Abstract
Objective
We previously demonstrated that carboxypeptidase B (CPB) protects against joint erosion in rheumatoid arthritis by inactivating complement component C5a. We also found that levels of CPB are abnormally high in the synovial fluid of individuals with another joint disease, osteoarthritis (OA). In this study, we investigated whether CPB has a role in the pathogenesis of OA.
Methods
We compared the development of OA in CPB-deficient (Cpb2−/−) mice and wild-type mice by subjecting them to medial meniscectomy and four months later histologically assessing cartilage damage, osteophyte formation, and synovitis in the mouse stifle joints. We measured levels of proCPB, proinflammatory cytokines, and complement components in synovial fluid samples from patients with symptomatic and radiographic knee OA. Finally, we used ELISA, flow cytometry, and hemolytic assays to assess the effect of CPB on formation of membrane attack complex (MAC)—a complement effector critical to OA pathogenesis.
Results
Cpb2−/− mice developed dramatically greater cartilage damage than wild-type mice (P<0.01) and had a greater number of osteophytes (P<0.05) and degree of synovitis (P<0.05). In synovial fluids from OA patients, high levels of proCPB were associated with high levels of proinflammatory cytokines and complement components, and levels of proCPB correlated positively with those of MAC. In in vitro complement activation assays, activated CPB suppressed the formation of MAC as well as MAC-induced hemolysis.
Conclusions
Our data suggest that CPB protects against inflammatory destruction of the joints in OA, at least in part by inhibiting complement activation.
INTRODUCTION
Carboxypeptidase B (CPB; also known as thrombin-activatable fibrinolysis inhibitor) is a basic carboxypeptidase that cleaves C-terminal basic residues (arginine or lysine) from protein and peptide substrates. It is encoded by the Cpb2 gene and produced primarily by the liver as a circulating plasma zymogen (proCPB) and becomes activated by the thrombin-thrombomodulin complex during thrombotic events. CPB was initially described as a fibrinolysis inhibitor because it can remove C-terminal lysines from partially digested fibrin and thereby reduce the binding of plasminogen and tissue plasminogen activator to the fibrin clot. CPB has since been shown to also cleave and inactivate several inflammatory proteins, namely C5a, C3a, bradykinin, and thrombin-cleaved osteopontin1. Its ability to modulate inflammatory substrates suggests that CPB may also function to suppress inflammation. Consistent with this notion, we previously found that CPB protects against rheumatoid arthritis by dampening C5a-mediated inflammation in synovial joints2.
Although historically viewed as a non-inflammatory degenerative disease, OA is also accompanied by low-grade inflammation in the joints3. Indeed, we recently discovered that the inflammatory complement system is critical to the pathogenesis of OA, with deficiency in the central complement component C5 or in a component of the downstream MAC (membrane attack complex) effector attenuating arthritis in mouse models of OA4. Here we investigate the role of CPB in OA by using mouse models of OA, in vitro analyses of human OA synovial fluid, and in vitro complement activation assays.
Materials and Methods
Surgical induction of mouse OA
We performed mouse studies under protocols approved by the Stanford Committee of Animal Research and in accordance with National Institutes of Health guidelines. Medial meniscectomy was performed as described5 on 16-week-old, male CPB-deficient mice (Cpb2−/−) or age-matched C57BL/6J control mice.
Histologic assessment of OA development in mice
Stifle joints from Cpb2−/− and control mice were stained with toluidine blue. Cartilage degeneration, osteophyte formation, and synovitis were evaluated as described4.
Measurement of proCPB, complement, and cytokines in OA synovial fluid
Synovial fluid samples were obtained from knee OA patients over age 45 and with radiographic KL score ≥3 under protocols approved by the Stanford Institutional Review Board (IRB) and with informed consent. ProCPB levels were measured by the Zymutest TAFI-Ag ELISA kit (Aniara). For measurement of soluble MAC (sC5b-9) and C3a, we used the BD OptEIA Human C5b-9 and Microvue C3a Plus EIA kits (Quidel). Analysis of cytokines was performed as described6.
Quantitation of sMAC by ELISA
10% normal human serum (NHS) was incubated with 20nM of activated CPB (American Diagnostica) in Hank’s balanced salt solution with or without 25μM of potato carboxypeptidase inhibitor (Sigma), and sMAC measured by ELISA.
Immunofluorescence visualization of sMAC adsorption
Glass coverslips were allowed to react with either 10% untreated NHS or NHS pre-treated with 35nM of activated CPB. Coverslips were washed, then stained with anti-human C5b-9 (Dako) or isotype control followed by Alexa Fluor 488-conjugated anti-mouse IgG (Invitrogen). Thirty-six high-powered fields per sample were imaged, and C5b-9 complexes with an area >0.1μm2 counted with ImageJ.
Flow cytometric analysis of C5b-9 deposition
Huh7 hepatoma cells were incubated in either 25% NHS or NHS pretreated with 35nM of activated CPB, and cells stained with anti-human C5b-9 followed by Alexa Fluor 488-conjugated anti-mouse IgG.
Hemolysis assay
Unsensitized rabbit erythrocytes were resuspended in gelatin veronal buffer (GVB) and incubated at 37°C for 60 minutes in 10% serum from 1-year-old Cpb2−/− or age-matched mice (n=5 per group) with or without bovine cartilage extract (Sigma) in GVB/MgEGTA buffer. Positive (100% lysis; water) and negative (0% lysis; GVB/MgEGTA buffer) controls were included. Absorbance of the supernatants was measured at 412nm, and hemolysis calculated relative to the positive control.
RESULTS
CPB protects mice against surgically induced OA
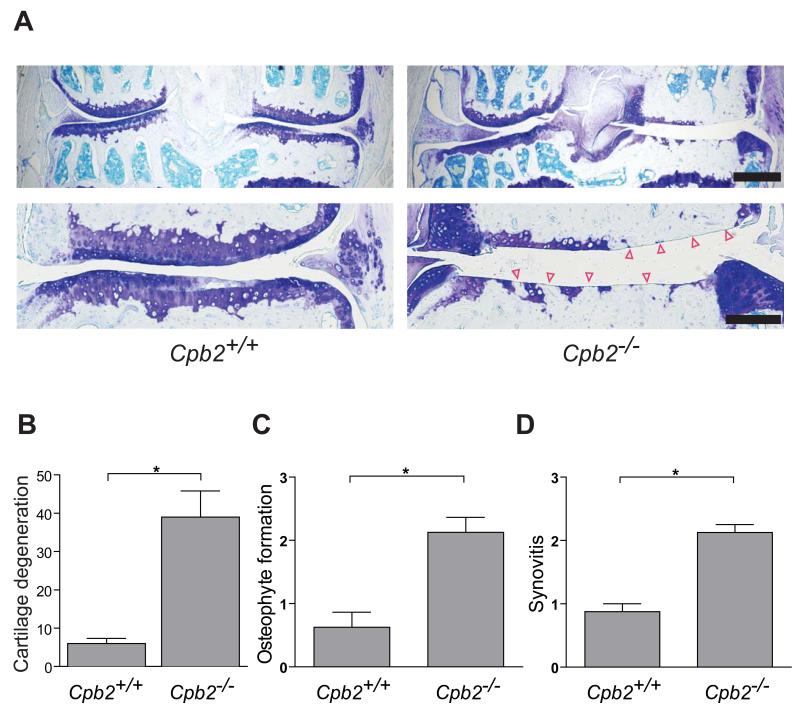
We used the medial meniscectomy mouse model of OA5 to investigate the role of CPB in the pathogenesis of OA. We performed medial meniscectomy on CPB-deficient (Cpb2−/−) mice and their wild-type counterparts and, sixteen weeks later, evaluated OA-like pathology in sections of their stifle joints. Compared to the operated joints of age-matched control mice, those of Cpb2−/− mice had a much greater degree of cartilage loss, osteophyte formation, and synovitis (Figure 1). These findings suggest that CPB protects against the development and progression of OA in mice.
Figure 1. ProCPB deficiency exacerbates OA in mice.
(A) Representative toluidine-blue-stained sections of the stifle joints of CPB-deficient (Cpb2−/−; backcrossed >9 generations to the C57BL/6J background) and wild-type C57BL/6J control mice (Cpb2+/+) surgically induced to develop OA by medial meniscectomy. Arrowheads indicate areas of total cartilage loss. Scale bar for the low (top) magnification images, 500 μm; scale bar for the high (bottom) magnification images, 200 μm. (B) Histologic quantification of cartilage degeneration, osteophyte formation, and synovitis in (A). Data represent the mean ± s.e.m; Cpb2−/−, n=4; Cpb2+/+, n=5). *P≤0.05 by Mann-Whitney test.
ProCPB levels correlate with levels of proinflammatory mediators in human osteoarthritic synovial fluid
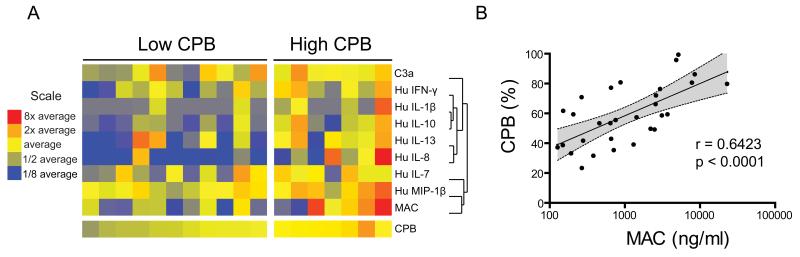
Levels of proinflammatory mediators are abnormally high in the synovial fluid of individuals with OA2. Because CPB negatively regulates local inflammatory responses by enzymatically cleaving and inactivating its proinflammatory substrates1, we investigated the association between levels of proCPB and levels of proinflammatory mediators in OA synovial fluid. To this end, we performed ELISA and multiplexed bead-based immunoassays to quantitate proCPB, proinflammatory cytokines, chemokines, and complement components in OA synovial fluid. Using the mean level of proCPB across all OA synovial fluid samples, we stratified the samples into low- and high-proCPB groups. Heatmap display of the levels of proinflammatory mediators identified by SAM as differentially expressed between the high- and low-proCPB groups showed that levels of several proinflammatory mediators, including IL-1β, IFNγ, IL-8, and macrophage inflammatory protein-1 beta, are higher in the high-proCPB group (Figure 2A). Levels of IL-10 and IL-13, cytokines with anti-inflammatory properties, were also higher in the high-proCPB group (2A). In the setting of chronic inflammation, such as in the OA joint, CPB may act in concert with these regulatory cytokines to prevent the destructive effects incurred by sustained proinflammatory activity.
Figure 2. Levels of proCPB correlate positively with levels of inflammatory mediators in OA synovial fluid.
(A) Heatmap display of inflammatory mediators whose levels differ significantly between OA synovial fluids from individuals with high levels of proCPB and those with low levels of proCPB (FDR<4.1%; Significance analysis of microarrays was used for identifying statistically significant differences). High- and low-proCPB groups were formed based on the mean proCPB level of all analyzed OA synovial fluids (64.8%). In the heatmap, blue represents a decrease relative to the mean value obtained in samples from OA patients, yellow no change, and red an increase. Cytokine and chemokine levels were measured with a multiplex bead-based immunoassay. ProCPB, C3a, and MAC were measured by ELISA. Heatmap columns represent individual OA patients, and rows represent individual inflammatory mediators. (B) Pearson’s correlation analysis of paired proCPB and MAC measurements in synovial fluid from each individual OA patient, with proCPB concentrations expressed as a percentage of that observed in normal pooled plasma. C3a, complement component 3a; MAC, membrane attack complex (C5b-9); IFNγ, interferon gamma; IL, interleukin; MIP-1β, macrophage inflammatory protein-1 beta.
Two effector components of the complement cascade, namely the anaphylatoxin C3a and the MAC (C5b-9), were significantly upregulated in OA synovial fluids with high levels of proCPB. Indeed, by analyzing paired values of proCPB and MAC for each synovial fluid sample, we found that levels of proCPB correlate positively with levels of MAC (Figure 2B, Pearson r=0.64, P<0.0001). This strong correlation suggests that CPB may control inflammation by regulating complement activation.
CPB inhibits MAC formation and MAC-mediated cell lysis
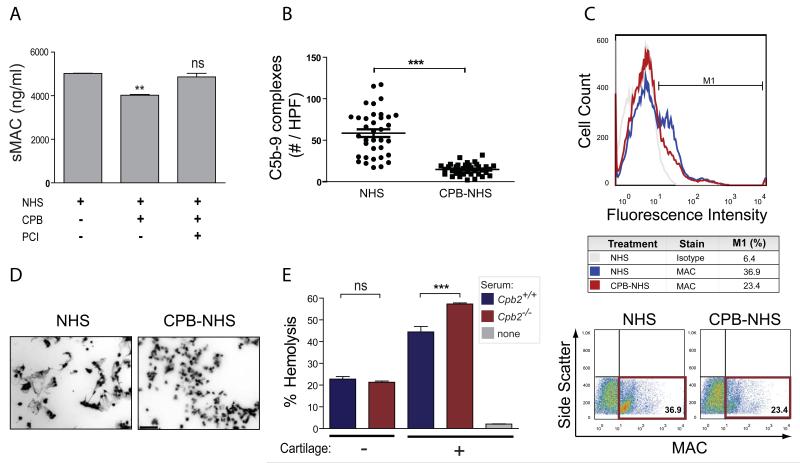
We previously reported a critical role for complement, in particular the MAC-mediated effector arm of the complement cascade, in the pathogenesis of OA4. Mice genetically deficient in complement effectors C5 or C6 (two components of MAC) are protected against surgically induced OA, whereas mice deficient in the MAC inhibitor CD59 suffer greater cartilage loss and accelerated OA development. Given the importance of MAC in OA pathogenesis4 and the strong correlation between levels of proCPB and MAC in OA synovial fluid (Figure 2B), we investigated whether inhibition of MAC assembly might be one mechanism by which CPB exerts its protective effects in OA. To evaluate the role of CPB in complement activation, we preincubated NHS with activated CPB and assessed its ability to activate complement relative to that of NHS that had not been preincubated with CPB (Figure 3A). CPB treatment suppressed soluble MAC (sMAC) formation in NHS (Figure 3A), an effect that was abolished by inactivation of CPB with potato carboxypeptidase inhibitor (PCI) (3A). We validated the in vitro formation of sMAC by immunostaining for C5b-9 complexes bound to glass coverslips that were allowed to react with either CPB-treated or untreated NHS. As predicted, markedly fewer C5b-9 complexes formed on glass coverslips incubated with CPB-treated NHS than on those incubated with untreated NHS (Figure 3B).
Figure 3. CPB inhibits complement activation in serum.
(A) ELISA analysis of soluble MAC (sMAC) formation in untreated normal human serum (NHS) or NHS treated with CPB or with CPB inactivated with potato carboxypeptidase inhibitor (PCI). Data are the mean ± s.d. of triplicates. (B) Immunostaining analysis of C5b-9 complexes (MAC) adsorbed to hydrophilic glass incubated with samples of untreated NHS or CPB-treated NHS (CPB-NHS). Number of complexes per 36 non-overlapping high-power fields are shown. (C) Flow cytometric analysis of MAC deposition on the surface of Huh7 cells incubated with 25% untreated NHS or CPB-NHS. Cells were stained with anti-C5b-9 or mouse IgG2a isotype control, followed by a goat anti-mouse IgG-488 secondary. Representative dot plots and percentages of MAC+ cells are shown. (D) DAPI (4′,6-diamidino-2-phenylindole) staining of nuclei in Huh7 cells incubated with untreated NHS or CPB-NHS. Cells incubated with CPB-NHS contain intact nuclei, whereas cells incubated with untreated NHS contain elongated nuclei with dispersed chromatin fibers. Scale bar, 100 m. (E) Hemolysis of rabbit red blood cells by 10% (v/v) sera from CPB-deficient mice or C57BL/6J wild-type mice in the presence or absence of cartilage extract. *P≤0.05, *** P≤0.001 by one-way ANOVA with Bonferroni post-hoc correction. Data are the mean ± s.d. of triplicates and representative of three or more independent experiments.
Upon activation of the complement cascade, MAC readily forms channels in the membranes of target cells, resulting in either cell signaling or cell lysis, depending on the concentration of MAC7. We therefore evaluated the ability of CPB to regulate MAC assembly at the surface of Huh7 hepatocarcinoma cells. Consistent with our previous findings with sMAC (Figures 3A and B), MAC deposition was markedly lower on Huh7 cells incubated with CPB-treated NHS than on Huh7 cells incubated with untreated NHS (Figure 3C). Moreover, Huh7 cells incubated with CPB-treated NHS contained intact nuclei, in stark contrast to the disrupted appearance of nuclei from cells incubated with untreated NHS (Figure 3D), suggesting that CPB inhibits MAC-mediated cell lysis.
To further confirm our findings, we compared the ability of serum from Cpb2−/− mice and serum from wild-type C57BL/6J mice to activate complement in a modified AH50 assay. In this assay, complement activity was determined by measuring the hemolysis of rabbit red blood cells (RBCs) after addition of CPB-deficient or wild-type mouse sera. There was no significant difference in hemolytic activity when RBCs reacted with CPB-deficient or wild-type sera alone (Figure 3E). However, when we added cartilage ECM components to the serum to increase complement activation above that attained spontaneously, sera from CPB-deficient mice lysed RBCs more efficiently than did sera from wild-type mice. A hallmark of cartilage degeneration in OA is the breakdown and progressive loss of extracellular matrix (ECM) components into the synovial space, and some of these ECM components can induce formation of MAC from serum complement components4. These data suggest that circulating CPB exerts its anti-inflammatory or cytotoxic effects at least in part by suppressing or limiting complement activation triggered by cartilage ECM components.
DISCUSSION
In this study, we show that CPB is important in protecting against the development of OA and that it may do so, at least in part, by inhibiting complement activation in the synovial joints. We found that (i) genetic deficiency in CPB exacerbated OA in a mouse model; (ii) high levels of CPB are associated with high levels of proinflammatory cytokines in the synovial fluid of individuals with OA, and levels of CPB correlate positively with levels of MAC in these samples; and (iii) CPB inhibits the formation and activity of MAC in vitro.
While traditionally considered a plasma protein, proCPB is also produced and secreted by fibroblast-like synoviocytes (FLS)8. Moreover, thrombomodulin, a critical cofactor for thrombin-mediated activation of proCPB, is expressed on FLS8. These findings suggest that locally produced and activated CPB may act in concert with CPB from plasma exudates into the joint space to control inflammation in OA.
Several of the proinflammatory mediators that were associated with high levels of CPB are known to be secreted by multiple cell types in response to signaling by CPB substrates (e.g., C5a signaling through C5aR induces the production of IL-1 and IL-8). Because CPB cleavage of its substrates reduces their proinflammatory activity, the observed association suggests that levels of CPB may increase in OA synovial fluid as part of a homeostatic counter-regulatory mechanism to suppress inflammation in OA joints.
Our finding that levels of proCPB correlated positively with levels of MAC is particularly interesting in light of our previous finding that MAC has a critical role in OA pathogenesis4. We now show that treating human serum with activated CPB reduces the formation of soluble and cell-surface MAC, resulting in a reduction in RBC death. We propose that CPB-mediated inhibition of MAC in synovial joints likewise attenuates MAC-induced killing of synovial lining and cartilage cells. In addition, sublytic levels of the MAC can stimulate production of cytokines and other proinflammatory mediators7—including many of those associated with high levels of proCPB in OA synovial fluid—and thus suppression of MAC-induced inflammation could be another way in which CPB protects against joint destruction in OA.
CPB could conceivably inhibit MAC formation in several different ways. It could do so by suppressing activation of the alternative complement pathway, which is activated spontaneously when the highly reactive thioester bond of complement component C3b reacts with a hydroxyl or amine group on a protein’s surface. By cleaving C-terminal lysines1, CPB removes reactive primary amines from proteins and could in this way eliminate potential C3b-binding sites and thereby suppress activation of the alternative pathway. One candidate for such a target protein is fibrin, which contains many C-terminal lysines, is a known CPB substrate1, and can activate complement9. Indeed, fibrin deposition is a prominent finding in OA joint tissues10, and complement, including MAC, co-localizes with fibrin deposits at sites of tissue injury11. Another way in which CPB-mediated removal of C-terminal lysines could suppress activation of the alternative complement pathway is by generating a net negative charge at the protein surface: polyanions are known to strongly inhibit the alternative complement pathway12. Additionally, C-terminal lysines of fibrin act as docking sites for plasminogen, a fibrinolytic protein recently identified as an inhibitor of both the alternative and the classical complement pathways13. Cleavage of fibrin by CPB would preclude the binding of plasminogen to fibrin, leaving plasminogen free to interact with and inhibit complement components.
The mouse medial meniscectomy model is relevant to human OA because humans who have undergone medial meniscectomy have a significantly increased risk of developing OA14. Further, arthroscopic partial meniscectomy in humans is associated with chemokine-driven inflammation15.
Our findings suggest that CPB serves as an important anti-inflammatory mediator that protects against inflammatory joint destruction following injury or iatrogenic removal of the meniscus. Thus, we propose that CPB downregulates inflammation and cytotoxicity in synovial joints, and that it does so in part by suppressing the formation of MAC.
Financial support
C.M.L. is supported by the Stanford Medical Scientist Training Program (MSTP). J.J.S.’s work on this project was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A2005003). J.S. is supported by a VA Career Development Award. This work was also supported by a VA Research Rehabilitation and Development (RR&D) Award, NIH NIAID R01-AI085268, and NIH NHLBI Proteomics Center N01-HV-00242 to W.H.R.
Footnotes
Conflict of interest: The authors declare no competing interests.
REFERENCES
- 1.Leung LL, Myles T, Nishimura T, Song JJ, Robinson WH. Regulation of tissue inflammation by thrombin-activatable carboxypeptidase B (or TAFI) Mol Immunol. 2008;45(16):4080–3. doi: 10.1016/j.molimm.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song JJ, Hwang I, Cho KH, Garcia MA, Kim AJ, Wang TH, et al. Plasma carboxypeptidase B downregulates inflammatory responses in autoimmune arthritis. J Clin Invest. 2011;121(9):3517–27. doi: 10.1172/JCI46387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213(3):626–34. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q, Rozelle AL, Lepus CM, Scanzello CR, Song JJ, Larsen DM, et al. Identification of a central role for complement in osteoarthritis. Nature medicine. 2011;17(12):1674–9. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadri A, Funck-Brentano T, Lin H, Ea HK, Hannouche D, Marty C, et al. Inhibition of bone resorption blunts osteoarthritis in mice with high bone remodelling. Ann Rheum Dis. 2010;69(8):1533–8. doi: 10.1136/ard.2009.124586. [DOI] [PubMed] [Google Scholar]
- 6.Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther. 2012;14(1):R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohana-Kashtan O, Ziporen L, Donin N, Kraus S, Fishelson Z. Cell signals transduced by complement. Mol Immunol. 2004;41(6-7):583–97. doi: 10.1016/j.molimm.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Sharif SA, Du X, Myles T, Song JJ, Price E, Lee DM, et al. Thrombin-activatable carboxypeptidase B cleavage of osteopontin regulates neutrophil survival and synoviocyte binding in rheumatoid arthritis. Arthritis Rheum. 2009;60(10):2902–12. doi: 10.1002/art.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oikonomopoulou K, Ricklin D, Ward PA, Lambris JD. Interactions between coagulation and complement--their role in inflammation. Seminars in immunopathology. 2012;34(1):151–65. doi: 10.1007/s00281-011-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberg JB, Pippen AM, Greenberg CS. Extravascular fibrin formation and dissolution in synovial tissue of patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 1991;34(8):996–1005. doi: 10.1002/art.1780340809. [DOI] [PubMed] [Google Scholar]
- 11.Rampersad R, Barton A, Sadovsky Y, Nelson DM. The C5b-9 membrane attack complex of complement activation localizes to villous trophoblast injury in vivo and modulates human trophoblast function in vitro. Placenta. 2008;29(10):855–61. doi: 10.1016/j.placenta.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiler JM, Linhardt RJ. Comparison of the activity of polyanions and polycations on the classical and alternative pathways of complement. Immunopharmacology. 1989;17(2):65–72. doi: 10.1016/0162-3109(89)90051-9. [DOI] [PubMed] [Google Scholar]
- 13.Barthel D, Schindler S, Zipfel PF. Plasminogen is a complement inhibitor. The Journal of biological chemistry. 2012;287(22):18831–42. doi: 10.1074/jbc.M111.323287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41(4):687–93. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Scanzello CR, McKeon B, Swaim BH, DiCarlo E, Asomugha EU, Kanda V, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 2011;63(2):391–400. doi: 10.1002/art.30137. [DOI] [PMC free article] [PubMed] [Google Scholar]