) Vector plasmid and pGL4.74 (hRluc/TK) vector, using Fugene HD transfection reagent (Promega, Madison, WI, USA) according to the manufacturer's protocol. Twenty-four hours after transfection, cells were stimulated with histamine for 2 hours, harvested, and luciferase activity detected using a dual-luciferase reporter assay system (Promega, Madison, WI) according to the manufacturer's instructions. Firefly luciferase activity was normalized to Renilla luciferase activity, and the level of induction was reported.
Statistical analysis
Results are shown as means±SE The statistical significance of differences between groups was assessed by one-way analysis of variance (ANOVA) for factorial comparisons and by Tukey's multiple comparison test. Triplicate wells were prepared for each condition in each experiment.
RESULTS
The expression of histamine receptors in nasal fibroblasts
To identify the histamine receptors expressed in nasal fibroblasts, RT-PCR of H1R, H2R, H3R, and H4R was performed. Although nasal fibroblasts constitutively expressed all histamine receptor subtypes, H1R expression was higher than that of the other subtypes (Fig. 1).
Fig. 1.
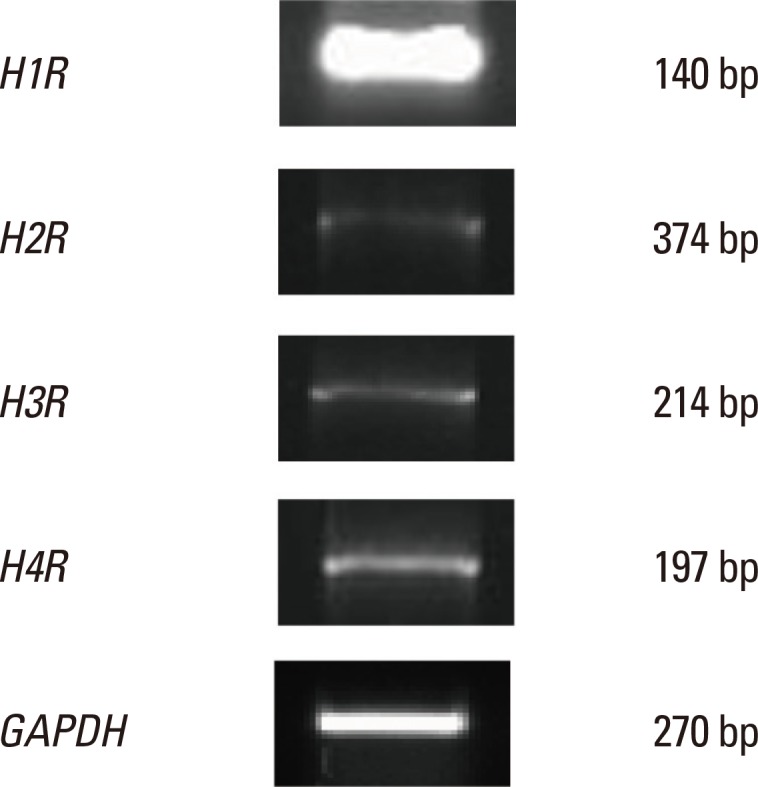
Messenger RNA levels of histamine receptors in nasal fibroblasts. Gene expression levels were determined by RT-PCR.
Effect of histamine on IL-6 production
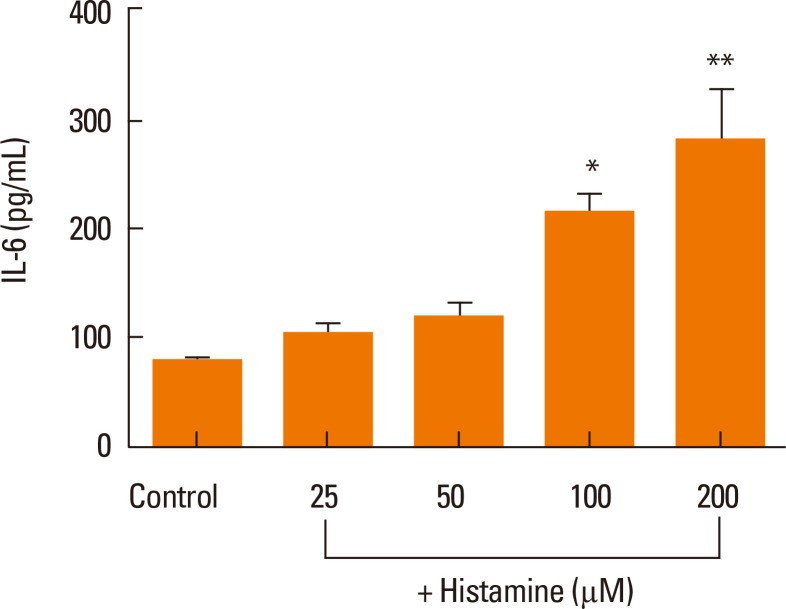
Next, we examined whether histamine stimulates IL-6 production in nasal fibroblasts using an ELISA. Nasal fibroblasts were treated with up to 200 µM histamine for 24 hours. IL-6 protein expression increased significantly in response to histamine stimulation in a dose-dependent manner (Fig. 2).
Fig. 2.
ELISA of the effects of histamine on IL-6 production in nasal fibroblasts. Values are expressed as the means±SE of independent experiments. *P<0.05 vs control, **P<0.01 vs control.
Effect of H1R antagonist on IL-6 production in histamine-stimulated nasal fibroblasts
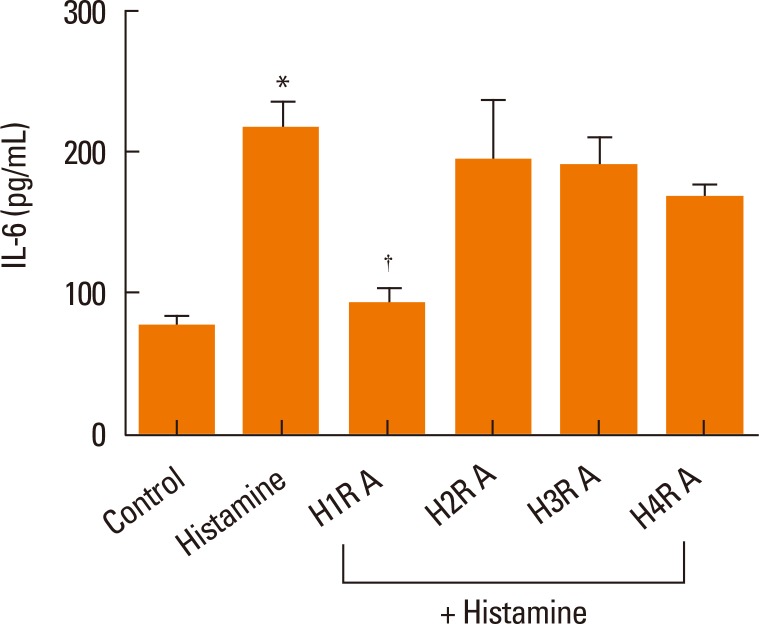
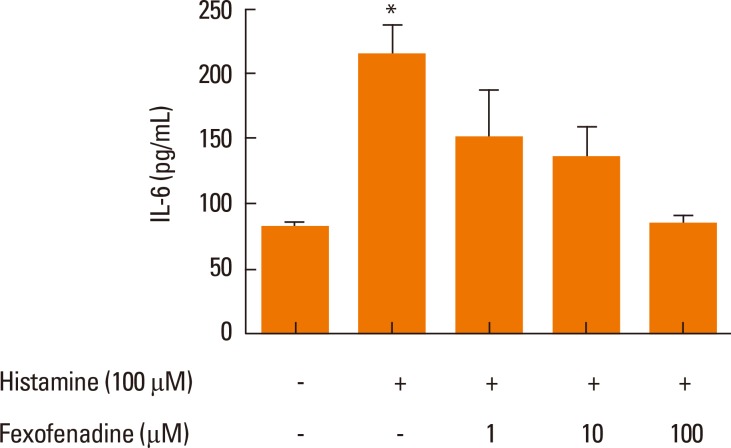
To identify the histamine receptors involved in IL-6 production, nasal fibroblasts were pretreated with histamine receptor antagonists for H1R (fexofenadine, 100 µM), H2R (ranitidine, 50 µM), H3R (clobenpropit, 50 µM), and H4R (JNJ7777120, 100 µM) for 2 hours, and then stimulated with histamine (100 µM) for 24 hours. Fexofenadine significantly decreased IL-6 production, whereas ranitidine, clobenpropit, and JNJ7777120 had no effect (Fig. 3). These assays were then expanded to assess the role of fexofenadine concentration on IL-6 production. Cells were pretreated with 1, 10, or 100 µM fexofenadine for 1 hour and then stimulated with histamine (100 µM) for 24 hours. Fexofenadine inhibited the expression of IL-6 protein in a dose-dependent manner (Fig. 4).
Fig. 3.
The effect of histamine receptor antagonists on IL-6 production in histamine-stimulated nasal fibroblasts was determined by ELISA. Nasal fibroblasts were pretreated with antagonists for H1R (H1RA, fexofenadine), H2R (H2RA, ranitidine), H3R (H3RA clobenpropit), and H4R (H4RA, JNJ7777120) for 2 hours prior to histamine (100 µM) stimulation. Values are expressed as the means±SE of independent experiments. *P<0.05 vs control. †P<0.05 vs histamine alone.
Fig. 4.
A dose-response analysis of H1R antagonist fexofenadine on IL-6 production was determined by ELISA. Values are expressed as the means±SE of independent experiments. *P<0.05 vs control.
Effect of an H1R antagonist on mitogen-activated protein kinase (MAPK) activity
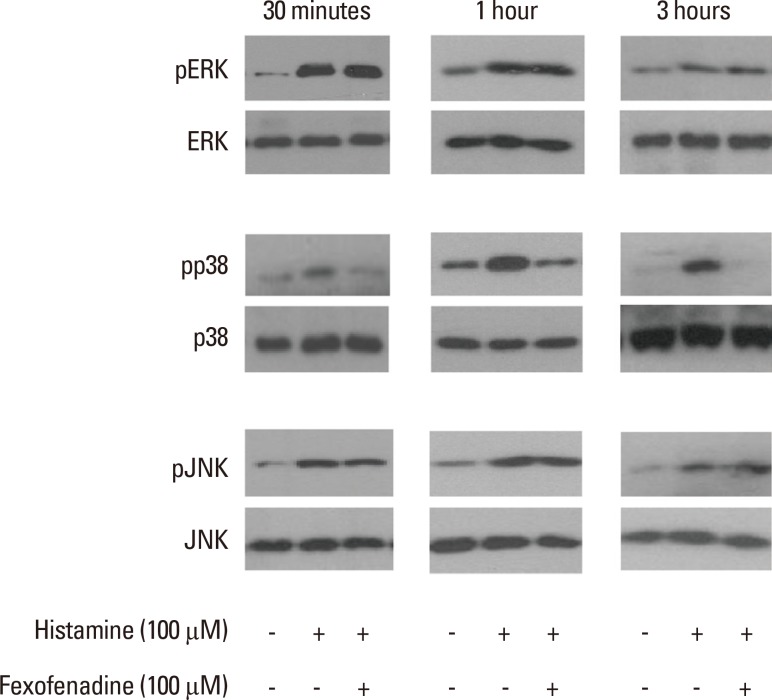
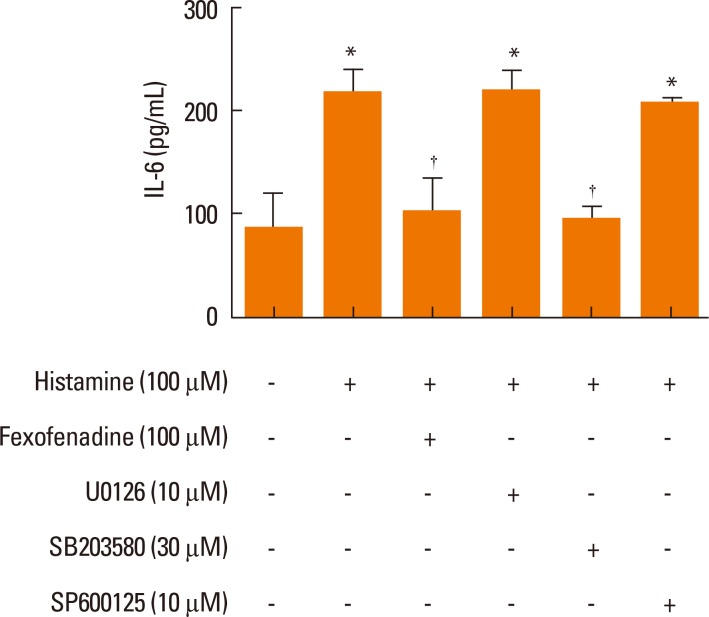
To explore the effect of fexofenadine on MAPK signaling pathways in histamine-stimulated nasal fibroblasts, 3 MAP kinases (p38, extracellular signal-regulated kinase [ERK], and c-Jun N-terminal kinase [JNK]) were evaluated by Western blotting. Cells were pretreated with fexofenadine (100 µM) for 1 hour, and then stimulated with histamine (100 µM) for 24 hours. Histamine increased the expression of phosphorylated p38 (pp38), pERK, and pJNK. Fexofenadine effectively blocked pp38 activation in histamine-induced nasal fibroblasts, but had no effect on either pERK or pJNK, indicating that the effect of fexofenadine on histamine-induced IL-6 production was mediated by the p38 pathway (Fig. 5). Inhibitors of ERK (U0126, 10 µM), p38 (SB203580, 30 µM), and JNK (SP600125, 10 µM) were used as positive controls.
Fig. 5.
The effect of H1R antagonist fexofenadine on the phosphorylation of mitogen-activated protein kinases (ERK, p38, JNK) was evaluated by Western blotting (representative of independent experiments).
U0126, ERK inhibitor; SB203580, p38 inhibitor; SP600125, JNK inhibitor.
Effect of p38 inhibition on IL-6 production in histamine-stimulated nasal fibroblasts
Next, we confirmed the effect of p38 inhibition on IL-6 production in histamine-stimulated nasal fibroblasts by ELISA. Cells were pretreated with fexofenadine for 1 hour, and then stimulated with histamine for 24 hours. Increased expression of IL-6 following histamine-stimulation was markedly suppressed in the presence of 30 µM SB203580 (Fig. 6). Neither U0126 nor SP600125 inhibited IL-6 production in histamine-stimulated nasal fibroblasts, consistent with our Western blot results.
Fig. 6.
Effect of p38 inhibition on IL-6 production in histamine-stimulated nasal fibroblasts. Values are expressed as the means±S.E. *P<0.05 vs control. †P<0.05 vs histamine alone.
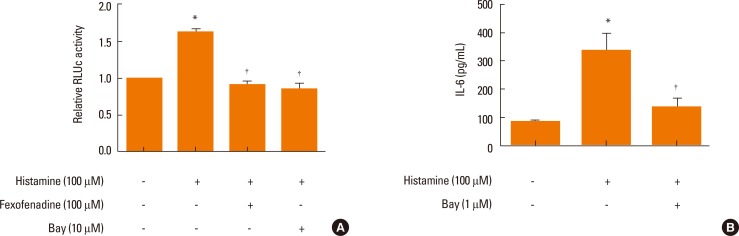
Effect of an H1R antagonist on IL-6 production and NF-κB expression in histamine-stimulated nasal fibroblasts
Histamine regulated HBp17/FGFBP-1 expression via the NF-κB binding site was evaluated using a luciferase reporter assay in which luciferase activity was driven by the HBp17/FGFBP-1 promoter. We observed a significant increase in HBp17/FGFBP-1 promoter activity following exposure to histamine (Fig. 7A). To confirm the effect of NF-κB inhibition on IL-6 production, we examined whether inhibition of NF-κB (Bay, 1 µM) could prevent histamine-induced IL-6 production in nasal fibroblasts using an ELISA. Cells were pretreated with fexofenadine and Bay for 1 hour, and then stimulated with histamine for 24 hours. Bay significantly blocked the increased production of IL-6 in histamine-stimulated nasal fibroblasts (Fig. 7B), consistent with a role for NF-κB in IL-6 production.
Fig. 7.
Effects of an H1R antagonist on NF-κB expression (A) and IL-6 production (B) in histamine-stimulated nasal fibroblasts. Values are expressed as the means±SE. *P<0.05 vs control. †P<0.05 vs histamine alone.
DISCUSSION
Here, we examined the effect of histamine on nasal fibroblasts in vitro. Our results indicate a clear link between histamine signaling and IL-6 production in nasal fibroblasts, mediated primarily through H1R. Of the 4 selective histamine receptor antagonists tested, only fexofenadine, an H1R antagonist, inhibited production of IL-6. These effects were mediated via the p38 MAPK and NF-κB pathways. The p38 MAPK inhibitor SB203580, and the NF-κB inhibitor Bay, significantly diminished the effect of histamine on IL-6 secretion, while the selective blockage of H1R inhibited the activation of p38 MAPK and NF-κB. In contrast, JNK and ERK inhibitors showed no effect on IL-6 production in nasal fibroblasts.
Degranulation of mast cells is the most critical initiating event in acute allergic reactions.13 Mediators produced by mast cells can be divided into preformed mediators, newly synthesized lipid mediators, and cytokines/chemokines.14 Of these, histamine is one of the most potent preformed mediators. Exposure to allergen results in the explosive degranulation of histamine from mast cells, leading to itching, sneezing, nasal discharge, and swelling in the nose due to its effects on smooth muscle, endothelial cells, nerve endings, and mucous secretion.15 However, histamine also modulates immune responses by interacting with inflammatory cells in ways that extend beyond the above effects.16 For example, histamine stimulation of H2R on peripheral monocytes suppresses IL-12 production and stimulates IL-10 secretion.17 Histamine also induces production of granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-8, and IL-6 in native human epidermal keratinocytes.18 In this study of nasal fibroblasts, IL-6 production was stimulated by histamine via H1R.
Fibroblasts are the most abundant cell type in normal connective tissue. The most well-known roles of fibroblasts are synthesis, degradation, and remodeling of the extracellular matrix in both wound repair and scar formation in many different tissues.19 However, fibroblasts also possess a number of other functional properties.20 One of these is production of cytokines, including GM-CSF, IL-1α, IL-1β, IL-6, IL-8, and IL-10.21 In this study, we demonstrated that fibroblasts produce IL-6 in response to histamine. The implications of this work are significant in terms of our understanding of allergy and infection. The network of interactions between inflammatory cells and structural components in the microenvironment is thought to result in chronic inflammation in upper airway inflammatory diseases, such as allergic rhinitis, with fibroblasts acting as a key source of cytokines and chemokines.22
IL-6 is a multifunctional cytokine with a wide range of biological activities in immune regulation, hematopoiesis, inflammation, and oncogenesis.24 It is produced by a number of cell types, including fibroblasts, macrophages, dendritic cells, T and B lymphocytes, endothelial cells, glial cells, and keratinocytes in response to a variety of external stimuli.6 There is abundant evidence that the immunomodulatory function of IL-6 is closely related to allergic response. In one study, asthmatic patients showed higher plasma IL-6 concentrations than normal controls.9 In examining the cytokine production of the nasal mucosa, a significant increase in IL-6 was found in nasal lavage samples of allergic rhinitis.25,26 Although more work is needed to fully define the role of IL-6, control of Th1/Th2 differentiation is strongly suggested.27
In conclusion, we showed that IL-6 production was stimulated by histamine via H1R followed by downstream activation of p38 MAPK and NF-κB. This is the first study demonstrating the effects of histamine and H1R on IL-6 production in nasal fibroblasts. These results suggest that antihistamines are likely to affect multiple cytokines, such as IL-6, in addition to blocking the more common histamine effects associated with allergic inflammation. Further testing, including in vivo studies, will be required to verify these findings.
ACKNOWLEDGMENTS
This study was funded by the Bumsuk Academic Scholarship Foundation.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Howarth PH, Salagean M, Dokic D. Allergic rhinitis: not purely a histamine-related disease. Allergy. 2000;55(Suppl 64):7–16. doi: 10.1034/j.1398-9995.2000.00802.x. [DOI] [PubMed] [Google Scholar]
- 2.Mygind N, Secher C, Kirkegaard J. Role of histamine and antihistamines in the nose. Eur J Respir Dis Suppl. 1983;128:16–20. [PubMed] [Google Scholar]
- 3.Shirasaki H, Kanaizumi E, Seki N, Himi T. Localization and upregulation of the nasal histamine H1 receptor in perennial allergic rhinitis. Mediators Inflamm. 2012;2012:951316. doi: 10.1155/2012/951316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rotrosen D, Gallin JI. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J Cell Biol. 1986;103:2379–2387. doi: 10.1083/jcb.103.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 6.Adkinson NF, Jr, Bochner BS, Busse WW, Holgate ST, Lemanske RF, Jr, Simons FE. Middleton's allergy: principles and practice. 7th ed. New York (NY): Mosby/Elsevier; 2009. [Google Scholar]
- 7.Ohshima S, Saeki Y, Mima T, Sasai M, Nishioka K, Nomura S, Kopf M, Katada Y, Tanaka T, Suemura M, Kishimoto T. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci U S A. 1998;95:8222–8226. doi: 10.1073/pnas.95.14.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doganci A, Sauer K, Karwot R, Finotto S. Pathological role of IL-6 in the experimental allergic bronchial asthma in mice. Clin Rev Allergy Immunol. 2005;28:257–270. doi: 10.1385/CRIAI:28:3:257. [DOI] [PubMed] [Google Scholar]
- 9.Wong CK, Ho CY, Ko FW, Chan CH, Ho AS, Hui DS, Lam CW. Proinflammatory cytokines (IL-17, IL-6, IL-18, and IL-12) and Th cytokines (IFN-γ, IL-4, IL-10, and IL-13) in patients with allergic asthma. Clin Exp Immunol. 2001;125:177–183. doi: 10.1046/j.1365-2249.2001.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Triggiani M, Gentile M, Secondo A, Granata F, Oriente A, Taglialatela M, Annunziato L, Marone G. Histamine induces exocytosis and IL-6 production from human lung macrophages through interaction with H1 receptors. J Immunol. 2001;166:4083–4091. doi: 10.4049/jimmunol.166.6.4083. [DOI] [PubMed] [Google Scholar]
- 11.Delneste Y, Lassalle P, Jeannin P, Joseph M, Tonnel AB, Gosset P. Histamine induces IL-6 production by human endothelial cells. Clin Exp Immunol. 1994;98:344–349. doi: 10.1111/j.1365-2249.1994.tb06148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denburg JA, Gauldie J, Dolovich J, Ohtoshi T, Cox G, Jordana M. Structural cell-derived cytokines in allergic inflammation. Int Arch Allergy Appl Immunol. 1991;94:127–132. doi: 10.1159/000235343. [DOI] [PubMed] [Google Scholar]
- 13.Galli SJ. New concepts about the mast cell. N Engl J Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- 14.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Repka-Ramirez MS, Baraniuk JN. Histamine in health and disease. Clin Allergy Immunol. 2002;17:1–25. [PubMed] [Google Scholar]
- 16.Falus A, Merétey K. Histamine: an early messenger in inflammatory and immune reactions. Immunol Today. 1992;13:154–156. doi: 10.1016/0167-5699(92)90117-p. [DOI] [PubMed] [Google Scholar]
- 17.Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, Wilder RL. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol. 1998;161:2586–2593. [PubMed] [Google Scholar]
- 18.Matsubara M, Tamura T, Ohmori K, Hasegawa K. Histamine H1 receptor antagonist blocks histamine-induced proinflammatory cytokine production through inhibition of Ca2+-dependent protein kinase C, Raf/MEK/ERK and IKK/IκB/NF-κB signal cascades. Biochem Pharmacol. 2005;69:433–449. doi: 10.1016/j.bcp.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Bousquet J, Jacot W, Vignola AM, Bachert C, Van Cauwenberge P. Allergic rhinitis: a disease remodeling the upper airways? J Allergy Clin Immunol. 2004;113:43–49. doi: 10.1016/j.jaci.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 20.Evans RA, Tian YC, Steadman R, Phillips AO. TGF-beta1-mediated fibroblast-myofibroblast terminal differentiation-the role of Smad proteins. Exp Cell Res. 2003;282:90–100. doi: 10.1016/s0014-4827(02)00015-0. [DOI] [PubMed] [Google Scholar]
- 21.Pang G, Couch L, Batey R, Clancy R, Cripps A. GM-CSF, IL-1α, IL-1β, IL-6, IL-8, IL-10, ICAM-1 and VCAM-1 gene expression and cytokine production in human duodenal fibroblasts stimulated with lipopolysaccharide, IL-1α and TNF-α. Clin Exp Immunol. 1994;96:437–443. doi: 10.1111/j.1365-2249.1994.tb06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denburg JA, Dolovich J, Ohtoshi T, Cox G, Gauldie J, Jordana M. The microenvironmental differentiation hypothesis of airway inflammation. Am J Rhinol. 1990;4:29–32. [Google Scholar]
- 23.Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A, Tsunasawa S, Sakiyama F. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 24.Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22:347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 25.Bachert C, Hauser U, Prem B, Rudack C, Ganzer U. Proinflammatory cytokines in allergic rhinitis. Eur Arch Otorhinolaryngol. 1995;252(Suppl 1):S44–S49. doi: 10.1007/BF02484434. [DOI] [PubMed] [Google Scholar]
- 26.Gosset P, Malaquin F, Delneste Y, Wallaert B, Capron A, Joseph M, Tonnel AB. Interleukin-6 and interleukin-1α production is associated with antigen-induced late nasal response. J Allergy Clin Immunol. 1993;92:878–890. doi: 10.1016/0091-6749(93)90066-o. [DOI] [PubMed] [Google Scholar]
- 27.Diehl S, Rincón M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002;39:531–536. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]