Abstract
Nucleotide-binding oligomerization domain-containing protein 2 (NOD2), an intracellular pattern recognition receptor, induces autophagy on detection of muramyl dipeptide (MDP), a component of microbial cell walls. The role of bacteria and NOD2 signaling toward ischemia/reperfusion (I/R)–induced intestinal injury response is unknown. Herein, we report that I/R-induced intestinal injury in germ-free (GF) C57BL/6 wild-type (WT) mice is worse than in conventionally derived mice. More important, microbiota-mediated protection against I/R-induced intestinal injury is abrogated in conventionally derived Nod2−/− mice and GF Nod2−/− mice. Also, WT mice raised in specific pathogen-free (SPF) conditions fared better against I/R-induced injury than SPF Nod2−/− mice. Moreover, SPF WT mice i.p. administered 10 mg/kg MDP were protected against injury compared with mice administered the inactive enantiomer, l-MDP, an effect lost in Nod2−/− mice. However, MDP administration failed to protect GF mice from I/R-induced intestinal injury compared with control, a phenomenon correlating with undetectable Nod2 mRNA level in the epithelium of GF mice. More important, the autophagy-inducer rapamycin protected Nod2−/− mice against I/R-induced injury and increased the levels of LC3+ puncta in injured tissue of Nod2−/− mice. These findings demonstrate that NOD2 protects against I/R and promotes wound healing, likely through the induction of the autophagy response.
The epithelium lining the intestinal track is composed of a single layer sheet of epithelial cells that provides nutrient absorption, hormone secretion, and innate immune sampling of luminal contents.1,2 In addition, the epithelium provides a physical barrier between the host and gut microbes, where intestinal epithelial cells (IECs) are stitched together by tight junctions that maintain the architecture of the epithelial sheet and prevent uncontrolled access of luminal content (eg, microbes and dietary toxins) to subepithelial tissues.3 The epithelium is preserved by the homeostatic migration and proliferation of IECs from the base of the intestinal crypts to tips of the villi. Events that disrupt this equilibrium could have deleterious consequences for the host, as seen in patients experiencing intestinal ischemia.4
Ischemia occurs when blood supply to the small bowel is occluded, which is followed by reperfusion, the return of blood flood flow, and simultaneous re-oxygenation of the tissue. During ischemia, an imbalance of metabolic demand and supply results in hypoxic response with activation of hypoxia-inducible factor-1 as well as cell death programs,5 autophagy,6–8 and necrosis (organelle swelling and plasma membrane rupturing).4,9 Paradoxically, the restoration of blood flow causes the release of inflammatory mediators, such as IL-6, tumor necrosis factor-α, and IL-1β, which exacerbate the injury.4 As a result, extra-intestinal organs, such as liver and the lung, may experience inflammatory activation and fatal multiorgan dysfunction syndrome. In the clinic, causes of intestinal ischemia/reperfusion (I/R)–induced injury include atherosclerosis, hypotension, blood clots, hernias, cardiac and mesenteric surgery, venous thrombosis, and necrotizing enterocolitis.
Nucleotide-binding oligomerization domain-containing protein 2 (NOD2) is a pattern-recognition receptor whose function is the intracellular reconnaissance of pathogen-associated molecular patterns. NOD2 is important for the recognition of muramyl dipeptide, MDP, a component of peptidoglycan that is present in the cell walls of Gram-positive and Gram-negative bacteria. Loss-of-function mutations of NOD2 have been associated with Crohn's disease and, recently, NOD2-associated autoinflammatory disease.10 The mechanism by which NOD2 maintains intestinal homeostasis has yet to be clearly defined, although a current paradigm suggests an involvement of this innate sensor in controlling microbial composition,11,12 likely through expression of antimicrobial peptides from Paneth cells.13 In addition, NOD2 is implicated in other important biological responses, such as inflammasome activation14 and autophagy.6 More important, in a preclinical model of necrotizing enterocolitis, NOD2 signaling was shown to protect against hypoxic stress through down-regulation of the Toll-like receptor (TLR) 4 pathway.15 However, the role of commensal bacteria and NOD2 signaling in intestinal I/R injury response has not been elucidated.
A balance between innate inflammatory responses and cytoprotective mechanisms dictates the extent of end-organ damage during I/R injury. During injury-induced hypoxic stress, cells undergo a prosurvival process called autophagy.16 This autophagic response occurs on inhibition of mammalian target of rapamycin, thereby inducing the encapsulation of cytoplasmic components in a double membrane (autophagosome), which is delivered to the lysosome for degradation.16 In hepatic ischemia, autophagy has been shown to be a protective mechanism that favors cell survival and proliferation,8 two key processes in epithelial injury response. Interestingly, NOD2 recruits ATG16L1 to the plasma membrane to initiate autophagosome formation in response to MDP and at the site of Shigella flexneri entry.17 However, the role of commensal bacteria–induced autophagy in the context of hypoxic stress and intestinal damage is currently unknown.
Herein, we investigated the role of microbes and NOD2 signaling in I/R-induced intestinal injury using germ-free (GF) and conventionally derived (CONV-D) Nod2−/− mice. We demonstrate that microbes are important for optimal intestinal response to injury, an effect mediated by NOD2 signaling.
Materials and Methods
Mice
Wild-type (WT) and Nod2−/− mice (C57BL/6 background) were maintained in GF conditions at the National Gnotobiotic Rodent Resource Center at University of North Carolina at Chapel Hill (Chapel Hill, NC), the gnotobiotic Facility at the University of Florida (Gainesville, FL), or in specific pathogen-free (SPF) conditions in a standard animal facility. To study the impact of microbiome on I/R injury, cohorts of GF WT and GF Nod2−/− mice were split, such that one group was CONV-D by transfer to SPF conditions for 4 weeks, whereas another group was kept in GF conditions.
I/R-Induced Injury
I/R-induced injury was performed as previously described.18 Briefly, mice were anesthetized by 2% isoflurane. To minimize pain during surgery and reperfusion, mice were injected with 10 mg/kg ketamine and 0.1 mg/kg buprenorphine injected s.c. A midline laparotomy was performed. Then, the peripheral branches of coronal mesenteric artery and collateral blood flow were occluded using 50-g aneurysm clips (Kent Scientific, Torrington, CN) to generate a 3- to 5-cm region of ischemic ileum adjacent to the cecum. Hematoxylin was used to mark the edges of ischemic tissue to allow harvesting ischemic tissue and adjacent healthy tissue from the same mouse for comparison. This control is more appropriate than the sham operated on control mice, because sham operated on mice do not undergo a systemic reaction to I/R-induced injury.18,19 The clips were removed after 60 minutes of ischemia, and the mice were maintained in a heated room for a variable amount of time (0, 1.5, or 3 hours) without anesthesia for the reperfusion phase of injury. Mice were administered 10 mg/kg MDP (Invitrogen, San Diego, CA) or 10 mg/kg inactive enantiomer l-MDP (Invitrogen) 24 hours before injury. Rapamycin (3 mg/kg; LC Laboratories, Woburn, MA) or its vehicle [dimethyl sulfoxide (DMSO)] was administered 1 hour before injury. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill and the University of Florida.
Murine Sample Collection and Histological Evaluation
Ileal sections were dissected, splayed, Swiss rolled, fixed in 30% phosphate-buffered formalin for 24 hours, and then embedded in paraffin. Damage severity was evaluated using hematoxylin and eosin (H&E)–stained sections by a blinded investigator (M.M.). The scoring system is based on an IEC apoptosis/necrosis system, where a score of 1 signified a loss of only the villus tips; 2, loss of 50% of the villus; 3, a loss of the entire villus, but with maintenance of the crypt; and 4, complete loss of the epithelial layer, as previously described.20 The ischemic tissue was divided into four quarters, and a score was given to each quarter separately and added to generate a final damage score.
Electron Microscopy
Ileal sections were fixed in 4% paraformaldehyde overnight at 4°C. Sections were fixed for 1 hour in potassium ferrocyanide–reduced osmium, followed by dehydration through a graded series of ethanol. Then, they were embedded in Polybed 812 epoxy resin (Polysciences, Warrington, PA). Transverse sections (1 μm thick) were cut at several locations along the epithelium, stained with 1% toluidine blue and 1% sodium borate, and examined by light microscopy to confirm the region of interest. Ultrathin sections were cut with a diamond knife (70 to 80 nm thick), mounted on 200 mesh copper grids, and stained with 4% aqueous uranyl acetate for 15 minutes, followed by Reynolds' lead citrate for 8 minutes. The sections were analyzed using a LEO EM-910 transmission electron microscope (Carl Zeiss SMT, Peabody, MA), operating at an accelerating voltage of 80 kV. Digital images were taken using a Gatan Orius SC 1000 charged-coupled device Camera and Digital Micrograph software version 3.11.0 (Gatan, Pleasanton, CA). All images were acquired at room temperature.
RNA Isolation, RT-PCR, and Real-Time Quantitative PCR
RNA isolation from IECs, ileal tissues, and subsequent cDNA amplification and analysis were performed as previously described.19,21 The following primers were used to amplify Nod2: forward, 5′-CCAGCGTCTTTGGCCATTCAACAT-3′; and reverse, 5′-TTGAGCTCATCCAGTGCTTGGAGT-3′. The purity of IECs was confirmed using the IEC-specific gene product Villin (forward, 5′-CCCACGCAAAGAACTGAAGG-3′; reverse, 5′-TCCCGTCATCCACCATTTTC-3′), β-actin (forward, 5′-TTACCAACTGGGACGACATG-3′; reverse, 5′-CTGGGGTGTTGAAGGTCTC-3′), as previously described.19 The PCR product was run on a 2% agarose gel. The following primers were used to amplify Xbp1: forward, 5′-AAACAGAGTAGCAGCGCAGACTGC-3′; and reverse, 5′-TCCTTCTGGGTAGACCTCTGGGAG-3′.22 The PCR product was run on a 3% agarose gel to resolve the spliced variant. For quantitative real-time PCR, relative fold changes of Il6, Il1, and Tnfα were determined using the ΔΔCT calculation method, as previously described.23 Values were normalized to the internal controls, β-actin and Gapdh. Primers were as follows: Gapdh (forward, 5′-GGTGAAGGTCGGAGTCAACGGA-3′; reverse, 5′-GAGGGATCTCGCTCCTGGAAGA-3′), β-actin (forward, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′; reverse, 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′), Il6 (forward, 5′-CGGAGGCTTGGTTACACATGTT-3′; reverse, 5′-CTGGCTTTGTCTTTCTTGTTATC-3′), Tnfα (forward, 5′-ATGAGCACAGAAAGCATGATC-3′; reverse, 5′-TACAGGCTTGTCACTCGAAT-3′), Il1b (forward, 5′-GCCCATCCTCTGTGACTCAT-3′; reverse, 5′-AGGCCACAGGTATTTTGTCG-3′).23,24
Cell Culture and Immunocytochemistry
Human colonic HCT-116 cells were grown in 60-mm tissue culture dishes to 70% to 80% confluency. Cells were treated with 10 μg/mL pepstatin A (MP Pharmaceuticals, Santa Ana, CA) and 10 μg/mL E-64-d (Peptide Institute, Osaka, Japan) 1 hour before exposure to 1 hour of normoxia or hypoxia [1% O2, hypoxic glove box (Coy Labs, Grass Lake, MI)] and stimulated by 5 μg/mL rapamycin (LC Laboratories). Cells were fixed and permeabilized, as previously described.25 The primary antibody for LC3 (1:200; number 4108; Cell Signaling, Danvers, MA) was used according to the manufacturer's specifications, followed by fluorescein isothiocyanate–conjugated goat anti-rabbit secondary antibody (1:1000; number A-11008; Invitrogen). Images were acquired using a Zeiss 710 microscope and Zeiss Zen software version 2009 (Carl Zeiss, Thornwood, NY). Then, puncta (green fluorescent protein) and cells (DAPI; number H-1200; Vector Laboratories, Burlingame, CA) were counted using ImageJ software version 1.48u4 (Wayne. S. Rasband, NIH, Bethesda, MD).
Immunofluorescence
WT healthy and injured ileal tissues were deparaffinized using ethanol and xylenes. Antigen retrieval was performed by using citrate buffer (pH 6) in a pressure cooker. Primary LC3 antibody (1:200; number 4108; Cell Signaling, Danvers, MA) was incubated overnight according to manufacturer's specifications, followed by Alexa Fluor 647 goat anti-rabbit secondary antibody (1:1000; Invitrogen). DAPI (number H-1200; Vector Laboratories, Burlingame, CA) was used to counterstain the sections. Images were acquired by a Leica SPA microscope (Leica Microsystems Inc., Buffalo Grove, IL). Analysis of LC3 puncta was performed using ImageJ software, generating a basal cutoff of LC3 puncta (threshold, 10), then using the Analyze Particle feature for counting to generate a mean of positive pixels per cell (%) ± SEM of three fields of view per sample.26 Immunofluorescence experiments were performed using this secondary antibody instead of the one previously mentioned, because of the change in confocal microscope.
Statistical Analysis
Unless specifically noted, statistical analyses were performed using GraphPad Prism version 5.0a (GraphPad, La Jolla, CA). Comparisons of mouse studies were made with nonparametric analysis of variance and then a U-test. All graphs depict means ± SEM. Experiments were considered statistically significant if P < 0.05.
Results
Microbes Protect WT Mice, But Not Nod2−/− Mice, against Intestinal I/R-Induced Injury
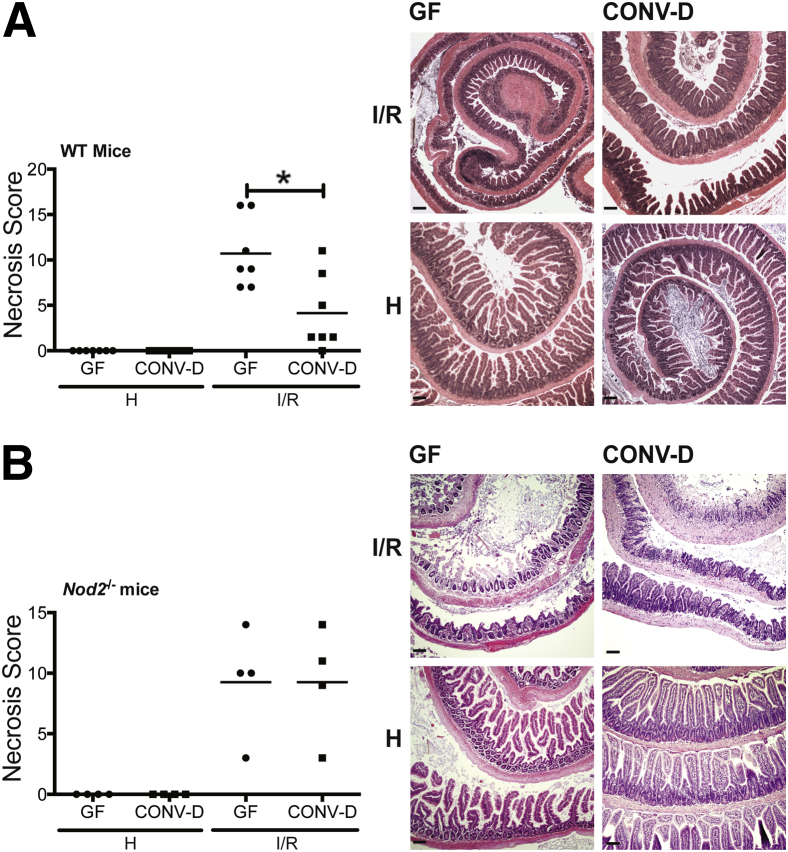
Antibiotic treatments have recently been shown to ameliorate host response to I/R-induced injury.27 However, antibiotic exposure disrupts a large segment of the microbial community and damages epithelial barrier function, adding a confounding element to the approach.28 To avoid this limitation, we used GF WT and Nod2−/− mice to test the relationship between microbes and NOD2 on I/R-induced injury. Cohorts of GF WT and GF Nod2−/− mice were separated into two groups: one that remained in GF conditions and another that was CONV-D by transfer to an SPF housing facility for 4 weeks. Mice were subjected to 1 hour of ischemia, followed by 3 hours of reperfusion, at which point ischemic and healthy portions of the ileum were harvested for histological analysis. Although GF and CONV-D mice exhibited a comparable degree of injury after 1 hour of ischemia (data not shown), CONV-D WT mice displayed attenuated necrosis compared with GF WT mice after reperfusion (scores: 10.7 ± 1.5 versus 4.1 ± 1.6; P < 0.05) (Figure 1A). Histological representation of tissue injury showed that GF mice displayed midvillus denudement of the epithelium, whereas CONV-D mice showed restituted, nascent villi (Figure 1A). Interestingly, there was no difference in injury between GF Nod2−/− mice and CONV-D Nod2−/− mice after I/R-induced injury (scores: 9.3 ± 2.3 versus 9.2 ± 2.3) (Figure 1B). These results suggest that microbes harbor protective function during I/R-induced injury.
Figure 1.
Microbial signaling protects against I/R-induced injury. Cohorts of GF, WT mice and GF, Nod2−/− mice were split into two groups. One group remained in germ free (GF) conditions, whereas the other was CONV-D with commensal microbes in a SPF facility. Mice were subjected to 60 minutes of ischemia, followed by 3 hours of reperfusion. Necrosis was assessed using an established necrosis scoring system. Histological intestinal damage scores of individual mice are depicted (means ± SEM), and representative images of H&E-stained ileal sections of WT (A) and Nod2−/− (B) mice are shown. Results are representative of two independent experiments (n ≥ 4 per group). ∗P < 0.05. Scale bar = 100 μm (A and B). H, healthy; I/R, 1 hour ischemia, followed by 3 hours’ reperfusion.
NOD2 Signaling Protects against I/R-Induced Injury
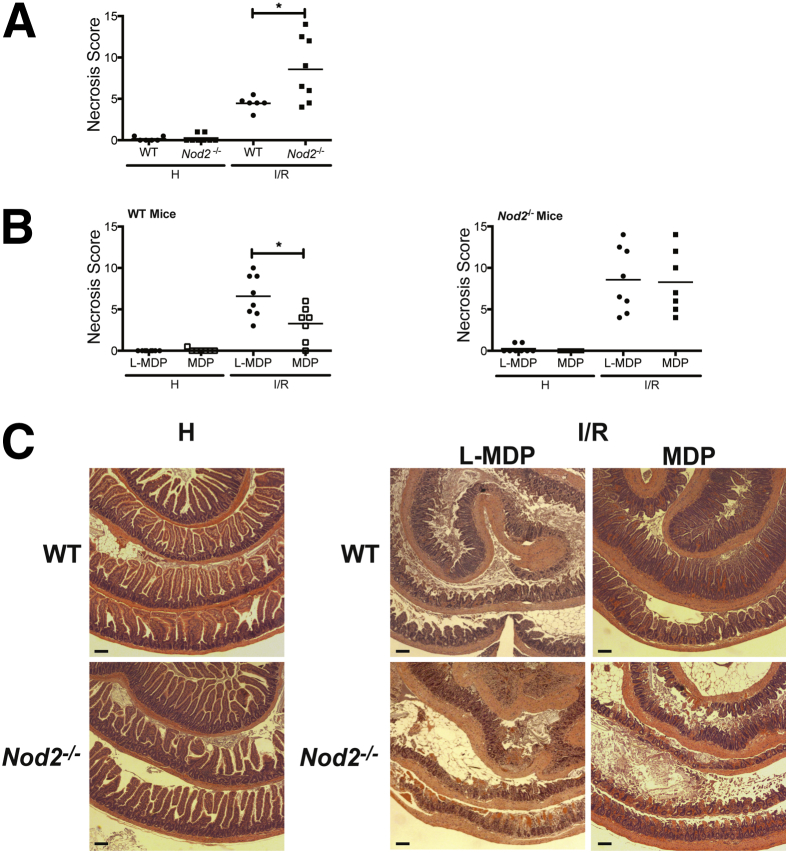
To test the protective capacity of NOD2 signaling, we subjected cohorts of WT and Nod2−/− mice raised to I/R-induced injury in the presence of the NOD2 agonist MDP or its inactive enantiomer, l-MDP. We first performed a time course (up to 6 hours of reperfusion) to better define the kinetics of I/R injury response (data not shown). On the basis of these findings, the reperfusion time was shortened to 1.5 hours to better evaluate the modulatory effect of bacteria and NOD2 signaling on injury response. We previously used this timeline to measure the modulatory effect of the opioid agonist Dermorphin [D-Arg2, Lys4] (1-4) amide (DALDA) on I/R-induced injury.12,18 The extent of necrosis between WT and Nod2−/− mice was comparable after 0.5 and 1 hour of ischemia (Supplemental Figure S1). However, WT mice exhibited improved recovery outcome over Nod2−/− mice after reperfusion (scores: 4.5 ± 0.3 versus 8.6 ± 1.4; P < 0.05) (Figure 2A). More important, i.p. administration of 10 mg/kg MDP, but not the inactive enantiomer, l-MDP, protected WT mice against I/R-induced injury (scores: 6.6 ± 0.9 versus 3.3 ± 0.8; P < 0.05) (Figure 2B), whereas it failed to protect Nod2−/− mice (scores: 8.3 ± 1.4 versus 8.6 ± 1.4) (Figure 2B). The improved intestinal response after MDP treatment in the WT mice is noted by the restitution of the villi, whereas Nod2−/− mice showed epithelium denudement of the villi, leaving only the crypts (Figure 2C).
Figure 2.
MDP signaling protects against I/R-induced injury. WT and Nod2−/− mice were subjected to ileal ischemia for 1 hour, followed by 1.5 hours of reperfusion. NOD2 ligand, 1 mg/kg MDP, or 1 mg/kg of its enantiomer, l-MDP, was injected i.p. in WT mice 24 hours before I/R exposure. Healthy and injured tissues were collected, Swiss rolled, and stained with H&E. Necrosis was assessed using an established necrosis scoring system. A and B: Histological intestinal damage scores of individual mice are depicted. C: Representative images of H&E-stained ileal sections. Results are representative of three independent experiments (n ≥ 4 per group). Data are given as means ± SEM (A and B). ∗P < 0.05. Scale bar = 100 μm (C). H, healthy; I/R, 1 hour ischemia, followed by 1.5 hours’ reperfusion.
Microbially Induced Expression of NOD2 in IECs Is Necessary for Protection against Injury
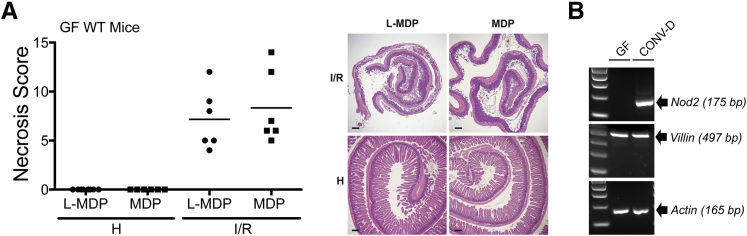
A previous report showed that Nod2 expression in the intestine is lower in GF mice than SPF mice.12 Therefore, we hypothesized that MDP-mediated protective effects would be ablated in GF mice because of impaired NOD2 expression. Indeed, MDP-administered GF mice were not protected from I/R-induced injury compared with l-MDP controls (scores: 7.2 ± 3.0 versus 8.3 ± 3.7) (Figure 3A), a phenomenon correlating with lack of detectable Nod2 mRNA in IECs isolated from the terminal ileum of GF mice (Figure 3B). Transferring GF mice in SPF condition for 4 weeks resulted in detectable NOD2 mRNA expression (Figure 3B), suggesting that bacteria regulate Nod2 expression and signaling. Taken together, these results suggest that Nod2 expression/signaling is dependent on commensal microbes signaling, likely mediating protection against I/R-induced injury.
Figure 3.
Microbial induction of NOD2 expression in IECs is necessary for protection against injury. A: Germ free (GF), WT mice were subjected to ileal ischemia for 1 hour, and followed by 3 hours of reperfusion. NOD2 ligand, 1 mg/kg MDP, or 1 mg/kg of its enantiomer, l-MDP, was injected i.p. in mice 24 hours before I/R exposure. Healthy and injured tissue was collected, Swiss rolled, and stained with H&E. Necrosis was assessed using an established necrosis scoring system. Histological intestinal damage scores of individual mice are depicted (left), and representative images of H&E-stained ileal sections are shown (right). B: IECs were isolated from GF and CONV-D WT mice, and Nod2 mRNA level was assessed using RT-PCR. Actin and Villin mRNAs were amplified as loading and isolation efficacy, respectively. Scale bar = 100 μm (B). H, healthy; I, ischemia only; I/R, 1 hour ischemia, followed by 3 hours’ reperfusion.
I/R-Induced Injury Causes Hypoxic Stress and Autophagy in WT and Nod2−/− Mice
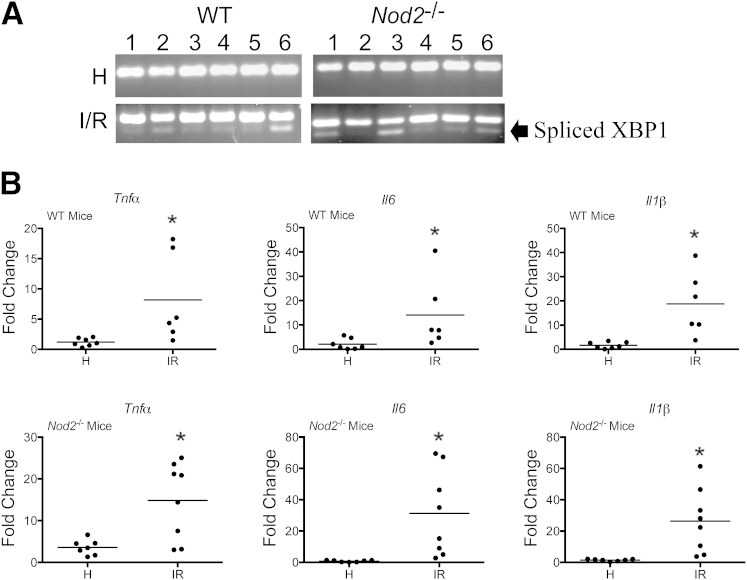
Further evaluation of the injury using electron microscopy analysis showed cytoplasmic thinning, mitochondrial swelling, and autophagosome and late endosome formation, all cellular characteristics of hypoxic tissues29,30 (Supplemental Figure S2). In related models of I/R-induced injury, the endoplasmic reticulum (ER) stress marker XPB1 has been shown to accumulate in hypoxic tissues.31,32 We evaluated the expression of spliced Xbp1 mRNA in injured tissues from WT mice and compared it with adjacent healthy tissue. We observed an accumulation of spliced Xbp1 mRNA in the injured tissue of both WT and Nod2−/− mice (Figure 4A). In addition, cytokine expression has been used as an indicator of hypoxia in I/R-induced injury,33 as well as in related models of hypoxia in vitro34 and in vivo.6–8,35 Therefore, we evaluated Tnfα, Il-6, and Il-1β mRNA accumulation in ileal tissue of WT and Nod2−/− mice that underwent I/R-induced injury. As expected, I/R-induced injury increased Tnfα, Il-6, and Il-1β mRNA accumulation in WT and Nod2−/− mice compared to cytokine levels in adjacent healthy tissue to the site of ischemia (Figure 4B). No noticeable differences were observed in ER-stress response (Xbp1 or cytokines) between WT and Nod2−/− mice, suggesting that increased tissue damage in Nod2−/− mice (Figure 2) is not related to the extent of ER-stress response.
Figure 4.
I/R-induced injury induces ER stress in WT and Nod2−/− mice. WT and Nod2−/− mice were subjected to I/R-induced injury. A: RT-PCR of ER-stress response marker, XBP1, was performed and resolved on a 3% agarose gel. B: Il1b, Il6, and Tnfα mRNAs from healthy and injured ileal tissues were determined using ABI Prism 7900HT (Life Technologies, Grand Island, NY). Data were processed using the ΔΔCT method, normalized to β-actin, and set relative to healthy tissue. Results are representative of three independent experiments. ∗P < 0.05 (n ≥ 4 per group). H, healthy; I/R, 1 hour ischemia, followed by 1.5 hours’ reperfusion.
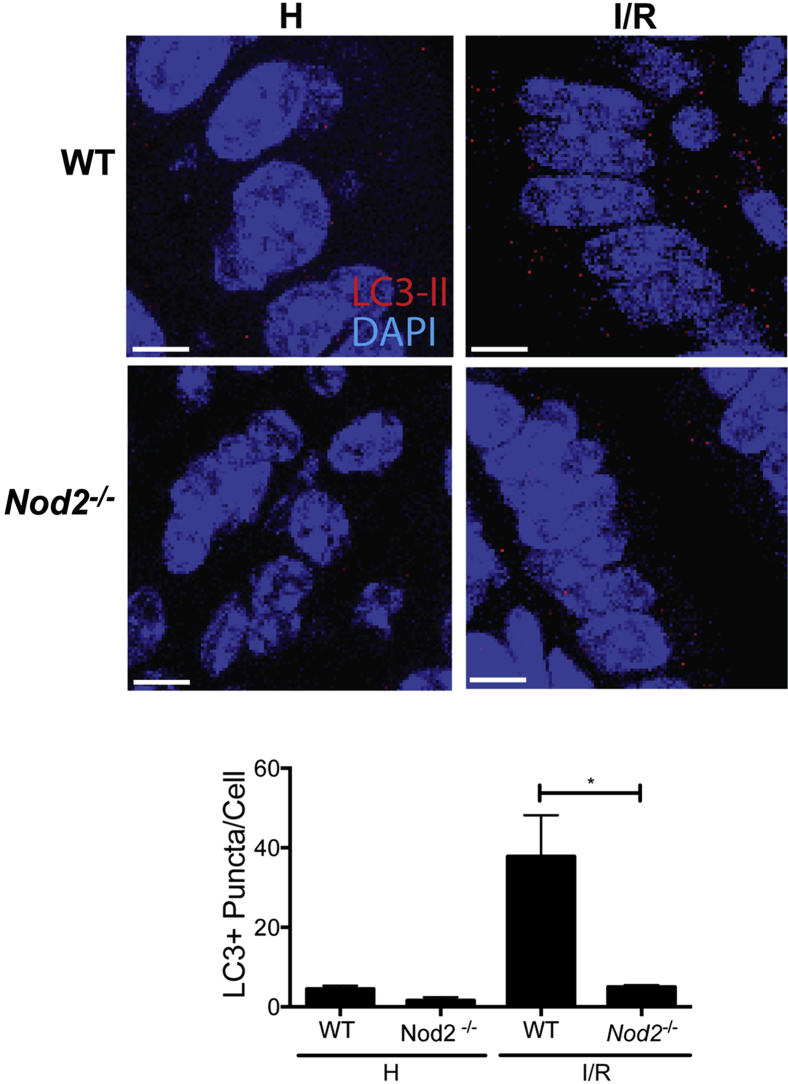
Recent studies show that hypoxic stress in the intestinal mucosa occurs during I/R-induced injury,4 which associates with increased expression of autophagy marker, LC3, in the small bowel.29 Therefore, we determined LC3 levels in WT and Nod2−/− mice exposed to I/R conditions. At homeostasis, healthy tissue of WT and Nod2−/− mice show low detectable amounts of cytoplasmic LC3-positive (LC3+) puncta. On I/R-induced injury, the amount of LC3+ puncta per cell increased substantially in the epithelium of WT mice (37.8 ± 10.37 puncta per cell), compared with Nod2−/− mice (5.0 ± 0.4 puncta per cell) (Figure 5). Taken together, these results indicate that I/R-induced injury is associated with ER stress and induction of autophagy, a phenomenon dependent on NOD2 signaling.
Figure 5.
I/R-induced injury induces autophagy through NOD2. Immunofluorescence staining of LC3 was performed on paraffin-embedded healthy and injured ileal tissue from WT and Nod2−/− mice. NOD2 ligand, 1 mg/kg MDP, or 1 mg/kg of its enantiomer, l-MDP, was injected i.p. in mice 24 hours before I/R exposure. The average number of LC3+ puncta per cell in the injured and healthy portions of the terminal ileum of untreated WT and Nod2−/− mice and corresponding representative images of the intestinal epithelium of mice. ∗P < 0.05 (results are representative of three fields of view per sample). Scale bar = 5 μm. H, healthy; I/R, 1 hour ischemia, followed by 1.5 hours’ reperfusion.
Rapamycin Rescues Autophagy-Mediated Protection in Nod2−/− Mice, But Not WT Mice, from I/R-Induced Injury
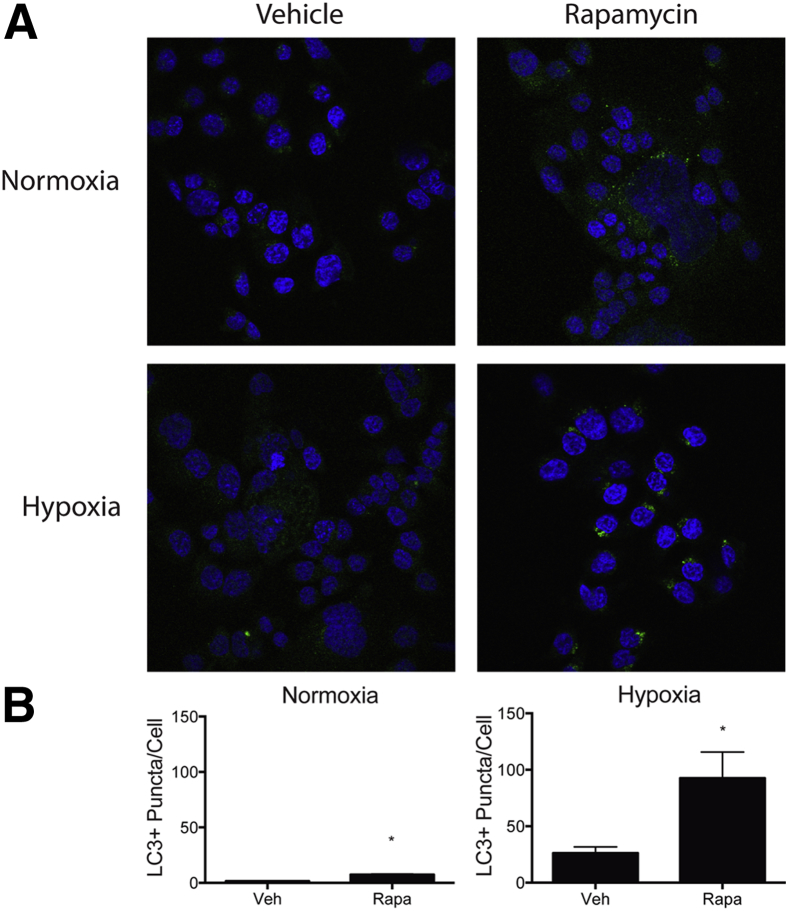
The lack of Nod2 mRNA expression in GF IECs and increased expression of the innate sensor after bacterial colonization, which correlated with protection against I/R-induced injury, suggest that these cells may be important for injury response. We hypothesized that inducing the autophagy response in IECs would alleviate I/R-induced injury in Nod2−/− mice. We first tested whether the autophagy inducer, rapamycin, could modulate hypoxia-induced autophagy using a reductionist in vitro system. Rapamycin has previously been shown to attenuate I/R-induced injury in the gut, pancreas, and heart.8,36,37 Human colonic epithelial HCT116 cells, which constitutively expressed NOD2, were exposed to hypoxic conditions (1% O2) using a Coy Labs hypoxic glove box and then treated with 500 nmol/L rapamycin for 1 hour. To monitor autophagic flux, the cells were pretreated for 1 hour with proteasome inhibitors E-64-d and Pepstatin A and autophagy response was evaluated by measuring LC3+ puncta per cell using immunofluorescence staining. As expected, the baseline of LC3+ puncta was elevated under hypoxic stress. Remarkably, hypoxic cells exposed to rapamycin displayed a strong increase in the number of LC3+ puncta per cell, compared with un-stimulated conditions (Figure 6).
Figure 6.
Rapamycin (Rapa) enhances hypoxia-induced autophagy in human colon colorectal carcinoma HCT116 cells. HCT116 cells were treated with 10 μg/mL pepstatin A and 10 μg/mL E-64-d 1 hour before exposure to 1 hour of normoxia or hypoxia (1% O2, Coy Labs hypoxic glove box) and stimulated by 5 μg/mL Rapa. LC3+ puncta (green fluorescent protein) and cells (DAPI) were counted using ImageJ software. A: Representative images of LC3+ puncta in HCT116 cells stimulated with Rapa in normoxic and hypoxic conditions. B: The average number of puncta per cell of cells stimulated with Rapa in normoxic and hypoxic conditions. Results are representative of three independent experiments. ∗P < 0.05. Veh, vehicle.
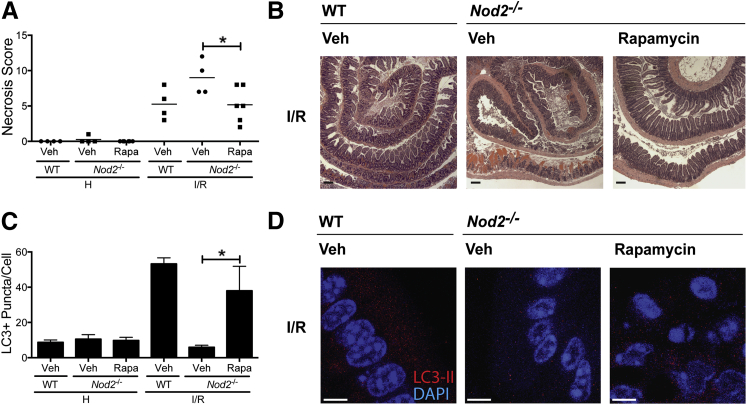
To specifically determine the beneficial effect of autophagic response on I/R injury, we evaluated whether rapamycin could rescue Nod2−/− mice from exacerbated I/R-induced injury. Cohorts of WT and Nod2−/− mice were i.p. administered 3 mg/kg rapamycin or vehicle (DMSO) 1 hour before I/R-induced injury and tissue damage evaluated using H&E staining. Interestingly, rapamycin decreased I/R-induced injury in Nod2−/− mice compared with the level of injury observed in vehicle-treated mice (scores: 9.0 ± 1.2 versus 5.2 ± 1.0; P < 0.05), as shown by the nascent villi of the restituted epithelium (Figure 7, A and B). WT mice trended toward protection against injury, but the effect of rapamycin was marginal, likely due to functional NOD2 signaling in these mice (data not shown). In addition, rapamycin treatment increased the number of detectable LC3+ puncta per cell in Nod2−/− mice (37.9 ± 13.9 puncta per cell) compared with the number in vehicle-treated mice (5.9 ± 2.0 puncta per cell), suggesting that NOD2 contributes to autophagy response that fosters the recovery of the intestinal epithelium (Figure 7, C and D). Overall, our findings indicate that intestinal bacteria contribute to epithelial cell recovery from I/R-induced damage through NOD2 signaling and improved autophagy response.
Figure 7.
Rapamycin (Rapa)–induced autophagy protects Nod2−/− mice against I/R-induced injury. WT and Nod2−/− mice were subjected to ileal ischemia, followed by 1.5 hours of reperfusion. Autophagy-inducer, Rapa (3 mg/kg), or its vehicle (Veh), DMSO, was injected i.p. in Nod2−/− 1 hour before I/R exposure. Healthy and injured tissues were collected, Swiss rolled, and stained with H&E. Necrosis was assessed using an established necrosis scoring system. A: Histological intestinal damage scores of individual. B: Representative images of H&E-stained ileal sections. Results are representative of two independent experiments (n ≥ 4 per group). C and D: The average number of LC3+ puncta per cell and representative images of the intestinal epithelium of mice administered either Rapa or Veh. Results are representative of three independent fields of view per sample. Data are given as means ± SEM (A). ∗P < 0.05. Scale bars: 100 μm (B); 5 μm (C and D). H, healthy; I/R, 1 hour ischemia, followed by 1.5 hours of reperfusion; Veh, vehicle.
Discussion
Intestinal I/R-induced injury results from a wide range of pathological conditions, such as inflammation, infection, atherosclerotic clots, and surgery, and may lead to fatal multiple organ dysfunction syndrome.4 Because epithelial injury occurs in the context of a complex and rich microbiota, which significantly affects various intestinal functions, such as innate and adaptive immune system toning, epithelial homeostasis, and nutrient metabolism,38 one could posit that microbes would influence injury response. Already, microbial sensors have been linked to I/R injury response,39,40 although microbial contribution to this phenotype has not been clearly established. Herein, we directly address this possibility and show that the microbiota is an essential component to optimal epithelial recovery from injury. In addition, the beneficial effect of the microbiota requires the presence of NOD2, a finding consistent with a recent report showing that NOD2 engages cytoprotective programs within intestinal stem cells after chemical-induced damage.41 Nod2-deficient mice, unlike WT mice, showed exacerbated injury response, even when transfer from GF to SPF conditions. On colonization, the microbiota induced the expression of Nod2 through stimulation of other PRRs, which activate NF-κB in vivo,42,43 a key transcription factor involved in NOD2 expression.44 At a mechanistic level, NOD2 may be essential to activate a proper autophagic response after hypoxia exposure because rapamycin partially rescues Nod2−/− mice from I/R-induced injury.
The microbiota performs multiple tasks in the gut, including pathogen exclusion, immune system toning, and metabolic functions.45 Several reports demonstrate that the microbiota also contributes to various aspects of barrier function.46 For example, GF animals have decreased rates of epithelial cell turnover in the small bowel, as well as decreased TLR and antimicrobial peptide expression.47 In addition, GF mice exhibited heightened dextran sodium sulfate (DSS)-,48 radiation-,49 and thermal-induced injury,50 when compared with CONV-D mice. Our findings that recovery from I/R-induced injury is impaired in GF mice compared with CONV-D mice add to the growing evidence that microbes play a protective role against a multitude of injury insults.
The microbiota could benefit the host against injury through several mechanisms, including activation of the PRR signaling pathway (eg, TLR2 or TLR4)51 and production of bacterial-derived metabolites, such as short chain fatty acids.52 Because CONV-D Nod2−/− mice do not show protection against I/R-induced injury relative to GF Nod2−/− mice, and MDP greatly improved injury recovery in WT mice, but not Nod2−/− mice, we conclude that the presence of this innate sensor, and not microbial metabolites, is essential for microbiota-mediated injury response. Regarding PRR pathways, TLR4 signaling has been shown to protect against I/R-induced intestinal injury.40 Moreover, lipopolysaccharide (LPS)/TLR4 signaling increases NOD2 mRNA expression in macrophages.53 A similar regulatory system was shown to induce NOD2 expression and function in other epithelial cells.54 Whether TLR4-mediated intestinal protection is mediated through NOD2 function is currently unknown. Interestingly, i.p. LPS injection in GF mice rapidly induces (approximately 4 hours) Nod2 mRNA accumulation in IECs (E.P.-C. and C.J., personal observation). Because MDP failed to protect GF mice from I/R-induced injury, it is tempting to speculate that bacteria/LPS-induced Nod2 expression in GF mice is required for MDP-mediated effect. The interplay between TLR4 and NOD2 signaling in mediating host response to intestinal injury remains to be defined.
Our findings, however, are contrary to previous studies using antibiotics as a means to modulate bacterial load.27 This discrepancy highlights the complexity behind the host-microbe relationship and suggests that perturbations to the existing microbial community by antibiotics affect the underlying signaling mechanisms implicated in epithelial restitution. For instance, the extirpation of specific beneficial microbial taxa and the bloom of resistant organisms likely affect the production of metabolic products that modulate the host's response to injury.55,56 Whether antibiotics alter a selective group of bacteria implicated in tissue injury is currently unknown. Antibiotics reduce type 17 helper T-cell (Th17) lymphocyte populations in the small bowel in the hosts.57 Because Th17 cells contribute to intestinal I/R injury,58 antibiotics may benefit epithelial restitution through depletion of Th17-associated pathogenic effects. Nevertheless, the extent to which antibiotics interfere with microbial signaling requires further study.
The autophagy response is the culmination of hypoxia and ER stress during ischemia. Hypoxia has been previously shown to be an outcome of intestinal I/R-induced injury.59 Moreover, autophagy resulting from hypoxia is a protective mechanism in the heart36,60 and in I/R-induced kidney injury.61 During the hour-long ischemic insult, the damage to the intestinal epithelium is similar between WT and Nod2−/− mice. Both WT and Nod2−/− mice are able to effectively activate ER-stress response programs, as seen by Xbp1 splicing, electron microscopy, and the induction of inflammatory mediators Il1β, Il6, and Tnfα. However, the presence of NOD2 is necessary for effective epithelial restitution after ischemia, likely through induction of autophagy. Hypoxia-induced autophagy is potentiated by microbial signaling through NOD2, as demonstrated by the higher abundance of LC3 puncta in the epithelium of injured WT mice compared with Nod2−/− mice. In addition, increased LC3+ puncta is observed in the epithelium of rapamycin-treated Nod2−/− mice, with a concomitant improved epithelial injury response. The exact role of autophagy in I/R-induced injury is not clear but could represent an essential cellular adaptation, maintaining cellular energy balance when access to external nutrients and oxygen is ablated, as in the case for ischemic tissues.
Microbial dysbiosis has been linked to various diseases, including inflammatory bowel diseases62 and colorectal cancer,63 and one could postulate that defective injury response observed in Nod2−/− mice is due to altered microbiome composition. Interestingly, DSS-induced colitis and colitis-associated colorectal cancer in Nod2−/− mice could be transmitted to WT mice by fecal transplantation,64 suggesting that microbial dysbiosis in Nod2−/− mice could transfer disease phenotype. However, whether NOD2 signaling influences microbial composition is controversial because Shanahan et al65 demonstrated that Nod2−/− mice and cohoused WT littermates displayed comparable AMP expression patterns and identical antimicrobial activity against commensal and pathogenic bacteria. In addition, microbial composition was not influenced by NOD2 status.66 Because we observed that GF Nod2−/− and GF WT mice showed enhanced I/R-induced injury, it is unlikely that this response is due to a dysbiotic microbiome. The fact that MDP partially restores host response to injury in WT mice indicates that microbial products, rather than alteration in microbial composition, are responsible for the beneficial effect.
Recently, Mühlbauer et al19 showed that specific removal of MyD88 signaling from IECs attenuates intestinal injury, suggesting that this pathway promotes intestinal injury. Because MyD88 is a key signaling protein implicated in activation of numerous microbial detection systems (TLR2, TLR4, TLR5, and TLR9), our finding that bacteria use NOD2 to mediate a protective function highlights the complex interaction between bacteria, innate signaling, and intestinal homeostasis. It is likely that an ensemble of forward, feedback, and negative signaling cascades co-exist in the intestine to regulate intestinal homeostasis and response to various insults, including I/R. Differential engagement of this complex signaling network would dictate the final physiological outcome (deleterious or beneficial).
In summary, this study identified a protective role for microbial signaling through NOD2 in I/R-induced injury. The microbiota protects the intestinal epithelium from injury by inducing Nod2 expression and signaling in IECs, which induces the autophagy response. Although NOD2 deficiency worsens injury, rapamycin rescues epithelial restitution and the autophagy response in Nod2−/− mice. These findings indicate that NOD2 may induce the canonical autophagy response as a cytoprotective response to injury, allowing cells to survive and recover from the insult. Further studies using genetically engineered mice will delineate the NOD2 signaling compartment (IEC versus immune cells) involved in injury response as well as define signaling molecules downstream of NOD2 by which autophagy is in this process. On the basis of the absence of Nod2 mRNA in the IECs of GF mice and induction of Nod2 message in IECs after colonization, we predict that NOD2-derived IEC signaling is the main cellular compartment mediating intestinal recovery to I/R injury.
Acknowledgments
We thank Dr. Balfour Sartor and Maureen Bower (National Gnotobiotic Rodent Resource Center at University of North Carolina) as well as Stephanie Cormier (Gnotobiotic Facility at the University of Florida) for assistance with GF mice (NIH P40 R018603). Histology was performed at the Center for Gastrointestinal Biology and Disease histology core (P30 DK034987). We thank Sarah Tomkovich, Dr. Ye Yang, and Dr. Xiaolun Sun for editorial assistance.
Footnotes
Supported by NIH grants R01DK047700 and R01DK073338 (C.J.). GF mouse work was supported in part by NIH grant P40 R018603, and the Center for Gastrointestinal Biology and Disease histology core was supported in part by NIH grant P30 DK034987 (both to University of North Carolina at Chapel Hill).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosures: None declared.
Supplemental Data
WT and Nod2−/− mice show comparable ischemic injury. WT and Nod2−/− mice were subjected to ileal ischemia for 0.5 or 1 hour. Healthy and injured tissues were collected, Swiss rolled, and stained with H&E. Necrosis was assessed using an established necrosis scoring system. Histological intestinal damage scores of individual mice are depicted. Scale bar = 100 μm. H, healthy; I, ischemia.
I/R-induced injury induces ER-stress in WT and Nod2−/− mice. WT and Nod2−/− mice were subjected to ileal ischemia for 30 minutes. Healthy and damaged tissues were collected in 4% paraformaldehyde and prepared for electron microscopy analysis. AP, autophagosome; EE, early endosome; H, healthy; LE, late endosome; M, mitochondria.
References
- 1.Abreu M.T. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 2.Prescott D., Lee J., Philpott D.J. An epithelial armamentarium to sense the microbiota. Semin Immunol. 2013;25:323–333. doi: 10.1016/j.smim.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Karrasch T., Jobin C. Wound healing responses at the gastrointestinal epithelium: a close look at novel regulatory factors and investigative approaches. Z Gastroenterol. 2009;47:1221–1229. doi: 10.1055/s-0028-1109766. [DOI] [PubMed] [Google Scholar]
- 4.Eltzschig H.K., Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farber A., Connors J.P., Friedlander R.M., Wagner R.J., Powell R.J., Cronenwett J.L. A specific inhibitor of apoptosis decreases tissue injury after intestinal ischemia-reperfusion in mice. J Vasc Surg. 1999;30:752–760. doi: 10.1016/s0741-5214(99)70115-1. [DOI] [PubMed] [Google Scholar]
- 6.Travassos L.H., Carneiro L.A.M., Girardin S., Philpott D.J. Nod proteins link bacterial sensing and autophagy. Autophagy. 2010;6:409–411. doi: 10.4161/auto.6.3.11305. [DOI] [PubMed] [Google Scholar]
- 7.Matsui Y., Kyoi S., Takagi H., Hsu C.-P., Hariharan N., Ago T., Vatner S.F., Sadoshima J. Molecular mechanisms and physiological significance of autophagy during myocardial ischemia and reperfusion. Autophagy. 2008;4:409–415. doi: 10.4161/auto.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evankovich J., Zhang R., Cardinal J.S., Zhang L., Chen J., Huang H., Beer-Stolz D., Billiar T.R., Rosengart M.R., Tsung A. Calcium/calmodulin-dependent protein kinase IV limits organ damage in hepatic ischemia-reperfusion injury through induction of autophagy. Am J Physiol Gastrointest Liver Physiol. 2012;303:G189–G198. doi: 10.1152/ajpgi.00051.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sala-Mercado J.A., Wider J., Reddy Undyala V.V., Jahania S., Yoo W., Mentzer R.M., Gottlieb R.A., Przyklenk K. Profound cardioprotection with chloramphenicol succinate in the swine model of myocardial ischemia-reperfusion injury. Circulation. 2010;122:S179–S184. doi: 10.1161/CIRCULATIONAHA.109.928242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correa R.G., Milutinovic S., Reed J.C. Roles of NOD1 (NLRC1) and NOD2 (NLRC2) in innate immunity and inflammatory diseases. Biosci Rep. 2012;32:597–608. doi: 10.1042/BSR20120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rehman A., Sina C., Gavrilova O., Häsler R., Ott S., Baines J.F., Schreiber S., Rosenstiel P. Nod2 is essential for temporal development of intestinal microbial communities. Gut. 2011;60:1354–1362. doi: 10.1136/gut.2010.216259. [DOI] [PubMed] [Google Scholar]
- 12.Petnicki-Ocwieja T., Hrnčíř T., Liu Y.-J., Biswas A., Hudcovic T., Tlaskalová-Hogenová H., Kobayashi K.S. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci U S A. 2009;106:15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wehkamp J., Harder J., Weichenthal M., Schwab M., Schäffeler E., Schlee M., Herrlinger K.R., Stallmach A., Noack F., Fritz P., Schröder J.M., Bevins C.L., Fellermann K., Stange E.F. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu L.-C., Ali S.R., McGillivray S., Tseng P.-H., Mariathasan S., Humke E.W., Eckmann L., Powell J.J., Nizet V., Dixit V.M., Karin M. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A. 2008;105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson W.M., Sodhi C.P., Russo A., Siggers R.H., Afrazi A., Gribar S.C., Neal M.D., Dai S., Prindle T., Jr., Branca M., Ma C., Ozolek J., Hackam D.J. Nucleotide-binding oligomerization domain-2 inhibits toll-like receptor-4 signaling in the intestinal epithelium. Gastroenterology. 2010;139:904–917. doi: 10.1053/j.gastro.2010.05.038. 917.e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizushima N., Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Travassos L.H., Carneiro L.A.M., Ramjeet M., Hussey S., Kim Y.-G., Magalhães J.G., Yuan L., Soares F., Chea E., Le Bourhis L., Boneca I.G., Allaoui A., Jones N.L., Nuñez G., Girardin S.E., Philpott D.J. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2009;11:55–62. doi: 10.1038/ni.1823. [DOI] [PubMed] [Google Scholar]
- 18.Goldsmith J.R., Perez-Chanona E., Yadav P.N., Whistler J., Roth B., Jobin C. Intestinal epithelial cell-derived μ-opioid signaling protects against ischemia reperfusion injury through PI3K signaling. Am J Pathol. 2013;182:776–785. doi: 10.1016/j.ajpath.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mühlbauer M., Perez-Chanona E., Jobin C. Epithelial cell-specific MyD88 signaling mediates ischemia/reperfusion-induced intestinal injury independent of microbial status. Inflamm Bowel Dis. 2013;19:2857–2866. doi: 10.1097/01.MIB.0000435445.96933.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jilling T., Lu J., Jackson M., Caplan M.S. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatr Res. 2004;55:622–629. doi: 10.1203/01.PDR.0000113463.70435.74. [DOI] [PubMed] [Google Scholar]
- 21.Uronis J.M., Mühlbauer M., Herfarth H.H., Rubinas T.C., Jones G.S., Jobin C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One. 2009;4:e6026. doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue X. Tumor necrosis factor (TNF) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNF. J Biol Chem. 2005;280:33917–33925. doi: 10.1074/jbc.M505818200. [DOI] [PubMed] [Google Scholar]
- 23.Sun X., Threadgill D., Jobin C. Campylobacter jejuni induces colitis through activation of mammalian target of rapamycin signaling. Gastroenterology. 2012;142:86–95. doi: 10.1053/j.gastro.2011.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joo Y.-E., Karrasch T., Mühlbauer M., Allard B., Narula A., Herfarth H.H., Jobin C. Tomato lycopene extract prevents lipopolysaccharide-induced NF-kappaB signaling but worsens dextran sulfate sodium-induced colitis in NF-kappaBEGFP mice. PLoS One. 2009;4:e4562. doi: 10.1371/journal.pone.0004562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanida I., Ueno T., Kominami E. LC3 and autophagy. Methods Mol Biol. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- 26.Potze L., Mullauer F.B., Colak S., Kessler J.H., Medema J.P. Betulinic acid-induced mitochondria-dependent cell death is counterbalanced by an autophagic salvage response. Cell Death Dis. 2014;5:e1169. doi: 10.1038/cddis.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshiya K., Lapchak P.H., Thai T.H., Kannan L., Rani P., Lucca J.J.D., Tsokos G.C. Depletion of gut commensal bacteria attenuates intestinal ischemia/reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1020–G1030. doi: 10.1152/ajpgi.00239.2011. [DOI] [PubMed] [Google Scholar]
- 28.van Ampting M.T.J., Schonewille A.J., Vink C., Brummer R.J.M., van der Meer R., Bovee-Oudenhoven I.M.J. Damage to the intestinal epithelial barrier by antibiotic pretreatment of Salmonella-infected rats is lessened by dietary calcium or tannic acid. J Nutr. 2010;140:2167–2172. doi: 10.3945/jn.110.124453. [DOI] [PubMed] [Google Scholar]
- 29.Neal M.D., Sodhi C.P., Dyer M., Craig B.T., Good M., Jia H., Yazji I., Afrazi A., Richardson W.M., Beer-Stolz D., Ma C., Prindle T., Grant Z., Branca M.F., Ozolek J., Hackam D.J. A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J Immunol. 2013;190:3541–3551. doi: 10.4049/jimmunol.1202264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeda H., Nagai H., Takemura G., Shintani-Ishida K., Komatsu M., Ogura S., Aki T., Shirai M., Kuwahira I., Yoshida K.-I. Intermittent-hypoxia induced autophagy attenuates contractile dysfunction and myocardial injury in rat heart. Biochim Biophys Acta. 2013;1832:1159–1166. doi: 10.1016/j.bbadis.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Karar J., Dolt K.S., Qadar Pasha M.A. Endoplasmic reticulum stress response in murine kidney exposed to acute hypobaric hypoxia. FEBS Lett. 2008;582:2521–2526. doi: 10.1016/j.febslet.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Thuerauf D.J. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res. 2006;99:275–282. doi: 10.1161/01.RES.0000233317.70421.03. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y.-Z., Wu C.-C., Huang Y.-C., Huang C.-Y., Yang C.-Y., Lee T.-C., Chen C.-F., Yu L.C.-H. Neutrophil priming by hypoxic preconditioning protects against epithelial barrier damage and enteric bacterial translocation in intestinal ischemia/reperfusion. Lab Invest. 2012;92:783–796. doi: 10.1038/labinvest.2012.11. [DOI] [PubMed] [Google Scholar]
- 34.Clambey E.T., McNamee E.N., Westrich J.A., Glover L.E., Campbell E.L., Jedlicka P., de Zoeten E.F., Cambier J.C., Stenmark K.R., Colgan S.P., Eltzschig H.K. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A. 2012;109:E2784–E2793. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riggle K.M., Rentea R.M., Welak S.R., Pritchard K.A., Oldham K.T., Gourlay D.M. Intestinal alkaline phosphatase prevents the systemic inflammatory response associated with necrotizing enterocolitis. J Surg Res. 2013;180:21–26. doi: 10.1016/j.jss.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen H.H., Mekkaoui C., Cho H., Ngoy S., Marinelli B., Waterman P., Nahrendorf M., Liao R., Josephson L., Sosnovik D.E. Fluorescence tomography of rapamycin-induced autophagy and cardioprotection in vivo. Circ Cardiovasc Imaging. 2013;6:441–447. doi: 10.1161/CIRCIMAGING.112.000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wen S.-H., Li Y., Li C., Xia Z.-Q., Liu W.-F., Zhang X.-Y., Lei W.-L., Huang W.-Q., Liu K.-X. Ischemic postconditioning during reperfusion attenuates intestinal injury and mucosal cell apoptosis by inhibiting jak/stat signaling activation. Shock. 2012;38:411–419. doi: 10.1097/SHK.0b013e3182662266. [DOI] [PubMed] [Google Scholar]
- 38.Sartor R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 39.Nace G.W., Huang H., Klune J.R., Eid R.E., Rosborough B.R., Korff S., Li S., Shapiro R.A., Stolz D.B., Sodhi C.P., Hackam D.J., Geller D.A., Billiar T.R., Tsung A. Cellular-specific role of toll-like receptor 4 in hepatic ischemia-reperfusion injury in mice. Hepatology. 2013;58:374–387. doi: 10.1002/hep.26346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tatum P.M., Jr., Harmon C.M., Lorenz R.G., Dimmitt R.A. Toll-like receptor 4 is protective against neonatal murine ischemia-reperfusion intestinal injury. J Pediatr Surg. 2010;45:1246–1255. doi: 10.1016/j.jpedsurg.2010.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nigro G., Rossi R., Commere P.H., Jay P., Sansonetti P.J. The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe. 2014;15:792–798. doi: 10.1016/j.chom.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Magness S.T., Jijon H., Van Houten Fisher N., Sharpless N.E., Brenner D.A., Jobin C. In vivo pattern of lipopolysaccharide and anti-CD3-induced NF-kappa B activation using a novel gene-targeted enhanced GFP reporter gene mouse. J Immunol. 2004;173:1561–1570. doi: 10.4049/jimmunol.173.3.1561. [DOI] [PubMed] [Google Scholar]
- 43.Karrasch T., Kim J.-S., Mühlbauer M., Magness S.T., Jobin C. Gnotobiotic IL-10-/-;NF-kappa B(EGFP) mice reveal the critical role of TLR/NF-kappa B signaling in commensal bacteria-induced colitis. J Immunol. 2007;178:6522–6532. doi: 10.4049/jimmunol.178.10.6522. [DOI] [PubMed] [Google Scholar]
- 44.Strober W., Murray P.J., Kitani A., Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2005;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 45.Packey C.D., Sartor R.B. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr Opin Infect Dis. 2009;22:292–301. doi: 10.1097/QCO.0b013e32832a8a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guzman J.R., Conlin V.S., Jobin C. Diet, microbiome, and the intestinal epithelium: an essential triumvirate? Biomed Res Int. 2013;2013:425146. doi: 10.1155/2013/425146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitajima S., Morimoto M., Sagara E., Shimizu C., Ikeda Y. Dextran sodium sulfate-induced colitis in germ-free IQI/Jic mice. Exp Anim. 2001;50:387–395. doi: 10.1538/expanim.50.387. [DOI] [PubMed] [Google Scholar]
- 49.Packey C.D., Ciorba M.A. Microbial influences on the small intestinal response to radiation injury. Curr Opin Gastroenterol. 2010;26:88–94. doi: 10.1097/MOG.0b013e3283361927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deitch E.A. Thermal injury promotes bacterial translocation from the gastrointestinal tract in mice with impaired T-cell–mediated immunity. Arch Surg. 1986;121:97–101. doi: 10.1001/archsurg.1986.01400010111015. [DOI] [PubMed] [Google Scholar]
- 51.Aprahamian C.J., Lorenz R.G., Harmon C.M., Dimmit R.A. Toll-like receptor 2 is protective of ischemia–reperfusion-mediated small-bowel injury in a murine model. Pediatr Crit Care Med. 2008;9:105–109. doi: 10.1097/01.PCC.0000288717.44702.C0. [DOI] [PubMed] [Google Scholar]
- 52.Aguilar-Nascimento J.E., Salomão A.B., Nochi R.J., Jr., Nascimento M., Neves Jde S. Intraluminal injection of short chain fatty acids diminishes intestinal mucosa injury in experimental ischemia-reperfusion. Acta Cir Bras. 2006;21:21–25. doi: 10.1590/s0102-86502006000100006. [DOI] [PubMed] [Google Scholar]
- 53.Tsai W.-H., Huang D.-Y., Yu Y.-H., Chen C.-Y., Lin W.-W. Dual roles of NOD2 in TLR4-mediated signal transduction and -induced inflammatory gene expression in macrophages. Cell Microbiol. 2011;13:717–730. doi: 10.1111/j.1462-5822.2010.01567.x. [DOI] [PubMed] [Google Scholar]
- 54.Mühlbauer M., Cheely A.W., Yenugu S., Jobin C. Regulation and functional impact of lipopolysaccharide induced Nod2 gene expression in the murine epididymal epithelial cell line PC1. Immunology. 2008;124:256–264. doi: 10.1111/j.1365-2567.2007.02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sturm A., Dignass A.U. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol. 2008;14:348–353. doi: 10.3748/wjg.14.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho I., Yamanishi S., Cox L., Methé B.A., Zavadil J., Li K., Gao Z., Mahana D., Raju K., Teitler I., Li H., Alekseyenko A.V., Blaser M.J. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ivanov I.I., Frutos Rde L., Manel N., Yoshinaga K., Rifkin D.B., Sartor R.B., Finlay B.B., Littman D.R. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edgerton C., Crispín J.C., Moratz C.M., Bettelli E., Oukka M., Simovic M., Zacharia A., Egan R., Chen J., Dalle Lucca J.J., Juang Y.-T., Tsokos G.C. IL-17 producing CD4+ T cells mediate accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. Clin Immunol. 2009;130:313–321. doi: 10.1016/j.clim.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kannan K.B., Colorado I., Reino D., Palange D., Lu Q., Qin X., Abungu B., Watkins A., Caputo F.J., Xu D.Z., Semenza G.L., Deitch E.A., Feinman R. Hypoxia-inducible factor plays a gut-injurious role in intestinal ischemia reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2011;300:G853–G861. doi: 10.1152/ajpgi.00459.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoo B.H., Wu X., Derouet M., Haniff M., Eskelinen E.-L., Rosen K. Hypoxia-induced downregulation of autophagy mediator Beclin-1 reduces the susceptibility of malignant intestinal epithelial cells to hypoxia-dependent apoptosis. Autophagy. 2009;5:1166–1179. doi: 10.4161/auto.5.8.10167. [DOI] [PubMed] [Google Scholar]
- 61.Jiang M., Wei Q., Dong G., Komatsu M., Su Y., Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82:1271–1283. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kostic A.D., Xavier R.J., Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146:1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwabe R.F., Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Couturier-Maillard A., Secher T., Rehman A., Normand S., De Arcangelis A., Haesler A., Huot L., Grandjean T., Bressenot A., Delanoye-Crespin A., Gaillot O., Schreiber S., Lemoine Y., Ryffel B., Hot D., Nùñez G., Chen G., Rosenstiel P., Chamaillard M. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest. 2013;123:700–711. doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shanahan M.T., Carroll I.M., Grossniklaus E., White A., von Furstenberg R.J., Barner R., Fodor A.A., Henning S.J., Sartor R.B., Gulati A.S. Mouse Paneth cell antimicrobial function is independent of Nod2. Gut. 2014;63:903–910. doi: 10.1136/gutjnl-2012-304190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robertson S.J., Zhou J.Y., Geddes K., Rubino S.J., Cho J.H., Girardin S.E., Philpott D.J. Nod1 and Nod2 signaling does not alter the composition of intestinal bacterial communities at homeostasis. Gut Microbes. 2013;4:222–231. doi: 10.4161/gmic.24373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
WT and Nod2−/− mice show comparable ischemic injury. WT and Nod2−/− mice were subjected to ileal ischemia for 0.5 or 1 hour. Healthy and injured tissues were collected, Swiss rolled, and stained with H&E. Necrosis was assessed using an established necrosis scoring system. Histological intestinal damage scores of individual mice are depicted. Scale bar = 100 μm. H, healthy; I, ischemia.
I/R-induced injury induces ER-stress in WT and Nod2−/− mice. WT and Nod2−/− mice were subjected to ileal ischemia for 30 minutes. Healthy and damaged tissues were collected in 4% paraformaldehyde and prepared for electron microscopy analysis. AP, autophagosome; EE, early endosome; H, healthy; LE, late endosome; M, mitochondria.