Abstract
Acetaminophen (APAP) overdose results in acute liver failure and has limited treatment options. Previous studies show that stimulating liver regeneration is critical for survival after APAP overdose, but the mechanisms remain unclear. In this study, we identified major signaling pathways involved in liver regeneration after APAP-induced acute liver injury using a novel incremental dose model. Liver injury and regeneration were studied in C57BL/6 mice treated with either 300 mg/kg (APAP300) or 600 mg/kg (APAP600) APAP. Mice treated with APAP300 developed extensive liver injury and robust liver regeneration. In contrast, APAP600-treated mice exhibited significant liver injury but substantial inhibition of liver regeneration, resulting in sustained injury and decreased survival. The inhibition of liver regeneration in the APAP600 group was associated with cell cycle arrest and decreased cyclin D1 expression. Several known regenerative pathways, including the IL-6/STAT-3 and epidermal growth factor receptor/c-Met/mitogen-activated protein kinase pathways, were activated, even at APAP600, where regeneration was inhibited. However, canonical Wnt/β-catenin and NF-κB pathways were activated only in APAP300-treated mice, where liver regeneration was stimulated. Furthermore, overexpression of a stable form of β-catenin, where serine 45 is mutated to aspartic acid, in mice resulted in improved liver regeneration after APAP overdose. Taken together, our incremental dose model has identified a differential role of several signaling pathways in liver regeneration after APAP overdose and highlighted canonical Wnt signaling as a potential target for regenerative therapies for APAP-induced acute liver failure.
Acetaminophen (APAP) is one of the most widely used over-the-counter analgesic and antipyretic drugs in the world. APAP is safe at therapeutic doses, but overdose can cause acute liver failure (ALF). In fact, APAP overdose is associated with 56,000 emergency department visits and 26,000 hospitalizations every year in the United States.1 The only pharmacological intervention, at present, is N-acetyl cysteine (precursor of glutathione), which is successful only if given within a few hours after APAP overdose.2 An ultimate option is liver transplantation, which is complicated by issues such as donor availability, long-term immunosuppression, and exorbitant costs.3
Previous studies suggest that liver regeneration after APAP overdose plays a critical role in determination of outcome of injury.4–7 α-Feto protein, a marker of liver regeneration, was found to be associated with better survival rate in patients with APAP-induced ALF.4 Several other studies in animal models suggest that timely stimulation of liver regeneration, such as with stem cell factor6 and vascular endothelial growth factor,7 improves survival after APAP overdose in mice. These studies highlight stimulating liver regeneration in APAP-induced patients with ALF as a plausible therapeutic option. However, the mechanisms of liver regeneration after APAP-induced ALF are not well known. Although liver regeneration has been extensively studied in the past,8 most of the studies are on the basis of a partial hepatectomy (PHX) model, a mechanistically different model from APAP-induced ALF.
Data on hepatotoxicants, in general, suggest that liver regeneration follows the principles of dose-response.9 Studies indicate that liver regeneration after toxic injury to liver increases proportionately to injury but only up to a threshold dose. Doses higher than the threshold dose actually inhibit liver regeneration, resulting in progression of injury to ALF and death.9–11 These studies have suggested that at higher doses, regeneration is inhibited because of blockade in critical proregenerative signaling pathways.9,11,12 On the basis of this principle, we developed a novel incremental dose model to delineate the mechanisms of liver regeneration after APAP-induced acute liver injury (ALI). We used two doses of APAP, a lower dose (300 mg/kg), after which liver regeneration is intact, and a higher dose (600 mg/kg), after which liver regeneration is inhibited. We performed a comprehensive analysis of several signaling pathways known to be involved in liver regeneration and identified pathways that are potentially important for liver regeneration after APAP-induced ALI; these pathways can be targeted therapeutically.
Materials and Methods
Animals, Treatment, and Tissue Harvesting
Two-month-old male C57BL/6 mice, purchased from Jackson Laboratories (Bar Harbor, ME), were used in these studies. Mice overexpressing a stable form of β-catenin, where serine 45 is mutated to aspartic acid (S45D), have been described before.13 Details of generation and characterization of these mice have been published previously.13 All animals were housed in Association for Assessment and Accreditation of Laboratory Animal Care–accredited facilities at the University of Kansas Medical Center (Kansas City, KS) under a standard 12-hour light/dark cycle with access to chow and water ad libitum. The Institutional Animal Care and Use Committee at University of Kansas Medical Center approved all studies. Mice were fasted 12 hours before administration of APAP. APAP was dissolved in warm 0.9% saline, and mice were treated with either 300 or 600 mg/kg APAP, i.p. Food was returned to the mice after APAP treatment. Mice (n = 3 to 7) were sacrificed at 0, 0.5, 1, 3, 6, 12, 24, 48, 72, and 96 hours after APAP treatment by cervical dislocation under isoflurane anesthesia, and blood and livers were collected. The S45D and respective wild-type (WT) littermates were treated with either 300 or 600 mg/kg APAP and sacrificed at 6 and 24 hours (APAP300 study) or 24 and 48 hours (APAP600 study) after APAP administration, followed by blood and liver collection. Serum samples were obtained from the blood and used for analysis of alanine aminotransferase (ALT) activity using commercially available kits (ThermoFisher Scientific, Pittsburgh, PA). Liver sections and nuclear and cytoplasmic protein extracts were prepared as described previously.14
Antibodies
All primary antibodies obtained from Cell Signaling Technologies (Danvers, MA) for Western blot analysis and their catalog numbers are provided in Table 1. Active β-catenin antibody was obtained from EMD Millipore Corporation (Billerica, MA) (catalog number 05-665). All secondary antibodies used for Western blot analysis were obtained from Cell Signaling Technologies. Biotinylated secondary antibodies for immunohistochemistry (IHC) were purchased from Jackson Immunoresearch (West Grove, PA).
Table 1.
Antibodies Used in This Study and Their Catalog Numbers
| Antibody | Catalog no.∗ |
|---|---|
| Cyclin D1 | 2978 |
| CDK4 | 2906 |
| Phospho-Rb (Ser807/811) | 9308 |
| PCNA | 2586 |
| p21 | 2947 |
| p27 | 3688 |
| β-Catenin | 8480 |
| Phospho-β-catenin (Ser45/Thr41) | 9565 |
| Phospho-β-catenin (Ser33/37/Thr41) | 9561 |
| GSK-3β | 9315 |
| Phospho-GSK-3β (Ser9) | 9323 |
| Dvl2 | 3224 |
| JNK 1 | 3708 |
| Phospho-JNK (Thr183/Tyr185) | 4668 |
| c-Jun | 9165 |
| c-Fos | 2250 |
| STAT-3 | 4904 |
| Phospho–STAT-3 | 9145 |
| EGFR | 4267 |
| Phospho-EGFR (Tyr1068) | 3777 |
| AKT | 4691 |
| Phospho-AKT | 9271 |
| p38 | 9212 |
| Phospho-p38 | 9211 |
| ERK1/2 | 9102 |
| Phospho-EKR1/2 | 4376 |
| c-Met | 4560 |
| Phospho-Met (Tyr1234/1235) | 3077 |
| p65 | 8242 |
| Phospho-p65 (Ser536) | 3033 |
| IκB | 4812 |
| GAPDH | 2118 |
| Actin | 4970 |
GAPDH, glyceraldehye-3-phosphate dehydrogenase.
Antibodies were obtained from Cell Signaling Technologies (Danvers, MA).
Protein Isolation and Western Blot Analysis
Protein estimation and Western blot analysis were performed using pooled samples of protein extracts prepared from frozen liver tissues, as previously published, without any modifications.14
PCNA IHC
Paraffin-embedded liver sections (4 μm thick) were used for IHC detection of proliferating cell nuclear antigen (PCNA), as described before.14
Real-Time PCR
Total RNA was isolated from APAP300 and APAP600 livers using the TRIzol method, according to the manufacturer's protocol (Sigma, St. Louis, MO), and converted to cDNA, as previously described.5 mRNA levels of various genes were determined using real-time PCR analysis using commercially available TaqMan Gene Expression Assays (Life Technologies, previously known as Applied Biosystems, Carlsbad, CA) on the Applied Biosystems Prism 7300 Real-Time PCR Instrument, according to the manufacturer's protocol. Rplp0 gene expression in the same samples was used for data normalization, and data were expressed as fold change compared with 0 hour.
ChIP Data
Chromatin immunoprecipitation (ChIP) was done using whole liver tissue from APAP300 and APAP600 animals for the 12-hour time point, as described previously.15 Chromatin was isolated using approximately 200 mg of frozen liver tissue from three mice of each group. Isolated chromatin was then incubated with no antibody, 5 μg β-catenin (catalog number 610154; BD Transduction, Danvers, MA) or 5 μg p65 (catalog number 8242; Cell Signaling Technologies) antibodies for immunoprecipitation. Real-time PCR analysis was done using primers designed to cover known p65 and β-catenin binding sites on Ccnd1 promoter. Data were normalized with input DNA and represented as fold enrichment with respect to no antibody control. Primer sequences were as follows: forward, 5′-GCAGGACTTTGCAACTTCAAC-3′ and reverse, 5′-TTTCTCTGCCCGGCTTTG-3′.
Statistical Analysis
Data presented in the form of bar/line graphs show means ± SEM. A significant difference between groups was determined using Student's t-test. A difference between groups was considered statistically significant at P < 0.05.
Results
Sustained Injury and Inhibited Recovery after Higher Dose of APAP
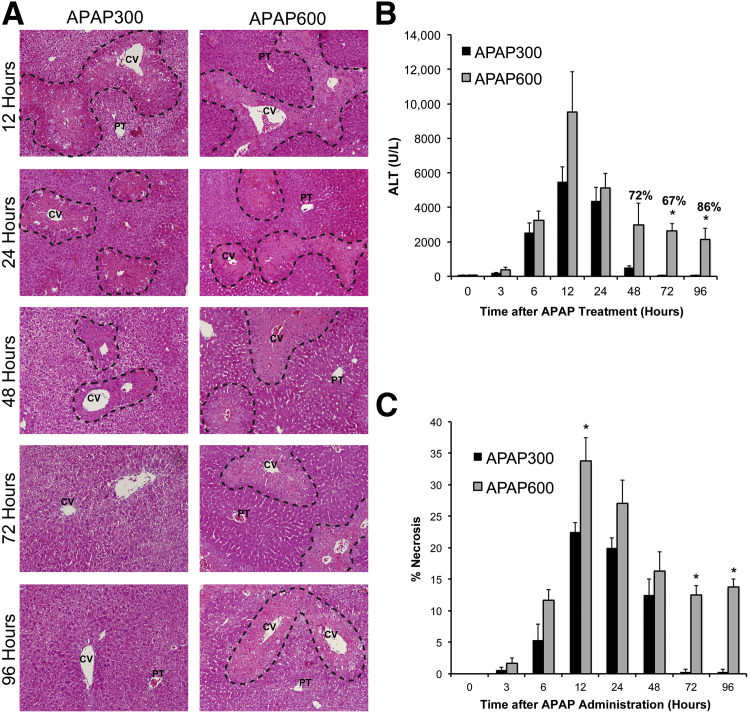
We compared liver injury at the two doses of APAP (APAP300 and APAP600) over the time course of 0 to 96 hours by studying serum ALT and histopathological analysis of liver sections (scoring sections for percentage necrosis) (Figure 1, A–C). Liver injury increased after APAP treatment and peaked at 12 hours after treatment at both doses. Liver injury was characterized by necrosis in the centrilobular region, a hallmark of APAP toxicity. Interestingly, there was no significant difference in injury up to 24 hours in both the groups, except area of necrosis was significantly higher at 12 hours in APAP600 compared with APAP300 group (Figure 1C). The overall trend showed moderately higher injury in APAP600 group up to 24 hours. However, there was a marked difference in progression of injury at later time points. APAP300 showed slight regression of injury at 48 hours and complete recovery at 72 and 96 hours, as evidenced by decreased serum ALT levels, complete recovery from necrosis, and 100% survival. In contrast, approximately 25% animals died at various time points from 48 to 96 hours in the APAP600 group (Figure 1B). Remaining animals showed sustained injury and were not recovered up to 96 hours. Injury was strikingly higher at 72 and 96 hours in the APAP600 group compared with the APAP300 group. In addition, we investigated other parameters of liver failure (serum glucose and bilirubin levels) in APAP300 and APAP600 mice, which displayed a similar pattern showing a significant difference between the two groups at later time points of 48 to 96 hours after APAP treatment (Supplemental Figure S1, A and B).
Figure 1.
Sustained liver injury and inhibited recovery after higher dose of APAP. A: Representative photomicrographs of H&E-stained liver sections with necrotic area outlined. B: Bar graph shows serum ALT levels with percentage survival specified over bars at time points where any mortality was observed. C: Bar graph shows percentage necrosis area on the basis of H&E-stained liver sections. All samples were collected from mice treated with either APAP300 or APAP600 at various time points up to 96 hours after APAP treatment. ∗P < 0.05 between two doses. CV, central vein; PT, portal triad.
Inhibited Liver Regeneration and Cell Cycle Arrest Correlate with Sustained Injury and Inhibited Recovery at a Higher Dose
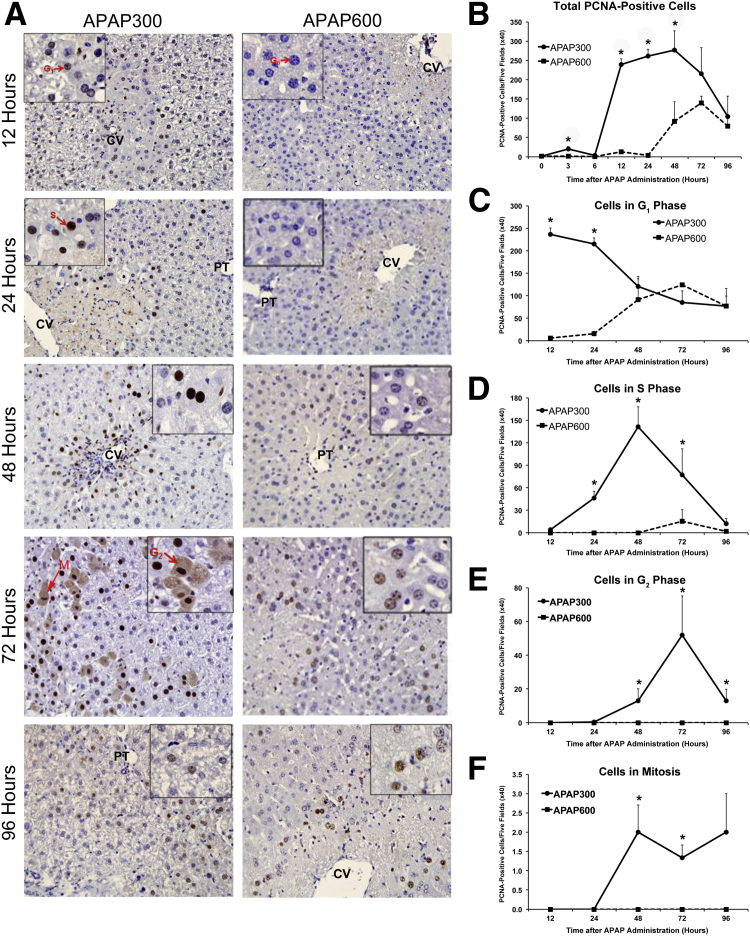
Next, we studied liver regeneration at the two doses of APAP using PCNA IHC (Figure 2A). Quantification of total PCNA-positive cells (Figure 2B) showed evidence of a few cells entering the cell cycle as early as 3 hours in APAP300, but a robust proliferation response with a dramatic increase in PCNA-positive cells was observed only from 12 hours after APAP treatment, specifically around necrotic areas. PCNA-positive cells remain elevated up to 48 hours and then progressively decreased at 72 and 96 hours. In contrast, APAP600 showed almost no PCNA-positive cells up to 24 hours after APAP administration. PCNA-positive cells started appearing at a much delayed time point of 48 hours onward in the APAP600 group and were significantly lower compared with peak cell proliferation observed in the APAP300 group. The PCNA data were corroborated by Ki-67 immunofluorescence staining (Supplemental Figure S2). Overall, there was significantly delayed and attenuated liver regeneration in the APAP600 group, which correlated with sustained injury, delayed recovery, and decreased survival at a higher dose.
Figure 2.
Inhibition of liver regeneration following higher doses of APAP. A: Representative photomicrographs of PCNA-stained liver sections. Insets: Cells in specific phase of cell cycle (arrows), indicating different phases of the cell cycle. G0, G1, and S cells show blue, light brown, and deep brown nuclear staining, respectively; G2 cells, diffused brown cytoplasmic staining; and M phase cells, deep blue chromosomal staining. B: Line graph shows total number of PCNA-positive cells per five high-power (×40) fields. C–F: Line graphs show total number of cells in G1 (C), S (D), G2 (E), and M (F) phases per five high-power (×40) fields, demonstrating cell cycle progression. All samples were collected from mice treated with either APAP300 or APAP600 over a time course of 0 to 96 hours after APAP treatment. ∗P < 0.05 between two doses. CV, central vein; PT, portal triad.
Interestingly, even at the time point of peak injury (12 hours after APAP treatment) at a higher dose, >50% of hepatocytes were still intact, as demonstrated by necrosis scoring. This suggests that a marked decrease in regeneration in the APAP600 dose is not due to lack of viable hepatocyte in the higher dose. This was further confirmed by hepatocyte nuclear factor (HNF) 4α staining as a marker of intact hepatocytes (Supplemental Figure S3). In fact, overall cellular death was not strikingly different at the two doses at early time points, where regeneration was initiated at a lower dose. Furthermore, a closer look at individual animal data revealed that, although there was no difference in liver injury in some animals between groups, there was a striking difference in regenerative response (data not shown).
Next, we quantified cells in a specific cell cycle phase by studying PCNA-stained sections in detail to further look into progression of cell cycle (Figure 2, C–F). In the APAP300 group, cells started entering the cell cycle at 12 hours, with numerous cells entering into the G1 phase from the G0 phase. At 24 hours, most of the cells were still in the G1 phase, with many cells progressed to the S phase. Most of the cells progressed to the S phase at 48 hours and further to the G2 phase by 72 hours. Mitoses were evident between 48 and 96 hours. By 96 hours, most cells returned to the quiescent state. In contrast, in the APAP600 group, almost all of the cells stayed in the G0 phase up to 24 hours. A few cells started entering the G1 phase only at a much delayed time point of 48 hours, and furthermore, these cells do not progress through the cell cycle at later time points and remain arrested at the G1 phase. No mitotic activity was observed at any time points in the APAP600 group. These results suggest that inhibition of entry into cell cycle and early cell cycle arrest in the remaining viable hepatocytes at higher dose are the reason for inhibited liver regeneration, rather than mere higher cell death.
Cell Cycle Regulators Correlate with Arrested Cell Cycle and Inhibited Liver Regeneration at a Higher Dose
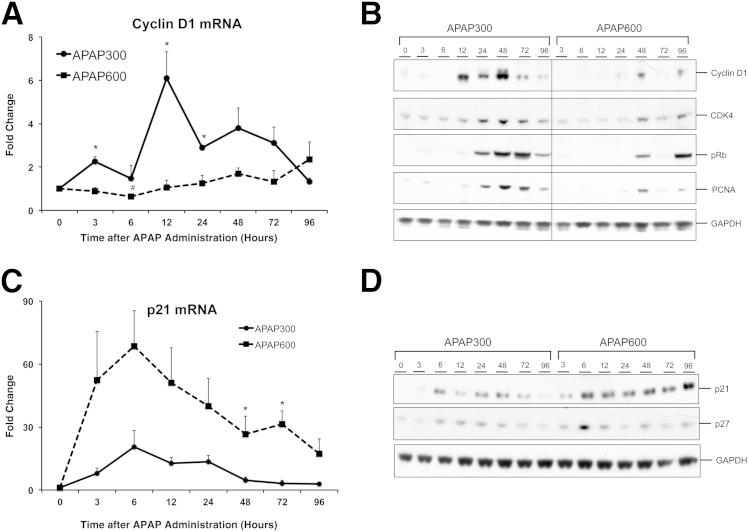
We further studied expression of various core cell cycle proteins, which regulate cell cycle entry. We first determined cyclin D1 levels, induction of which is the critical step that is required by hepatocytes for progressing through the G1 phase and committing to DNA replication.16 There was an early twofold induction of cyclin D1 mRNA at 3 hours, followed by a sharp peak (sixfold induction) at 12 hours, specifically in the APAP300 group, correlating with time of onset of the regenerative phase. Cyclin D1 mRNA level rapidly declined at 24 hours to approximately 2.5-fold and reached to basal level by 96 hours. In contrast, cyclin D1 mRNA did not increase significantly in the APAP600 group at any time point but showed slight induction in some animals at much delayed time points of 48 and 96 hours (Figure 3A). Moreover, we observed a significant decrease in cyclin D1 mRNA levels at 6 hours compared with basal levels in the APAP600 group, suggesting active inhibition of the cell cycle. Western blot analysis further corroborated mRNA data with an early and overall much higher increase in cyclin D1 protein only in APAP300 dose, correlating with PCNA protein expression (Figure 3B). Cyclin D1 binds to cyclin-dependent kinase (CDK) 4, and this complex, in turn, causes phosphorylation and inactivation of retinoblastoma (Rb), which ultimately leads to transcription of many cell cycle genes.16 CDK4 protein and phosphorylation of Rb followed a trend similar to cyclin D1 expression (Figure 3B).
Figure 3.
Cell cycle regulators correlate with inhibited liver regeneration at higher dose. Cyclin D1 (A) and p21 (C) mRNA expression, cyclin D1, CDK4, phospho-Rb, and PCNA (B) and p21 and p27 (D) protein expression in liver of mice treated with either APAP300 or APAP600. All samples were collected over a time course of 0 to 96 hours after APAP treatment. ∗P < 0.05 between two doses. GAPDH, glyceraldehye-3-phosphate dehydrogenase.
We further analyzed the dynamics of CDK inhibitors, p21 and p27, to elucidate if active cell cycle inhibition is involved at a higher dose (Figure 3, C and D). Interestingly, p21 mRNA was markedly induced at both the doses as early as 3 hours after APAP. However, induction was much higher and sustained up to 96 hours at a higher dose but decreased remarkably after 24 hours at a lower dose (Figure 3C). Protein expression of p21 also displayed a similar trend. The p27 protein also showed moderate induction at both doses, showing a pattern similar to p21, with notable higher expression at 6 hours after APAP treatment at higher dose, the time point preceding onset of normal liver regeneration response in regenerative dose (Figure 3D). These data suggest that active cell cycle inhibition is involved at a higher dose of APAP, where liver regeneration is inhibited.
Overall, early and robust induction of cyclin D1 at a lower dose resulted in timely liver regeneration and recovery. Inhibition of such a robust and timely response and sustained activation of cell cycle inhibitors at a higher dose correlated with cell cycle arrest, inhibited liver regeneration, progression of injury, and decreased survival. Next, we studied major upstream pathways, which are known to induce cyclin D1 and stimulate liver regeneration. For these studies, on the basis of the time course of regenerative response, we focused on time points up to 24 hours after APAP treatment, considering the importance of early regenerative response for successful recovery.
Growth Factor Signaling via EGFR and c-Met and MAPK Signaling Remain Highly Activated after High Dose of APAP
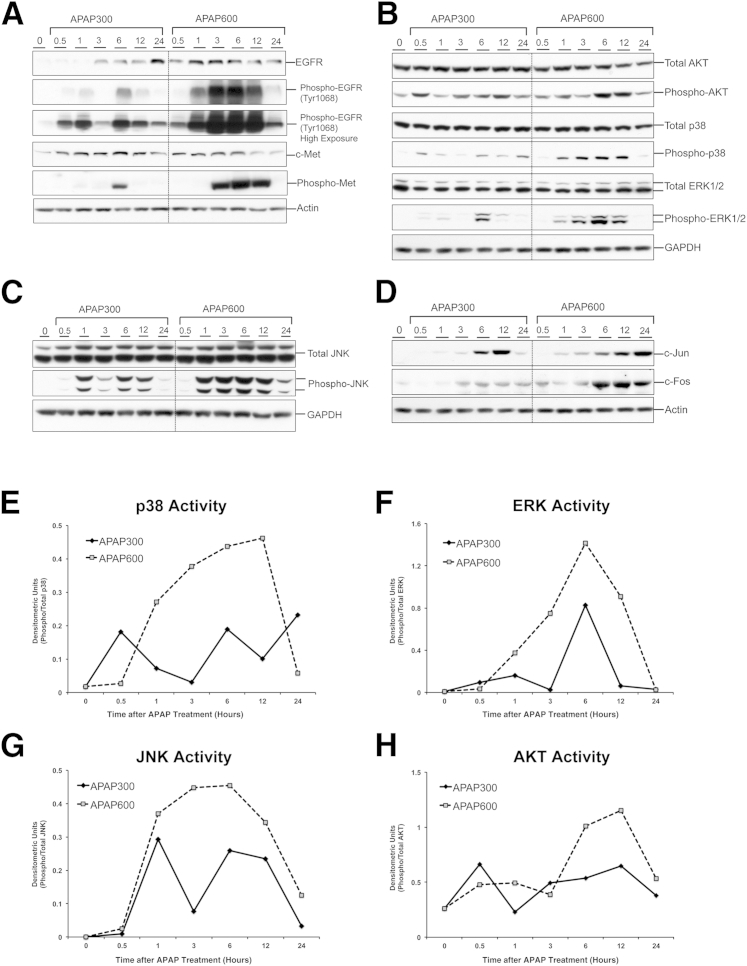
Epidermal growth factor (EGF) and hepatocyte growth factor (HGF) are considered the primary mitogens for hepatocytes and are critical for liver regeneration after PHX.8 EGF and HGF act primarily through activation of EGF receptor (EGFR) and c-Met receptor, respectively.8 This leads to, among others, activation of mitogen-activated protein kinase (MAPK) signaling, culminating in cell proliferation. However, the importance of these growth factors in liver regeneration after APAP-induced ALI is not known. We found that the protein expression of EGFR and c-Met was increased at both the doses (Figure 4A). To determine activation status of these receptors, we investigated the expression of phosphorylated forms of EGFR and c-Met. There was remarkable activation of EGFR as early as 0.5 hours after APAP300 treatment, attaining a peak at 6 hours, the time point preceding onset of marked liver regeneration response, and then progressively decreased at later time points. Interestingly, a similar trend was observed for the APAP600 group, with much higher and sustained response in a dose-dependent manner (Figure 4A). Similar results were observed for c-Met activation, for which we found marked phosphorylation only at 6 hours after APAP300 treatment, but much higher and sustained phosphorylation after APAP600 treatment, starting as early as 3 hours and maintained until 12 hours (Figure 4A). Further studies on downstream MAPK signaling revealed that AKT, p38, extracellular signal–regulated kinase (ERK) 1/2, and c-Jun N-terminal kinase (JNK) total protein levels remained unchanged after both the doses. However, a dose-dependent increase in phosphorylation (activation) of all these kinases, including AKT, p38, ERK1/2, and JNK, was observed after APAP treatment (Figure 4, B, C, and E–H). Activation of all these kinases was higher in the APAP600 group compared with the APAP300 group. Furthermore, a similar trend was observed for expression of downstream transcription factors, c-Jun and c-Fos (Figure 4D). Overall, these results suggest that signaling through growth factor and downstream MAPKs remains activated even at the dose of APAP where liver regeneration is inhibited. Thus, lack of activation of these signaling factors may not be the reason for failed liver regeneration at a higher dose.
Figure 4.
Growth factor signaling via EGFR and c-Met and MAPK signaling remain highly activated after high dose of APAP. Western blot analysis of EGFR, phospho-EGFR (normal and high exposures), c-Met, phospho-Met (A), total AKT, phospho-AKT, total p38, phospho-p38, total ERK1/2, phospho-ERK 1/2 (B), total JNK, phospho-JNK (C), and c-Jun and c-Fos (D) using total liver extract of mice liver treated with either APAP300 or APAP600. Densitometric analysis shows p38 (E), ERK1/2 (F), JNK (G), and AKT (H) activation. All samples were collected over a time course of 0 to 24 hours after APAP treatment. GAPDH, glyceraldehye-3-phosphate dehydrogenase.
Apart from involvement in cell proliferation and liver regeneration pathways,17 JNK activation is also considered as one of the important steps in mediating APAP overdose–induced liver injury.18 Phosphorylation of JNK and its translocation to mitochondria early in APAP toxicity leads to exacerbation of mitochondrial oxidant stress.18 Despite previous attempts, the role of JNK activation in liver regeneration after APAP overdose remains undefined and should be further evaluated.19
Differential Activation of Cytokine Pathways (IL-6/STAT-3 and TNF-α/NF-κB) during Liver Regeneration after APAP Overdose
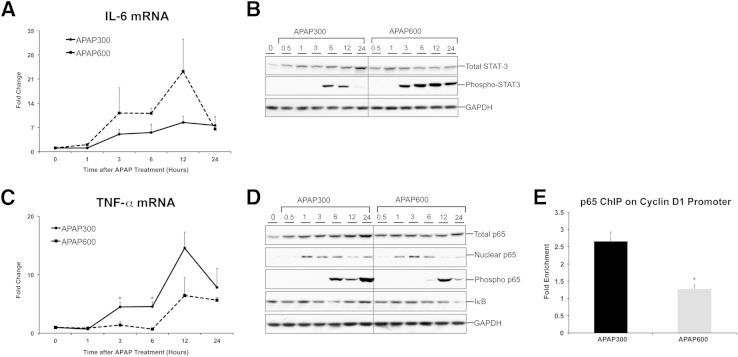
Activation of cytokine pathways [IL-6/STAT-3 and tumor necrosis factor (TNF)-α/NF-κB] is considered as an important priming event that contributes to initiation of early stages of liver regeneration after PHX.16 However, their role in liver regeneration after APAP overdose is not known. IL-6 mRNA was induced at both the doses of APAP as early as 3 hours after APAP treatment and remains induced up to 24 hours, showing a peak at 12 hours after APAP treatment (Figure 5A). Interestingly, IL-6 induction was greater at a higher dose of APAP, at which regeneration was inhibited. IL-6 binding to its receptor at the cellular membrane leads to phosphorylation and nuclear translocation of STAT-3, which ultimately results in transcription of many target genes. Corroborating IL-6 data, we observed significantly higher phosphorylation of STAT-3 in APAP600 mice, which showed less regeneration (Figure 5B). Although STAT-3 phosphorylation was transient (appeared at 6 and 12 hours) in the APAP300 group, it started early (at 3 hours) and maintained until 24 hours in the APAP600 group. Although total STAT-3 levels were induced after APAP treatment in both doses, no significant difference between the groups was observed. Overall, these data suggest that, similar to growth factor signaling, IL-6 signaling remains intact, even at a nonregenerative dose of APAP; thus, it may not explain failed liver regeneration at a higher dose.
Figure 5.
Differential activation of cytokine pathways during liver regeneration after APAP overdose. IL-6 (A) and TNF-α (C) mRNA expression in liver of mice treated with either APAP300 or APAP600. Western blot analysis of total STAT-3, phospho STAT-3 (B), total, nuclear, phosphorylated p65, and IκB (D) using total liver extract (unless specified) of mice treated with either APAP300 or APAP600. All samples were collected over a time course of 0 to 24 hours after APAP treatment. E: ChIP analysis shows p65 binding to cyclin D1 promoter at 12 hours after either APAP300 or APAP600 treatment. ∗P < 0.05 between two doses. GAPDH, glyceraldehye-3-phosphate dehydrogenase.
Next, we determined the status of NF-κB signaling during liver regeneration after APAP-induced ALI using our incremental dose model (Figure 5, C and D). TNF-α binding to its receptor ultimately results in stabilization and nuclear translocation of transcription factor NF-κB (consisting of p65 as one of its subunits). Cyclin D1 is a known target of NF-κB.20 Interestingly, TNF-α was induced at early time points (3 and 6 hours), preceding the regenerative phase, specifically in a lower (regenerative) dose, and its mRNA level peaked at 12 hours after APAP treatment. In contrast, TNF-α was not induced until 12 hours after APAP in a higher dose and overall showed much lower induction compared with APAP300 dose (Figure 5C). Furthermore, there was a marked increase in phosphorylation of p65 at Ser536, known to enhance transactivation potential of p65,21 in the APAP300 group preceding regenerative phase (6 hours after APAP) and sustained until 24 hours (Figure 5D). Although the APAP600 group also showed an increase in Ser536 phosphorylation, it was only at 12 hours after APAP and was overall lower than in the APAP300 group. Although marked nuclear translocation of p65 was observed at both the doses, the increase in nuclear p65 was sustained in the APAP300 group until 24 hours after APAP, but declined sharply at 12 and 24 hours after APAP in the APAP600 group. Total p65 protein expression was increased in both the groups at all of the time points compared with basal levels, and IκB protein level did not appear to change at any dose (Figure 5D).
Overall, activation of NF-κB signaling correlated with stimulation of liver regeneration at a lower dose, and we observed an indication of inhibited activation of NF-κB signaling at a higher dose, where liver regeneration was inhibited. Considering cyclin D1 is a known target of p-65, we wanted to directly compare p-65 binding with cyclin D1 promoter at the two doses using ChIP assay (Figure 5E). Interestingly, we observed an approximately 2.5-fold higher binding of p-65 to cyclin D1 promoter in the APAP300 compared with the APAP600 group, 12 hours after APAP treatment, which is the time point of peak induction of cyclin D1. These data suggest that inhibited activation of NF-κB signaling may be one of the reasons for inhibited cyclin D1 induction and inhibited liver regeneration at a higher dose.
Activation of Wnt/β-Catenin Signaling Is Inhibited after a Higher Dose of APAP
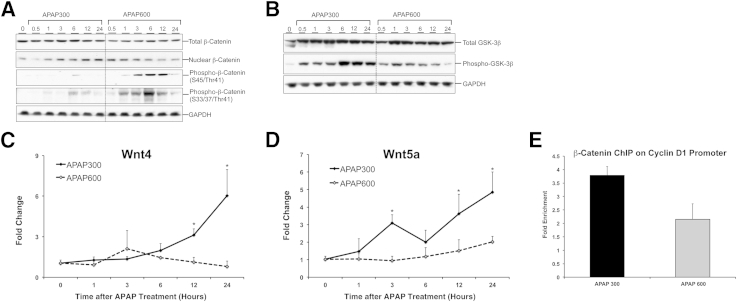
Previous reports have demonstrated involvement of β-catenin activation in liver regeneration after PHX and APAP-induced ALI.5,22 Herein, we further analyzed the role of Wnt/β-catenin signaling in detail using our incremental dose model. Although total β-catenin protein remained unchanged in both the groups at all time points, a substantial increase in nuclear β-catenin was observed at 12 and 24 hours after APAP overdose in the APAP300 group (Figure 6A). In contrast, APAP600 exhibited an initial increase in nuclear β-catenin at 0.5 and 1 hour after APAP treatment, which decreased at later time points. The decline in nuclear β-catenin was consistent with a substantial increase in two inactive forms of β-catenin (Ser45/Thr41 phosphorylated and Ser33/37/Thr41 phosphorylated) in APAP600 mice.
Figure 6.
Activation of Wnt/β-catenin signaling is inhibited after a higher dose of APAP. Western blot analysis of total β-catenin, nuclear β-catenin, phospho-β-catenin (S45/Thr41), phospho-β-catenin (S33/37/Thr41) (A) and total GSK-3β and phospho-GSK-3β (B). Total liver extracts (unless specified) of mice treated with either APAP300 or APAP600 were used for Western blot analyses. mRNA expression of Wnt4 (C) and Wnt5a (D) in liver of mice treated with either APAP300 or APAP600. All samples were collected over a time course of 0 to 24 hours after APAP treatment. E: ChIP analysis shows β-catenin binding to cyclin D1 promoter at 12 hours after either APAP300 or APAP600 treatment. ∗P < 0.05 between two doses. GAPDH, glyceraldehye-3-phosphate dehydrogenase.
To further study the upstream mechanism, we determined expression of total and phosphorylated (inactive) forms of glycogen synthase kinase-3β (GSK3β), which regulates β-catenin degradation in canonical Wnt signaling (Figure 6B). Data indicate significant inactivation of GSK3β, as shown by a marked increase in phospho-GSK3β protein in APAP300 mice between 6 and 24 hours after APAP overdose, correlating with β-catenin activation. Interestingly, a higher dose showed initial GSK3β inactivation to some extent (less compared with APAP 300), but this inactivation markedly decreased during later phase. The decreased inactivation (or increased activation) of GSK3β at 6 to 24 hours after APAP treatment in the APAP600 group is consistent with an increase in phosphorylated β-catenin species (inactive). Next, we determined protein expression of Dvl, which acts upstream of GSK3β in canonical Wnt signaling and is activated by Wnt binding to Frizzled (Fzd) receptor. A significant increase in Dvl expression was observed in the APAP300 group at 12 and 24 hours after APAP. In contrast, Dvl expression increased early after APAP600 dose but declined during the regenerative phase (Supplemental Figure S4).
To determine whether expression of any of the Wnts and Fzds is induced during APAP-induced liver regeneration, we quantified mRNA of all 11 Wnts and 10 Fzld genes known to be expressed in the liver.23 The data indicate that Wnt4 (Figure 6C) and Wnt5a (Figure 6D) mRNA exhibited significant induction in the APAP300 group during regenerative phase (12 and 24 hours). However, none of the Wnt mRNAs were induced at any time point after APAP600 dose. Among the Fzd genes, Fzd7 was significantly induced only in the APAP300 group between 3 and 12 hours, whereas Fzd8 showed an opposite trend, with slight but significant induction in the APAP600 group at 12 and 24 hours (Supplemental Figure S4). All other Fzd genes remained unaffected in both groups.
Overall, we observed stimulation of Wnt/β-catenin signaling specifically in a regenerating dose, correlating with induction of cyclin D1, which is a known target of β-catenin.24 Furthermore, activation of Wnt/β-catenin signaling was inhibited at a higher dose, correlating with inhibited cyclin D1 induction. Finally, we directly checked if there is a difference in β-catenin binding to cyclin D1 promoter at the two doses using ChIP assay. We observed an approximately twofold higher enrichment of β-catenin binding to cyclin D1 promoter in APAP300 compared with APAP600 group, 12 hours after APAP treatment, which is the time point of peak induction of cyclin D1 (Figure 6E). Taken together, these data suggest that inhibited activation of Wnt/β-catenin signaling may be one of the reasons for inhibited cyclin D1 induction and inhibited liver regeneration at a higher dose.
Increased Liver Regeneration in Mice Overexpressing β-catenin after APAP Overdose
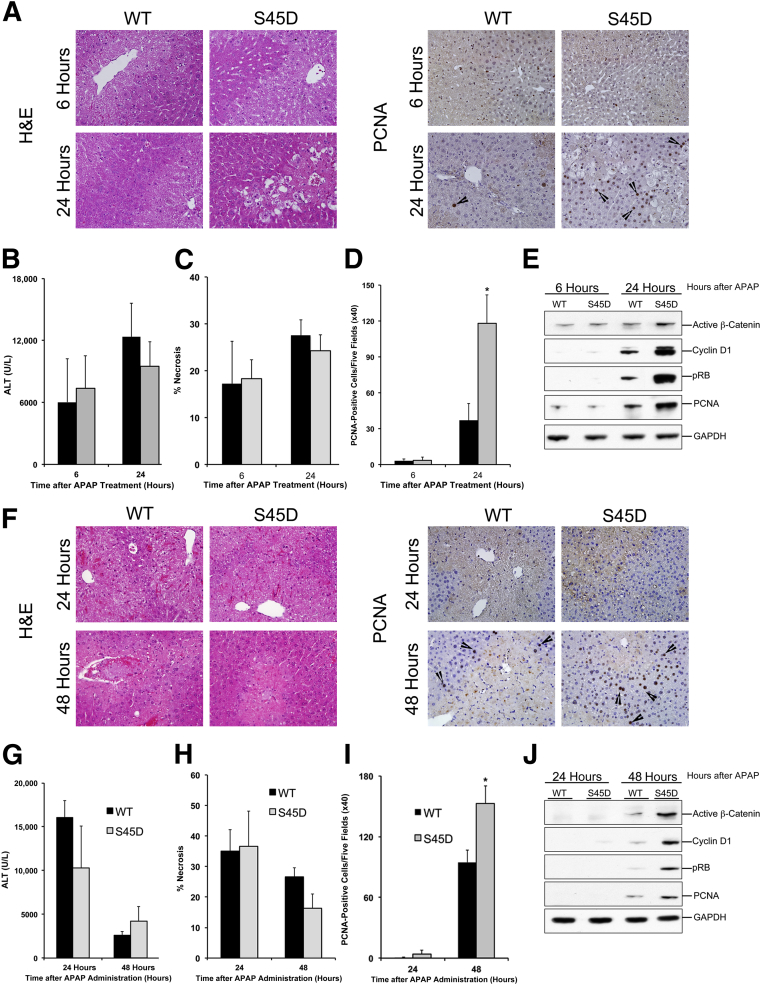
Our studies using the incremental dose model identified Wnt/β-catenin signaling as a potential target for stimulating liver regeneration after APAP overdose. We further confirmed the role of β-catenin in stimulation of liver regeneration after APAP overdose using mice overexpressing S45D. Preliminary analysis showed moderately higher total β-catenin in the S45D mice, which is consistent with previous studies (Supplemental Figure S5A).13 Further analysis indicated no significant difference in hepatic CYP2E1, the main enzyme involved in APAP bioactivation, in S45D mice compared with WT mice (Supplemental Figure S5A). Preliminary studies indicated that the WT control mice for the S45D strain, which are the S45D negative littermates, are more susceptible to APAP than C57BL/6 mice. Therefore, we initially used a dose of 300 mg/kg APAP in this study, which was sufficient to show attenuated liver regeneration response in WT mice. WT and S45D mice were treated with 300 mg/kg APAP, and liver injury and regeneration were analyzed at 6 and 24 hours after APAP treatment. Serum ALT levels and necrosis scoring demonstrated similar liver injury in WT and S45D mice at both the time points after APAP (Figure 7, A–C), suggesting the injury process is not altered in S45D mice. Although WT mice showed only a few PCNA-positive hepatocytes at 24 hours after APAP treatment, liver regeneration was remarkably improved in S45D mice, as indicated by higher PCNA-positive hepatocytes in S45D mice at 24 hours after APAP treatment, compared with WT mice (Figure 7D). This was further substantiated by PCNA protein expression, which displayed a similar pattern (Figure 7E). Western blot analysis indicated significantly higher activated β-catenin in S45D mice at 24 hours after APAP treatment (Figure 7E). An increase in activated β-catenin was accompanied by increased cyclin D1 and phosphorylation of Rb protein in S45D mice at 24 hours after APAP overdose (Figure 7E). Real-time PCR analysis indicated increased gene expression of cyclin D1 and axin 2, the known targets of β-catenin, in S45D mice after APAP treatment, compared with WT mice at 24 hours after APAP overdose (Supplemental Figure S5, B and C). Next, we studied liver regeneration in S45D mice after a higher dose of APAP (600 mg/kg) (Figure 7, F–J). Neither WT nor S45D mice showed any significant liver regeneration at 24 hours after APAP. However, S45D mice showed higher PCNA-positive cells (with higher PCNA protein expression) compared with WT mice (Figure 7, F, I, and J) at 48 hours after APAP treatment. This was further substantiated by higher cyclin D1 protein expression and downstream phosphorylation of Rb protein in S45D mice compared with WT mice at 48 hours after APAP treatment (Figure 7J). Similar to APAP300 dose, both WT and S45D mice showed similar injury at 24 and 48 hours after APAP600 treatment (Figure 7, F–H). These data show that overexpression of β-catenin resulted in faster and higher liver regeneration after APAP overdose.
Figure 7.
Overexpression of stable form of β-catenin improves liver regeneration after APAP overdose. A: Representative photomicrographs of H&E- and PCNA-stained liver sections from WT and S45D mice treated with 300 mg/kg APAP. B–D: Bar graphs show serum ALT levels (B), percentage necrosis area (C), and PCNA counts (D) in WT (black bars) and S45D (gray bars) mice treated with APAP300. E: Western blot analysis of active β-catenin, cyclin D1, pRb, and PCNA using total liver extracts in WT and S45D mice treated with APAP300. F: Representative photomicrographs of H&E- and PCNA-stained liver sections from WT and S45D mice treated with APAP600. Bar graphs show serum ALT levels (G), percentage necrosis area (H), and PCNA counts (I) in WT (black bars) and S45D (gray bars) mice treated with APAP600. J: Western blot analysis of active β-catenin, cyclin D1, pRb, and PCNA using total liver extracts in WT and S45D mice treated with APAP600. ∗P < 0.05 between WT and S45D group. Arrowhead represents PCNA-positive cells. GAPDH, glyceraldehye-3-phosphate dehydrogenase.
Discussion
APAP overdose is the foremost cause of ALF in the United States, contributing to approximately 46% of all ALF cases.25 Treatment options after APAP-induced ALF are extremely limited. Extensive research on chemicals and drugs that induce liver injury has shown that compensatory liver regeneration plays a critical role in determination of final outcome of injury. It is known that timely stimulation of regeneration leads to regression of injury, whereas failing to regenerate culminates in progression of injury and death.9,11,26 Several recent clinical and animal studies showing similar findings after APAP overdose suggest that stimulating liver regeneration can be a potential treatment option for APAP-induced ALF.5–7 However, our knowledge regarding mechanisms of liver regeneration after APAP-induced injury is extremely limited, and there is a critical need to study these mechanisms systematically.
Dynamics of liver regeneration after toxic insult have been extensively studied for many toxicants, such as thioacetamide,11 carbon tertrachloride,27 and chloroform.26 These studies demonstrated that liver regeneration follows classic rules of dose-response such that regeneration increases with dose of toxicant proportionate to injury. This occurs until a threshold dose beyond which the ability of liver to regenerate declines, leading to progression of injury.9 On the basis of this principle, we developed an incremental dose model to study mechanisms of liver regeneration after APAP-induced ALI.
Herein, we used two doses of APAP, a lower dose (300 mg/kg) that caused extensive liver injury but also significant compensatory regeneration, leading to regression of injury and spontaneous recovery, and a higher dose (600 mg/kg) that caused sustained injury, compromised recovery, and decreased survival. We observed marked inhibition of liver regeneration at a higher dose, highlighting the importance of liver regeneration in overall survival after APAP overdose, supporting previous findings.5–7 The marked inhibition of regeneration was not due to lack of viable hepatocytes at the higher dose. Necrosis score analysis and IHC staining for HNF4α, which stains for viable hepatocytes, demonstrated that >50% hepatocytes were viable in APAP600 dose at peak injury. In fact, overall cellular death was not strikingly different at the two doses at early time points, where regeneration was initiated at lower dose and inhibited at higher dose. These data and further analysis of cell cycle phases indicated that the inhibition of regeneration was not simply because of too much cell death but due to inhibited cell cycle entry and cell cycle arrest at an early stage in viable cells surrounding the necrotic zone at a higher dose. Higher cellular stress in viable hepatocytes may be a possible explanation for this inhibited liver regeneration at a higher dose. This observation also distinguishes the APAP incremental dose model from 90% PHX model, in which the primary reason behind decreased liver regeneration is lack of critical mass required to initiate regeneration.
Our most remarkable finding was that cyclin D1 mRNA and protein expression increased in the lower dose preceding the start of regenerative phase, but this was completely inhibited in the higher dose. These data further support that initial entry into the cell cycle is inhibited at a higher dose, underlining the importance of induction of cyclin D1 in regulation of liver regeneration after APAP-induced ALI. Furthermore, we found higher and sustained induction of p21 protein at a higher dose, supporting possible contribution of active inhibition of cell cycle in cell cycle arrest at higher dose due to elevated cellular stress. Similar results were reported previously after ethanol treatment in the PHX model, where ethanol inhibited cyclin D1, induced p21, and impaired liver regeneration.28 Interestingly, there was moderate induction of p21, even at the regenerative dose, suggesting possible role of p21 in coordinating liver regeneration. Recent study demonstrating role reversal of p21 in liver regeneration, depending on degree of injury, certainly supports this possibility.29 Further studies are required to determine the exact role of p21 in liver regeneration after APAP overdose.
Several regenerative pathways, such as growth factors/MAPKs, TNF-α/NF-κB, Wnt/β-catenin, and IL-6/STAT-3, are known to induce cyclin D1 and hepatocyte proliferation.8 We determined the status of some of these pathways in our model to look further into the mechanism behind differential expression of cyclin D1 at both the doses. There was remarkable activation of growth factor receptors (c-Met and EGFR), downstream MAPKs (ERK1/2, p38, and JNK), and IL-6/STAT-3 signaling at the regenerative dose, but, interestingly, activation was much higher and sustained at the nonregenerative dose. Previous studies suggest that some of these pathways may be important for normal liver regeneration after APAP overdose, where animals spontaneously regenerate. For instance, liver regeneration was found to be impaired in IL-6 knockout mice after moderate APAP overdose.30 Further confirmatory studies are required to demonstrate the role of some of these pathways in liver regeneration after APAP overdose.
However, our data clearly indicate, for the first time to our knowledge, that inability to activate these signaling pathways is not limiting regeneration at a higher dose, where animals are not able to recover spontaneously, because these pathways are already highly activated at this dose. Additional stimulation of these pathways may not be a good strategy from a therapeutic standpoint. This also questions administration of growth factors as a therapeutic strategy after APAP-induced ALF. This observation is consistent with previous reports that administration of growth factors did not affect liver regeneration in dogs after APAP-induced ALF.31 Also, patients who died after APAP-induced ALF were found to have a higher amount of HGF circulating in plasma.32
These data produce two possibilities. First, an active inhibitory signaling is blocking liver regeneration at a higher dose, counteracting this regenerative signaling. Activation of cell cycle inhibitors (eg, p21) is one such signaling pathway that we observed in this study. Moreover, some of the known regenerative pathways, which are highly activated in APAP600, may be actively involved in inhibiting regeneration. For instance, overactivation of p38 has been associated with decreased regeneration in the PHX model.28,33 Also, p38 can directly inhibit cyclin D1 expression.34 Similarly, impaired regeneration after PHX in ob/ob mice with fatty liver or in mice with hyper-stimulated IL-6 signaling was correlated with sustained activation and overactivation of STAT-3.35,36 Furthermore, sustained activation of these mediators was correlated with induction of p21 and inhibition of cyclin D1, which is consistent with our data.
The second possibility is that specific critical pathways apart from the growth factor–mediated proregenerative signaling regulate liver regeneration after APAP overdose. Previous studies have shown that the Wnt/β-catenin pathway plays a critical role in liver regeneration, in general, and after APAP overdose, in particular.5,22 Consistent with these data, we observed activation of canonical Wnt signaling at the regenerative dose. More interestingly, β-catenin activation was inhibited at a higher dose, where liver regeneration was inhibited. We also observed TNF-α/NF-κB signaling activation at the regenerative dose, supporting previous studies showing a role of TNF receptor in liver regeneration after APAP overdose.37,38 Furthermore, previous data showed increased NF-κB DNA binding after APAP treatment, which was correlated positively with regeneration in mice.39,40 Interestingly, similar to β-catenin signaling, our results indicated that activation of TNF-α/NF-κB signaling is inhibited at a higher dose, where recovery is compromised. Both β-catenin and NF-κB are known to transcriptionally regulate and induce cyclin D1 directly.20,24 Our ChIP data support the important role of such regulation in liver regeneration after APAP overdose. Our findings implicate that inhibited activation of Wnt/β-catenin and TNF-α/NF-κB signaling may be a contributing factor to inhibited liver regeneration after severe APAP overdose and, thus, could be a potential target for stimulating liver regeneration therapeutically.
Overactivation of β-catenin is known to accelerate liver regeneration after PHX.13,22 Furthermore, our previous study showed correlation of β-catenin activation with higher spontaneous liver regeneration and survival in patients with APAP-induced ALF.5 Herein, we observed that mice overexpressing a mutated form of β-catenin have better liver regeneration after low and high doses of APAP. Our data, for the first time to our knowledge, indicated that stimulating canonical Wnt signaling could be a viable approach for improving liver regeneration in patients with APAP-induced ALF.
In summary, our findings using a novel comparative model have demonstrated that high doses of APAP will inhibit liver regeneration because of active inhibition of cell cycle progression and/or by lack of stimulation via critical promitogenic pathways. Some interesting potential pathways that may be involved in active inhibition of liver regeneration include p38 MAPK and IL-6 signaling and require further detailed investigation. Furthermore, this study has revealed canonical Wnt signaling and NF-κB signaling as potential therapeutic targets to stimulate liver regeneration after APAP overdose. Finally, our studies indicate that concomitant inhibition of several inhibitory pathways and activation of various stimulatory pathways may have a promising future in developing regenerative therapies for APAP-induced patients with ALF.
Footnotes
Supported by NIH grants P20 RR021940, 5T32ES007079-34, and 1R01DK098414, an American Association for the Study of Liver Diseases/American Liver Foundation Liver Scholar Award, and grant 1R01DK098414.
Disclosures: None declared.
Supplemental Data
Serum glucose (A) and total bilirubin (B) levels in mice treated with either APAP300 or APAP600 over a time course of 0 to 96 hours. ∗P < 0.05 between two doses.
Ki-67 immunofluorescence performed on frozen liver sections of mice treated with APAP300 and APAP600 and scarified at 24 hours after APAP treatment. Arrowheads indicate Ki-69 positive cells.
HNF4α IHC on paraffin-embedded liver sections from APAP600-treated mice at 12 and 24 hours after APAP treatment.
Western blot (A) and densitometric (B) analyses of Dvl using total liver extracts from mice treated with either APAP300 or APAP600. Real-time PCR analysis of Fzd7 (C) and Fzd8 (D) mRNA in livers of mice treated with APAP300 and APAP600. ∗P < 0.05 between two doses. GAPDH, glyceraldehye-3-phosphate dehydrogenase.
A: Total β-catenin and CYP2E1 blots in S45D mice before APAP treatment. Changes in cyclin D1 (B) and axin 2 (C) mRNA at 24 hours after APAP300 treatment to WT and S45D mice. ∗P < 0.05 between two doses. GAPDH, glyceraldehye-3-phosphate dehydrogenase.
References
- 1.Nourjah P., Ahmad S.R., Karwoski C., Willy M. Estimates of acetaminophen (Paracetomal)-associated overdoses in the United States. Pharmacoepidemiol Drug Saf. 2006;15:398–405. doi: 10.1002/pds.1191. [DOI] [PubMed] [Google Scholar]
- 2.Athuraliya T.N., Jones A.L. Prolonged N-acetylcysteine therapy in late acetaminophen poisoning associated with acute liver failure: a need to be more cautious? Crit Care. 2009;13:144. doi: 10.1186/cc7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keeffe E.B. Liver transplantation: current status and novel approaches to liver replacement. Gastroenterology. 2001;120:749–762. doi: 10.1053/gast.2001.22583. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt L.E., Dalhoff K. Alpha-fetoprotein is a predictor of outcome in acetaminophen-induced liver injury. Hepatology. 2005;41:26–31. doi: 10.1002/hep.20511. [DOI] [PubMed] [Google Scholar]
- 5.Apte U., Singh S., Zeng G., Cieply B., Virji M.A., Wu T., Monga S.P. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol. 2009;175:1056–1065. doi: 10.2353/ajpath.2009.080976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu B., Colletti L.M. Stem cell factor and c-kit are involved in hepatic recovery after acetaminophen-induced liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G45–G53. doi: 10.1152/ajpgi.00024.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donahower B.C., McCullough S.S., Hennings L., Simpson P.M., Stowe C.D., Saad A.G., Kurten R.C., Hinson J.A., James L.P. Human recombinant vascular endothelial growth factor reduces necrosis and enhances hepatocyte regeneration in a mouse model of acetaminophen toxicity. J Pharmacol Exp Ther. 2010;334:33–43. doi: 10.1124/jpet.109.163840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michalopoulos G.K. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehendale H.M. Tissue repair: an important determinant of final outcome of toxicant-induced injury. Toxicol Pathol. 2005;33:41–51. doi: 10.1080/01926230590881808. [DOI] [PubMed] [Google Scholar]
- 10.Anand S.S., Murthy S.N., Vaidya V.S., Mumtaz M.M., Mehendale H.M. Tissue repair plays pivotal role in final outcome of liver injury following chloroform and allyl alcohol binary mixture. Food Chem Toxicol. 2003;41:1123–1132. doi: 10.1016/s0278-6915(03)00066-8. [DOI] [PubMed] [Google Scholar]
- 11.Mangipudy R.S., Chanda S., Mehendale H.M. Tissue repair response as a function of dose in thioacetamide hepatotoxicity. Environ Health Perspect. 1995;103:260–267. doi: 10.1289/ehp.95103260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apte U.M., Limaye P.B., Desaiah D., Bucci T.J., Warbritton A., Mehendale H.M. Mechanisms of increased liver tissue repair and survival in diet-restricted rats treated with equitoxic doses of thioacetamide. Toxicol Sci. 2003;72:272–282. doi: 10.1093/toxsci/kfg021. [DOI] [PubMed] [Google Scholar]
- 13.Nejak-Bowen K.N., Thompson M.D., Singh S., Bowen W.C., Jr., Dar M.J., Khillan J., Dai C., Monga S.P. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology. 2010;51:1603–1613. doi: 10.1002/hep.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe A., Thomas A., Edwards G., Jaseja R., Guo G.L., Apte U. Increased activation of the Wnt/beta-catenin pathway in spontaneous hepatocellular carcinoma observed in farnesoid X receptor knockout mice. J Pharmacol Exp Ther. 2011;338:12–21. doi: 10.1124/jpet.111.179390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walesky C., Gunewardena S., Terwilliger E.F., Edwards G., Borude P., Apte U. Hepatocyte-specific deletion of hepatocyte nuclear factor-4alpha in adult mice results in increased hepatocyte proliferation. Am J Physiol Gastrointest Liver Physiol. 2013;304:G26–G37. doi: 10.1152/ajpgi.00064.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 17.Schwabe R.F., Bradham C.A., Uehara T., Hatano E., Bennett B.L., Schoonhoven R., Brenner D.A. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology. 2003;37:824–832. doi: 10.1053/jhep.2003.50135. [DOI] [PubMed] [Google Scholar]
- 18.Jaeschke H., McGill M.R., Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourdi M., Korrapati M.C., Chakraborty M., Yee S.B., Pohl L.R. Protective role of c-Jun N-terminal kinase 2 in acetaminophen-induced liver injury. Biochem Biophys Res Commun. 2008;374:6–10. doi: 10.1016/j.bbrc.2008.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guttridge D.C., Albanese C., Reuther J.Y., Pestell R.G., Baldwin A.S., Jr. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viatour P., Merville M.P., Bours V., Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Nejak-Bowen K.N., Monga S.P. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Semin Cancer Biol. 2011;21:44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng G., Awan F., Otruba W., Muller P., Apte U., Tan X., Gandhi C., Demetris A.J., Monga S.P. Wnt'er in liver: expression of Wnt and frizzled genes in mouse. Hepatology. 2007;45:195–204. doi: 10.1002/hep.21473. [DOI] [PubMed] [Google Scholar]
- 24.Torre C., Benhamouche S., Mitchell C., Godard C., Veber P., Letourneur F., Cagnard N., Jacques S., Finzi L., Perret C., Colnot S. The transforming growth factor-alpha and cyclin D1 genes are direct targets of beta-catenin signaling in hepatocyte proliferation. J Hepatol. 2011;55:86–95. doi: 10.1016/j.jhep.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Lee W.M., Squires R.H., Jr., Nyberg S.L., Doo E., Hoofnagle J.H. Acute liver failure: summary of a workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anand S.S., Soni M.G., Vaidya V.S., Murthy S.N., Mumtaz M.M., Mehendale H.M. Extent and timeliness of tissue repair determines the dose-related hepatotoxicity of chloroform. Int J Toxicol. 2003;22:25–33. doi: 10.1080/10915810305074. [DOI] [PubMed] [Google Scholar]
- 27.Rao P.S., Mangipudy R.S., Mehendale H.M. Tissue injury and repair as parallel and opposing responses to CCl4 hepatotoxicity: a novel dose-response. Toxicology. 1997;118:181–193. doi: 10.1016/s0300-483x(97)03617-2. [DOI] [PubMed] [Google Scholar]
- 28.Koteish A., Yang S., Lin H., Huang J., Diehl A.M. Ethanol induces redox-sensitive cell-cycle inhibitors and inhibits liver regeneration after partial hepatectomy. Alcohol Clin Exp Res. 2002;26:1710–1718. doi: 10.1097/01.ALC.0000036923.77613.59. [DOI] [PubMed] [Google Scholar]
- 29.Buitrago-Molina L.E., Marhenke S., Longerich T., Sharma A.D., Boukouris A.E., Geffers R., Guigas B., Manns M.P., Vogel A. The degree of liver injury determines the role of p21 in liver regeneration and hepatocarcinogenesis in mice. Hepatology. 2013;58:1143–1152. doi: 10.1002/hep.26412. [DOI] [PubMed] [Google Scholar]
- 30.James L.P., Lamps L.W., McCullough S., Hinson J.A. Interleukin 6 and hepatocyte regeneration in acetaminophen toxicity in the mouse. Biochem Biophys Res Commun. 2003;309:857–863. doi: 10.1016/j.bbrc.2003.08.085. [DOI] [PubMed] [Google Scholar]
- 31.Francavilla A., Azzarone A., Carrieri G., Cillo U., Van Thiel D., Subbottin V., Starzl T.E. Administration of hepatic stimulatory substance alone or with other liver growth factors does not ameliorate acetaminophen-induced liver failure. Hepatology. 1993;17:429–433. [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes R.D., Zhang L., Tsubouchi H., Daikuhara Y., Williams R. Plasma hepatocyte growth factor and biliprotein levels and outcome in fulminant hepatic failure. J Hepatol. 1994;20:106–111. doi: 10.1016/s0168-8278(05)80475-1. [DOI] [PubMed] [Google Scholar]
- 33.Horimoto M., Fulop P., Derdak Z., Wands J.R., Baffy G. Uncoupling protein-2 deficiency promotes oxidant stress and delays liver regeneration in mice. Hepatology. 2004;39:386–392. doi: 10.1002/hep.20047. [DOI] [PubMed] [Google Scholar]
- 34.Lavoie J.N., L'Allemain G., Brunet A., Muller R., Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 35.Torbenson M., Yang S.Q., Liu H.Z., Huang J., Gage W., Diehl A.M. STAT-3 overexpression and p21 up-regulation accompany impaired regeneration of fatty livers. Am J Pathol. 2002;161:155–161. doi: 10.1016/S0002-9440(10)64167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wustefeld T., Rakemann T., Kubicka S., Manns M.P., Trautwein C. Hyperstimulation with interleukin 6 inhibits cell cycle progression after hepatectomy in mice. Hepatology. 2000;32:514–522. doi: 10.1053/jhep.2000.16604. [DOI] [PubMed] [Google Scholar]
- 37.Chiu H., Gardner C.R., Dambach D.M., Durham S.K., Brittingham J.A., Laskin J.D., Laskin D.L. Role of tumor necrosis factor receptor 1 (p55) in hepatocyte proliferation during acetaminophen-induced toxicity in mice. Toxicol Appl Pharmacol. 2003;193:218–227. doi: 10.1016/j.taap.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 38.James L.P., Kurten R.C., Lamps L.W., McCullough S., Hinson J.A. Tumour necrosis factor receptor 1 and hepatocyte regeneration in acetaminophen toxicity: a kinetic study of proliferating cell nuclear antigen and cytokine expression. Basic Clin Pharmacol Toxicol. 2005;97:8–14. doi: 10.1111/j.1742-7843.2005.pto_97102.x. [DOI] [PubMed] [Google Scholar]
- 39.Yang R., Zhang S., Kajander H., Zhu S., Koskinen M.L., Tenhunen J. Ringer's lactate improves liver recovery in a murine model of acetaminophen toxicity. BMC Gastroenterol. 2011;11:125. doi: 10.1186/1471-230X-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang R., Miki K., He X., Killeen M.E., Fink M.P. Prolonged treatment with N-acetylcystine delays liver recovery from acetaminophen hepatotoxicity. Crit Care. 2009;13:9. doi: 10.1186/cc7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serum glucose (A) and total bilirubin (B) levels in mice treated with either APAP300 or APAP600 over a time course of 0 to 96 hours. ∗P < 0.05 between two doses.
Ki-67 immunofluorescence performed on frozen liver sections of mice treated with APAP300 and APAP600 and scarified at 24 hours after APAP treatment. Arrowheads indicate Ki-69 positive cells.
HNF4α IHC on paraffin-embedded liver sections from APAP600-treated mice at 12 and 24 hours after APAP treatment.
Western blot (A) and densitometric (B) analyses of Dvl using total liver extracts from mice treated with either APAP300 or APAP600. Real-time PCR analysis of Fzd7 (C) and Fzd8 (D) mRNA in livers of mice treated with APAP300 and APAP600. ∗P < 0.05 between two doses. GAPDH, glyceraldehye-3-phosphate dehydrogenase.
A: Total β-catenin and CYP2E1 blots in S45D mice before APAP treatment. Changes in cyclin D1 (B) and axin 2 (C) mRNA at 24 hours after APAP300 treatment to WT and S45D mice. ∗P < 0.05 between two doses. GAPDH, glyceraldehye-3-phosphate dehydrogenase.