Abstract
Drug-induced osteonecrosis of the jaw (ONJ) is a detrimental intraoral lesion that often occurs after dental-related interventions in patients undergoing treatment with bisphosphonates or denosumab, the neutralizing human anti–receptor activator of NF-κB ligand (RANKL) antibody (Ab). The cause of ONJ by these drugs has been speculated to their direct effects on osteoclasts. However, the extent to which osteoclasts contribute to ONJ pathogenesis remains controversial. Herein, by using a tooth-extraction mouse model with i.v. administration of mouse anti-RANKL Ab or the bisphosphonate zoledronate (ZOL), we show that unresorbed bone due to impaired formation or suppressed functions of osteoclasts, respectively, is associated with ONJ development. After tooth extraction, ONJ-like lesions developed 50% in the anti-RANKL Ab-treated mice and 30% in the ZOL-treated mice. Nonviable and unresorbed bone was found more in anti-RANKL Ab-treated mice compared with mice receiving ZOL. All mice receiving anti-RANKL Ab had an undetectable tartrate-resistant acid phosphatase (TRAP) level in the serum and no TRAP-positive osteoclasts at the extracted sockets, whereas ZOL-treated mice had a decreased TRAP level without altering the numbers of TRAP-positive osteoclasts. Interestingly, the absence of newly formed woven bone in the extracted sockets was evident in ONJ-like lesions from both anti-RANKL Ab- and ZOL-treated mice. Our study suggests that the lack of osteoclasts' bone-resorptive functions by these drugs and suppression of woven bone formation after dental trauma may be associated with ONJ development.
CME Accreditation Statement: This activity (“ASIP 2014 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2014 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Bisphosphonates (BPs) and denosumab (Dmab) are anti-resorptive drugs that are clinically being used to treat bone-related diseases, such as osteoporosis or cancers with bone metastasis. Users of these drugs are known to be at higher risk of developing osteonecrosis of the jaw (ONJ), specifically in the oral cavity after dental interventions, which is clinically defined as exposed necrotic bone and unclosed overlaying oral mucosa for at least 8 weeks.1 In particular, ONJ incidence in patients with cancer (eg, breast, prostate, or multiple myeloma) receiving i.v. BPs is reported to range from 2% to 15%,2 although ONJ development in osteoporotic patients still remains controversial. Two recent randomized clinical trials comparing the bisphosphonate zoledronate (ZOL)- with Dmab-treated patients with cancer revealed that the incidences of BP- and Dmab-related ONJ (BRONJ and DRONJ, respectively) were comparable in these two groups,3,4 suggesting that ONJ induced by both drugs is a considerable clinical complication that compromises patients' quality of life.
Several animal models have been established for BRONJ to examine the ONJ pathophysiological characteristics by BPs,5–8 and these studies unequivocally showed that osteoclasts (bone-resorbing cells that are critically important for bone remodeling9) are the key mediators in causing ONJ. Nonetheless, direct evidence of osteoclasts’ involvement and the extent to which osteoclasts contribute to ONJ pathogenesis remains elusive, presumably due to the pleotropic effects of BPs. Indeed, it remains unclear whether ONJ development by BPs is associated directly with their inhibitory effects on osteoclasts' bone-resorptive functions or indirectly with aberrant osteoclasts' behaviors after BP uptake (eg, secretion of cytokines to the surrounding environment). Current literature also remains inconclusive with respect to the presence, numbers, and location of mature osteoclasts.7,8,10 Cytotoxic effects of BPs on cells other than osteoclasts (eg, endothelial cells or oral mucosal cells)2 further complicate roles of osteoclasts in the ONJ pathophysiological characteristics in the BRONJ models.
Although BPs are small molecules that may affect cells other than osteoclasts, Dmab is a fully human monoclonal antibody (Ab) that is highly specific to osteoclasts by targeting receptor activator of NF-κB ligand (RANKL), which plays a central role in osteoclastogenesis affecting the formation, function, and survival of osteoclasts.11 Clinically, Dmab has less severe adverse effects, such as gastrointestinal tract ulceration or kidney dysfunction, when compared with BPs.9 In the human RANKL knock-in mice model, it was demonstrated that Dmab treatment resulted in >95% reduction in serum tartrate-resistant acid phosphatase (TRAP)-5b in as early as 7 days,12 indicating that Dmab significantly inhibits the formation of mature osteoclasts in vivo. Because Dmab also induces ONJ in the absence of osteoclast formation without significant cytotoxic effects, it is possible that ONJ development is primarily associated with structural defects (eg, unresorbed bone surfaces) as a result of impaired osteoclasts' functions or formation by BPs or Dmab, respectively, leading to incomplete healing after dental-related traumas, such as tooth extraction or local inflammation.
To examine experimentally whether osteoclasts are the required mediators in ONJ development, we specifically inhibited osteoclastogenesis in vivo by using the neutralizing mouse anti-RANKL Ab and established the DRONJ mouse model. We compared this DRONJ mouse model with that of BRONJ and examined the involvement of osteoclasts in ONJ development induced by these anti-resorptive drugs.
Materials and Methods
Animals
Eight-week-old female C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and kept in a pathogen-free vivarium in the University of California–Los Angeles, Division of Laboratory Animal Medicine. All experiments were performed according to the approved institutional guidelines from the Chancellor's Animal Research Committee (number 2011-062).
Mouse Model for BRONJ and DRONJ
For the DRONJ mouse model, two groups of female C57BL/6 mice (n = 20 per group) were i.v. injected with 250 μg of anti-mouse RANKL monoclonal Ab (IK22-5)13 or control rat IgG (Sigma-Aldrich, St. Louis, MO) in phosphate-buffered saline (PBS) through the tail veins biweekly for 4 weeks. For the BRONJ mouse model, 125 μg/kg ZOL (Zometa; Novartis Oncology, East Hanover, NJ) or vehicle (Veh) solution (0.9% NaCl saline) was also i.v. administered biweekly for 4 weeks (n = 10 per group). Under general anesthesia using ketamine/xylazine (100 and 5 mg/kg, respectively), the maxillary first molar was atraumatically extracted 1 week after the initial injection. Three weeks after the tooth extraction, the mice were sacrificed to harvest blood. The maxilla and femur were also harvested from each mouse and fixed with 4% paraformaldehyde in PBS, pH 7.4, at 4°C overnight and stored in 70% ethanol solution. After micro-computed tomography (μCT) scanning, these tissues were decalcified with 5% EDTA and 4% sucrose in PBS, pH 7.4. Decalcification continued for 2 to 3 weeks at 4°C, and the decalcification solution was changed daily. Tissue samples were sent to the UCLA Translational Procurement Core Laboratory and processed for paraffin embedding.
μCT Scan and Three-Dimensional Volumetric Analysis
Areas of interest on the maxilla and femur were subjected to μCT scanning (Scanco μCT 40; Scanco Medical, Brüttisellen, Switzerland) using a voxel size of 20 μm3 and a 0.5-mm aluminum filter. Image analysis was performed using μCT software version 6.1 (Scanco Medical). μCT scans were performed at 55 kVp and 145 μA with an integration time of 200 milliseconds using a cylindrical tube (field of view/diameter, 20.48 mm). Resolution was set to medium (1024 × 1024 × 148 pixels). Two-dimensional slices from each maxilla or femur were combined using the μCT software version 6.1 (Scanco Medical) to form a three-dimensional reconstruction. Reconstructions were manipulated so that the viewing angles could provide an accurate representation of bone resorption after 3 weeks of healing. Several parameters were determined quantitatively using the Scanco software on three-dimensional reconstructions of the collected femurs. Bone volume (BV; mm3) and the total volume (TV; mm3) were measured to obtain percentage bone volume (BV/TV). The trabecular number (1/mm), trabecular thickness (mm), and trabecular separation (Tb.Sp; mm) were also determined on reconstruction of each femur. Newly formed bone in the tooth-extracted sites was quantified by selecting a volume containing the mesial, distal buccal, and distal lingual roots. Mean volumes are displayed as percentage BV/TV. The scanning and analyses of data abide by the published guidelines for assessing bone microstructure in rodents.14
Histochemical Staining
TRAP staining was performed as described previously.15 Briefly, EDTA-decalcified tissue sections were deparaffinized at 60°C for 30 minutes. The slides were rehydrated and incubated for 1 hour in 37°C with the TRAP staining solution, according to manufacturer's protocol (Acid Phosphatase Kit; Sigma-Aldrich, Inc., St. Louis, MO). The slides were counterstained with hematoxylin solution and mounted with medium (Permount; Fisher Scientific, Tustin, CA).
Histomorphometric Analysis
To evaluate the degree of ONJ-like lesions, we quantified the amounts of empty lacunae and necrotic bone. Three samples from control IgG-treated group and anti-RANKL Ab–treated group, with or without ONJ-like lesions, were randomly selected, and four sections (1, 6, 11, and 16) per sample were stained with hematoxylin and eosin (H&E). For each section that contains an area of interest covering first molar extracted sites horizontally from the buccal plates toward the palatal suture (approximately 1.4 mm) and vertically from the tip of the extracted site to the bottom of the palatal bone (approximately 0.8 mm), the digital images of each section were obtained using the microscope (model DP72; Olympus, Center Valley, PA) at ×100 magnification. The percentage of necrotic bone was defined as an area that contains five or more empty lacunae per 1 mm2 divided by total bone areas. The number of empty lacunae was also evaluated per total bone area (#/mm2). The total bone surface area was measured using ImageJ software version 1.48 (NIH, Bethesda, MD).
Blood Chemistry
Serum samples collected from each mouse were stored in −20°C and thawed once. The presence of TRAP-5b was detected by sandwich ELISA using the MouseTRAP Kit (Immunodiagnostic Systems, Fountain Hills, AZ). The procedure was performed according to the manufacturer's protocol, and serum samples were assayed in triplicate. Serum levels of alkaline phosphatase (ALP) were determined by the UCLA Division of Laboratory Animal Medicine Diagnostic Laboratory.
Statistical Analysis
The measures of key outcomes (eg, percentage bone volume, trabecular number, trabecular thickness, TRAP-5b, ALP, empty lacunae, or necrotic percentages) were expressed as means ± SD. A two-group t-test was used to compare the means of outcomes between the groups. A two-way table χ2 test was used to evaluate epithelial wound closure 3 weeks after the tooth extraction. P < 0.05 was considered statistically significant.
Results
Anti-RANKL Ab or ZOL Treatment Induces ONJ-Like Lesions in Mice
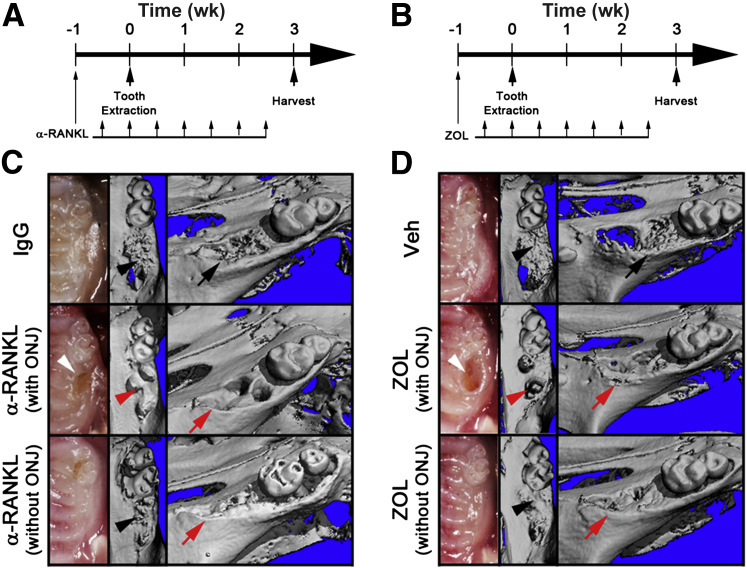
To directly evaluate the involvement of osteoclasts in ONJ development, we used anti-RANKL Ab or ZOL in a tooth extraction mouse model. Biweekly administration of anti-RANKL Ab and ZOL for 4 weeks increased bone mass, as demonstrated by bone morphometric analysis (Supplemental Figure S1), confirming systemic effects of anti-RANKL Ab and ZOL. To induce ONJ, the maxillary first molar was extracted 1 week after initial anti-RANKL Ab treatment. Anti-RANKL Ab was continually administered for an additional 3 weeks, after which the mice were sacrificed (Figure 1A). Typical wound closure after an atraumatic tooth extraction usually occurs within 3 weeks (Supplemental Figure S2). Clinical presentation at the extracted sites after 3 weeks showed complete epithelial closure in 90% (18 of 20) mice treated with control IgG (Figure 1C and Table 1). In contrast, only 40% (8 of 20) of the mice in the anti-RANKL Ab-treated group had complete epithelial closure (Figure 1C and Table 1), indicating that there are statistically significant differences in epithelial wound closure (P = 0.0009). However, in the BRONJ mouse model (Figure 1B), there was no statistically different epithelial wound closure (P = 0.6392); 70% (7 of 10) of Veh-treated mice and 60% (6 of 10) of ZOL-treated mice showed complete epithelial closure (Figure 1D and Table 1).
Figure 1.
Establishment of the osteonecrosis of the jaw (ONJ) mouse models for DRONJ and BRONJ. The schematic timelines for the DRONJ (A) and BRONJ (B) models. C and D: Three weeks after tooth extraction, maxillae were harvested, clinical presentations were imaged (left panels), and μCT scans of the maxillae were taken. The occlusal views (middle panels) and the angled views (right panels) are shown. The black arrows resorbed alveolar ridges (C and D); black arrowheads (C and D), new bone in socket; red arrows (C and D), unresorbed alveolar ridges; red arrowheads (C and D), no bone in socket; white arrowheads (C and D), open wound.
Table 1.
Summary of ONJ-Like Lesions in DRONJ and BRONJ Models
| Animal groups | Complete epithelial closure (%) | Delayed epithelial closure (%) | Breached connective tissues (%) | Pseudoepitheliomatous hyperplasia (%) | Osteonecrosis (%) |
|---|---|---|---|---|---|
| IgG (n = 20) | 18 (90) | 2 (10) | 0 | 0 | 0 |
| α-RANKL Ab (n = 20) | 8 (40) | 12 (60) | 11 (55) | 10 (50) | 10 (50) |
| Veh (n = 10) | 7 (70) | 3 (30) | 0 | 0 | 0 |
| ZOL (n = 10) | 6 (60) | 4 (40) | 3 (30) | 3 (30) | 3 (30) |
Ab, antibody; RANKL, receptor activator of NF-κB ligand; Veh, vehicle; ZOL, zoledronate.
μCT scans at the tooth-extracted sites revealed new bone formation in the sockets as well as alveolar ridge resorption around the sockets when there is complete wound closure in both IgG- or anti-RANKL Ab-treated mice (Figure 1C). In contrast, anti-RANKL Ab-treated mice without wound closure exhibited unresorbed alveolar ridges and lack of bone formation within the sockets (Figure 1C). Similar results were obtained in ZOL-treated groups (Figure 1D). These data indicate that both anti-RANKL Ab and ZOL cause a significant delay in wound closure after tooth extraction.
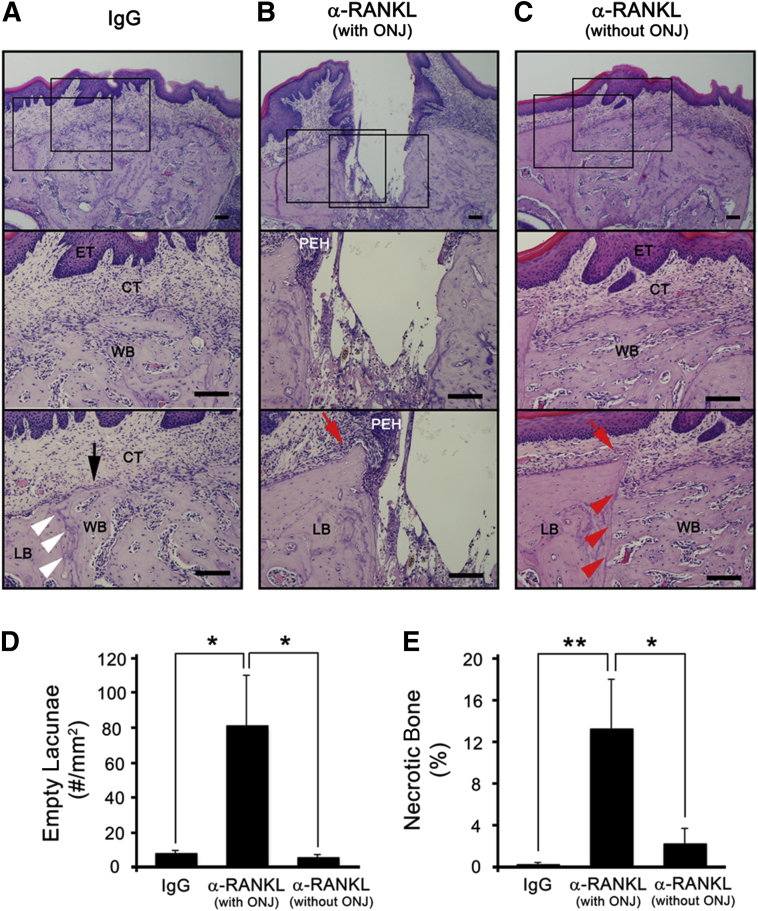
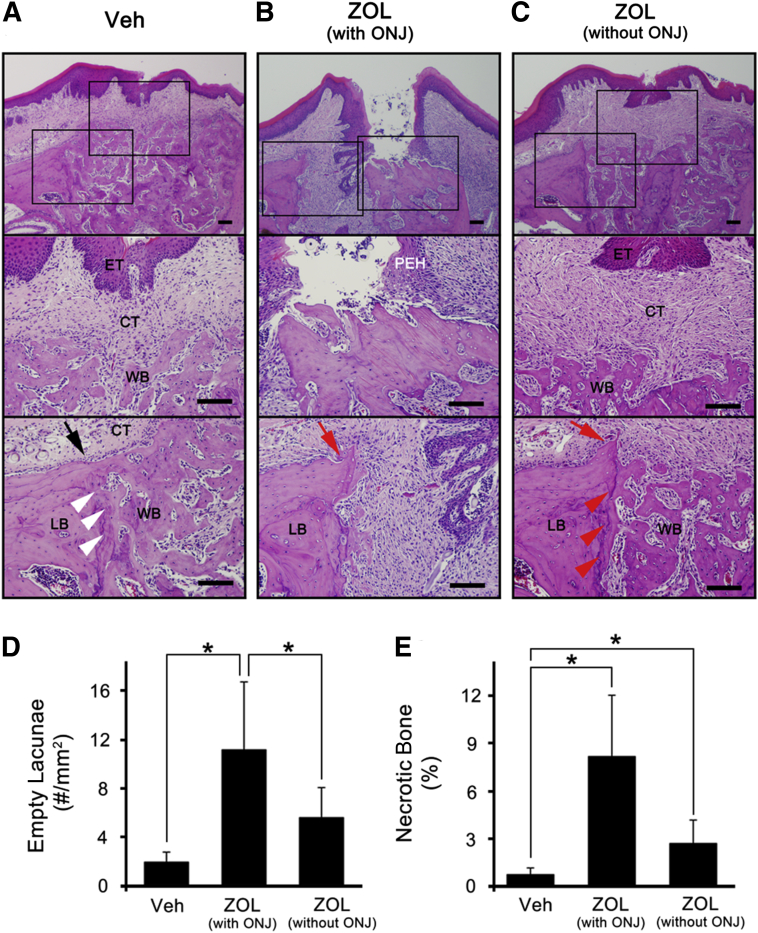
Further histological examination of these tooth-extracted sites revealed that none of the control IgG-treated mice had exposed bone; all mice had connective tissue directly above the bone-filled sockets regardless of epithelial tissue closure (Figure 2A). On the other hand, 50% of the anti-RANKL Ab-treated mice exhibited typical ONJ phenotypes, including the following: i) breached oral mucosal tissues, ii) absence of connective tissue layers, iii) denuded necrotic bone with empty lacunae, and iv) pseudoepitheliomatous hyperplasia invading onto the bone surfaces (Figure 2B). Unlike the control groups, unresorbed sharp alveolar ridges were prominently evident in anti-RANKL Ab-treated mice (Figure 2B), suggesting that bone remodeling was inhibited. The numbers of empty lacunae and the percentage of necrotic bone were all significantly higher in the anti-RANKL Ab-treated mice that exhibited denuded bone compared with the control group [7.68 ± 1.80 versus 80.86 ± 29.50 (P = 0.013) for empty lacunae and 0.19 ± 0.23 versus 13.16 ± 4.69 (P = 0.0087) for necrotic bone], indicating that anti-RANKL Ab treatment causes ONJ-like lesions (Figure 2, D and E). The remaining 50% of the anti-RANKL Ab-treated mice were healed, as demonstrated by closed epithelial and connective tissues (Figure 2C), exhibiting no differences in the numbers of empty lacunae (5.20 ± 1.88, P = 0.17) and the percentage of necrotic bone (2.18 ± 1.50, P = 0.085) when compared with those from the control group, suggesting that the anti-RANKL Ab-treated mice that healed did not exhibit ONJ-like lesions. In the BRONJ model, the similar patterns were observed (Figure 3, A–E) [1.88 ± 0.82 versus 11.06 ± 5.55 (P < 0.047) for empty lacunae and 0.69 ± 0.47 versus 8.22 ± 3.87 (P = 0.029) for necrotic bone].
Figure 2.
Anti-RANKL Ab treatment induces osteonecrosis of the jaw (ONJ)-like lesions. H&E-stained tissue at tooth-extracted sites from mice i.v. administered with IgG (A) as well as anti-RANKL Ab that induced (B) or did not induce (C) ONJ-like lesions. The numbers of empty lacunae per mm2 (D) and the percentage of necrotic bones (E) were quantified. ∗P < 0.05, ∗∗P < 0.01. Scale bars: 100 μm (A–C). The black arrow (A) indicates resorbed alveolar ridge; red arrows (B and C), unresorbed ridge; white (A) and red (C) arrowheads, margin between existing lamellar bone and newly formed woven bone. CT, connective tissue; ET, epithelial tissue; LB, lamellar bone; PEH, pseudoepitheliomatous hyperplasia; WB, woven bone.
Figure 3.
Zoledronate (ZOL) treatment induces osteonecrosis of the jaw (ONJ)-like lesions. H&E-stained tissue at tooth-extracted sites from mice i.v. administered with Veh (A) or ZOL that induced (B) or did not induce (C) ONJ-like lesions. Quantification of the numbers of empty lacunae per mm2 (D) and the percentage of necrotic bones (E). ∗P < 0.05. Scale bars: 100 μm (A–C). The black arrow (A) indicates the resorbed alveolar ridge; red arrows (B and C), unresorbed ridge; white (A) and red (C) arrowheads, margin between existing lamellar bone and newly formed woven bone CT, connective tissue; ET, epithelial tissue; LB, lamellar bone; PEH, pseudoepitheliomatous hyperplasia; WB, woven bone.
Anti-RANKL Ab But Not ZOL Induces ONJ in the Absence of TRAP-Positive Osteoclasts
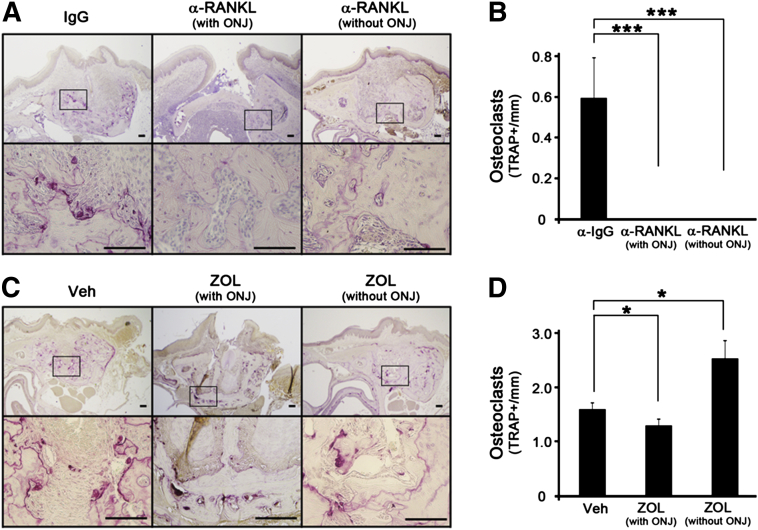
To examine the presence of osteoclasts at the tooth-extracted sites, we harvested the mice 2 weeks after extraction, during which bone healing, including osteoclastic activity, is expected to be ongoing. Mature osteoclasts positive to TRAP staining were identified and were evident around the tooth-extracted sites in both Veh- or IgG-treated mice (Figure 4, A and C). However, all mice that received anti-RANKL Ab showed no TRAP-positive osteoclasts regardless of ONJ development (Figure 4, A and B). In sharp contrast, ZOL-treated mice with ONJ-like lesions showed a similar degree of TRAP-positive staining, if not slightly less, than those in the control group (Figure 4, C and D), suggesting that physical presence of osteoclasts is not associated with ONJ development.
Figure 4.
Mature osteoclasts are absent in anti-RANKL Ab-treated, but not in zoledronate (ZOL)-treated, mice. Three weeks after initial i.v. administration and 2 weeks after tooth extraction, the maxillae were harvested and EDTA decalcified. A: TRAP staining was performed at the tooth-extracted sites from mice i.v. administered with IgG or anti-RANKL Ab that induced or did not induce ONJ-like lesions. B: Quantification of the numbers of TRAP+ osteoclasts. C and D: Similar experiments were performed in mice treated with Veh and ZOL. ∗P < 0.05, ∗∗∗P < 0.001. Scale bar = 100 μm (A and C).
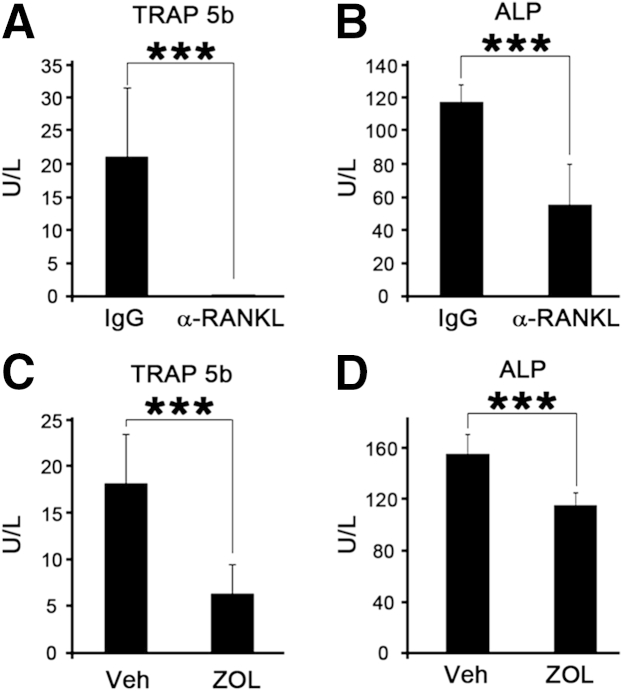
TRAP and ALP Markers Are Systemically Diminished in Both Anti-RANKL Ab- and ZOL-Treated Mice
To examine whether anti-RANKL Ab or ZOL suppressed the bone remodeling process, including osteoclastic and osteoblastic activity, we collected the blood samples and measured the serum concentration of TRAP-5b and ALP, biochemical markers for bone resorption and formation, respectively.16 In anti-RANKL Ab-treated mice, TRAP-5b in serum was notably suppressed to undetectable levels (P < 0.0001) (Figure 5A). Similarly, TRAP-5b was also decreased in ZOL-treated mice (P < 0.0001) (Figure 5C), albeit to a lesser degree than those in anti-RANKL Ab-treated mice. ALP was also decreased significantly in both anti–RANKL-treated mice (P < 0.0001) (Figure 5B) and ZOL-treated mice (P < 0.0001) (Figure 5D), indicating bone remodeling in both anti–RANKL- and ZOL-treated mice is suppressed.
Figure 5.
Anti-RANKL Ab or zoledronate (ZOL) treatment diminishes bone remodeling in vivo. Blood was collected from the control IgG- or anti-RANKL Ab-treated mice 4 weeks after initial administration, and the serum levels of TRAP 5b (A) or ALP (B) were evaluated using ELISAs. The serum levels of TRAP 5b (C) and ALP (D) in mice treated with Veh or ZOL. ∗∗∗P < 0.001.
Inhibition of New Bone Formation in the Tooth-Extracted Sockets Is Associated with ONJ Lesions
Osteoclast-osteoblast coupling is an important bone remodeling process at the bone surfaces during wound healing.17 The fact that bone remodeling is suppressed in both anti-RANKL Ab- and ZOL-treated mice suggests that ONJ may be associated with bone formation in the tooth-extracted socket. Indeed, our μCT scan images (Figure 1) and histological sections (Figures 2 and 3) suggested that lack of new bone formation within the extracted sockets is associated with incomplete wound closure of the overlying soft tissues and bone necrosis. To determine whether the lack of new bone formation in the tooth-extracted socket is associated with ONJ development, we quantified the newly formed bone within the extracted socket using μCT analysis. In both BRONJ and DRONJ models, new bone formation within the extracted sockets was significantly inhibited in an ONJ-specific manner (Figure 6), indicating that lack of newly formed bone is associated with ONJ lesions.
Figure 6.
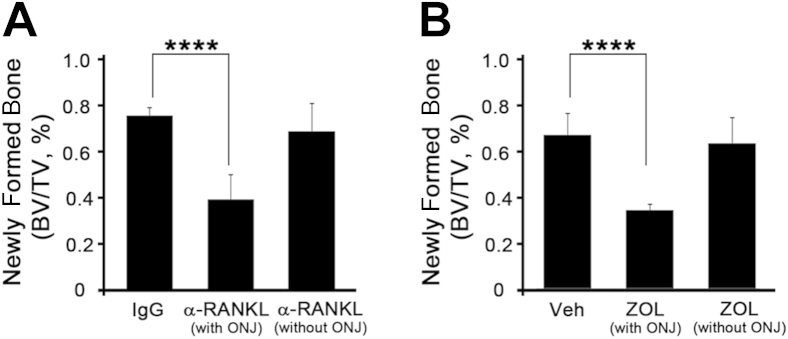
Newly formed bone in the tooth-extracted sockets is inhibited in osteonecrosis of the jaw (ONJ) lesions. Newly formed bone in the tooth-extracted sockets was quantified using the micro computed tomography scan from the DRONJ (A) or BRONJ (B) mouse model. ∗∗∗∗P < 0.0001.
Discussion
Long-term use of BPs has been shown to increase the incidence of developing ONJ after dental-related interventions.1,18 Osteoclasts have been known for their key roles in mediating ONJ because BPs improve bone density by inhibiting bone resorption at the clinical level. However, a direct link between inhibition of osteoclasts' resorptive functions and ONJ development as well as oral cavity specificity is lacking, presumably due to the pleotropic effects of BPs. Dmab, another class of anti-resorptive drug, is a fully human monoclonal Ab that specifically targets RANKL and is known to induce ONJ.19 Herein, by developing a mouse model for DRONJ and comparing it with BRONJ, we found that the suppression of osteoclasts' resorptive function by these drugs is essential in inducing ONJ.
The primary role of mature osteoclasts is to resorb bone matrix.20 However, roles other than bone resorption have been suggested, such as modulating the differentiation of nearby cells or secreting inflammatory cytokines to induce immune responses.21 Indeed, recent studies suggest that aberrant functions of osteoclasts may contribute to ONJ development. In particular, Ephrin/Eph signaling plays an important role in osteoclast-osteoblast interactions.22 It was shown that alendronate enhances expression of ephrinB1 as well as ephB1 and ephB3 in osteoclasts and osteoblasts, respectively, to inhibit osteoblast differentiation, which may explain the pathophysiological characteristics of ONJ.23 It has also been postulated that isopentenyl diphosphate released from BP-induced apoptotic osteoclasts at the bone surface provokes oral innate immunity through γδ T cells and mediates a potential pathophysiological mechanism for ONJ.6 These data support speculation that dysfunctional osteoclasts affected by BPs (eg, osteoclasts undergoing BP-mediated apoptosis) may play etiological roles in developing ONJ.
Interestingly, ONJ was developed in the absence of mature osteoclasts in the DRONJ model (Figures 2 and 4A), which is consistent with a recent finding,24 suggesting that the aberrant behaviors of BP-affected osteoclasts (eg, osteoclasts undergoing apoptosis) may not be associated with ONJ development. Rather, unresorbed bone surfaces due to impaired osteoclast functions or formation by BPs or Dmab are directly associated with ONJ development.
In the BRONJ mouse model, histological examination revealed that only 30% of mice treated with ZOL developed ONJ-like lesions (Figure 5A). The incidence of ONJ development by BP in other animal models ranges from 15% to 50%, depending on the method, duration, and amount of drugs delivered.6,17 For mouse models in particular, the incidence of ONJ development when treated with BP was 20% to 30%,5,15 which is similar to the incidence we obtained (Table 1).
In contrast, incidence of ONJ lesions in anti-RANKL Ab-treated mice was higher than that in ZOL-treated mice (50% versus 30%) (Table 1). Such difference is, in part, due to the fact that osteoclastogenesis was completely abolished in the DRONJ model, whereas it was only partially suppressed in the BRONJ model (Figures 4, A and B, and 5A).
Compared with our animal models, previous clinical studies reveal minimal differences between the incidence of ONJ by BPs and Dmab (1.3% versus 1.5%, respectively).3,4 Dmab was recently introduced in 2011 for clinical use, and clinical studies about ONJ development in Dmab users are still accumulating. Nonetheless, recent studies suggest that ONJ may occur in a relatively shorter period of time, even within a year, in Dmab users.25–27 Because Dmab is known to be more potent than BP in inhibiting resorptive function of osteoclasts, the onset of ONJ by Dmab may require careful monitoring in the clinic.
Surprisingly, complete inhibition of osteoclastogenesis by anti-RANKL Ab treatment did not induce ONJ in all mice. Despite complete absence of mature osteoclasts at the tooth-extracted sites (Figure 4, A and B), 50% of the anti-RANKL Ab-treated mice were healed. Such observation implies that, although required, the lack of osteoclast's resorptive function mediated by BPs and Dmab is not sufficient to induce ONJ. Further parallel comparisons between the clinical presentations and μCT scanning images of tooth-extracted sites in both BRONJ and DRONJ mice models revealed a striking correlation between new bone formation within the sockets and complete wound closure of oral mucosa (Figures 2, 3, and 6), suggesting that newly formed bone (alias woven bone) may play a key role during the healing process in the oral cavity.
Woven bone formation is known to occur in two ways: appositional formation from the existing bone surfaces primarily mediated by the osteoclast-osteoblast coupling mechanism, and de novo formation without any existing bone surfaces.28 This dual mode of new bone formation is known to play an important role during healing in the oral cavity after dental interventions, such as implant surgery.29 In the context of wound healing after tooth extraction, appositional bone formation mandates tight coupling between initial bone resorption mediated by mature osteoclasts and subsequent bone formation by recruiting osteoblasts at the resorbed sites.17 As such, a defect in this coupling process due to impaired osteoclasts' resorptive function in the presence of BP and Dmab may interfere and inhibit appositional woven bone formation, leaving de novo bone formation as the only source of new bone formation in the extracted sockets. Consistent with this notion, a closer examination reveals a clear demarcation at the interface between the existing bone and newly formed bone (Figures 2C and 3C), suggesting that woven bone formation during healing after extraction in the presence of anti-resorptive agents was primarily mediated by de novo bone formation.
The oral cavity is a unique entity because bone is situated immediately beneath the oral mucosal tissues and there are no remarkable anatomical structures (eg, fascia, muscle, and fat) between these soft and hard tissues. For this anatomical reason, healing of the soft and hard tissues, so-called osteomucosal healing, usually occurs simultaneously in the oral cavity after trauma (eg, tooth extraction). Indeed, early stages of woven bone formation at healing sites require deposition of collagens onto which mineralization occurs.30 Collagens are integral parts of extracellular matrixes not only for bone formation but also for connective tissue formation onto which epithelial cells migrate to close the wounded sites. Therefore, it is tempting to speculate that woven bone formation plays a critical role in the osteomucosal healing process by physically bridging soft and hard tissues in the oral cavity.
Recent studies suggest that bacterial infection and inflammatory responses may play an integral part of the ONJ pathophysiological characteristics because bacterial colonization in the ONJ lesions is inevitably always present both at the clinical level and animal models.8,30–33 Therefore, it is also possible that inability to remove bacteria-infected bone by osteoclasts impairs the body's ability to resolve the infected bone, ultimately leading to bone death. As such, the absence of woven bone may be a result, rather than a cause, of osteonecrosis. The precise role of woven bone formation at or near the bacteria-infected bone warrants closer examination.
Although we put our efforts to compare ONJ induced by BP and anti-RANKL Ab, there are several limitations to such direct comparison. First, we used only a single dose of each drug (eg, 250 μg of anti-mouse RANKL monoclonal Ab or 125 μg/kg of ZOL). Second, effects of anti-RANKL Ab are rapid, whereas those of ZOL are relatively slow as it accumulates in bone. Inevitably, these dose and duration effects of anti-resorptive agents are also challenging to standardize in the clinical settings. As such, careful interpretation should be considered when comparing ONJ incidence by BPs and Dmab.
In conclusion, we have developed a mouse model for DRONJ and compared it with the BRONJ mouse model to test our hypothesis that inhibition of bone resorption by osteoclasts plays a direct etiological role in inducing ONJ by these anti-resorptive agents, BP and Dmab. We also showed that woven bone formation in the extracted sockets is associated with ONJ lesions. Many BP and Dmab users taking high doses are in life-threatening conditions with metastatic bone cancer. Therefore, further examination is necessary as to whether therapeutic modalities that enhance woven bone formation in the absence of osteoclasts' bone-resorptive functions by BP and Dmab may provide means to intervene and prevent ONJ development.
Acknowledgment
We thank the UCLA Translational Procurement Core Laboratory for their expedited and cooperative services.
Footnotes
Supported in part by National Institute of Dental and Craniofacial Research/NIH grants R03DE021114 and R01DE023348 (R.H.K.), Dean's Faculty Research Seed grant (R.H.K.), and Chancellor's grant (N.-H.P.).
Disclosures: None declared.
Supplemental Data
Anti-RANKL antibody (Ab) or zoledronate (ZOL) treatment improves bone mass. A: Femurs were obtained from mice treated with control IgG or anti-RANKL Ab for 4 weeks, and micro computed tomography (μCT) scan was used to examine bone morphometric indices. C: Three-dimensional volumetric analysis was performed to obtain percentage bone volume (BV/TV, percent), trabecular number (Tb.N; 1/mm), trabecular thickness (Tb.Th; mm), and trabecular separation (Tb.Sp; mm). B: Femurs were also obtained from mice treated with Veh or ZOL for 4 weeks, and the relevant μCT scan data were obtained. D: Summary of three-dimensional volumetric analysis. ∗∗∗∗P < 0.0001.
A time-dependent wound healing process after tooth extraction in mice. Clinical presentation (A) and H&E staining (B) of the tooth-extracted sockets.
References
- 1.Ruggiero S.L., Dodson T.B., Assael L.A., Landesberg R., Marx R.E., Mehrotra B., American Association of Oral and Maxillofacial Surgeons American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws–2009 update. J Oral Maxillofac Surg. 2009;67(Suppl):2–12. doi: 10.1016/j.joms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Reid I.R., Cornish J. Epidemiology and pathogenesis of osteonecrosis of the jaw. Nat Rev Rheumatol. 2011;8:90–96. doi: 10.1038/nrrheum.2011.181. [DOI] [PubMed] [Google Scholar]
- 3.Stopeck A.T., Lipton A., Body J.J., Steger G.G., Tonkin K., de Boer R.H., Lichinitser M., Fujiwara Y., Yardley D.A., Viniegra M., Fan M., Jiang Q., Dansey R., Jun S., Braun A. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 4.Henry D.H., Costa L., Goldwasser F., Hirsh V., Hungria V., Prausova J., Scagliotti G.V., Sleeboom H., Spencer A., Vadhan-Raj S., von Moos R., Willenbacher W., Woll P.J., Wang J., Jiang Q., Jun S., Dansey R., Yeh H. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 5.Bi Y., Gao Y., Ehirchiou D., Cao C., Kikuiri T., Le A., Shi S., Zhang L. Bisphosphonates cause osteonecrosis of the jaw-like disease in mice. Am J Pathol. 2010;177:280–290. doi: 10.2353/ajpath.2010.090592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hokugo A., Christensen R., Chung E.M., Sung E.C., Felsenfeld A.L., Sayre J.W., Garrett N., Adams J.S., Nishimura I. Increased prevalence of bisphosphonate-related osteonecrosis of the jaw with vitamin D deficiency in rats. J Bone Miner Res. 2010;25:1337–1349. doi: 10.1002/jbmr.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aghaloo T.L., Kang B., Sung E.C., Shoff M., Ronconi M., Gotcher J.E., Bezouglaia O., Dry S.M., Tetradis S. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J Bone Miner Res. 2011;26:1871–1882. doi: 10.1002/jbmr.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguirre J.I., Akhter M.P., Kimmel D.B., Pingel J.E., Williams A., Jorgensen M., Kesavalu L., Wronski T.J. Oncologic doses of zoledronic acid induce osteonecrosis of the jaw-like lesions in rice rats (Oryzomys palustris) with periodontitis. J Bone Miner Res. 2012;27:2130–2143. doi: 10.1002/jbmr.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baron R., Ferrari S., Russell R.G. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48:677–692. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Kuroshima S., Go V.A., Yamashita J. Increased numbers of nonattached osteoclasts after long-term zoledronic acid therapy in mice. Endocrinology. 2012;153:17–28. doi: 10.1210/en.2011-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogado C.E., Zanchetta M.B., Boailchuk J.A., Massari F.E., Zanchetta J.R. Denosumab: what's new? Curr Osteoporos Rep. 2011;9:12–19. doi: 10.1007/s11914-010-0040-1. [DOI] [PubMed] [Google Scholar]
- 12.Kostenuik P.J., Nguyen H.Q., McCabe J., Warmington K.S., Kurahara C., Sun N., Chen C., Li L., Cattley R.C., Van G., Scully S., Elliott R., Grisanti M., Morony S., Tan H.L., Asuncion F., Li X., Ominsky M.S., Stolina M., Dwyer D., Dougall W.C., Hawkins N., Boyle W.J., Simonet W.S., Sullivan J.K. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J Bone Miner Res. 2009;24:182–195. doi: 10.1359/jbmr.081112. [DOI] [PubMed] [Google Scholar]
- 13.Kamijo S., Nakajima A., Ikeda K., Aoki K., Ohya K., Akiba H., Yagita H., Okumura K. Amelioration of bone loss in collagen-induced arthritis by neutralizing anti-RANKL monoclonal antibody. Biochem Biophys Res Commun. 2006;347:124–132. doi: 10.1016/j.bbrc.2006.06.098. [DOI] [PubMed] [Google Scholar]
- 14.Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 15.Kikuiri T., Kim I., Yamaza T., Akiyama K., Zhang Q., Li Y., Chen C., Chen W., Wang S., Le A.D., Shi S. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J Bone Miner Res. 2010;25:1668–1679. doi: 10.1002/jbmr.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naylor K., Eastell R. Bone turnover markers: use in osteoporosis. Nat Rev Rheumatol. 2012;8:379–389. doi: 10.1038/nrrheum.2012.86. [DOI] [PubMed] [Google Scholar]
- 17.Feng X., McDonald J.M. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121–145. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizzoli R., Reginster J.Y. Adverse drug reactions to osteoporosis treatments. Expert Rev Clin Pharmacol. 2011;4:593–604. doi: 10.1586/ecp.11.42. [DOI] [PubMed] [Google Scholar]
- 19.Aghaloo T.L., Felsenfeld A.L., Tetradis S. Osteonecrosis of the jaw in a patient on Denosumab. J Oral Maxillofac Surg. 2010;68:959–963. doi: 10.1016/j.joms.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Fattore A., Teti A., Rucci N. Bone cells and the mechanisms of bone remodelling. Front Biosci (Elite Ed) 2012;4:2302–2321. doi: 10.2741/543. [DOI] [PubMed] [Google Scholar]
- 21.Boyce B.F., Yao Z., Zhang Q., Guo R., Lu Y., Schwarz E.M., Xing L. New roles for osteoclasts in bone. Ann N Y Acad Sci. 2007;1116:245–254. doi: 10.1196/annals.1402.084. [DOI] [PubMed] [Google Scholar]
- 22.Zhao C., Irie N., Takada Y., Shimoda K., Miyamoto T., Nishiwaki T., Suda T., Matsuo K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu E., Tamasi J., Partridge N.C. Alendronate affects osteoblast functions by crosstalk through EphrinB1-EphB. J Dent Res. 2012;91:268–274. doi: 10.1177/0022034511432170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aghaloo T.L., Cheong S., Bezouglaia O., Kostenuik P., Atti E., Dry S.M., Pirih F.Q., Tetradis S. RANKL inhibitors induce osteonecrosis of the jaw in mice with periapical disease. J Bone Miner Res. 2014;29:843–854. doi: 10.1002/jbmr.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bone H.G., Chapurlat R., Brandi M.L., Brown J.P., Czerwinski E., Krieg M.A., Mellström D., Radominski S.C., Reginster J.Y., Resch H., Ivorra J.A., Roux C., Vittinghoff E., Daizadeh N.S., Wang A., Bradley M.N., Franchimont N., Geller M.L., Wagman R.B., Cummings S.R., Papapoulos S. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab. 2013;98:4483–4492. doi: 10.1210/jc.2013-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pichardo S.E., Kuypers S.C., van Merkesteyn J.P. Denosumab osteonecrosis of the mandible: a new entity? a case report. J Craniomaxillofac Surg. 2013;41:e65–e69. doi: 10.1016/j.jcms.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Diz P., López-Cedrún J.L., Arenaz J., Scully C. Denosumab-related osteonecrosis of the jaw. J Am Dent Assoc. 2012;143:981–984. doi: 10.14219/jada.archive.2012.0323. [DOI] [PubMed] [Google Scholar]
- 28.Gorski J.P. Is all bone the same? distinctive distributions and properties of non-collagenous matrix proteins in lamellar vs. woven bone imply the existence of different underlying osteogenic mechanisms. Crit Rev Oral Biol Med. 1998;9:201–223. doi: 10.1177/10454411980090020401. [DOI] [PubMed] [Google Scholar]
- 29.Berglundh T., Abrahamsson I., Lang N.P., Lindhe J. De novo alveolar bone formation adjacent to endosseous implants. Clin Oral Implants Res. 2003;14:251–262. doi: 10.1034/j.1600-0501.2003.00972.x. [DOI] [PubMed] [Google Scholar]
- 30.Del Fattore A., Teti A., Rucci N. Bone cells and the mechanism of bone remodelling. Front Biosci (Elite Ed) 2012;4:2302–2321. doi: 10.2741/543. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S.K., Gorur A., Schaudinn C., Shuler C.F., Costerton J.W., Sedghizadeh P.P. The role of microbial biofilms in osteonecrosis of the jaw associated with bisphosphonate therapy. Curr Osteoporos Rep. 2010;8:40–48. doi: 10.1007/s11914-010-0008-1. [DOI] [PubMed] [Google Scholar]
- 32.Mawardi H., Giro G., Kajiya M., Ohta K., Almazrooa S., Alshwaimi E., Woo S.B., Nishimura I., Kawai T. A role of oral bacteria in bisphosphonate-induced osteonecrosis of the jaw. J Dent Res. 2011;90:1339–1345. doi: 10.1177/0022034511420430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li D., Gromov K., Proulx S.T., Xie C., Li J., Crane D.P., Søballe K., O'Keefe R.J., Awad H.A., Xing L., Schwarz E.M. Effects of antiresorptive agents on osteomyelitis: novel insights into the pathogenesis of osteonecrosis of the jaw. Ann N Y Acad Sci. 2010;1192:84–94. doi: 10.1111/j.1749-6632.2009.05210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anti-RANKL antibody (Ab) or zoledronate (ZOL) treatment improves bone mass. A: Femurs were obtained from mice treated with control IgG or anti-RANKL Ab for 4 weeks, and micro computed tomography (μCT) scan was used to examine bone morphometric indices. C: Three-dimensional volumetric analysis was performed to obtain percentage bone volume (BV/TV, percent), trabecular number (Tb.N; 1/mm), trabecular thickness (Tb.Th; mm), and trabecular separation (Tb.Sp; mm). B: Femurs were also obtained from mice treated with Veh or ZOL for 4 weeks, and the relevant μCT scan data were obtained. D: Summary of three-dimensional volumetric analysis. ∗∗∗∗P < 0.0001.
A time-dependent wound healing process after tooth extraction in mice. Clinical presentation (A) and H&E staining (B) of the tooth-extracted sockets.