Abstract
Jagriti Innovations developed a collaboration tool in partnership with the Cure2Children Foundation that has been used by health professionals in Italy, Pakistan, and India for the collaborative management of patients undergoing bone marrow transplantation (BMT) for thalassemia major since August 2008. This online open-access database covers data recording, analyzing, and reporting besides enabling knowledge exchange, telemedicine, capacity building, and quality assurance. As of February 2014, over 2400 patients have been registered and 112 BMTs have been performed with outcomes comparable to international standards, but at a fraction of the cost. This approach avoids medical emigration and contributes to local healthcare strengthening and competitiveness. This paper presents the experience and clinical outcomes associated with the use of this platform built using open-source tools and focusing on a locally pertinent tertiary care procedure—BMT.
Keywords: Bone Marrow Transplantation, Database Management Systems, International Cooperation, Beta-Thalassemia/Prevention & Control, Beta-Thalassemia/Surgery, Integrated Advanced Information Management Systems
Introduction
Hemoglobinopathies, particularly thalassemia and sickle cell disease, represent the most frequent life-threatening non-communicable disorders of children with several million cases worldwide.1 2 Most children with these disorders live in resource-limited settings and die before reaching adolescence, have a very poor quality of life, and pose a major emotional and financial burden to their families.3
The only available definitive cure is bone marrow transplantation (BMT),4 which not only restores normal life expectancy and health-related quality of life,5 6 but is also highly cost-effective. In fact, the cost of BMT is equivalent to a few years of non-curative supportive care.7 8
Severe hemoglobinopathies are prevalent in a wide geographical belt extending from Africa to the Middle East, South Asia, and the Pacific Islands, where the majority of the world's children live. In these areas, there is a dire shortage of centers offering a cure for hemoglobinopathies.9 Focused international cooperative initiatives among transplant professionals and scientific societies can save many lives and contribute to sustainable local healthcare strengthening.10 11
The reduction of poverty, global changes in the distribution of wealth, and access to information exchange technologies have created unprecedented opportunities and the need for focused cooperation and training in tertiary healthcare. The development of dedicated information technology (IT) platforms promoting open and creative collaborations according to shared principles and visions is increasingly critical for appropriate knowledge exchange, capacity building, and quality assurance.
Jagriti Innovations, in partnership with the Cure2Children Foundation, has developed a collaboration tool based on an open-source software used by health professionals in Italy, Pakistan, and India for the prospective collaborative management of patients undergoing BMT for thalassemia major since August 2008.10 11
BMT is a medical rather than surgical procedure involving several months of inpatient and outpatient care. The platform covers data recording, analyzing, and reporting needs for all stages of BMT including enrollment, selection, preparation, the actual transplant, and follow-ups. The platform enables the various centers to collaborate while aiding in knowledge exchange, capacity building, and quality assurance.
Methods
The free and open-source LAMP (Linux, Apache, MYSQL, and PHP) software stack was utilized to create the platform in association with Drupal CMS (http://www.drupal.org) as the framework for application development. The platform is accessible through web browsers and consequently works independently of the underlying operating system on a range of internet-enabled devices. Traceability was built into the platform to enable tracking of each change. All data are stored securely on the remote server, which is backed up periodically.
Access-controlled zones were created for individuals associated with any given center to upload, share, and manage their center's records. Access levels were created to allow consultants and other professionals, including multidisciplinary experts who may not be available locally, from various collaborating centers, to share knowledge and provide general as well as patient-specific advice. All centers had full control over institutional patient data and role-based permissions (eg, physicians, nurses, data managers, administrators), thus enabling user access to view or modify (create, edit, delete) records. The system was specifically designed to allow health professionals to monitor patients from multiple centers as a consulting tool and learning opportunity according to granted permissions. The platform allowed for the collection of patients’ information and progress through forms for enrollment data, general medical evaluations, disease-specific summaries, notes, socioeconomic profiles, files, and histocompatibility results. Investigations and records can be updated by multiple users in various locations. For example, advanced tests like compatibility analysis (HLA typing) are often performed centrally, and assessed by experienced transplant physicians before the child is given final clearance for BMT. A detailed pre-calculated patient-specific treatment plan designed according to good clinical practice12 is attached to each patient and reviewed by at least two physicians and two nurses. At present this is not a full computerized physician order entry (CPOE), but rather protocol- and patient-specific template MS Excel files in which only patient code, date of birth, weight, height, and date of transplant need to be entered and from which a PDF document is generated, checked centrally, and attached to the patient record. Each treatment plan/order sheet is thus reviewed by a BMT consultants and again by primary physicians locally. The printout is signed by the responsible physician and provided to nurses as the sole order sheet. Space is available for each nurse to sign once drugs have been administered. At discharge the completed treatment plan is scanned and uploaded to the patient record. Daily clinical forms are used to monitor patients’ progress through pre- and post-transplant phases and include vital signs, lab reports, transfusions, medications, complications, nursing notes, and physicians’ notes. Daily medications in clinical form and inventory provide an additional tool that will allow consistency checks with the computerized treatment plan. A clinical form in tabular display of essential daily parameters including weight, maximal temperature, blood counts, liver and renal function tests to be monitored during the critical phase of BMT is provided for at-a-glance clinical assessment. The system also offers charts plotting height and weight z-scores based on the ‘WHO child growth standards’,13 which is most relevant for both pre-transplant and follow-up assessments.
Authorized users can subscribe to any given patient record and receive email notifications whenever that record is updated or modified. The system users can generate status reports related to relevant parameters such as lack of critical pre-BMT tests, requirement for an evaluation within a defined time from BMT, or relevant alerts— for example, allergies, hepatitis, or HIV positivity. Thus, the system checks the child's eligibility for BMT, indicates the presence of matched siblings, warns of any missing critical enrollment data, tracks BMT preparation status, monitors post-transplant critical parameters, and highlights any abnormalities including missed labs. A child's follow-up height and weight are monitored according to standard gender- and age-specific parameters. Each time there is a change in the patient's status, concerned healthcare professionals are immediately notified by email. The same professionals also receive periodic emails indicating the patient's status on all parameters that need attention.
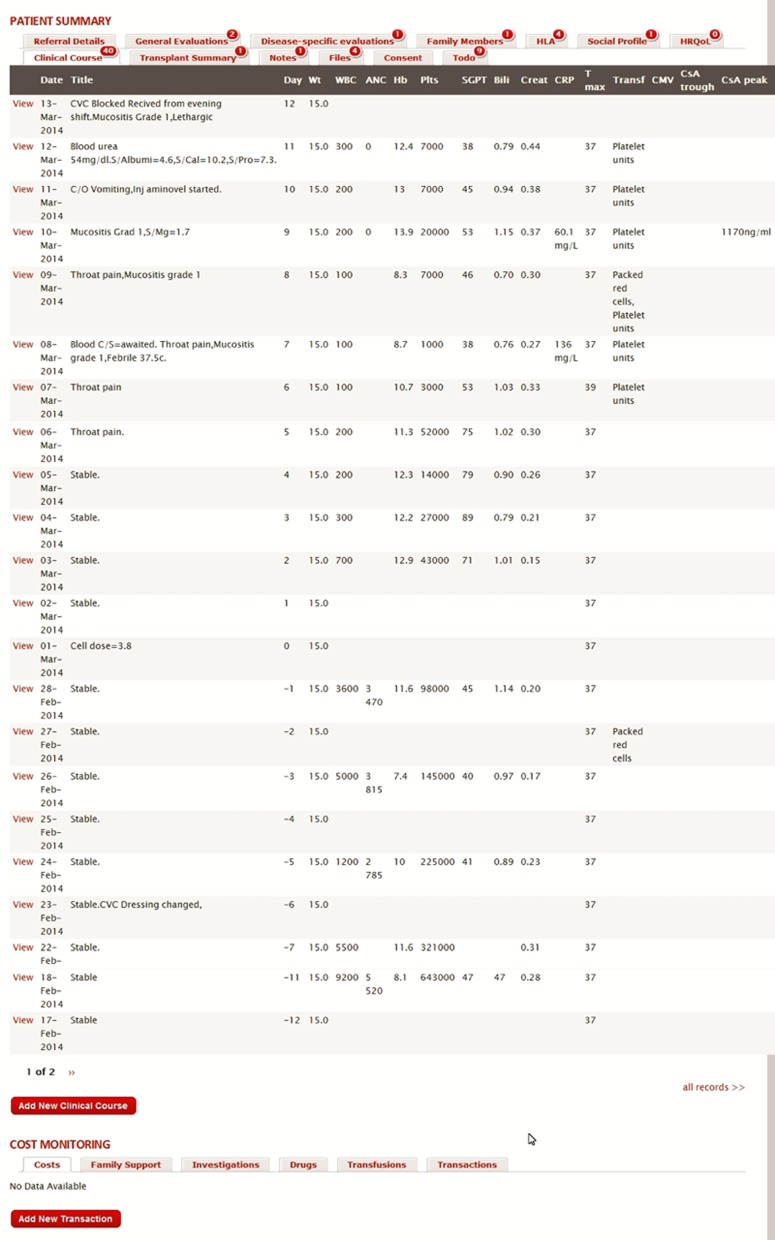
The system allows patient records to be grouped by customizable study categories, risk categories, treatment regimens, centers, disease, and complications. Survival curves for specific patient groups selectable through configurable filters can be generated. Figure 1 is a snapshot of a patient record.
Figure 1.
Snapshot of a patient record on the database.
Collaboration and knowledge-exchange tools built into the application include private/group messaging, forums, user profiles and directories, file sharing, and a task management system that are all accessible through a single interface. The system also allows for sharing manuals, protocols, references, policies, and procedures.
A personal-user dashboard enables a user-centric view of the system with all important links and patient-related bookmarks in one place. The site was designed to be intuitive and user friendly. No specific face-to-face training was required for users to start on the system. The system also provides the user with help notes and an inbuilt support desk in case they need assistance. The system enables users to use either standard international or a conventional system of units.
Role-specific professional development plans and self-assessment tools are incorporated in the user directory and are tailored to core competencies as specified by international standards for hematopoietic transplant unit accreditation.14
A drug and consumables inventory is under development. The aim is to track critical supplies stock and expiration dates as well as transplant-related costs. The availability of BMT-specific drugs within the network of collaborating institutions can also be monitored. For example, if a given drug is close to its expiration date, it is made available to partner institutions, thus improving cost-containment. The system facilitates submission of transplant data to international registries and scientific societies— for example, the Center for International Blood and Marrow Transplant Research (CIBMTR) or the European Blood and Marrow Transplant group (EBMT)—as well as compliance with quality assurance and international standards of accreditation by the Federation for the Accreditation of Cellular Therapy (FACT) or the Joint Accreditation Commission ICT-EBMT (JACIE).
Results
As of February 2014, over 2400 patients have been registered, more than 1600 individuals have been tested for histocompatibility, and 112 BMTs have been performed in children with thalassemia major. In children with a compatible sibling, actuarial thalassemia-free survival is 71% in the whole group and 84% in the 75 low-risk cases (no hepatomegaly and age <7 years); transplant-related mortality is 9%. This outcome is consistent with results obtained in affluent countries where the cost of BMT is on average 10 times higher. This online collaboration tool was instrumental for obtaining very good results, even in the start-up phase of the BMT centers. A screening and prevention program is incorporated into the project, with 2700 extended family members of affected children who have been offered cascade screening and counseling and 50 pregnant mothers who were offered targeted prenatal diagnosis.
Given the prospective and clinically oriented nature of this database and its implementation as a cooperation and capacity-building tool, compliance with data input has been quite good.
The overall development cost of this IT platform was approximately US$100 000. The funding for the platform was largely provided by the Cure2Children Foundation, a non-profit non-governmental organization promoting local access to cure for children with cancer and severe blood disorders in low- and middle-income countries (LMICs).15
Discussion
To our knowledge this is the largest online prospective collaborative IT platform focused on cure of childhood life-threatening disorders by BMT.
The experience over 5 years of registering more than 2400 patients performing 112 BMTs, with results comparable to high-income countries at a fraction of the cost, is highly encouraging. These achievements would not have been possible without the IT tool herein described and its capability to support intensive daily cooperation between consultants and in-training local health professionals.
Investing in collaborations promoting sustainable tertiary care and higher medical education is becoming increasingly important as healthcare globalizes. Medical ‘tourism’ has the potential to decrease soaring domestic healthcare costs in affluent countries and strengthen healthcare in emerging ones. We have evidence that, at least in low-risk matched-related BMTs for severe thalassemia, results within our partner institutions are comparable to international standards. This however is not enough; outcomes need to be appropriately documented, validated, and submitted to international registries and scientific societies. Moreover, consistent standards have to be guaranteed by a proper quality assurance procedure as per international agencies such as FACT or JACIE. At present there are no centers in LMIC which are accredited or even eligible for international accreditation even if, as a result of large patient loads, there might be great expertise on specific diseases curable by BMT, such as thalassemia. For example, Pakistan has at least 100 times the incidence of thalassemia compared to the West, and many cases have a compatible sibling donor due to large average family size. As a result, many more BMTs for young thalassemic children with a compatible donor are currently carried out in LMICs compared to Europe or North America.16 The IT platform described herein facilitates international accreditation and it seems reasonable to assume that if quality standards are assured, expertise is higher, and costs are much lower, there might be the potential for patient attraction. In fact, developing tools that facilitate international cooperation, quality assurance, and the implementation of appropriate global standards of care in LMICs will be critical to reassure national health systems, insurance agencies, and individual patients. Why should western insurance or national health systems refuse to cover a patient willing to go to India or Pakistan to centers that have much more experience on specific diseases, for example, thalassemia, where BMT costs one-tenth that in their native country, and appropriate quality standards are assured? The strengthening of tertiary care in LMICs will also decrease the hemorrhage of financial resources to wealthy countries by local families seeking a cure for their child with a life-threatening disorder. Moreover, ‘brain drain’ is an increasing threat to healthcare sustainability in developing countries,17 where insufficient professional development and motivation may contribute to high physician and nurse emigration rates.
We believe that the strengths of the IT platform we employ lie in its prospective, clinical management-oriented nature, and the focus on a medically, ethically, and financially justified advanced medical procedure. In fact, BMT has the potential to save the lives of many children, and is a unique opportunity for tertiary care development in many LMICs where increased financial resources are not matched by a parallel growth in local professional skills.
The system could also help to find unrelated donors by interfacing with international donor registries. In conclusion, we believe that the Jagriti–Cure2Children Foundation collaborative database is a unique example of how open-source IT platforms focused on locally pertinent tertiary care procedures offer a major opportunity to save lives, relieve emotional and financial distress in many families, and strengthen healthcare in developing countries.
Acknowledgments
We are very grateful to Julia Challinor for manuscript revision.
Footnotes
Contributors: The manuscript has been read and approved by all the authors.
Funding: This work was partially supported by Fondazione Monte dei Paschi di Siena, Siena, Italy and by the Cure2Children Foundation, Florence, Italy.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood 2010;115:4331–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO | Sickle-cell disease and other haemoglobin disorders [Internet]. [cited 2010 Apr 9]. http://www.who.int/mediacentre/factsheets/fs308/en/
- 3.Payne KA, Rofail D, Baladi J-F, et al. Iron chelation therapy: clinical effectiveness, economic burden and quality of life in patients with iron overload. Adv Ther 2008;25:725–42 [DOI] [PubMed] [Google Scholar]
- 4.Angelucci E. Hematopoietic stem cell transplantation in thalassemia. Hematology 2010:456–62. http://www.ncbi.nlm.nih.gov/pubmed/21239835 [DOI] [PubMed] [Google Scholar]
- 5.La Nasa G, Caocci G, Efficace F, et al. Long-term health-related quality of life evaluated more than 20 years after hematopoietic stem cell transplantation for thalassemia. Blood 2013;122:2262–70 [DOI] [PubMed] [Google Scholar]
- 6.Bernaudin F, Socie G, Kuentz M, et al. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood 2007;110:2749–56 [DOI] [PubMed] [Google Scholar]
- 7.Leelahavarong P, Chaikledkaew U, Hongeng S, et al. A cost-utility and budget impact analysis of allogeneic hematopoietic stem cell transplantation for severe thalassemic patients in Thailand. BMC Health Serv Res 2010;10:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho W-L, Lin K-H, Wang J-D, et al. Financial burden of national health insurance for treating patients with transfusion-dependent thalassemia in Taiwan. Bone Marrow Transplant 2006;37:569–74 [DOI] [PubMed] [Google Scholar]
- 9.Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA 2010;303:1617–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta PA, Faulkner LB. Hematopoietic cell transplantation for thalassemia: a global perspective. Biol Blood Marrow Transplant 2013;19(1 Suppl):S70–3 [DOI] [PubMed] [Google Scholar]
- 11.Faulkner LB, Uderzo C, Masera G. International cooperation for the cure and prevention of severe hemoglobinopathies. J Pediatr Hematol Oncol 2013;35:419–23 [DOI] [PubMed] [Google Scholar]
- 12.Meisenberg BR, Wright RR, Brady-Copertino CJ. Reduction in Chemotherapy Order Errors With Computerized Physician Order Entry. JOP [Internet]. 2013 Sep 3 [cited 2013 Sep 4]. http://jop.ascopubs.org/content/early/2013/09/03/JOP.2013.000903 [DOI] [PubMed]
- 13. WHO | The WHO Child Growth Standards [Internet]. [cited 2010 Apr 9]. http://www.who.int/childgrowth/en/
- 14.McGrath E. International Standards for Cellular Therapy Product Collection, Processing and Administration Accreditation Manual, FACT-JACIE 5th edition [Internet]. 2013. [cited 2013 Jun 10]. http://www.jacie.org/standards/interim-standards
- 15.Cure2Children Foundation | Supporting children with Thalassemia and Cancer [Internet]. [cited 2011 Feb 13]. http://www.cure2children.org/
- 16.Sabloff M, Chandy M, Wang Z, et al. HLA-matched sibling bone marrow transplantation for β-thalassemia major. Blood 2011;117:1745–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullan F. The metrics of the physician brain drain. N Engl J Med 2005;353:1810–18 [DOI] [PubMed] [Google Scholar]