Abstract
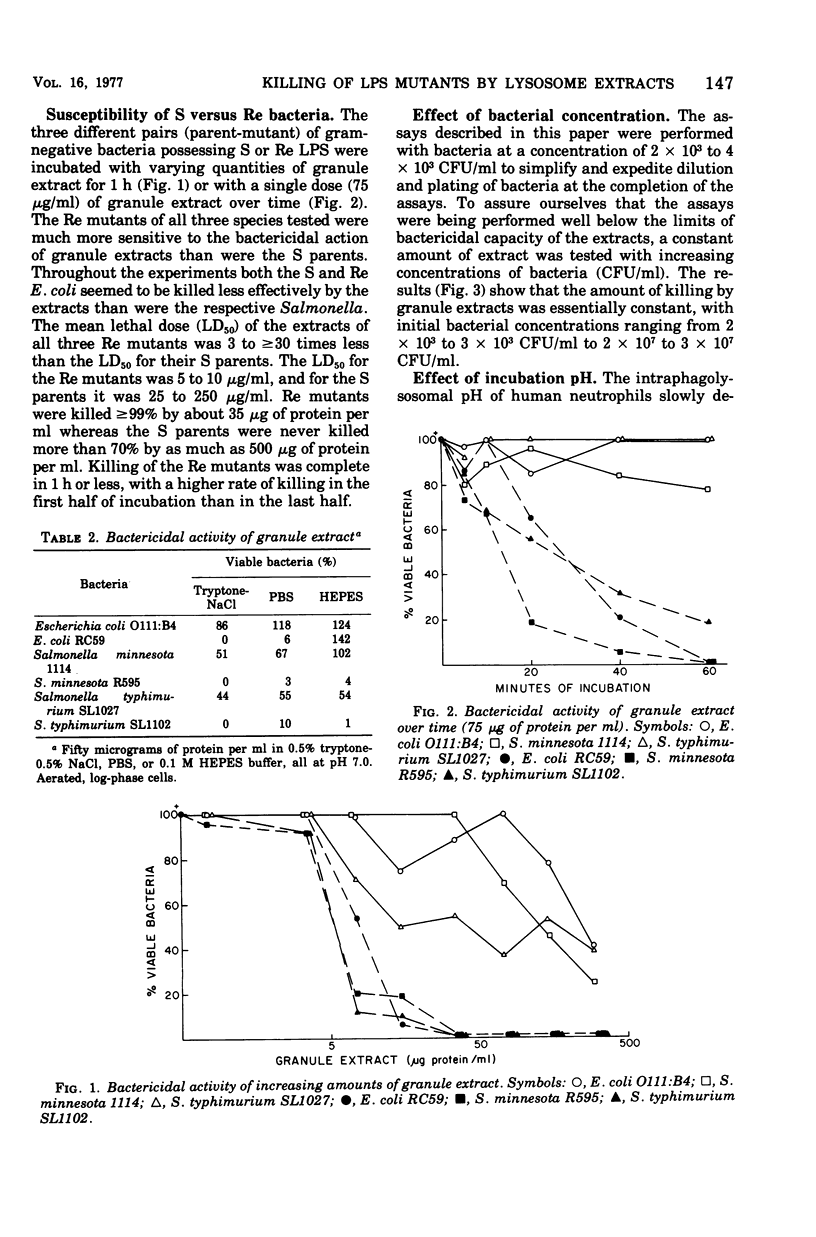
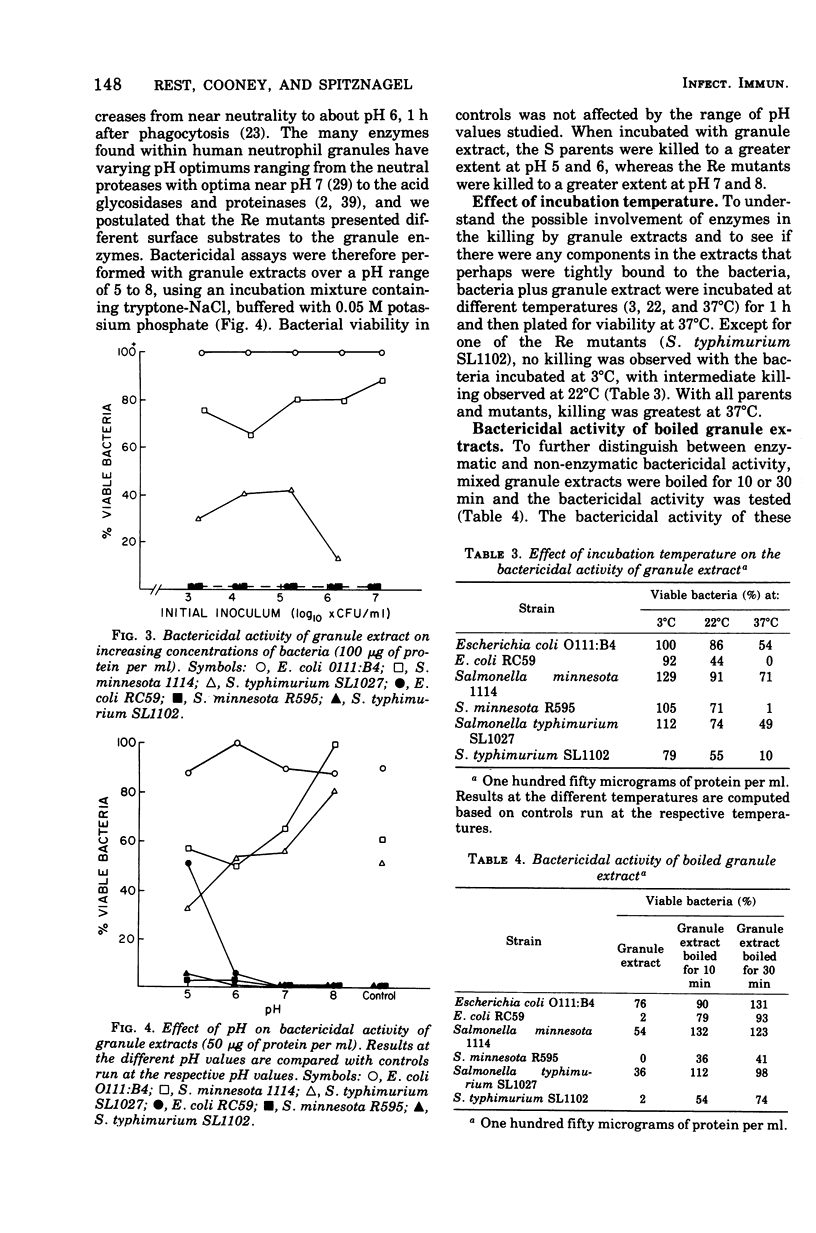
Acetate extracts of purified human neutrophil granules (a mixed population containing specific and azurophil granules) were dialyzed against phosphate-buffered saline (pH 7.0) and tested for bactericidal activity against smooth parent and rough mutant, gram-negative bacteria. Rough (Re) mutants of Escherichia coli, Salmonella typhimurium, and Salmonella minnesota were exquisitely more sensitive to extracts of human polymorphonuclear leukocyte granules than were their smooth (S) parents. The mean lethal dose (LD50) for the parent strains was 25 to 50 μg of granule protein per ml. As much as 500 μg of extract per ml failed to kill 100% of the S parents. The LD50 for the rough mutants was 1.5 to 2.0 μg of the same granule extract per ml; 100% killing occurred with 5 to 10 μg of lysosomal protein per ml. Conditions affecting the growth of the bacteria greatly affected their sensitivity to the granule extracts. Granule extract killed bacteria grown with aeration to log phase 10 to 15 times more efficiently than the same bacteria grown to stationary phase under static conditions. The bactericidal incubation mixture also influenced results, in that greater killing occurred with tryptone than with phosphate or N-2-hydroxyethyl piperazine-N′-2-ethanesulfonic acid-buffered saline. Bactericidal activity depended on lysosomal protein concentration, time, and temperature. Boiled lysosomal fractions failed to kill the S parents but retained 20 to 50% of their ability to kill the Re mutants. Parents (smooth) were killed more efficiently at pH 5 to 6, whereas their Re mutants were killed more efficiently at pH 7 to 8.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerdite S., Mooney C., Weiss J., Franson R., Elsbach P. Early and discrete changes in permeability of Escherichia coli and certain other gram-negative bacteria during killing by granulocytes. J Exp Med. 1974 Aug 1;140(2):396–409. doi: 10.1084/jem.140.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune K., Spitznagel J. K. Peroxidaseless chicken leukocytes: isolation and characterization of antibacterial granules. J Infect Dis. 1973 Jan;127(1):84–94. doi: 10.1093/infdis/127.1.84. [DOI] [PubMed] [Google Scholar]
- Elsbach P. On the interaction between phagocytes and micro-organisms. N Engl J Med. 1973 Oct 18;289(16):846–852. doi: 10.1056/NEJM197310182891610. [DOI] [PubMed] [Google Scholar]
- Friedberg D., Friedberg I., Shilo M. Interaction of Gram-Negative Bacteria with the Lysosomal Fraction of Polymorphonuclear Leukocytes II. Changes in the Cell Envelope of Escherichia coli. Infect Immun. 1970 Mar;1(3):311–318. doi: 10.1128/iai.1.3.311-318.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg D., Shilo M. Interaction of Gram-Negative Bacteria with the Lysosomal Fraction of Polymorphonuclear Leukocytes I. Role of Cell Wall Composition of Salmonella typhimurium. Infect Immun. 1970 Mar;1(3):305–310. doi: 10.1128/iai.1.3.305-310.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone G. P., Walton E. Effect of iron on the bactericidal proteins from rabbit polymorphonuclear leukocytes. Nature. 1970 Aug 22;227(5260):849–851. doi: 10.1038/227849a0. [DOI] [PubMed] [Google Scholar]
- Good R. A., Quie P. G., Windhorst D. B., Page A. R., Rodey G. E., White J., Wolfson J. J., Holmes B. H. Fatal (chronic) granulomatous disease of childhood: a hereditary defect of leukocyte function. Semin Hematol. 1968 Jul;5(3):215–254. [PubMed] [Google Scholar]
- Holmes B., Park B. H., Malawista S. E., Quie P. G., Nelson D. L., Good R. A. Chronic granulomatous disease in females. N Engl J Med. 1970 Jul 30;283(5):217–221. doi: 10.1056/NEJM197007302830501. [DOI] [PubMed] [Google Scholar]
- Janoff A., Blondin J. The effect of human granulocyte elastase on bacterial suspensions. Lab Invest. 1973 Oct;29(4):454–457. [PubMed] [Google Scholar]
- Janoff A., Blondin J. The role of elastase in the digestion of E. coli proteins by human polymorphonuclear leukocytes. I. Experiments in vitro. Proc Soc Exp Biol Med. 1974 Apr;145(4):1427–1430. doi: 10.3181/00379727-145-38027. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lahav M., Ne'eman N., Adler E., Ginsburg I. Effect of leukocyte hydrolases on bacteria. I. Degradation of 14C-labeled Streptococcus and Staphylococcus by leukocyte lysates in vitro. J Infect Dis. 1974 May;129(5):528–537. doi: 10.1093/infdis/129.5.528. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Functional aspects of a second mechanism of candidacidal activity by human neutrophils. J Clin Invest. 1972 Oct;51(10):2566–2572. doi: 10.1172/JCI107073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman M. B., Steward J. P., Roantree R. J. Characterization of the virulence and antigenic structure of Salmonella typhimurium strains with lipopolysaccharide core defects. Infect Immun. 1976 Jun;13(6):1539–1542. doi: 10.1128/iai.13.6.1539-1542.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L. Intraphagosomal pH of human polymorphonuclear neutrophils. Proc Soc Exp Biol Med. 1970 Jun;134(2):447–449. doi: 10.3181/00379727-134-34810. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XII. Hydrogen peroxide-myeloperoxidase bactericidal system in the phagocyte. J Bacteriol. 1967 Nov;94(5):1425–1430. doi: 10.1128/jb.94.5.1425-1430.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medearis D. N., Jr, Camitta B. M., Heath E. C. Cell wall composition and virulence in Escherichia coli. J Exp Med. 1968 Sep 1;128(3):399–414. doi: 10.1084/jem.128.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- Muschel L. H., Larsen L. J. The sensitivity of smooth and rough gram-negative bacteria to the immune bactericidal reaction. Proc Soc Exp Biol Med. 1970 Jan;133(1):345–348. doi: 10.3181/00379727-133-34472. [DOI] [PubMed] [Google Scholar]
- Ng A. K., Chang C. M., Chen C. H., Nowotny A. Comparison of the chemical structure and biological activities of the glycolipids of Salmonella minnesota R595 and Salmonella typhimurium SL1102. Infect Immun. 1974 Oct;10(4):938–947. doi: 10.1128/iai.10.4.938-947.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Antibacterial activity of cationic proteins from human granulocytes. J Clin Invest. 1975 Nov;56(5):1118–1124. doi: 10.1172/JCI108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. Inhibition of bacteria by lactoferrin and other iron-chelating agents. Biochim Biophys Acta. 1968 Dec 23;170(2):351–365. doi: 10.1016/0304-4165(68)90015-9. [DOI] [PubMed] [Google Scholar]
- Padgett G. A., Hirsch J. G. Lysozyme: its absence in tears and leukocytes of cattle. Aust J Exp Biol Med Sci. 1967 Oct;45(5):569–570. doi: 10.1038/icb.1967.56. [DOI] [PubMed] [Google Scholar]
- Penniall R., Spitznagel J. K. Chicken neutrophils: oxidative metabolism in phagocytic cells devoid of myeloperoxidase. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5012–5015. doi: 10.1073/pnas.72.12.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe E. A., Jones R. J. Intracellular killing of different strains of Pseudomonas aeruginosa by human leucocytes. Br J Exp Pathol. 1974 Aug;55(4):336–343. [PMC free article] [PubMed] [Google Scholar]
- SPRICK M. G. Phagocytosis of M. tuberculosis and M. smegmatis stained with indicator dyes. Am Rev Tuberc. 1956 Oct;74(4):552–565. doi: 10.1164/artpd.1956.74.4.552. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., MacAlister T., Costerton J. W., Cheng K. J. Permeability of lipopolysaccharide-deficient (rough) mutants of Salmonella typhimurium to antibiotics, lysozyme, and other agents. Can J Microbiol. 1974 Aug;20(8):1135–1145. doi: 10.1139/m74-176. [DOI] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Dalldorf F. G., Leffell M. S., Folds J. D., Welsh I. R., Cooney M. H., Martin L. E. Character of azurophil and specific granules purified from human polymorphonuclear leukocytes. Lab Invest. 1974 Jun;30(6):774–785. [PubMed] [Google Scholar]
- Tamaki S., Matsuhashi M. Increase in sensitivity to antibiotics and lysozyme on deletion of lipopolysaccharides in Escherichia coli strains. J Bacteriol. 1973 Apr;114(1):453–454. doi: 10.1128/jb.114.1.453-454.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Oliver R. C., Barrett A. J. Lysis and killing of bacteria by lysosomal proteinases. Infect Immun. 1976 Aug;14(2):555–563. doi: 10.1128/iai.14.2.555-563.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh I. R., Spitznagel J. K. Distribution of lysosomal enzymes, cationic proteins, and bactericidal substances in subcellular fractions of human polymorphonuclear leukocytes. Infect Immun. 1971 Aug;4(2):97–102. doi: 10.1128/iai.4.2.97-102.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Isolation of polymorphonuclear leukocyte granules from rabbit bone marrow. Lab Invest. 1971 Mar;24(3):237–245. [PubMed] [Google Scholar]


