Abstract
Objectives
Measurement of the incidence of the human immunodeficiency virus (HIV) is very important for epidemiological studies. Here, we determined the recency period with the AxSYM avidity assay and the BED-capture enzyme immunoassay (BED-CEIA) in Korean seroconverters.
Methods
Two hundred longitudinal specimens from 81 seroconverters with incident HIV infections that had been collected at the Korea National Institute of Health were subjected to the AxSYM avidity assay (cutoff = 0.8) and BED-CEIA (cutoff = 0.8). The statistical method used to estimate the recency period in recent HIV infections was nonparametric survival analyses. Sensitivity and specificity were calculated for 10-day increments from 120 days to 230 days to determine the recency period.
Results
The mean recency period of the avidity assay and BED-CEIA using a survival method was 158 days [95% confidence interval (CI), 135–181 days] and 189 days (95% CI, 170–208 days), respectively. Based on the use of sensitivity and specificity, the mean recency period for the avidity assay and BED-CEIA was 150 days and 200 days, respectively.
Conclusion
We determined the recency period to estimate HIV incidence in Korea. These data showed that the nonparametric survival analysis often led to shorter recency periods than analysis of sensitivity and specificity as a new method. These findings suggest that more data from seroconverters and other methodologies are needed to determine the recency period for estimating HIV incidence.
Keywords: avidity assay, BED-CEIA, HIV incidence, Korea, recency period, seroconverter
1. Introduction
Measurement of the incidence of the human immunodeficiency virus (HIV) is very important for epidemiological studies because it can provide basic data for: (1) determining which medication to use, (2) detecting changes in patterns of HIV infection, and (3) evaluating the effects of education programs on HIV prevention in vulnerable groups. However, the traditional method of estimating incidence by following longitudinal cohorts is time-consuming and expensive.
As a result, for some time, several attempts have been made to calculate HIV incidence using cross-sectional studies [1]. The United States Centers for Disease Control and Prevention (CDC) conducts laboratory-based surveillance on HIV incidence on a national level by developing diagnostic kits for identifying recent infections and using a formula to estimate HIV incidence with data collected from its Serologic Testing Algorithm for Recent HIV Seroconversion project [2].
After a person is infected with HIV, a certain amount of time must pass before a threshold amount of antibody is produced. That time interval is called the recency period and is considered to be a key variable for estimation of HIV incidence [3]. Recency periods vary among individuals and by race, so a study to estimate the recency period should be conducted first to accurately measure HIV incidence in the Korean population [4].
The recency period, for the purpose of a particular test, is the mean time interval between the estimated time of HIV infection and an arbitrary time at which the given threshold of the assay is attained. In the current study, we estimated the mean recency period to determine HIV incidence in Korea using longitudinal data and specimens from seroconverters whose time of HIV infection could be estimated reliably.
2. Materials and methods
2.1. Specimens
We looked at HIV seroconverters who were designated as “indeterminate” in the initial HIV antibody test, but who later converted to being “positive” in the repeat test among HIV-infected individuals detected from 2005 to 2012. Among them, 81 individuals with at least two specimens—classified as “indeterminate specimens” prior to the confirmation of HIV infection and the confirmatory positive specimen—were finally selected. We also included their specimens, which were collected for immunoassay prior to treatment after they were confirmed to be HIV-positive. A total of 200 specimens from these 81 individuals were used. The largest number of specimens from a single individual was four and the mean number of specimens per individual was 2.5.
2.2. Collection of specimens from HIV seroconverters
In South Korea, if a sample is HIV-positive in a screening laboratory, the sample is referred to the Institute for Health and the Environment (IHE) for confirmatory testing. For samples for which the IHE cannot make a definitive diagnosis, a designation of “indeterminate” is assigned, and the sample sent to the Division of AIDS of the Korea National Institute of Health (KNIH) for testing. If the KNIH test result remains “indeterminate”, a notification is made to the screening laboratory to instruct it to collect another blood sample after a certain period of time, and to send it to the KNIH. The notified laboratory sends the next blood sample directly to the KNIH for retesting [5]. Through such processes, the KNIH identified HIV seroconverters and collected their data and specimens.
2.3. Test methods
Two assays were used in the current study to estimate recency periods: AxSYM avidity assay and BED-capture enzyme immunoassay (BED-CEIA). The avidity assay is designed to measure antibody avidity using a modified fourth-generation enzyme-linked immunosorbent assay (ELISA). When a reactive specimen is treated with 1.5 M guanidine, hydrogen bonds are destroyed, which inhibits secondary reactions [6]. The specimens were assayed by automated AxSYM HIV Ag/Ab Combo (Abbott Diagnostics Division, Wiesbaden, Germany). We defined avidity to be the ratio of the strength of antigen–antibody (Ag–Ab) binding in specimens treated with guanidine to that in specimens not treated with guanidine [6,7]. For the BED-CEIA, normalized optical density (OD-n) for each specimen was calculated based on the principle that HIV-specific immunoglobulin (Ig)G can be detected by a plate coated with goat-antihuman-IgG and the BED-Biotin peptide gp41 [8]. The stated cutoff value in the kit insert of the commercially available BED-CEIA was 0.8. The cutoff value for the avidity assay was set at 0.8 in a study conducted to develop avidity assays for HIV-infected persons in Korea [9].
2.4. Determination of the recency period
The term recency period was used in the current study to refer to the time span between HIV infection and a specified assay cutoff. A total of 200 specimens from 81 persons with incident HIV infections were tested by the avidity assay and BED-CEIA. In both assays, we estimated the expected time for the OD-n level in HIV-positive individuals to reach the 0.8 cutoff. All analyses were done using the SAS software (version 9.3; SAS Institute, Inc., Cary, NC).
2.5. Estimation of the recency period using statistical method
The Kaplan–Meier method [10] was applied to the results of the avidity assay and BED-CEIA. We used a cutoff value of 0.8 for the avidity assay and BED-CEIA for censoring in the Kaplan–Meier analysis. A nonparametric method was used because it was assumed that recency periods do not form a normal distribution. The cumulative probability that the test results would be lower than the cutoff value for a certain period of time was calculated to estimate the mean recency period. This coincides with the time when the test value will surpass the cutoff value. For each of the 81 patients, the date of first collection of the blood specimen was referred to as “time since HIV infection” (“HIV infection period”). Based on the patterns of the Ag–Ab response found in the test results of the first specimen, patients were categorized into one of six laboratory stages of primary HIV infection [11,12]. According to the Fiebig classification, the period of HIV infection is divided into seven stages based on the emergence of viral markers such as HIV RNA, ELISA antigen, ELISA antibody, and Western blot. That is: eclipse phase (10 days), stage I (17 days), stage II (22 days), stage III (25 days), stage IV (31 days), stage V (101 days), and stage VI (open-ended).
2.6. Estimation of the recency period using sensitivity and specificity
Specimens with results of the avidity assay and BED-CEIA of ≤0.8 (cutoff) were regarded to be recent infections. With 10-day periods between 120 days and 230 days since the first HIV-positive test, we distinguished recent infection from long-standing infection. From the data, sensitivity and specificity were calculated to estimate the recency period. Sensitivity was defined as the proportion of specimens at or below the threshold day for recent infection among specimens classified as recent infections by new assays looking at incidence. Specificity was defined as the proportion of specimens above the threshold day for recent infection among specimens classified as long-standing infections by new assays looking at incidence. We took into account sensitivity and specificity to make an optimal estimation of the recency period.
3. Results
3.1. Test results
Some of the basic information about the 81 patients (sex; age at the time of diagnosis of HIV infection; year of confirmation of HIV diagnosis; stages of infection) is shown in Table 1. Ninety-seven percent of the patients were male. For most of the patients, infection was detected when they were aged in their 20s (31%) or 30s (28%). Twenty-six individuals (32%) were identified between 2011 and 2012, whereas 23 individuals (28%) were reported between 2009 and 2010. The number of patients in stage II (22 days after infection) at the time of the first positive test was 33 (41%). The number of patients in stage IV (31 days after infection) was 38 (47%). Except for two patients, 79 (98%) were estimated to be in the 1st month of HIV infection. In 49 individuals, immunoassays performed within 6 months after HIV-positive test showed a CD4+ cell count of <200 in 16.7% of the cases and 350–499 in 35.4% of the cases and the mean CD4+ cell count was 369. The mean results of the avidity assay and BED-CEIA of indeterminate specimens were 0.311 and 0.065, respectively. The mean tracking duration for individuals was 92 days (data not shown).
Table 1.
Characteristics of HIV seroconverters (N = 81) in South Korea.a
| Number of persons (%) | |
|---|---|
| Sex | |
| Male | 77 (95.1) |
| Female | 4 (4.9) |
| Age (y) | |
| <20 | 4 (4.9) |
| 20–29 | 26 (32.1) |
| 30–39 | 21 (25.9) |
| 40–49 | 15 (18.5) |
| ≥50 | 14 (17.3) |
| Unknown | 1 (1.2) |
| Year | |
| 2005–2006 | 15 (18.5) |
| 2007–2008 | 17 (21.0) |
| 2009–2010 | 23 (28.4) |
| 2011–2012 | 26 (32.1) |
| Stage | |
| Eclipse phase (10 d) | – |
| I (17 d) | 2 (2.5) |
| II (22 d) | 33 (40.7) |
| III (25 d) | 6 (7.4) |
| IV (31 d) | 38 (46.9) |
| V (101 d) | 2 (2.5) |
| VI (open-ended) | – |
| Initial CD4+cell countb | |
| < 200 | 8 (16.7) |
| 200–349 | 13 (27.1) |
| 350–499 | 17 (35.4) |
| ≥500 | 11 (22.9) |
HIV = human immunodeficiency virus.
Age denotes the age at the time of diagnosis of HIV infection; Year denotes the year of confirmation of HIV diagnosis; and CD4+ cell counts denotes the data from immunoassay results undertaken <6 mo after HIV-positive confirmation test.
These percentages were calculated after excluding samples with missing values.
3.2. Estimation of the recency period
3.2.1. Estimation of the recency period using survival analyses
Figure 1 shows how the results of the avidity assay and BED-CEIA changed in each of the 81 test patients in relation to time since HIV infection. The avidity assay was carried out on 191 specimens from 79 patients after excluding two patients with only one longitudinal observation each. All 200 specimens from 81 patients were tested with the BED-CEIA. Table 2 contains the results of Kaplan–Meier analyses undertaken on each set of assay results to estimate the recency period. The recency period of the avidity assay was 158 days [95% confidence interval (CI); 135–181 days]. The recency period of the BED-CEIA was estimated to be 189 days (95% CI; 170–208 days).
Figure 1.
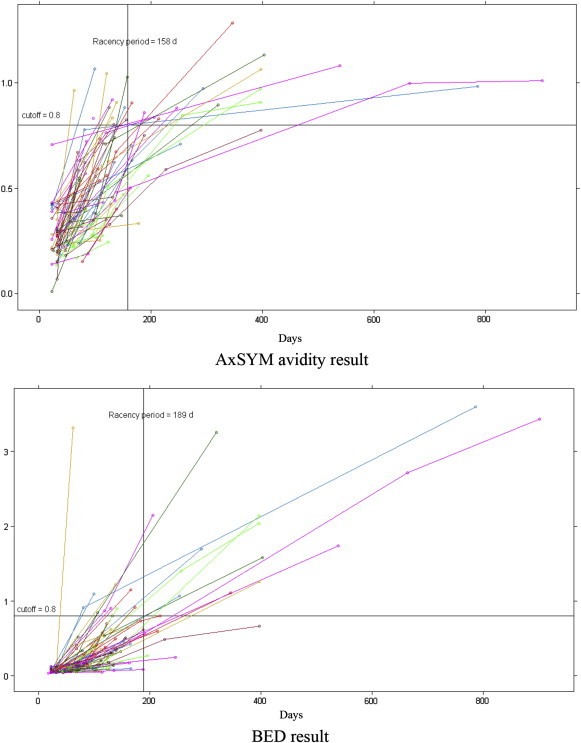
Distribution in results of the AxSYM avidity index and BED-capture enzyme immunoassay by human immunodeficiency virus seroconverters.
Table 2.
Mean recency period at the AxSYM avidity assay and BED-capture enzyme immunoassay.
| Assay | Cutoff value | Mean recency period (d) | 95% CI | |
|---|---|---|---|---|
| Avidity | 0.8 | 158 | 135 | 181 |
| BED-CEIA | 0.8 | 189 | 170 | 208 |
CEIA = capture enzyme immunoassay; CI = confidence interval.
3.2.2. Estimation of the recency period using sensitivity and specificity
We distinguished recent infection from long-standing infection by rearranging recency periods into 10-day intervals from 120 days to 230 days based on the calculation of the time interval between HIV infection and until the cutoff value was reached (Table 3). Sensitivity and specificity for individual recency periods are also shown in Table 3. When sensitivity and specificity were considered together and both values reached the highest values two values, the recency periods in the avidity assay and in BED-CEIA, were 150 days and 200 days, respectively.
Table 3.
Use of different recency periods of the AxSYM avidity assay and BED-capture enzyme immunoassay to identify recent human immunodeficiency virus infections.
| Recency period (d) | Avidity assay |
BED-CEIA |
||
|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | |
| 120 | 82.0 | 86.2 | 79.5 | 80.8 |
| 130 | 86.5 | 75.9 | 84.8 | 76.9 |
| 140 | 91.0 | 69.0 | 88.9 | 69.2 |
| 150 | 92.5 | 69.0 | 90.1 | 65.4 |
| 160 | 93.2 | 58.6 | 92.4 | 65.4 |
| 170 | 95.5 | 55.2 | 94.2 | 61.5 |
| 180 | 96.2 | 55.2 | 94.7 | 57.7 |
| 190 | 97.0 | 51.7 | 96.5 | 57.7 |
| 200 | 97.7 | 51.7 | 97.7 | 57.7 |
| 210 | 97.7 | 48.3 | 97.7 | 53.8 |
| 220 | 97.7 | 44.8 | 98.2 | 50.0 |
| 230 | 98.5 | 44.8 | 98.8 | 50.0 |
CEIA = capture enzyme immunoassay.
4. Discussion
In the current study, we estimated the recency period by analyzing specimens from HIV seroconverters in the Korean population to establish the optimal recency period for the measurement of HIV incidence in Korea. Assay results for new incidence were subjected to Kaplan–Meier analyses and to assessments using sensitivity and specificity. The results of Kaplan–Meier analyses suggested that the recency periods of the avidity assay and BED-CEIA were 158 days and 189 days, respectively. Based on the use of sensitivity and specificity, the recency periods for the avidity assay and BED-CEIA were 150 days and 200 days, respectively.
The recency period of the BED-CEIA (which is currently in widespread use for the detection of early HIV infection) is 155 days [8]. Recently, the US CDC added new patients to predict the recency period of the BED-CEIA, and estimated it to be 197 days, which is longer than before. This was because of the inclusion of more races and subtypes in the specimens, providing proof that the recency period can differ depending on the characteristics of test patients. Even in patients with the same subtype, recency periods change among different races [1].
Recency periods can also differ depending on the assay used and the method of data analysis. The recency period of a less sensitive assay is 129 days (95% CI, 109–149 days) [9] whereas that of the avidity assay is 141 days (95% CI, 119–160 days) [10]. The same phenomenon was observed in the current study because the recency period of the BED-CEIA was longer than that of the avidity assay. With regard to the method of data analyses, the linear mixed effects (LME) regression model generated a slightly longer recency period than Kaplan–Meier analyses [176 days (95% CI, 164–188 days) versus 162 days (95% CI, 152–172 days)], which highlights the need to choose an appropriate analysis method that fits data characteristics [1].
Given that recency periods can vary depending on race, HIV subtype, the assay, and method of data analyses, calculating an optimal recency period that can accommodate the characteristics of the test centers of a given country is important. Thus, on the basis that the patients are of Korean race and have subtype-B infection, we calculated the mean recency period using two assays and two methods of analyses. Considering the relatively small number of seroconverter specimens available in Korea and the shorter tracking period, we decided to undertake Kaplan–Meier analyses. In addition, sensitivity and specificity were calculated for each recency period after an assumption about recency periods was made. In general, sensitivity and specificity are used to determine the type of assay kit and cutoff value of the test. Moreover, most of our data tended to show high sensitivity and low specificity because of short infection periods and, considering that the BED-CEIA produces many false-positive results, we prioritized specificity over sensitivity in determination of the recency period.
There was no significant difference between the recency periods determined by survival analyses and by sensitivity and specificity. The recency period of the BED-CEIA at a cutoff value of 0.8 differed between 189 days (95% CI, 170–208 days) and 200 days depending on the analysis methods used. In both cases, the recency period was similar to the 197-day period set by the US CDC upon consideration of more recent findings. Our analyses regarding the estimation of recency period had two advantages. First, in most cases, the estimated date of HIV infection was taken to be the midpoint between the last HIV-negative test and the first HIV-positive test after tracking the test history of each individual. However, we estimated the date of HIV infection by looking at the level of Ag–Ab response at the time of the first HIV-positive test rather than studying the patient's test history and, thus, could reduce the error margin of prediction significantly. Second, for estimation of recency periods, we tried to use the analysis of sensitivity and specificity as a new method.
The current study had three main limitations. First, selection bias occurred in choosing 81 patients among 342 seroconverters detected in Korea until 2012. Also, the number of test patients was small. Second, because of the limited number of longitudinal observations per patient, we could not use the LME regression model, which is a parametric method. Third, with 84% of specimens concentrated in recency periods of ≤5 months, only a small number of specimens could be classified as late infections. This led to a lower specificity than sensitivity because specificity was defined as the ability to correctly detect longstanding HIV infections.
Despite these limitations, however, the current study was important because it was the first attempt to determine the recency period in Korea and allowed for the measurement of HIV incidence in this country.
Conflicts of interests
The authors declare that they have no competing interests.
Acknowledgments
The current study was supported by the project “Estimation of HIV incidence based on the recent HIV infection assays in Korea (2011-N51001-00)” of Intramural Research Grant from the Korea National Institute of Health.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Parekh B.S., Hanson D.L., Hargrove J. Determination of mean recency period for estimation of HIV type 1 incidence with the bed-capture EIA in persons infected with diverse subtypes. AIDS Res Hum Retroviruses. 2011 Mar;27(3):265–273. doi: 10.1089/aid.2010.0159. [DOI] [PubMed] [Google Scholar]
- 2.Prejean J., Song R., Hernandez A. HIV Incidence Surveillance Group. Estimated HIV incidence in the United States, 2006–2009. PLoS One. 2011 Aug;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parekh B.S., Hu D.J., Vanichseni S. Evaluation of a sensitive/less-sensitive testing algorithm using the 3A11-LS assay for detecting recent HIV seroconversion, among individuals with HIV-1 subtype B or E infection in Thailand. AIDS Res Hum Retroviruses. 2001 Mar;17(5):453–458. doi: 10.1089/088922201750102562. [DOI] [PubMed] [Google Scholar]
- 4.Parekh B.S., Kennedy M.S., Dobbs T. Quantitative detection of increasing HIV type I antibodies after seroconversion: a simple assay for detecting recent HIV infection and estimating incidence. AIDS Res Hum Retroviruses. 2002 Mar;18(4):295–307. doi: 10.1089/088922202753472874. [DOI] [PubMed] [Google Scholar]
- 5.Korea Centers for Diseases Control and Prevention. HIV/AIDS Guideline. Korea Centers for Disease Control and Prevention; Osong: 2012. [Google Scholar]
- 6.Wang J.S., Kim N.Y., Sim H.J. The Korean Society for AIDS; 2012. Establishment of modified serological testing for the diagnose of recent HIV infections to estimate HIV incidence in Korea. [Google Scholar]
- 7.Suligoi B., Galli C., Massi M. Precision and accuracy of a procedure for detecting recent human immunodeficiency virus infections by calculating the antibody avidity index by an automated immunoassay-based method. J Clin Microbiol. 2002 Nov;40(11):4015–4020. doi: 10.1128/JCM.40.11.4015-4020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calypte HIV-1 BED incidence EIA (IgG-Capture HIV-EIA): enzyme immunoassay for population estimates of HIV-1 incidence. Calypte Biomedical Corporation; Portland, Oregon: 2005. [Google Scholar]
- 9.Kim N.Y., Yu H.K., Wang J.S. Congress of the Korean Society for Chemotherapy and the Korean Society of Infectious Diseases. 2013 Oct. Approach for avidity index assay to detect recent HIV infection in Korea. abstract no.D1. [Google Scholar]
- 10.Kalbfleisch J.D., Prentice R.L. John Wiley & Sons, Inc.; New York: 1980. The statistical analysis of failure time data. [Google Scholar]
- 11.Levy J.A. 3rd ed. ASM Press; Washington: 2007. HIV and the Pathogenesis of AIDS. [Google Scholar]
- 12.Keele B.F., Giorgi E.E., Salazar-Gonzalez J.F. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008 May;105(21):7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]


