Abstract
Age-related macular degeneration (AMD) is a common disease that can result in severe visual impairment. Abnormal regulation of the complement system has been implicated in its pathogenesis, and CFH polymorphisms contribute substantially to risk. How these polymorphisms exert their effects is poorly understood. We performed enzyme-linked immunosorbent assay (ELISA) analysis on young, aged, and AMD choroids to determine the abundance of the membrane attack complex (MAC) and performed immunofluorescence studies on eyes from 117 donors to evaluate the MAC in aging, early AMD, and advanced AMD. Morphometric studies were performed on eyes with high- or low-risk CFH genotypes. ELISA confirmed that MAC increases significantly with aging and with AMD. MAC was localized to Bruch’s membrane and the choriocapillaris and was detectable at low levels as early as 5 years of age. Hard drusen were labeled with anti-MAC antibody, but large or confluent drusen and basal deposits were generally unlabeled. Labeling of retinal pigment epithelium was observed in some cases of advanced AMD, but not in early disease. Eyes homozygous for the high-risk CFH genotype had thinner choroids than low-risk homozygotes (P < 0.05). These findings suggest that increased complement activation in AMD and in high-risk genotypes can lead to loss of endothelial cells in early AMD. Treatments to protect the choriocapillaris in early AMD are needed.
Age-related macular degeneration (AMD) is a complex disease that frequently results in loss of visual acuity. AMD, the most common cause of irreversible blindness in the elderly, is projected to affect 3 million Americans by 2020.1,2 Clinically, the early stages of AMD are characterized by structural abnormalities in the posterior pole that include an abnormal appearance of the retinal pigment epithelium (RPE) and the presence of drusen, which are extracellular deposits that form between the RPE and its blood supply, the choriocapillaris.3 Although for most patients early AMD does not progress to severe end-stage disease, a subset of patients will develop extensive degeneration of photoreceptor cells and RPE cells in the macula, caused by either pathological invasion of new blood vessels from the choroid into the sub-RPE or subretinal spaces [ie, choroidal neovascularization (CNV)] or by idiopathic loss of the macular RPE and photoreceptor cells (ie, geographic atrophy).
Although our understanding of the pathophysiology of AMD is incomplete, recent genome-wide association studies and candidate gene approaches have led to the identification of various polymorphisms that affect the risk of AMD at all stages. These include single-nucleotide polymorphisms in a number of genes encoding members and regulators of the complement pathway, including C3, CFI, C2 and/or CFB, and CFH (recently reviewed by Khandhadia et al4). One polymorphism in the CFH gene (rs1061170) increases risk of AMD by approximately twofold to sevenfold, depending on the population studied.5–8 This variant results in the substitution of histidine for tyrosine at amino acid residue 402. The effect of this polymorphism in the human eye is not well understood, although adults harboring the Y402H polymorphism show increased choroidal C-reactive protein9 and increased membrane attack complex (MAC).10
Formation of the MAC is the final event in the terminal portion of the complement cascade and results from the binding of C5b to plasma complement proteins C6, C7, C8, and multiple molecules of C9. MAC forms transmembrane channels that lead to cell lysis and death. The MAC has been found in drusen of older eyes with AMD.11 However, the relative abundance and distribution of MAC in aging, early AMD, and advanced AMD have not been comprehensively studied. Inhibition of MAC components such as C6 can inhibit CNV,12 and other complement pathway inhibitors are in active clinical trials for the treatment of AMD.13 Because it is the ultimate downstream effector of the complement pathway, understanding the role of the MAC in the pathophysiology of AMD is important for the development of new therapies.
We evaluated the MAC in a large series of donor eyes. MAC was present in Bruch’s membrane and choriocapillaris in very young eyes, but the concentration increased with age; we observed the highest levels in eyes with AMD. We further evaluated the MAC in a series of eyes from young and old donors, and from donors with early and advanced AMD. Although in early AMD the MAC is associated exclusively with the choriocapillaris, in advanced AMD the RPE may be exposed as well. Morphometric experiments suggest that high-risk CFH genotypes may contribute to thinning or atrophy of the choroid. Overall, these studies suggest that choroidal endothelial cells are targets of the MAC and that approaches to prevent their injury from complement-mediated lysis may be useful in the treatment of AMD.
Materials and Methods
Human Donor Eyes
Whole globes from human donors were obtained from the Iowa Lions Eye Bank (Iowa City, IA). Full consent for research was obtained from the donor’s next of kin in all cases, and all experiments were performed in accordance with the Declaration of Helsinki.
Eyes were processed within 9.5 hours of death (range, 1 hour 42 minutes to 9 hours 15 minutes). For biochemical studies, a 6-mm juxtamacular, inferotemporal punch was acquired. Neural retina and RPE–choroid layers were collected separately and snap-frozen in liquid nitrogen, before long-term storage at −80°C. Macular punches and/or superotemporal wedges were collected from each eye and preserved in 4% paraformaldehyde in phosphate-buffered saline within 8 hours of death. After 2 hours of fixation, eyes were washed in phosphate-buffered saline and then were cryoprotected in sucrose and embedded in sucrose–optimal cutting temperature medium, as described by Barthel and Raymond.14
Quantification of Soluble C5b-9/MAC
Samples were chosen for MAC quantification from a collection of frozen juxtamacular punches of RPE–choroid, centered approximately 7 mm temporal to the fovea. Ten RPE–choroid samples were selected from each of three groups: young (mean age, 39.6 years; range, 21 to 48 years); aged, with a clinical and/or histological diagnosis of dry AMD (mean age, 87.1 years; range, 77 to 99 years); and age-matched control, without AMD (mean age, 82.8 years; range, 71 to 96 years) (Table 1). Of the 30 samples studied, 2 samples in the AMD group were new punches from donor eyes reported previously.10 Samples were homogenized for 90 seconds using a Kontes disposable pestle (Thermo Fisher Scientific, Waltham, MA) and motorized tissue grinder (Sigma-Aldrich, St. Louis, MO) in 30 μL of phosphate-buffered saline with 1% Triton X-100 and protease inhibitors (cOmplete kit; Roche Diagnostics, Indianapolis, IN). Total protein concentration was determined using the Lowry method (Bio-Rad Laboratories, Hercules, CA); 30 μg of each sample was loaded in duplicate wells into a MAC enzyme-linked immunosorbent assay (ELISA) plate (MicroVue SC5b-9 Plus enzyme immunoassay kit; Quidel, San Diego, CA), and MAC levels were determined according to the manufacturer’s instructions.
Table 1.
Characteristics of Donor Eyes Used in Enzyme-Linked Immunosorbent Assay Study
| Donor | Donor age (years) | MAC concentration (ng/mL) | Cause of death | D–P time (hours) | Allele |
|
|---|---|---|---|---|---|---|
| Y402H | V62I | |||||
| Young (<50 years of age) | ||||||
| 1 | 23 | 2.75 | Methane asphyxia | 6.75 | HY | VV |
| 2 | 48 | 18.68 | Cancer | 6.5 | HY | VV |
| 3 | 45 | 15.67 | Respiratory failure | 6.5 | HY | VV |
| 4 | 21 | 30.71 | Hodgkin lymphoma | 6 | HY | VV |
| 5 | 34 | 26.78 | Intercranial hemorrhage | 6.5 | HY | VV |
| 6 | 46 | 4.46 | Cardiopulmonary arrest | <8 | YY | IV |
| 7 | 46 | 45.34 | Pneumonia | 2.75 | YY | II |
| 8 | 43 | 34.19 | Lung cancer, renal failure | 5.25 | HY | IV |
| 9 | 46 | 19.97 | Renal disease | 6.5 | HY | VV |
| 10 | 45 | 1.64 | Pneumonia | 6.75 | YY | IV |
| Aged control, without AMD | ||||||
| 11 | 77 | 38.77 | Pulmonary embolism | 5.5 | YY | VV |
| 12 | 71 | 28.72 | Intracerebral hemorrhage | 7 | HY | IV |
| 13 | 82 | 83.68 | Heart failure | 5.5 | YY | II |
| 14 | 83 | 68.07 | Respiratory failure | 5.75 | YY | IV |
| 15 | 96 | 30.16 | Respiratory failure | 7 | HY | IV |
| 16 | 85 | 47.80 | Metastatic bladder cancer | 4.75 | HY | VV |
| 17 | 79 | 52.29 | Congestive heart failure | 5.25 | HY | IV |
| 18 | 95 | 43.95 | Stroke | 7 | YY | VV |
| 19 | 82 | 49.05 | NA | 6 | YY | II |
| 20 | 78 | 43.72 | Lung cancer | 6 | YY | IV |
| Aged AMD | ||||||
| 21 | 89 | 185.44 | NA | 4.5 | HH | VV |
| 22 | 88 | 221.13 | Myocardial infarction | 6 | HH | VV |
| 23 | 99 | 26.96 | Stroke | 5.75 | YY | IV |
| 24 | 78 | 113.91 | Respiratory failure | 5 | HH | VV |
| 25∗ | 77 | 89.88 | NA | 6 | HH | VV |
| 26∗ | 85 | 114.65 | Acute coronary syndrome | 7 | HY | VV |
| 27 | 82 | 40.67 | Stroke | 5.5 | HY | IV |
| 28 | 93 | 165.16 | Urosepsis | 5.5 | HY | VV |
| 29 | 89 | 70.34 | Congestive heart failure | 7.25 | HY | VV |
| 30 | 91 | 77.24 | Perforated bowel | 5.5 | HY | IV |
AMD, age-related macular degeneration; D–P time, maximum time between death and preservation of eye in liquid nitrogen; NA, not available; sC5B-9, soluble terminal complement complex.
Earlier punches from these donor eyes were analyzed in a previous study.10
Immunohistochemistry
Sections were collected on a cryostat and dual-labeled with antibodies directed against a neoepitope present in activated complement C9 that is exposed during formation of the MAC (anti–C5b-9 complex; Dako, Carpinteria, CA) and the vascular marker Ulex europaeus agglutinin I (UEA-I; Vector Laboratories, Burlingame, CA), which labels fucosylated glycoconjugates on viable human endothelial cells. MAC and endothelial cells were detected using Alexa Fluor 488–conjugated goat anti-mouse IgG (Life Technologies, Carlsbad, CA) and avidin–Texas Red (Vector Laboratories), respectively. Immunohistochemistry and lectin histochemistry were performed as described previously.9,15 Eyes from 117 donors were evaluated (Table 2).
Table 2.
Characteristics of Donor Eyes Used for Immunohistochemistry
| Donor ID | Age (years) | AMD Dx | Cause of death | D–P time (hours) |
|---|---|---|---|---|
| 1 | 0 | None | Cardiac arrest | 7:00 |
| 2 | 1 | None | Cardiac arrest | 7:00 |
| 3 | 5 | None | Myelogenous leukemia | 8:06 |
| 4 | 21 | None | Hemorrhagic pancreatitis/leukemia | 5:23 |
| 5 | 21 | None | Hodgkin lymphoma | 6:03 |
| 6 | 28 | None | Metastatic carcinoma | 4:12 |
| 7 | 41 | None | Ovarian cancer | 4:50 |
| 8 | 42 | None | Anaplastic T-cell lymphoma/sepsis | 4:12 |
| 9 | 48 | None | Septic shock | 5:53 |
| 10 | 50 | None | Adenocarcinoma/respiratory failure | 7:35 |
| 11 | 53 | None | Cardiac arrest | 6:30 |
| 12 | 57 | None | Cardiogenic shock/heart disease | 5:50 |
| 13 | 59 | None | Lymphoma | 4:59 |
| 14 | 60 | None | Sepsis | 7:45 |
| 15 | 62 | None | Cardiac arrest | 7:18 |
| 16 | 63 | None | Myocardial infarction | 5:06 |
| 17 | 65 | None | Renal failure | 4:56 |
| 18 | 66 | None | Unknown | 6:32 |
| 19 | 67 | None | Lung cancer | 4:22 |
| 20 | 67 | None | Sepsis | 5:50 |
| 21 | 68 | AMD: OS GA | NA | 6:48 |
| 22 | 68 | None | Acute leukemia | 6:57 |
| 23 | 69 | AMD: OU dry | Respiratory failure | 7:22 |
| 24 | 70 | AMD: OU wet | NA | 3:54 |
| 25 | 70 | None | Acute respiratory failure | 5:57 |
| 26 | 70 | AMD: OD CNV | Cardiac arrest | 7:50 |
| 27 | 71 | None | Sepsis | 7:02 |
| 28 | 72 | None | Perforated bowel | 5:03 |
| 29 | 73 | None | Throat cancer | 5:45 |
| 30 | 74 | None | Ovarian cancer | 7:25 |
| 31 | 75 | None | NA | 5:15 |
| 32 | 76 | AMD: OU dry | Subdural hematoma | 4:44 |
| 33 | 76 | AMD: OU dry | Basal ganglion bleed | 6:01 |
| 34 | 76 | None | NA | 7:38 |
| 35 | 76 | AMD: OU dry | NA | 8:03 |
| 36 | 77 | None | Brain tumor | 5:07 |
| 37 | 77 | AMD: OD wet, OS dry | NA | 5:58 |
| 38 | 77 | AMD: OU dry | Sepsis | 6:35 |
| 39 | 77 | None | Aortic valve stenosis/congestive heart failure | 6:41 |
| 40 | 78 | AMD: OD dry | Respiratory failure 2 COPD | 4:07 |
| 41 | 78 | AMD | Respiratory failure | 4:56 |
| 42 | 78 | AMD: OU dry | Subarachnoid hemorrhage | 5:34 |
| 43 | 78 | None | Sepsis | 5:47 |
| 44 | 79 | None | Metastatic lung cancer | 4:50 |
| 45 | 79 | None | NA | 5:32 |
| 46 | 79 | None | Bladder cancer | 5:52 |
| 47 | 79 | None | NA | 6:26 |
| 48 | 79 | AMD: OU dry | NA | 6:47 |
| 49 | 79 | AMD: OD dry, OS wet | Lymphoma | 7:55 |
| 50 | 79 | AMD: OU dry | NA | NA |
| 51 | 80 | None | NA | 4:51 |
| 52 | 80 | AMD: OD dry | COPD | 4:53 |
| 53 | 80 | None | NA | 5:34 |
| 54 | 80 | None | Hemorrhagic shock | 8:00 |
| 55 | 81 | None | Cancer | 4:44 |
| 56 | 81 | None | NA | 6:02 |
| 57 | 81 | None | Renal failure | 6:30 |
| 58 | 82 | None | Respiratory failure | 5:12 |
| 59 | 82 | AMD: OU GA | Respiratory failure | 6:04 |
| 60 | 82 | None | Perforated bowel | 6:52 |
| 61 | 82 | None | Multisystem cancer | 8:20 |
| 62 | 83 | AMD: OU GA | NA | 5:24 |
| 63 | 83 | AMD: OU dry | Motor vehicle collision | 5:55 |
| 64 | 83 | None | NA | 6:32 |
| 65 | 83 | AMD: OU wet | Myocardial infarction | 8:40 |
| 66 | 84 | AMD: OU dry | Respiratory failure | 4:16 |
| 67 | 84 | None | Hypoxia due to congestive heart failure | 5:43 |
| 68 | 85 | None | NA | 6:56 |
| 69 | 86 | AMD: OU dry | NA | 3:13 |
| 70 | 86 | AMD: OS dry | Small bowel obstruction/renal insufficiency | 3:35 |
| 71 | 86 | None | Heart disease | 6:05 |
| 72 | 86 | AMD: OU dry | Myocardial infarction | 6:54 |
| 73 | 86 | AMD: OU wet | Colon cancer | 7:20 |
| 74 | 87 | AMD: OD GA, OS wet | Heart and respiratory failure | 4:37 |
| 75 | 87 | None | NA | 5:26 |
| 76 | 87 | None | Stroke | 5:30 |
| 77 | 87 | AMD: OU dry | Motor vehicle accident with head injury | 5:42 |
| 78 | 87 | AMD: OU wet | Myocardial infarction | 5:45 |
| 79 | 87 | AMD: OU GA | Prostate cancer | 8:50 |
| 80 | 88 | None | NA | 2:35 |
| 81 | 88 | AMD: OU dry | GI bleed | 4:12 |
| 82 | 88 | None | Respiratory failure | 5:35 |
| 83 | 88 | None | Complications after a fall | 5:37 |
| 84 | 88 | None | NA | 5:56 |
| 85 | 88 | AMD: OD GA, OS wet | Heart failure | 6:43 |
| 86 | 88 | None | Respiratory failure/sepsis | 8:10 |
| 87 | 88 | None | Cardiogenic shock | 9:15 |
| 88 | 88 | AMD: OU dry | Cerebrovascular accident | NA |
| 89 | 89 | None | NA | 4:20 |
| 90 | 89 | None | Stroke | 5:08 |
| 91 | 89 | AMD: OU dry | Congestive heart failure | 7:10 |
| 92 | 90 | None | Cardiac arrest | 4:27 |
| 93 | 90 | AMD: OD wet, OS dry | Cerebrovascular accident | 5:30 |
| 94 | 90 | None | Cerebrovascular accident | 6:46 |
| 95 | 90 | None | NA | 8:50 |
| 96 | 91 | None | Cerebrovascular accident | 5:06 |
| 97 | 91 | AMD: OD wet, OS early dry | NA | 5:10 |
| 98 | 91 | None | NA | 5:32 |
| 99 | 91 | AMD: OU dry | Aspiration pneumonia | 7:48 |
| 100 | 91 | AMD: OU wet | Intracerebral hemorrhage | NA |
| 101 | 92 | AMD: OU dry | Acute myocardial infarction | 4:25 |
| 102 | 92 | AMD: OU dry | Lung cancer | 5:25 |
| 103 | 92 | AMD: OU dry | Cardiac arrest | 7:49 |
| 104 | 93 | None | Respiratory failure | 5:50 |
| 105 | 93 | AMD: OU dry | Renal insufficiency | 6:30 |
| 106 | 94 | None | Congestive heart failure | 5:34 |
| 107 | 94 | None | Respiratory failure | 5:34 |
| 108 | 94 | AMD: OU dry | Cardiac arrest | 5:46 |
| 109 | 94 | AMD: OU dry | Cardiac arrest | 7:40 |
| 110 | 95 | AMD: OD GA | Stroke/atrial fibrillation | 6:20 |
| 111 | 95 | AMD: OU dry | Heart and renal failure | 6:35 |
| 112 | 96 | AMD: OU dry | NA | 5:00 |
| 113 | 98 | None | NA | 5:41 |
| 114 | 98 | AMD: OU dry | COPD | 6:14 |
| 115 | 98 | AMD: OU dry | Multisystem organ failure/renal failure | 6:17 |
| 116 | 99 | AMD: OD GA | NA | 1:42 |
| 117 | 100 | AMD: OD dry | Found unresponsive | 5:55 |
CNV, choroidal neovascularization; COPD, chronic obstructive pulmonary disease; D–P, maximum time between death and preservation in liquid nitrogen or fixative; Dx, diagnosis; GA, geographic atrophy; GI, gastrointestinal tract; NA, not available; OD, right eye; OS, left eye; OU, both eyes.
Genotyping
Genotyping for the Y402H allele (rs1061170) and the V62I allele (rs800292) of the CFH gene was performed on DNA from subsets of donor eyes using a microfluidics station (Fluidigm, South San Francisco, CA) with TaqMan (Life Technologies) reagents. DNA was isolated either from whole blood, collected during enucleation, or from extraocular muscle, using a DNeasy kit (Qiagen, Valencia, CA). Genotyping assays were performed as described previously.4,10
Morphometry of Choroidal Thickness
Eyes from 100 donors genotyped at the Y402H risk allele of CFH were used for quantitative analyses. The distribution was 43 donors homozygous for the low-risk allele (YY), 25 donors homozygous for the high-risk allele, and 32 donors heterozygous (HY). Cryostat sections were collected and assigned a random identifier by a masked observer. Choroidal thickness (measured from the outer edge of Bruch’s membrane to the inner surface of the sclera16) was quantified in sections from all donors using ImageJ software version 1.60_65 (NIH, Bethesda, MD). At least five measurements were taken from each section, and a single mean value was determined for each eye. Data were analyzed using linear regression in the R version 3.0.3 statistical computing environment.
Results
ELISA Analysis
The concentration of MAC was quantified in a series of punches of RPE–choroid from donor eyes. Standards included in the kit, ranging from 15 to 172 ng/mL, showed excellent correlation between concentration and absorbance (r2 > 0.99). For RPE–choroid samples, duplicate wells showed very good reproducibility (r2 > 0.989).
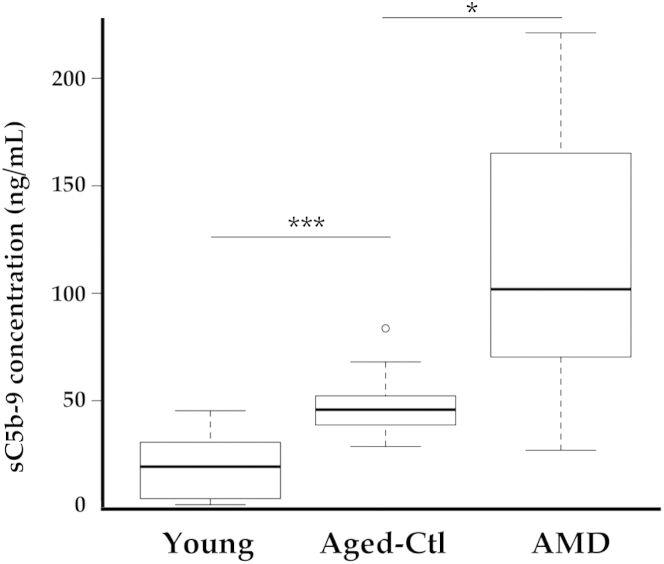
Samples from young donors had relatively low levels of MAC, and these levels were significantly lower than those in the aged control non-AMD group (P < 0.001) (Figure 1). Samples from those with AMD had variable but significantly higher levels of MAC than either age-matched control eyes (P < 0.05) or young eyes (P < 0.01). In both older age groups, MAC levels were significantly higher in eyes with one or more risk alleles (P < 0.05, Student’s t-test), as we have reported previously.10
Figure 1.
Enzyme-linked immunosorbent assay analysis of soluble C5b-9 MAC in young eyes, aged eyes without AMD (control), and eyes with atrophic AMD. Increased MAC levels were related to increased age (∗∗∗P < 0.001) and to diagnosis of AMD (∗P < 0.05). Data are expressed as box-and-whisker plots, indicating median, interquartile range, and minimum and maximum values; any outliers are indicated by individual symbols. n = 10 per group. AMD, age-related macular degeneration; Ctl, control; MAC, membrane attack complex.
MAC Immunofluorescence
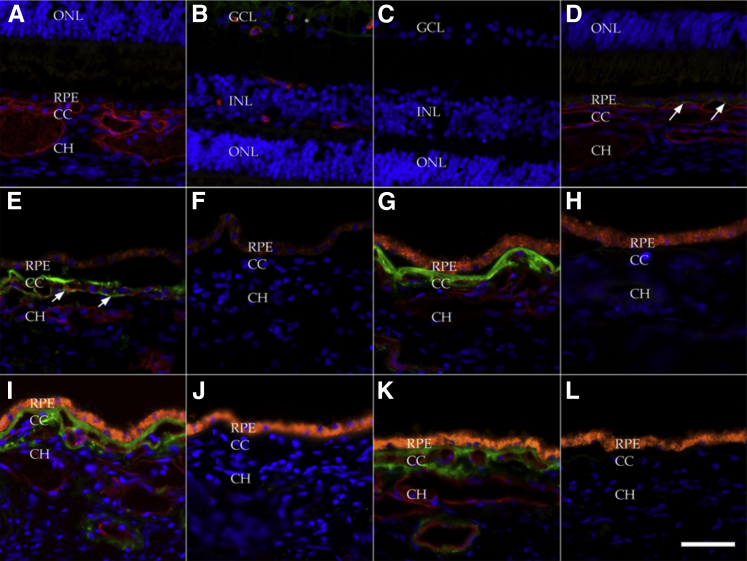
We evaluated a series of eyes from 117 human donors (Table 2). Consistent with previous report,17 MAC in aging maculae was predominantly localized to the outer aspect of Bruch’s membrane and in extracellular domains surrounding the choriocapillaris (Figure 2). The eye of one very young donor, a newborn, showed lack of labeling (Figure 2, A–C); this eye was also notable (compared with older donors) for lack of RPE lipofuscin. Interestingly, in this individual, robust MAC was detected in the inner retina, consistent with the proposed role of complement in developmental axon pruning.18 Bruch’s membrane and choriocapillaris from a 16-month–old donor (Figure 2D) showed tiny puncta of labeling, but were generally negative for MAC. Labeling in the eye of a 5-year-old donor who died of myelogenous leukemia showed modest MAC labeling, in a pattern that was predominantly on the inner aspect of Bruch’s membrane and did not wrap around the choriocapillaris endothelium (Figure 2, E and F). By 21 years of age, lipofuscin in the RPE was frequently already remarkable (data not shown). Donors in the third and fourth decade of life had detectable MAC both in Bruch’s membrane and around the outer aspect of the choriocapillaris (Figure 2, G and H). This distribution was also observed in the fifth decade (Figure 2, I and J), as well as in older donors without AMD (Figure 2, K and L).
Figure 2.
Localization of the membrane attack complex (MAC) in young (<50 years) and aged donor eyes without AMD: newborn (A–C), 16 months (D), 5 years (E and F), 32 years (G and H), 41 years (I and J), and 62 years (K and L). Sections were dual-labeled with anti-C5b-9 MAC antibody (green) and UEA-I lectin (red) (A, B, D, E, G, I, and K); the primary antibody and lectin were omitted for negative control (C, F, H, J, and L). Orange fluorescence of the RPE (G–L) indicates age-related lipofuscin accumulation. A–C: Eyes from a newborn infant showed no MAC labeling in Bruch’s membrane or choroid (A). Minor immunoreactivity was observed in the ganglion cell layer (B, asterisks; C, negative control) of this newborn, but in none of the older specimens. D: At 16 months of age, very minor labeling was observed as puncta (arrows) between the RPE and choriocapillaris. E: In a 5-year-old donor heterozygous for the Y402H variant, considerable MAC labeling (arrows) in Bruch’s membrane extended around the choriocapillaris. G–L: More robust labeling was observed in older eyes. Note the lack of choriocapillaris labeling and the constitutive RPE autofluorescence in the negative controls (H, J, and L). All images were processed identically. Scale bar = 50 μm. CC, choriocapillaris; CH, outer choroid; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium.
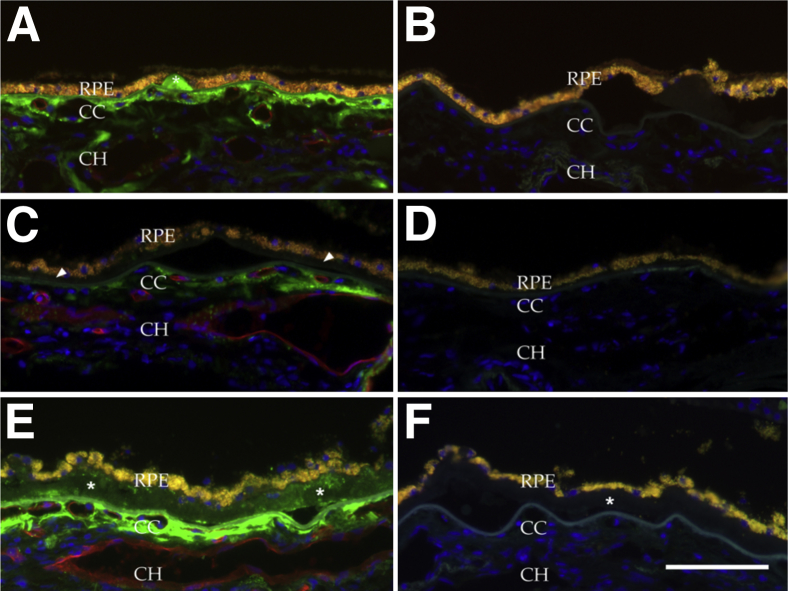
In eyes with early AMD, consistent with previous report,11,17,20 small, hard drusen were almost invariably labeled with anti-MAC antibody (Figure 3, A and B), whereas basal deposits and large confluent drusen were generally negative (Figure 3, C and D). Some large macular drusen showed punctate immunoreactivity (Figure 3, E and F). In contrast to younger eyes and aged control eyes, extension of the MAC-reactive domain often extended into the outer choroid (Figure 3A). Extramacular drusen were invariably positive for MAC, consistent with previous report.11,21
Figure 3.
Localization of the MAC in eyes with early AMD. A: MAC was localized to solitary, hard drusen (asterisk). C: By contrast, basal deposits characteristic of early AMD19 were generally unreactive with anti-MAC antibody (arrowheads). E: Modest punctate immunoreactivity was observed where drusen were confluent (asterisks). Note immunoreactive extracellular material extending into the outer choroid. B, D, and F: Secondary antibody controls from adjacent sections. Scale bar = 100 μm.
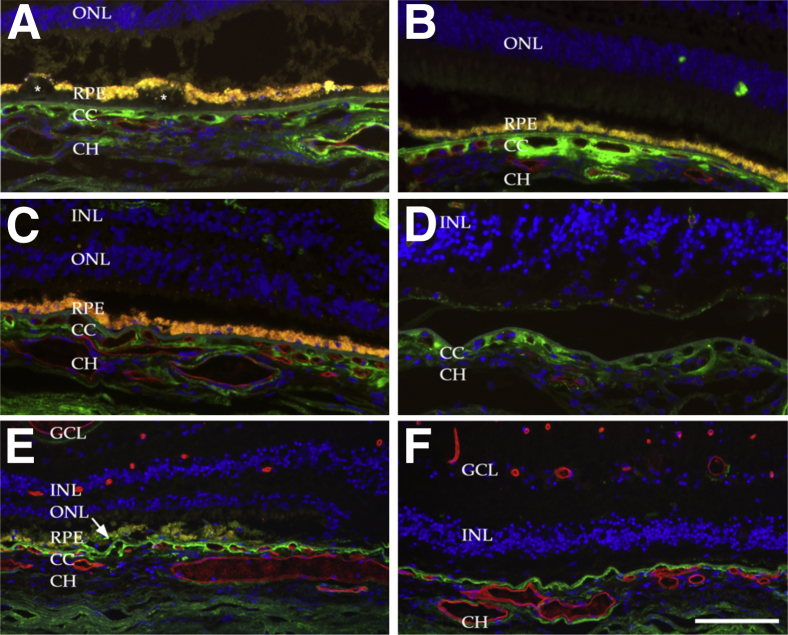
Eyes with advanced AMD were also evaluated. In eyes with geographic atrophy (n = 15 eyes from 11 donors), MAC was present outside of areas of RPE and photoreceptor loss in a pattern similar to that seen in early AMD (ie, choriocapillaris) (Figure 4, A–C), although reactivity on outer vessel walls was more notable in eyes with geographic atrophy (Figure 4A). In areas of extensive atrophy (Figure 4, D and F) the intensity of immunoreactivity at the choroiocapillaris–Bruch’s membrane interface was lower than elsewhere, although a moderate level of anti-MAC labeling was found to persist when RPE, photoreceptor, and choriocapillaris loss was complete (Figure 4F). In one case, near the interface of the healthy RPE and geographic atrophy, MAC deposition was observed on the RPE (Figure 4E).
Figure 4.
Localization of the MAC in the choriocapillaris of eyes with geographic atrophy. The choroid is thinner in eyes with advanced dry AMD than in control eyes.16 Shown are areas outside (A–C), within (D and F), and at the junction (E) of the central atrophy. A: Drusen deposits (asterisks), extensive MAC in the choriocapillaris layer, and vascular atrophy with loss of endothelium (ghost vessels) in an 83-year-old donor eye. B: Choriocapillaris atrophy, with nonreactive basal deposits, in an 82-year-old donor eye. C: An area of atrophy in an 89-year-old donor eye, with some thinning and attenuation of the ONL and shortening of the inner–outer segment. D: An area of central atrophy, with loss of the RPE, in an 87-year-old donor eye. Note the modest amount of RPE lipofuscin in a gliotic scar. MAC immunoreactivity was still present in the largely atrophied choriocapillaris. E and F: The atrophic interface (E) and the central atrophic zone (F) of an eye from a 68-year-old donor homozygous for the high-risk Y402H allele. Note localization of MAC to RPE cells near the interface (E, arrow) and persistence of MAC in the degenerated choroid in the area of central atrophy. Scale bar = 100 μm. CC, choriocapillaris; CH, outer choroid; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium.
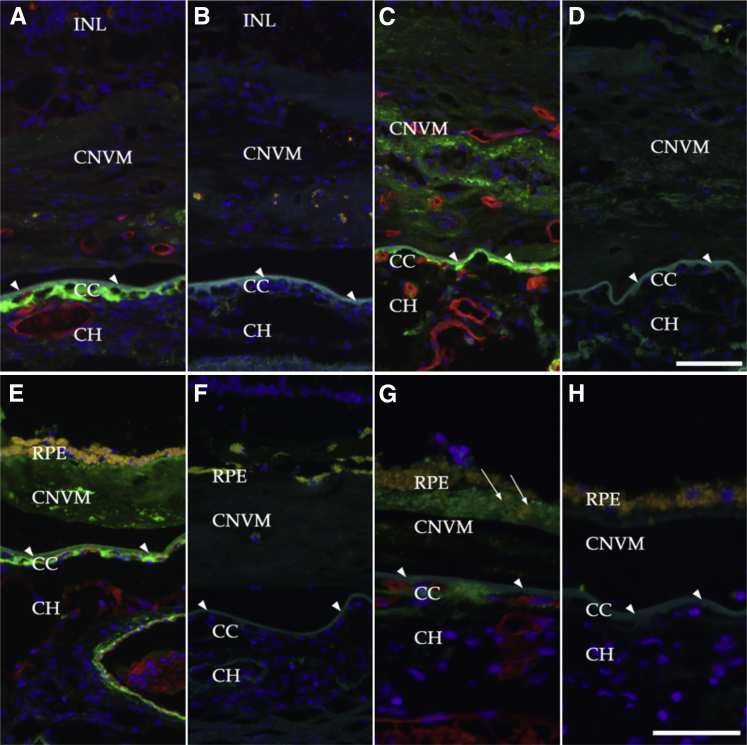
In addition, eyes from 11 donors with CNV were evaluated. Choroidal neovascular membranes, with or without subretinal fibrosis, were identified in eyes with RPE degeneration (Figure 5, A–D) or between the outer layers of Bruch’s membrane and an intact layer of RPE (type I or occult CNV) (Figure 5, E–H). Eyes with CNV showed persistent labeling of MAC at the level of the choriocapillaris even after degeneration of the endothelium was complete (Figure 5A). Eyes with CNV frequently show a detached layer of basal laminar deposits.22,23 Interestingly, in one donor with neovascularization, the MAC was present on or near the RPE (Figure 5, G and H), as well as in the outer aspect of the basal laminar deposits.24 This labeling of RPE with anti-MAC antibody was not observed in early AMD.
Figure 5.
Localization of MAC in eyes with choroidal neovascularization under dual labeling with anti-C9 neoepitope antibody (green) and the vascular-labeling lectin UEA-I (red). Sections were incubated with primary and secondary antibody (A, C, E, and G); adjacent unlabeled sections served as negative controls (B, D, F, and H). Bruch’s membrane is indicated by arrowheads. A and C: MAC persisted in domains surrounding the choriocapillaris even after extensive atrophy. MAC was also present in some choroidal neovascular membranes in association with dedifferentiated RPE cells (C, arrows). E: Punctate labeling could also be observed in subretinal fibrosis and beneath the RPE that resided on the surface of these fibrotic membranes. G: In an eye with type I or occult CNVM, MAC labeling of the RPE was robust (arrows). Scale bars: 50 μm (G and H); 75 μm (A–F). CC, choriocapillaris; CH, outer choroid; CNV, choroidal neovascularization; CNVM, choroidal neovascular membrane; INL, inner nuclear layer; RPE, retinal pigment epithelium.
Genotype and Choroidal Thickness
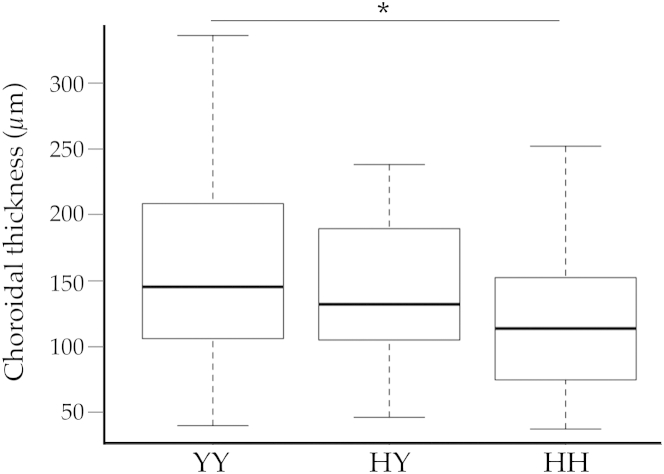
We hypothesized that the Y402H variant in CFH affects morphological features of the macula. Choroidal thickness measurements were collected in the macula in a masked fashion. The CFH high-risk H allele was associated with thinner choroids (Figure 6). Compared with eyes from donors homozygous for the low-risk Y allele, eyes from donors homozygous for the Y402H single-nucleotide polymorphism were 23.6% thinner (P < 0.05 for HH versus YY). Linear regression indicated a significant association between choroidal thickness and the number of copies of the H allele (P = 0.019).
Figure 6.
Choroidal thickness in donor eyes of the three Y402H CFH genotypes. Measurements of choroidal thickness were performed as described previously,16 and genotypes were determined for the Y402H allele (rs1061170). Eyes from donors homozygous for the high-risk H allele had thinner choroids than eyes from donors heterozygous or homozygous for the low-risk Y allele. Eyes homozygous for the H allele had significantly thinner choroids than those homozygous for the Y allele (P < 0.05), and there was a significant association (P = 0.019) between choroidal thickness and the number of copies of the H allele. Data are expressed as box-and-whisker plots, indicating median, interquartile range, and minimum and maximum values. n = 43 (YY); n = 32 (HY); n = 25 (HH). ∗P < 0.05.
Discussion
The complement system comprises an interacting set of evolutionarily ancient proteins, with members represented in animal phyla as far removed from humans and other vertebrates (Chordata) as the Cnidaria and Echinodermata.25 Metazoan species lacking complement genes (eg, Drosophila) are thought to have undergone selective loss of genes that were present in a shared ancestor.26 Although antimicrobial protection is the principal role for complement, roles for this system in tissue repair and regeneration have also been described.27 Although the complement system is a critical component of innate immunity, abnormal activation can lead to bystander injury of resident cells.28
The striking abundance of the MAC of complement in the human macular choriocapillaris has been reproducibly observed.8,17 Seth et al17 previously evaluated MAC semiquantitatively in aging eyes and found an increase in MAC abundance in aging. Hageman et al8 reported that MAC increases with aging and that its localization is predominantly in the macular region. Our immunofluorescence and ELISA results are in accord with these findings, although in fixed frozen sections using immunofluorescence we detected MAC at a much younger age and consistently in older eyes. Although the youngest donor eye in our cohort showed little or no labeling, we observed MAC as early as 2 years of age, with modest labeling by age 5 and striking accumulation by age 21. Thus, choriocapillaris endothelial cells are potentially exposed to some level of MAC for decades before any development of drusen or vision loss.
Given the role of the MAC in cell lysis and opsonization, its high concentration in the delicate tissues of the macula seems surprising, especially in eyes much too young to develop AMD and preceding the appearance of RPE lipofuscin. One possible explanation for complement activation in Bruch’s membrane and the choriocapillaris is that some beneficial role for this state of MAC formation offsets its risks for bystander injury, especially at younger ages. The photoreceptor–RPE–choriocapillaris interface is physiologically unusual. Photoreceptor cells, or at least rod cells, completely renew their outer segments approximately every 10 days, and thus 1/10 of the entire outer surface of the retina is phagocytosed and processed every day. Each RPE cell is responsible for the maintenance and phagocytosis of 12 to 40 photoreceptor outer segments.29 Assuming that the outer segment is 25 μm long and 1 μm wide, the RPE must remove and lyse a volume of approximately 2 μm3 per photoreceptor cell per day; at some eccentricities, this amounts to approximately as one red blood cell per RPE cell per day. That the RPE is able to perform this function, in addition to pumping fluid out of the retina, participating in retinal adhesion, and directing the visual cycle, suggests a highly efficient process.30,31 It is conceivable that the complement activation in Bruch’s membrane is an adaptive response to help opsonize and remove incompletely digested cellular debris. In this context, is notable that, in an in vitro model of RPE deposition of material into a porous substrate, the MAC was found to opsonize the debris.32 A similar event in vivo may facilitate clearance of Bruch’s membrane. The age-related accumulation coincides with other structural and molecular changes in Bruch’s membrane, including accumulation of lipids and advanced glycation end products.19,33–36
Moreover, the photoreceptor cells of the retina have long chain and very long chain fatty acid molecules with a restricted distribution.37 Docosahexaenoic acid, for example, can be metabolized into neuroprotective38,39 or pathogenic40,41 derivatives. Carboxyethylpyrrole-modified macromolecules are targets of autoantibodies in AMD, and lead to activation of the complement system in mouse.42 In light of the enrichment of docosahexaenoic acid in the retina, and its metabolism by the RPE, this pathway may explain the abundant complement activation in the aging macular choriocapillaris.
Unlike the major allele, CFH molecules harboring a histidine at residue 402 have an altered affinity for C-reactive protein (itself increased in the choroid of donors homozygous for the risk allele9), altered behavior in the presence of zinc,43 and impaired binding to glycosaminoglycans in Bruch’s membrane.44,45 In addition, the 402H form of CFH has a reduced affinity for Bruch’s membrane malondialdehyde.46 Moreover, eyes homozygous for the Y402H polymorphism have increased deposition of MAC.10 In light of the observed MAC assembly on RPE cells in advanced AMD in the present study, it is also of interest that cultured RPE cells harboring protective haplotypes are more resistant to MAC injury, compared with RPE cells with high-risk haplotypes.47
We also addressed the question of whether the increased MAC found in eyes with high-risk genotypes is associated with morphological changes in the macula. Masked studies of choroidal thickness showed evidence for choroidal atrophy associated with homozygosity for the Y402H polymorphism.
The finding that eyes with high-risk genotypes show loss of endothelial cells supports the notion that changes occur in the choroidal vasculature early in AMD pathogenesis. In previous gene expression studies with AMD and age-matched RPE–choroids using unbiased gene set enrichment criteria, we found that, as a group, endothelial cell–associated transcripts decline in eyes with early non-neovascular AMD,48 a finding that is consistent with proteomic,49 histopathologic,15,50–52 novel imaging,53,54 and blood flow55,56 studies.
The combined elements of localization of MAC to the choriocapillaris, increased choroidal MAC in high-risk genotypes,10 and evidence for the loss of vasculature in eyes with high-risk genotypes suggest a mechanism by which CFH risk alleles may contribute to AMD. By increasing the overall level of complement activation, CFH polymorphisms that result in impaired function or localization of the protein may allow for an increased level of MAC formation that is additive to the high complement activation of normal aging, which in turn leads to injury of the choriocapillaris endothelium in early AMD. Morphometric,15,57 biochemical,49 and gene expression48 studies support early loss of vascular endothelial cells in the choroid in early AMD.
The finding of robust MAC in small hard drusen, but less abundant or undetectable MAC in confluent drusen or basal deposits, suggests that complement may have the opportunity to stress the RPE overlying a druse early in its development, but that other, non-MAC–mediated responses are more important during drusen growth. Moreover, if MAC injury to the RPE up-regulates expression of drusen-associated gene products, as has been shown in vitro,58 this response is more likely in small hard drusen than in confluent drusen. We recently reported that MAC is present in the unusual laminated drusen-like deposits in an eye with Malattia Leventinese and suggested that these deposits have features of both hard drusen and basal deposits.20
Overall, the histological findings from our studies and those of others suggest that the choriocapillaris endothelium experiences more substantive challenge by the MAC than does the RPE. MAC was not observed on the RPE surface in either early AMD or age-matched control eyes, but exposure of RPE to MAC was observed in some cases of advanced AMD. An example of robust deposition of MAC on the RPE in an eye with neovascular AMD is shown in Figure 5G. A number of investigators have shown a requirement for complement activation, as well as a protective effect of complement deficiency or inhibition, in the development of experimental CNV in mice. Various mouse studies using cobra venom factor,12 targeted deletion of specific complement genes,59–61 and virally delivered62,63 or pharmaceutical64,65 complement inhibitors have all shown impaired formation of choroidal neovascular membranes, compared with controls. Moreover, cultured RPE cells respond to sublytic complement attack by increased synthesis of proinflammatory and proangiogenic molecules that may exacerbate the progression of AMD.58,66
In summary, aging and the high-risk H allele CFH genotypes are associated with increased MAC deposition at the level of the choriocapillaris. Eyes with AMD exhibit increased C-reactive protein and decreased complement factor H, consistent with a choroidal AMD microenvironment that favors complement activation.67 Choriocapillaris degeneration, whether due to MAC or some other source of injury, is notable in eyes with early AMD,15 and morphometric studies support a role for the CFH genotype in choroidal atrophy. Treatments that protect the choriocapillaris in early AMD, and the RPE in advanced AMD, would be beneficial in addressing this blinding disease.
Acknowledgments
We thank the donor families for their generosity, the staff of the Iowa Lions Eye Bank for their invaluable assistance in collection of donor eyes, Ms. Aditi Khanna for expert technical assistance, and Prof. Michael Holers (University of Colorado) for helpful discussions.
Footnotes
Supported in part by NIH grants R01EY017451 (R.F.M.), R01EY016822 (E.M.S.), and DP2-OD007483-01 (B.A.T.); the Howard Hughes Medical Institute (E.M.S.); The Elmer and Sylvia Sramek Charitable Foundation (R.F.M. and B.A.T.); the Doris Duke Clinical Research Fellowship Program (D.S.); an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness; the Hansjoerg E. J. W. Kolder, M.D., Ph.D., Professorship in Best Disease Research (R.F.M.); and the Stephen A. Wynn Foundation.
Disclosures: None declared.
References
- 1.Vannewkirk M.R., Nanjan M.B., Wang J.J., Mitchell P., Taylor H.R., McCarty C.A. The prevalence of age-related maculopathy: the Visual Impairment Project. Ophthalmology. 2000;107:1593–1600. doi: 10.1016/s0161-6420(00)00175-5. [DOI] [PubMed] [Google Scholar]
- 2.Friedman D.S., O’Colmain B.J., Muñoz B., Tomany S.C., McCarty C., de Jong P.T.V.M., Nemesure B., Mitchell P., Kempen J., Eye Diseases Prevalence Research Group Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [Erratum appeared in Arch Ophthalmol 2011, 129:1188] [DOI] [PubMed] [Google Scholar]
- 3.Bird A.C., Bressler N.M., Bressler S.B., Chisholm I.H., Coscas G., Davis M.D., de Jong P.T.V.M., Klaver C.C.W., Klein B.E.K., Klein R., Mitchell P., Sarks J.P., Sarks S.H., Soubrane G., Taylor H.R., Vingerling J.R., International ARM Epidemiological Study Group An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 4.Khandhadia S., Cipriani V., Yates J.R.W., Lotery A.J. Age-related macular degeneration and the complement system. Immunobiology. 2012;217:127–146. doi: 10.1016/j.imbio.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Haines J.L., Hauser M.A., Schmidt S., Scott W.K., Olson L.M., Gallins P., Spencer K.L., Kwan S.Y., Noureddine M., Gilbert J.R., Schnetz-Boutaud N., Agarwal A., Postel E.A., Pericak-Vance M.A. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 6.Klein R.J., Zeiss C., Chew E.Y., Tsai J.Y., Sackler R.S., Haynes C., Henning A.K., SanGiovanni J.P., Mane S.M., Mayne S.T., Bracken M.B., Ferris F.L., Ott J., Barnstable C., Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards A.O., Ritter R., 3rd, Abel K.J., Manning A., Panhuysen C., Farrer L.A. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 8.Hageman G.S., Anderson D.H., Johnson L.V., Hancox L.S., Taiber A.J., Hardisty L.I., Hageman J.L., Stockman H.A., Borchardt J.D., Gehrs K.M., Smith R.J., Silvestri G., Russell S.R., Klaver C.C., Barbazetto I., Chang S., Yannuzzi L.A., Barile G.R., Merriam J.C., Smith R.T., Olsh A.K., Bergeron J., Zernant J., Merriam J.E., Gold B., Dean M., Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson P.T., Betts K.E., Radeke M.J., Hageman G.S., Anderson D.H., Johnson L.V. Individuals homozygous for the age-related macular degeneration risk-conferring variant of complement factor H have elevated levels of CRP in the choroid. Proc Natl Acad Sci USA. 2006;103:17456–17461. doi: 10.1073/pnas.0606234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullins R.F., Dewald A.D., Streb L.M., Wang K., Kuehn M.H., Stone E.M. Elevated membrane attack complex in human choroid with high risk complement factor H genotypes. Exp Eye Res. 2011;93:565–567. doi: 10.1016/j.exer.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson L.V., Ozaki S., Staples M.K., Erickson P.A., Anderson D.H. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res. 2000;70:441–449. doi: 10.1006/exer.1999.0798. [DOI] [PubMed] [Google Scholar]
- 12.Bora P.S., Sohn J.H., Cruz J.M.C., Jha P., Nishihori H., Wang Y., Kaliappan S., Kaplan H.J., Bora N.S. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J Immunol. 2005;174:491–497. doi: 10.4049/jimmunol.174.1.491. [DOI] [PubMed] [Google Scholar]
- 13.Do D.V., Pieramici D.J., van Lookeren Campagne M., Beres T., Friesenhahn M., Zhang Y., Strauss E.C., Phase Ia Investigators A phase Ia dose-escalation study of the anti-factor D monoclonal antibody fragment FCFD4514S in patients with geographic atrophy. Retina. 2014;34:313–320. doi: 10.1097/IAE.0b013e3182979ddd. [DOI] [PubMed] [Google Scholar]
- 14.Barthel L.K., Raymond P.A. Improved method for obtaining 3-microns cryosections for immunocytochemistry. J Histochem Cytochem. 1990;38:1383–1388. doi: 10.1177/38.9.2201738. [DOI] [PubMed] [Google Scholar]
- 15.Mullins R.F., Johnson M.N., Faidley E.A., Skeie J.M., Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:1606–1612. doi: 10.1167/iovs.10-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sohn E.H., Khanna A., Tucker B.A., Abràmoff M.D., Stone E.M., Mullins R.F. Structural and biochemical analyses of choroidal thickness in human donor eyes. Invest Ophthalmol Vis Sci. 2014;55:1352–1360. doi: 10.1167/iovs.13-13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seth A., Cui J., To E., Kwee M., Matsubara J. Complement-associated deposits in the human retina. Invest Ophthalmol Vis Sci. 2008;49:743–750. doi: 10.1167/iovs.07-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens B., Allen N.J., Vazquez L.E., Howell G.R., Christopherson K.S., Nouri N., Micheva K.D., Mehalow A.K., Huberman A.D., Stafford B., Sher A., Litke A.M., Lambris J.D., Smith S.J., John S.W.M., Barres B.A. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 19.Sarks S., Cherepanoff S., Killingsworth M., Sarks J. Relationship of basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:968–977. doi: 10.1167/iovs.06-0443. [DOI] [PubMed] [Google Scholar]
- 20.Sohn E.H., Wang K., Thompson S., Riker M.J., Hoffmann J.M., Stone E.M., Mullins R.F. Comparison of drusen and modifying genes in autosomal dominant radial drusen and age-related macular degeneration. Retina. 2014 doi: 10.1097/IAE.0000000000000263. doi: 10.1097/IAE.0000000000000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullins R.F., Russell S.R., Anderson D.H., Hageman G.S. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- 22.Bressler S.B., Silva J.C., Bressler N.M., Alexander J., Green W.R. Clinicopathologic correlation of occult choroidal neovascularization in age-related macular degeneration. Arch Ophthalmol. 1992;110:827–832. doi: 10.1001/archopht.1992.01080180099035. [DOI] [PubMed] [Google Scholar]
- 23.Green W.R., Enger C. Age-related macular degeneration histopathologic studies: the 1992 Lorenz E. Zimmerman Lecture. 1992. Retina. 2005;25(5 Suppl):1519–1535. doi: 10.1097/00006982-200507001-00015. [DOI] [PubMed] [Google Scholar]
- 24.Lommatzsch A., Hermans P., Müller K.D., Bornfeld N., Bird A.C., Pauleikhoff D. Are low inflammatory reactions involved in exudative age-related macular degeneration? Morphological and immunohistochemical analysis of AMD associated with basal deposits. Graefes Arch Clin Exp Ophthalmol. 2008;246:803–810. doi: 10.1007/s00417-007-0749-4. [DOI] [PubMed] [Google Scholar]
- 25.Pinto M.R., Melillo D., Giacomelli S., Sfyroera G., Lambris J.D. Ancient origin of the complement system: emerging invertebrate models. Adv Exp Med Biol. 2007;598:372–388. doi: 10.1007/978-0-387-71767-8_26. [DOI] [PubMed] [Google Scholar]
- 26.Nonaka M., Kimura A. Genomic view of the evolution of the complement system. Immunogenetics. 2006;58:701–713. doi: 10.1007/s00251-006-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastellos D.C., Deangelis R.A., Lambris J.D. Complement-triggered pathways orchestrate regenerative responses throughout phylogenesis. Semin Immunol. 2013;25:29–38. doi: 10.1016/j.smim.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerr H., Richards A. Complement-mediated injury and protection of endothelium: lessons from atypical haemolytic uraemic syndrome. Immunobiology. 2012;217:195–203. doi: 10.1016/j.imbio.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snodderly D.M., Sandstrom M.M., Leung I.Y., Zucker C.L., Neuringer M. Retinal pigment epithelial cell distribution in central retina of rhesus monkeys. Invest Ophthalmol Vis Sci. 2002;43:2815–2818. [PubMed] [Google Scholar]
- 30.Finnemann S.C. Role of alphavbeta5 integrin in regulating phagocytosis by the retinal pigment epithelium. Adv Exp Med Biol. 2003;533:337–342. doi: 10.1007/978-1-4615-0067-4_42. [DOI] [PubMed] [Google Scholar]
- 31.Sethna S., Finnemann S.C. Analysis of photoreceptor rod outer segment phagocytosis by RPE cells in situ. Methods Mol Biol. 2013;935:245–254. doi: 10.1007/978-1-62703-080-9_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson L.V., Forest D.L., Banna C.D., Radeke C.M., Maloney M.A., Hu J., Spencer C.N., Walker A.M., Tsie M.S., Bok D., Radeke M.J., Anderson D.H. Cell culture model that mimics drusen formation and triggers complement activation associated with age-related macular degeneration. Proc Natl Acad Sci USA. 2011;108:18277–18282. doi: 10.1073/pnas.1109703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Handa J.T., Verzijl N., Matsunaga H., Aotaki-Keen A., Lutty G.A., Koppele te J.M., Miyata T., Hjelmeland L.M. Increase in the advanced glycation end product pentosidine in Bruch’s membrane with age. Invest Ophthalmol Vis Sci. 1999;40:775–779. [PubMed] [Google Scholar]
- 34.Ebrahimi K.B., Handa J.T. Lipids, lipoproteins, and age-related macular degeneration. J Lipids. 2011;2011:802059. doi: 10.1155/2011/802059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu X., Meer S.G., Miyagi M., Rayborn M.E., Hollyfield J.G., Crabb J.W., Salomon R.G. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003;278:42027–42035. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- 36.Curcio C.A., Johnson M., Rudolf M., Huang J.D. The oil spill in ageing Bruch membrane. Br J Ophthalmol. 2011;95:1638–1645. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu A., Chang J., Lin Y., Shen Z., Bernstein P.S. Long-chain and very long-chain polyunsaturated fatty acids in ocular aging and age-related macular degeneration. J Lipid Res. 2010;51:3217–3229. doi: 10.1194/jlr.M007518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bazan N.G., Calandria J.M., Serhan C.N. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51:2018–2031. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon W.C., Bazan N.G. Mediator lipidomics in ophthalmology: targets for modulation in inflammation, neuroprotection and nerve regeneration. Curr Eye Res. 2013;38:995–1005. doi: 10.3109/02713683.2013.827211. [DOI] [PubMed] [Google Scholar]
- 40.Salomon R.G., Hong L., Hollyfield J.G. Discovery of carboxyethylpyrroles (CEPs): critical insights into AMD, autism, cancer, and wound healing from basic research on the chemistry of oxidized phospholipids. Chem Res Toxicol. 2011;24:1803–1816. doi: 10.1021/tx200206v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hollyfield J.G., Perez V.L., Salomon R.G. A hapten generated from an oxidation fragment of docosahexaenoic acid is sufficient to initiate age-related macular degeneration. Mol Neurobiol. 2010;41:290–298. doi: 10.1007/s12035-010-8110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hollyfield J.G. Age-related macular degeneration: the molecular link between oxidative damage, tissue-specific inflammation and outer retinal disease: the Proctor lecture. Invest Ophthalmol Vis Sci. 2010;51:1275–1281. doi: 10.1167/iovs.09-4478. [DOI] [PubMed] [Google Scholar]
- 43.Nan R., Farabella I., Schumacher F.F., Miller A., Gor J., Martin A.C.R., Jones D.T., Lengyel I., Perkins S.J. Zinc binding to the Tyr402 and His402 allotypes of complement factor H: possible implications for age-related macular degeneration. J Mol Biol. 2011;408:714–735. doi: 10.1016/j.jmb.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark S.J., Perveen R., Hakobyan S., Morgan B.P., Sim R.B., Bishop P.N., Day A.J. Impaired binding of the age-related macular degeneration-associated complement factor H 402H allotype to Bruch’s membrane in human retina. J Biol Chem. 2010;285:30192–30202. doi: 10.1074/jbc.M110.103986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly U., Yu L., Kumar P., Ding J.D., Jiang H., Hageman G.S., Arshavsky V.Y., Frank M.M., Hauser M.A., Rickman C.B. Heparan sulfate, including that in Bruch’s membrane, inhibits the complement alternative pathway: implications for age-related macular degeneration. J Immunol. 2010;185:5486–5494. doi: 10.4049/jimmunol.0903596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weismann D., Hartvigsen K., Lauer N., Bennett K.L., Scholl H.P.N., Charbel Issa P., Cano M., Brandstätter H., Tsimikas S., Skerka C., Superti-Furga G., Handa J.T., Zipfel P.F., Witztum J.L., Binder C.J. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478:76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radu R.A., Hu J., Jiang Z., Bok D. Bisretinoid-mediated complement activation on retinal pigment epithelial cells is dependent on complement factor H haplotype. J Biol Chem. 2014;289:9113–9120. doi: 10.1074/jbc.M114.548669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitmore S.S., Braun T.A., Skeie J.M., Haas C.M., Sohn E.H., Stone E.M., Scheetz T.E., Mullins R.F. Altered gene expression in dry age-related macular degeneration suggests early loss of choroidal endothelial cells. Mol Vis. 2013;19:2274–2297. [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan X., Gu X., Crabb J.S., Yue X., Shadrach K., Hollyfield J.G., Crabb J.W. Quantitative proteomics: comparison of the macular Bruch membrane/choroid complex from age-related macular degeneration and normal eyes. Mol Cell Proteomics. 2010;9:1031–1046. doi: 10.1074/mcp.M900523-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhutto I., Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Aspects Med. 2012;33:295–317. doi: 10.1016/j.mam.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McLeod D.S., Grebe R., Bhutto I., Merges C., Baba T., Lutty G.A. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:4982–4991. doi: 10.1167/iovs.09-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lutty G., Grunwald J., Majji A.B., Uyama M., Yoneya S. Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration. Mol Vis. 1999;5:35. [PubMed] [Google Scholar]
- 53.Adhi M., Lau M., Liang M.C., Waheed N.K., Duker J.S. Analysis of the thickness and vascular layers of the choroid in eyes with geographic atrophy using spectral-domain optical coherence tomography. Retina. 2014;34:306–312. doi: 10.1097/IAE.0b013e3182993e09. [DOI] [PubMed] [Google Scholar]
- 54.Lee J.Y., Lee D.H., Lee J.Y., Yoon Y.H. Correlation between subfoveal choroidal thickness and the severity or progression of nonexudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:7812–7818. doi: 10.1167/iovs.13-12284. [DOI] [PubMed] [Google Scholar]
- 55.Grunwald J.E., Metelitsina T.I., Dupont J.C., Ying G.S., Maguire M.G. Reduced foveolar choroidal blood flow in eyes with increasing AMD severity. Invest Ophthalmol Vis Sci. 2005;46:1033–1038. doi: 10.1167/iovs.04-1050. [DOI] [PubMed] [Google Scholar]
- 56.Berenberg T.L., Metelitsina T.I., Madow B., Dai Y., Ying G.S., Dupont J.C., Grunwald L., Brucker A.J., Grunwald J.E. The association between drusen extent and foveolar choroidal blood flow in age-related macular degeneration. Retina. 2012;32:25–31. doi: 10.1097/IAE.0b013e3182150483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramrattan R.S., van der Schaft T.L., Mooy C.M., de Bruijn W.C., Mulder P.G., de Jong P.T. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857–2864. [PubMed] [Google Scholar]
- 58.Lueck K., Wasmuth S., Williams J., Hughes T.R., Morgan B.P., Lommatzsch A., Greenwood J., Moss S.E., Pauleikhoff D. Sub-lytic C5b-9 induces functional changes in retinal pigment epithelial cells consistent with age-related macular degeneration. Eye (Lond) 2011;25:1074–1082. doi: 10.1038/eye.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nozaki M., Raisler B.J., Sakurai E., Sarma J.V., Barnum S.R., Lambris J.D., Chen Y., Zhang K., Ambati B.K., Baffi J.Z., Ambati J. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci USA. 2006;103:2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bora N.S., Kaliappan S., Jha P., Xu Q., Sohn J.H., Dhaulakhandi D.B., Kaplan H.J., Bora P.S. Complement activation via alternative pathway is critical in the development of laser-induced choroidal neovascularization: role of factor B and factor H. J Immunol. 2006;177:1872–1878. doi: 10.4049/jimmunol.177.3.1872. [DOI] [PubMed] [Google Scholar]
- 61.Rohrer B., Coughlin B., Kunchithapautham K., Long Q., Tomlinson S., Takahashi K., Holers V.M. The alternative pathway is required, but not alone sufficient, for retinal pathology in mouse laser-induced choroidal neovascularization. Mol Immunol. 2011;48:e1–e8. doi: 10.1016/j.molimm.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramo K., Cashman S.M., Kumar-Singh R. Evaluation of adenovirus-delivered human CD59 as a potential therapy for AMD in a model of human membrane attack complex formation on murine RPE. Invest Ophthalmol Vis Sci. 2008;49:4126–4136. doi: 10.1167/iovs.08-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bora N.S., Jha P., Lyzogubov V.V., Kaliappan S., Liu J., Tytarenko R.G., Fraser D.A., Morgan B.P., Bora P.S. Recombinant membrane-targeted form of CD59 inhibits the growth of choroidal neovascular complex in mice. J Biol Chem. 2010;285:33826–33833. doi: 10.1074/jbc.M110.153130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rohrer B., Long Q., Coughlin B., Wilson R.B., Huang Y., Qiao F., Tang P.H., Kunchithapautham K., Gilkeson G.S., Tomlinson S. A targeted inhibitor of the alternative complement pathway reduces angiogenesis in a mouse model of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:3056–3064. doi: 10.1167/iovs.08-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Birke K., Lipo E., Birke M.T., Kumar-Singh R. Topical application of PPADS inhibits complement activation and choroidal neovascularization in a model of age-related macular degeneration. PLoS One. 2013;8:e76766. doi: 10.1371/journal.pone.0076766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kunchithapautham K., Rohrer B. Sublytic membrane-attack-complex (MAC) activation alters regulated rather than constitutive vascular endothelial growth factor (VEGF) secretion in retinal pigment epithelium monolayers. J Biol Chem. 2011;286:23717–23724. doi: 10.1074/jbc.M110.214593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhutto I.A., Baba T., Merges C., Juriasinghani V., McLeod D.S., Lutty G.A. C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration. Br J Ophthalmol. 2011;95:1323–1330. doi: 10.1136/bjo.2010.199216. [DOI] [PMC free article] [PubMed] [Google Scholar]