Abstract
Objective
Antibiotics are commonly recognized as non-indicated for acute bronchitis and upper respiratory tract infection (URI), yet their widespread use persists. Clinical decision support in the form of electronic warnings is hypothesized to prevent non-indicated prescriptions. The purpose of this study was to identify the effect of clinical decision support on a common type of non-indicated prescription.
Materials and methods
Using National Ambulatory Medical Care Survey data from 2006 to 2010, ambulatory visits with a primary diagnosis of acute bronchitis or URI and orders for antibiotic prescriptions were identified. Visits were classified on the basis of clinician report of decision-support use. Generalized estimating equations were used to assess the effect of decision support on likelihood of antibiotic prescription receipt, controlling for patient, provider, and practice characteristics.
Results
Clinician use of decision support increased sharply between 2006 (16% of visits) and 2010 (55%). Antibiotic prescribing for acute bronchitis and URI increased from ∼35% of visits in 2006 to ∼45% by 2010. Use of decision support was associated with a 19% lower likelihood of receiving an antibiotic prescription, controlling for patient, provider, and practice characteristics.
Discussion
In spite of the increased use of decision-support systems and the relatively fewer non-indicated antibiotic prescriptions resulting from the use of decision support, a secular upward trend in non-indicated antibiotic prescribing offset these improvements.
Conclusions
The overall effect of decision support suggests an important role for technology in reducing non-indicated prescriptions. Decision support alone may not be sufficient to eliminate non-indicated prescriptions given secular trends.
Keywords: Health information technology, Antibiotics, Acute bronchitis
Background and significance
The Health Information Technology for Economic and Clinical Health (HITECH) Act was passed in 2009 to spur adoption and ‘meaningful use’ of health information technology (health IT). As part of meaningful use requirements, eligible healthcare providers are required to electronically monitor for drug–drug and drug–allergy interactions and make use of clinical decision-support systems, which include electronic warning systems or alerts to highlight potential contraindications for prescriptions ordered.
Meaningful use of decision support may reduce medication errors and inappropriate and unnecessary prescriptions. This effect has been demonstrated in some cases, such as the substitution of generic for branded medication.1 Other studies have shown mixed impacts of decision-support use on non-indicated prescriptions for elderly patients and the impact of adding an electronic warning for medications with black-box warnings to an existing electronic medical record (EMR).2 3 However, an overall and nationally representative assessment of the impact of decision support on non-indicated prescriptions has not been conducted.
To better understand the overall impact of decision support, it is necessary to narrow the focus to a specific set of encounters and resulting prescriptions over the broadest possible population. In particular, prescriptions that provide little to no benefit to individual patients or to the population are especially concerning, as overuse not only provides no added value, but their systematic overuse may also actually detract value. One such example is non-indicated antibiotic prescriptions.
Antibiotics generally provide little to no benefit for most cases of acute bronchitis and upper respiratory tract infection (URI) 4–6 and are of particular concern because of rising levels of antibiotic-resistant microorganisms.7 Despite their ineffectiveness, such prescriptions remain common.8 9 Up to 50% of all antibiotic prescriptions are for non-clinically indicated viral respiratory infections.10 11
The American Academy of Pediatrics and the American College of Physicians have both issued guidelines for reducing antibiotic use for acute bronchitis and URI,4 12 and, beginning in 2008, the Healthcare Effectiveness Data and Information Set (HEDIS) has tracked antibiotic prescriptions for acute bronchitis through their NQF-endorsed ‘Avoidance of antibiotic treatment in adults with acute bronchitis’ measure.13 Additional distinctions have been made between broad- and narrow-spectrum antibiotics, with broad-spectrum antibiotics being of particular concern, as they may disproportionately contribute to antibiotic resistance.14
In spite of these widespread efforts to reduce use, antibiotic prescriptions for acute bronchitis and other respiratory tract infections persist.8 9 15 To date, the overall impact of decision support on the diversion of non-indicated prescriptions such as antibiotics for acute bronchitis or URI has not been assessed in a large, national sample.
Objective
This study seeks to strengthen the existing literature on the impact of health IT in ambulatory care through examination of the impact of decision support on antibiotic prescriptions for outpatient cases of acute bronchitis and URI. We use diffusion-of-innovation theory to guide our examination of the observed effects of decision support using separate cross-sectional samples for each year from 2006 to 2010. As this study uses 5 years of data, we also examine trends in the adoption of health IT by office-based ambulatory providers from 2006 through 2010 and clarify the extent to which the relationship of decision support and antibiotic prescribing changes over time.
Materials and methods
Logic model
Nyquist et al10 suggest four possible causes of outpatient antibiotic overprescribing: education, experience, expectations, and economics. We hypothesize that decision support acts to improve condition-specific education and enhance a clinician's previous experiences.16 Improvements in these areas through decision support should yield fewer antibiotic prescriptions for acute bronchitis or URI, leading to improved clinical performance and patient safety.
Several potential confounders are addressed through the study's logic model. Provider's use of other forms of health IT was assessed through provider's use of e-prescribing and provider's use of EMR, both of which may influence likelihood of decision-support use and antibiotic-prescribing practices.17–20
Several other factors can affect provider responses to decision-support alerts. Provider practice setting factors such as office type (ie, private practice, health maintenance organization (HMO), other) and physician specialty are related to likelihood of decision-support and health IT usage and to antibiotic-prescribing practices.8 21–24
Patient factors such as type of insurance used and patient race/ethnicity may also affect prescribing patterns through expectations and economic incentives and are associated with differential decision-support use.10 23 25–29 Additional factors such as patient age and the presence of pulmonary-related chronic conditions such as asthma or chronic obstructive pulmonary disease (COPD) were included as both are likely to affect a clinician's decision-making with respect to antibiotic prescriptions.8 10
Finally, relying on diffusion-of-innovation theory, we posit that the earliest adopters may be the most quality conscious, and that, if so, the impact of decision support on reducing non-indicated antibiotic prescriptions will be stronger for early adopters of decision support, and the positive benefit will diminish over time as later adopters use the innovation.30 We examine overall adoption trends by estimating the relationship between decision support and antibiotic prescriptions by year and consider whether the provider was an early adopter of the technology or among the later adopters.
Data and sample
Data from the 2006, 2007, 2008, 2009, and 2010 National Ambulatory Medical Care Survey (NAMCS) were used for this study. NAMCS is a nationally representative survey of non-federally employed, office-based providers of ambulatory medical care services.31 Data are collected from a nationally representative, stratified sample of clinicians on an annual basis. Each clinician provides data on his or her practice characteristics and a random sample of patient visits during a 1-week period. Sampling and data collection methods are described in detail elsewhere.32 The NAMCS sample is refreshed annually and there is no method to link responding clinicians or patients across years. Data for each year were combined to create the analytic sample, with indicator variables for each study year.
We used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) diagnosis codes to identify visits with a primary diagnosis of bronchitis or URI (acute bronchitis, ICD-9 code 466; bronchitis not otherwise specified, ICD-9 code 490; acute URI of multiple or unspecified sites, ICD-9 code 465; acute nasopharyngitis, ICD-9 code 460). These definitions are consistent with previous studies.8 10 25 33 A total of 3808 cases met these inclusion criteria.
Cases in which the patient's secondary diagnoses would indicate an antibiotic prescription were excluded. Exclusionary secondary diagnoses, ICD-9 codes, and the number of cases excluded by each are shown in detail in the online supplementary appendix. Exclusion criteria are consistent with previously published studies.8 25 33 A total of 491 cases were excluded, for a final sample size of 3317 cases.
Antibiotic prescriptions were identified using the National Committee for Quality Assurance HEDIS ‘Avoidance of antibiotic treatment for adults with acute bronchitis’ list.13 See online supplementary appendix for a complete list of antibiotics used in this study. Codes for each of these prescriptions were then matched to the NAMCS dataset using the NAMCS drug entry list and generic codes data. A board-certified internal medicine physician reviewed this coding strategy for accuracy and completeness.
Provider's use of decision support was assessed in the NAMCS provider survey with the question: ‘Are there warnings of drug interactions or contraindications provided?’ Response categories were ‘Yes’, ‘No’, ‘Unknown’, and ‘Turned off’. Respondents were considered to have the technology if they answered ‘Yes’; those who responded ‘Unknown’ or ‘Turned off’ were considered not to have decision support.
Multivariable models controlled for several factors outlined above in the logic model. Provider's use of e-prescribing and provider's use of EMR were assessed at the clinician level by NAMCS and were added as dichotomous variables. A categorical description of the clinician's office type was included with three categories: private practice, HMO, and other. Clinician specialty was also included as a three-category variable: general practitioner, pediatrics, and all others. Patient factor variables included insurance type (private, Medicare, Medicaid, self-pay, and other), age (0–4, 5–17, 18–64, 65+), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), and dichotomous variables for the presence of two pulmonary-related chronic conditions: asthma and COPD.
Analyses
To track trends in antibiotic prescribing over time, univariate statistics were compiled for each year. Survey weights were used in all to account for the complex NAMCS sampling design. Multivariable generalized estimating equations (GEE) with an exchangeable correlation structure were used to estimate the overall effect of decision support on receipt of antibiotics, controlling for the potential confounders discussed in the logic model above. The GEE models correct for clustering in prescribing patterns at the physician level and yield population-averaged estimates. GEE models used the binomial family and a logit link function.34
The main model used data from all 5 years (2006–2010). Post-estimation tests were performed to calculate marginal probabilities and risk ratios. Risk ratios were bootstrapped with 1000 repetitions using the percentile method.35
We estimated additional models to assess the sensitivity of the results to alternative model specifications. First, we ran models for each data-year separately in order to assess changes in the relation of decision-support and antibiotic-prescribing behavior over time. Second, in order to more closely examine the effect of decision support for early adopters versus later adopters, we ran a GEE model that contained an interaction term between use of decision support and the overall proportion of providers using decision support in the year in which the visit occurred. Early adopters may be more sympathetic to the goals of decision-support systems, and accordingly the effects of decision support may have been greater in earlier years than in later years as the technology became more widely adopted. Third, to estimate the effect of decision support on receipt of broad- versus narrow-spectrum antibiotics, we limited the sample to acute bronchitis/URI visits for which an antibiotic prescription was ordered (40% of visits) and ran a GEE model to assess whether use of decision support was associated with a change in likelihood of antibiotic prescription receipt. All coding and data analysis was performed using Stata V.13.1.
Results
The analytic sample is summarized in table 1. Of particular note are the large and statistically significant increases in the proportion of providers using decision support (16–55%, p<0.001), e-prescribing (13–56%, p<0.001), and EMR (14–47%, p<0.001) between 2006 and 2010. No other variables differed significantly across years at the p=0.05 level.
Table 1.
Characteristics of ambulatory care visits for acute bronchitis/URI, by year
| Variable | 2006 (%) | 2007 (%) | 2008 (%) | 2009 (%) | 2010 (%) | All years (%) |
|---|---|---|---|---|---|---|
| Patient received antibiotic prescription | 34.1 | 40.3 | 40.6 | 38.8 | 45.4 | 39.8 |
| Provider uses decision support* | 16.1 | 17.6 | 34.6 | 39.9 | 54.5 | 31.8 |
| Provider uses e-prescribing* | 13.2 | 16.2 | 30.4 | 35.9 | 55.6 | 29.4 |
| Provider uses EMR* | 14.5 | 18.7 | 25.1 | 36.2 | 47.3 | 27.9 |
| Patient insurance type | ||||||
| Private | 56.0 | 54.2 | 61.2 | 52.9 | 55.8 | 55.8 |
| Medicare | 7.6 | 13.6 | 13.6 | 14.1 | 12.8 | 12.4 |
| Medicaid | 25.5 | 21.0 | 17.9 | 27.2 | 23.6 | 23.2 |
| Self-pay | 2.6 | 4.7 | 3.4 | 3.1 | 2.4 | 3.3 |
| Other | 2.0 | 1.9 | 0.9 | 1.1 | 3.0 | 1.7 |
| Provider office type | ||||||
| Private practice | 85.4 | 87.9 | 89.4 | 90.1 | 86.3 | 87.9 |
| HMO | 2.0 | 1.8 | 1.3 | 1.2 | 2.8 | 1.8 |
| Other | 12.6 | 10.3 | 9.3 | 8.3 | 10.9 | 10.3 |
| Provider specialty | ||||||
| Pediatrics | 35.3 | 38.2 | 36.1 | 32.8 | 36.3 | 35.7 |
| General/family medicine | 42.4 | 36.6 | 41.5 | 47.4 | 37.1 | 41.1 |
| Other | 22.3 | 25.2 | 22.5 | 19.8 | 26.7 | 23.2 |
| Patient age (years) | ||||||
| 0–4 | 29.3 | 29.2 | 30.5 | 22.9 | 30.5 | 28.3 |
| 5–17 | 19.0 | 20.6 | 16.5 | 22.3 | 18.7 | 19.5 |
| 18–64 | 42.1 | 35.4 | 37.8 | 39.9 | 37.6 | 38.6 |
| >65 | 9.6 | 14.8 | 15.3 | 14.9 | 13.2 | 13.6 |
| Patient race | ||||||
| Non-Hispanic white | 62.6 | 63.4 | 67.4 | 69.0 | 66.1 | 65.6 |
| Non-Hispanic black | 12.2 | 10.7 | 9.8 | 10.6 | 12.2 | 11.1 |
| Hispanic | 17.1 | 14.6 | 13.7 | 15.6 | 15.5 | 15.3 |
| Other | 8.0 | 11.4 | 9.1 | 4.9 | 6.3 | 8.0 |
| Patient chronic condition(s) | ||||||
| None | 3.8 | 3.2 | 4.2 | 6.5 | 4.1 | 4.4 |
| Asthma | 7.8 | 8.9 | 9.4 | 8.4 | 10.9 | 9.0 |
| COPD | 28.7 | 24.7 | 23.3 | 26.9 | 19.9 | 24.9 |
| Unweighted cases (n) | 580 | 690 | 631 | 722 | 694 | 3317 |
*p<0.001.
COPD, chronic obstructive pulmonary disease; EMR, electronic medical record; HMO, health maintenance organization; URI, upper respiratory infection.
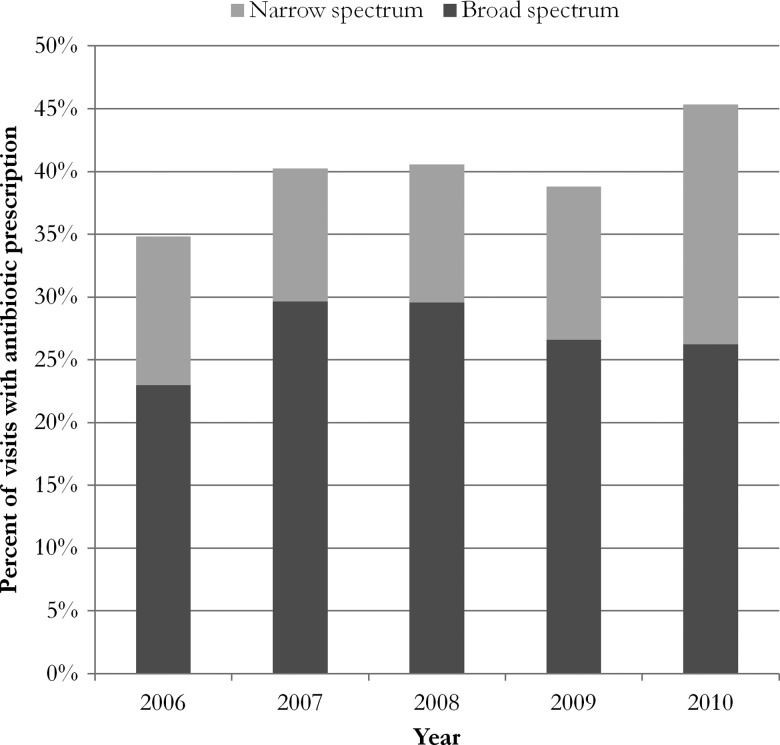
Figure 1 shows antibiotic prescription orders for outpatient visits with a primary diagnosis of acute bronchitis or URI. For the overall sample, 39.8% of acute bronchitis/URI visits resulted in antibiotic prescriptions, with some two-thirds of these antibiotic prescriptions for broad-spectrum antibiotics. There is no evidence that antibiotic prescribing for acute bronchitis/URI declined between 2006 and 2010. If anything, it would appear that there was an upward trajectory in overall prescribing across years, as the end points (2006 and 2010) were borderline significantly different (p=0.10). There were no significant changes in broad- versus narrow-spectrum composition or between year differences in either broad- or narrow-spectrum prescribing.
Figure 1.
Percentage of acute bronchitis or URI visits resulting in antibiotic prescription.
In adjusted analyses, use of decision support is associated with significantly reduced odds of antibiotic prescription receipt (OR 0.72, p=0.03). A full set of model estimates is shown in table 2. After adjustment for other covariates in the model and for the intra-provider correlation, the odds of a provider ordering an antibiotic prescription for acute bronchitis or URI visits are 0.72 times as great for providers who have decision support systems as for providers who do not have such systems (p<0.05).
Table 2.
ORs from GEE regression on receipt of antibiotic prescription
| Variable | OR |
|---|---|
| Provider uses decision support | 0.72* |
| Provider uses e-prescribing | 1.36 |
| Provider uses EMR | 1.02 |
| Provider specialty | |
| Pediatrics | Reference |
| General/family medicine | 2.07*** |
| Other | 1.95*** |
| Provider office type | |
| Private practice | Reference |
| HMO | 0.23** |
| Other | 0.90 |
| Patient insurance type | |
| Private | Reference |
| Medicare | 0.92 |
| Medicaid | 0.80* |
| Self-pay | 1.25 |
| Other | 0.77 |
| Patient age | |
| 0–4 | 0.62** |
| 5–17 | 0.85 |
| 18–64 | Reference |
| > 65 | 0.79 |
| Patient race | |
| Non-Hispanic white | Reference |
| Non-Hispanic black | 1.25 |
| Hispanic | 0.81 |
| Other | 0.54** |
| Patient chronic condition(s) | |
| Asthma | 0.86 |
| COPD | 2.78*** |
| NAMCS year | |
| 2006 | Reference |
| 2007 | 1.36* |
| 2008 | 1.55** |
| 2009 | 1.20 |
| 2010 | 1.82*** |
| Constant | 0.31*** |
*p<0.05;
**p<0.01;
***p<0.001.
COPD, chronic obstructive pulmonary disease; EMR, electronic medical record; GEE, generalized estimating equation; HMO, health maintenance organization; NAMCS, National Ambulatory Medical Care Survey.
Relative risk calculations for the sample (table 3) suggest that the overall relative risk of receiving an antibiotic prescription was associated with a decrease of some 19% when decision support was used. Stratifying the sample across each separate year shows little evidence of a differential effect across years. The year-specific estimates are similar in magnitude to the all-years estimate, but do not achieve statistical significance, reflecting lower sample size for the year-specific estimates.
Table 3.
Relative risk of receipt of antibiotic prescription with use of decision support, by year
| Year | Relative risk of receiving antibiotic prescription with use of decision support(95% CI*) |
|---|---|
| All years | 0.81 (0.66 to 0.96) |
| 2006 | 0.83 (0.35 to 1.53) |
| 2007 | 0.98 (0.68 to 1.46) |
| 2008 | 0.93 (0.63 to 1.37) |
| 2009 | 0.85 (0.59 to 1.22) |
| 2010 | 0.81 (0.59 to 1.14) |
*CIs bootstrapped using 1000 repetitions, percentile method shown.
The results of a separate GEE model that included an interaction term for the use of decision support and the overall proportion of providers using decision support in a given year were highly consistent with the overall analyses (see online supplementary appendix for full model results). In these analyses, the OR and SE for the decision-support coefficient was consistent with that observed in the main model (relative risk 0.69; bootstrapped 95% CI 0.49 to 0.90), while the coefficient for the interaction term was not statistically significant (relative risk for lowest vs highest value 1.32; bootstrapped 95% CI 0.93 to 1.85).
In GEE models assessing differences in the impact of narrow- versus broad-spectrum antibiotics, we found that clinician's use of decision support was only borderline significantly associated with an increased likelihood of broad- versus narrow-spectrum antibiotic receipt (p=0.11). Contrary to our hypothesis, however, the use of decision support was, if anything, positively associated with receipt of broad-spectrum antibiotic (relative risk 1.38; bootstrapped 95% CI 0.97 to 2.11).
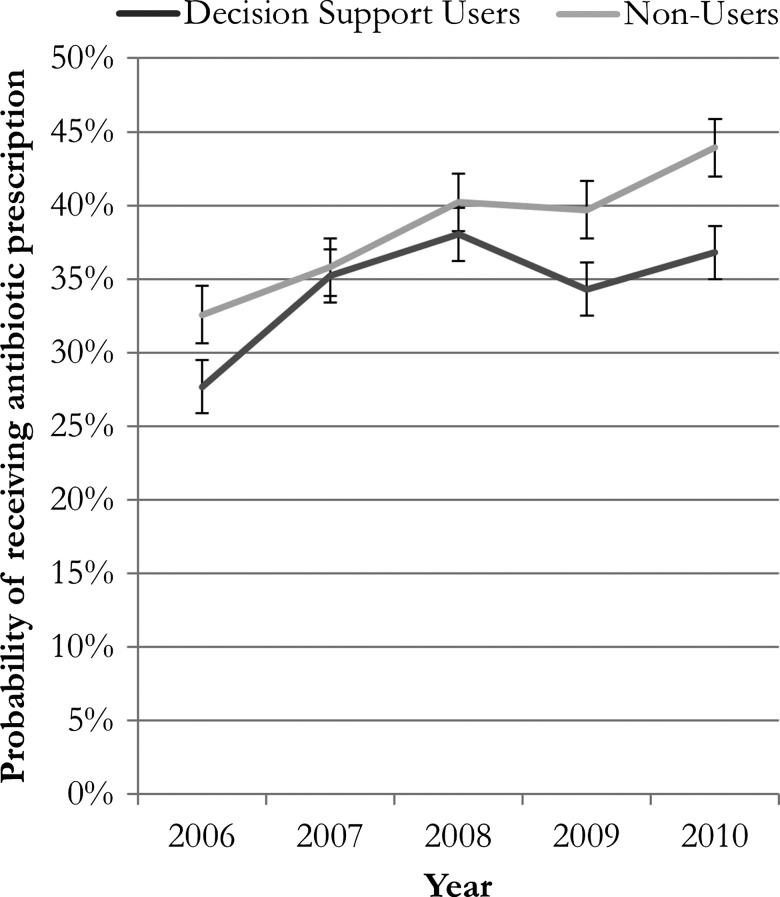
The trends in non-indicated antibiotic use (figure 2) indicate that, at any given point in time, decision support helps reduce the likelihood of antibiotic prescription for acute bronchitis or URI. There is an upward secular trend, however, for both decision-support and non-decision-support users. On net, as the composition of decision-support users changes, antibiotic rates remain the same, even though decision support reduces non-indicated prescriptions and is expanding.
Figure 2.
Adjusted probability of receiving antibiotic prescription, decision support users versus non decision support users.
Discussion
Our study—the first large-scale, nationally representative examination of the association of clinical decision support with orders for antibiotic prescriptions in cases of acute bronchitis or URI—found that, despite ongoing efforts aimed at reducing or eliminating prescriptions for such diagnoses, a substantial proportion—nearly 40%—of outpatient visits for acute bronchitis or URI result in a prescription for antibiotics. Despite public-awareness campaigns and guidelines, use of broad-spectrum antibiotics appeared to be at least as prevalent, if not more, than previously estimated,14 with no apparent downward trend. It is especially notable that no change in prescribing was observed before and after the 2008 introduction of a HEDIS measure in this area.
Consistent with the existing literature, NAMCS data from 2006 to 2010 reveal a sharp increase in clinician use of three forms of health IT: decision support, e-prescribing, and EMR. Each rose from approximately 10–15% prevalence in 2006 to over 50% by 2010. We believe this diffusion and the resulting diverse range of users is a strength of this study, as it enabled us to measure the effect of decision support on the early adopters using it in 2006 and the early/late majority using it in 2010.30
With respect to the impact of decision support on antibiotic prescriptions for acute bronchitis and URI, our results suggest that, after relevant patient and provider factors are accounted for, the use of decision-support systems is associated with a significantly lower likelihood of receiving an antibiotic prescription. Specifically, the likelihood of receiving an antibiotic prescription is 0.81 times as great for acute bronchitis or URI visits where the provider reports having decision support capabilities as for visits where the provider reports not having them. Even though decision support systems were becoming increasingly common from 2006 to 2010, an upward secular trend in antibiotic prescribing appears to have wiped out gains that may otherwise have accrued with wider use of decision support. More research will be required to understand the reasons for this upward secular trend.
To provide some context for the estimate of the effect of decision support on antibiotic prescribing, the data used in this study represent an average of approximately 830 million ambulatory encounters per year between 2006 and 2010. Nearly 27 million of these visits are for acute bronchitis or URI. With ∼40% of all such visits resulting in an order for an antibiotic prescription, more than 10 million such prescriptions occur annually. A 20% decline in acute bronchitis or URI antibiotic prescribing could translate to over one million fewer antibiotic prescriptions per year (assuming approximately half of clinicians use decision support, as in 2010) or potentially as many as two million fewer prescriptions annually if all clinicians were to use decision support.
Our estimate of the impact of decision support on prescribing is consistent with previous studies examining the impact of a specific clinical decision support system or technological intervention on prescribing behavior for acute bronchitis/URI36 37 and for other evidence-based prescription diversion efforts.38 The modest effect size of decision support we found is roughly comparable to other studies of individual clinical decision-support systems33 or of decision support plus community interventions39 on antibiotic-prescribing patterns. We hypothesized that decision support would influence two of the four potential reasons for antibiotic prescribing for acute bronchitis (education and experience, but not expectations or economics).10 An electronic warning may be insufficient to avert a prescription in the face of strong economic incentives or patient expectations for a prescription, although it may be enough to tip the scales in the absence of these factors. So a reduction of some 20% is not unreasonable.
Other forms of health IT, including EMRs and e-prescribing, did not have any significant impact on the likelihood of receiving an antibiotic prescription for acute bronchitis. All of the categorical patient- and clinician-level covariates included in our model were significant. We interpreted these results as evidence that the net effectiveness of decision support, as with many other forms of health IT, is associated with a range of patient- and clinician-level sociotechnical factors.40
This study expands on earlier research by providing an estimate of the overall impact of decision-support systems nationwide for patients with bronchitis. This estimate suggests that decision support is associated with a roughly 20% decline in the likelihood of antibiotic prescription, which could represent hundreds of thousands of averted prescriptions that are wasteful and in some cases harmful.
Decision support could make an especially important contribution given that acute bronchitis and URI visits account for ∼10 million antibiotic prescriptions per year. Recent analysis of all 260–270 million antibiotic prescriptions per year shows declines of ∼3% in overall prescribing from 2006 to 2010.41 Without further data, however, we cannot be certain whether the decline is due to changing opportunities to prescribe or changing tendencies to prescribe given the opportunity. For example, it could be that improvements in care have led to a reduction in conditions that require antibiotics. Our data suggest that, when the opportunity to prescribe is separated out from the tendency to prescribe given the opportunity, the tendency to prescribe is going up for outpatient acute bronchitis and URI visits.
To add context to our estimates, we also ran a model that included an interaction term between use of decision support and the overall proportion of providers using decision support in the year in which the visit took place. If we believe that the observed decision-support effect is not due to the warnings themselves but to some underlying construct, say attentiveness to quality, which is associated with both the likelihood of decision-support usage and the likelihood of antibiotic prescribing, then we would expect that the observed effect of decision support would wane as it became more widely used. We were able to observe this in our data as decision support became much more common between 2006 (16% of visits) and 2010 (55% of visits); we are thus able to observe in this dataset the early adopters using it in 2006 (ie, those most attentive to quality) and the later majority using it in 2010 (ie, those slightly less attuned to quality). In this scenario we would expect the observed relationship to attenuate over time. This model demonstrates that no such attenuation occurred for acute bronchitis visits between 2006 and 2010, as the effect of decision support was significant and similar in magnitude with and without the year-specific-usage interaction term.
Together these results suggest that there is no greater effect of decision-support systems among early adopters than among later adopters. This should strengthen confidence in the conclusion that the decision support itself is responsible for the observed effects and not an unmeasured covariate or a merely spurious correlation.
With respect to the types of antibiotic prescriptions ordered, we found no effect of decision support on broad- versus narrow-spectrum antibiotics. While we might have expected a decision-support capability to be especially beneficial in averting broad-spectrum prescriptions, as these are hypothesized to be especially problematic at the system level, there was no effect due to decision support. It is possible that the specific warnings or information currently being generated by clinicians’ systems do not adequately differentiate between broad- and narrow-spectrum agents.
Our study has relevant limitations to note. First, our measure of decision-support usage was limited by data available through NAMCS. IT system use is measured at the provider level rather than at the visit level. IT functionalities may or may not be used for a given visit,42–44 and our data do not contain information on the specific conditions or warnings generated by each clinician's system. Thus, we cannot be sure that all of the reported decision-support users were subject to the effect of decision support for antibiotic prescriptions. We are also not able to assess the nature and salience of the decision support provided by each clinician's system. Since we do not measure the specificity, visibility, or intrusiveness of alerts, we are mixing alerts that may be more or less effective at averting antibiotic prescriptions. This would result in an underestimate of the impact of a highly effective alert system. These measurement issues all tend to dilute the observed effect of decision support and may result in an understatement of the true impact of decision support. Second, we were not able to measure the sociotechnical and cultural factors that are often hypothesized to moderate the system effectiveness of health IT on patient safety.16 45 These contextual factors are notably absent from all large-scale datasets, so this limitation is not unique to our study.16 Third, although we utilized multiple years of cross-sectional data and carefully accounted for other relevant factors in our conceptual model, we are not able to demonstrate causality. Decision-support users and non-users may differ in other unmeasured respects. To address this, we have presented statistical and conceptual rationale in the discussion above for why spuriousness is not likely to be the cause of our findings. We also note that use of decision support has diffused rapidly throughout our study period. Whatever between-group differences existed in 2006 would have been at least partially diluted by later adopters who used decision support by 2010.30 Fourth, our study may have been limited by somewhat small samples for each year of data, limiting the sub-sample analyses possible. By pooling several years of data we were able to overcome this potential limitation, although we were thus unable to identify any trends over time in the impact of decision support on antibiotic-prescribing practices for acute bronchitis or URI.
Conclusion
Our results indicate that decision support can play a role in reducing antibiotic prescribing for acute bronchitis and URI. On the whole, there is a roughly 20% decrease in the likelihood of antibiotic prescribing for clinical decision-support users. An effect of this size might be expected to avert one million non-indicated antibiotic prescriptions annually, a major achievement and one that could help clinicians improve the care they deliver and their performance on at least one HEDIS measure. The end result of this is a reduction in inappropriate antibiotic use and a concomitant reduction in the spread of antibiotic-resistant bacteria.
In addition to the effect of decision support itself, several patient- and clinician-level variables also helped explain antibiotic-prescribing practices in the study's nationwide ambulatory care sample. These findings reiterate the complex and interdependent nature of prescriptions and emphasize the role that technology can play in achieving desired outcomes in conjunction with efforts aimed at other system stakeholders.
The magnitude of the reduction in likelihood of antibiotic prescribing for acute bronchitis or URI due to use of decision support underscores that this tool can play an important role in reducing non-indicated antibiotic prescriptions, but its use alone is unlikely to substantially reduce non-indicated antibiotic prescriptions or to substantively affect the likelihood of broad- versus narrow-spectrum prescribing. Quality improvement initiatives might therefore look to decision support as a tool to facilitate appropriate prescribing, but additional clinician and patient education efforts are likely warranted.
Supplementary Material
Acknowledgments
We gratefully acknowledge the valuable input and review on earlier drafts of this manuscript by Douglas Bell, MD, PhD and Paul Torrens, MD, MPH.
Footnotes
Collaborators: Douglas S Bell; Paul R Torrens.
Contributors: JMM conceived and designed the study. JMM was responsible for acquisition of data. All authors were responsible for data analysis and interpretation. JMM drafted the manuscript, and FJZ and HPR revised it critically for important intellectual content. HPR supervised the study. All authors have given final approval of the version to be published and agree to be accountable for all aspects of the work.
Funding: JMM received funding in partial support of this work from the Graduate Division of the University of California, Los Angeles.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Collaborators: Douglas S Bell and Paul R Torrens
References
- 1.Stenner SP, Chen Q, Johnson KB. Impact of generic substitution decision support on electronic prescribing behavior. J Am Med Inform Assoc 2010;17:681–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu DT, Seger DL, Lasser KE, et al. Impact of implementing alerts about medication black-box warnings in electronic health records. Pharmacoepidemiol Drug Saf 2011;20:192–202 [DOI] [PubMed] [Google Scholar]
- 3.Smith DH, Perrin N, Feldstein A, et al. The impact of prescribing safety alerts for elderly persons in an electronic medical record: an interrupted time series evaluation. Arch Intern Med 2006;166:1098–104 [DOI] [PubMed] [Google Scholar]
- 4.Dowell SF, Marcy SM, Phillips WR, et al. Principles of judicious use of antimicrobial agents for pediatric upper respiratory tract infections. Pediatrics 1998;101(Suppl 1): 163–5 [Google Scholar]
- 5.Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med 2001;134:521–9 [DOI] [PubMed] [Google Scholar]
- 6.Tan T, Little P, Stokes T. Antibiotic prescribing for self limiting respiratory tract infections in primary care: summary of NICE guidance. BMJ 2008;337:a437. [DOI] [PubMed] [Google Scholar]
- 7.Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis 2008;46:155–64 [DOI] [PubMed] [Google Scholar]
- 8.Mainous AG, III, Hueston WJ, Davis MP, et al. Trends in antimicrobial prescribing for bronchitis and upper respiratory infections among adults and children. Am J Public Health 2003;93:1910–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. J Am Med Assoc 2009;302:758–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyquist A, Gonzales R, Steiner JF, et al. Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis. J Am Med Assoc 1998;279:875–7 [DOI] [PubMed] [Google Scholar]
- 11.Cantrell R, Young AF, Martin BC. Antibiotic prescribing in ambulatory care settings for adults with colds, upper respiratory tract infections, and bronchitis. Clin Ther 2002;24:170–82 [DOI] [PubMed] [Google Scholar]
- 12.Snow V, Mottur-Pilson C, Gonzales R. Principles of appropriate antibiotic use for treatment of acute bronchitis in adults. Ann Intern Med 2001;134:518–20 [DOI] [PubMed] [Google Scholar]
- 13.National Quality Measures Clearinghouse. Avoidance of antibiotic treatment in adults with acute bronchitis: percentage of adults 18 to 64 years of age with a diagnosis of acute bronchitis who were not dispensed an antibiotic prescription. 2013. [cited 14 June 2013]; http://www.qualitymeasures.ahrq.gov/content.aspx?id=34647 [DOI] [PubMed]
- 14.Steinman MA, Landefeld CS, Gonzales R. Predictors of broad-spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA 2003;289:719–25 [DOI] [PubMed] [Google Scholar]
- 15.Evertsen J, Baumgardner DJ, Regnery A, et al. Diagnosis and management of pneumonia and bronchitis in outpatient Primary Care practices. Prim Care Respir J 2010;19:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paez K, Roper RA, Andrews RM. Health information technology and hospital patient safety: a conceptual model to guide research. Jt Comm J Qual Patient Saf 2013;39:415–25 [DOI] [PubMed] [Google Scholar]
- 17.Bell DS, Cretin S, Marken RS, et al. A conceptual framework for evaluating outpatient electronic prescribing systems based on their functional capabilities. J Am Med Inform Assoc 2004;11:60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Overhage JM, Perkins S, Tierney WM, et al. Controlled trial of direct physician order entry: effects on physicians’ time utilization in ambulatory primary care internal medicine practices. J Am Med Inform Assoc 2001;8:361–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanham HJ, Sittig DF, Leykum LK, et al. Understanding differences in electronic health record (EHR) use: linking individual physicians’ perceptions of uncertainty and EHR use patterns in ambulatory care. J Am Med Inform Assoc. 2014;21:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller RH, Sim I. Physicians’ use of electronic medical records: barriers and solutions. Health Aff 2004;23:116–26 [DOI] [PubMed] [Google Scholar]
- 21.Hsiao C-J, Hing E, Socey TC, et al. Electronic Medical Record/Electronic Health Record Systems of Office-based Physicians: United States, 2009 and Preliminary 2010 State Estimates. 2010. [cited 15 February 2011]; http://www.cdc.gov/nchs/data/hestat/emr_ehr_09/emr_ehr_09.htm
- 22.Jha AK, DesRoches CM, Campbell EG, et al. Use of electronic health records in US hospitals. N Engl J Med 2009;360:1628–38 [DOI] [PubMed] [Google Scholar]
- 23.Jha AK, DesRoches CM, Shields AE, et al. Evidence of an emerging digital divide among hospitals that care for the poor. Health Aff 2009;28:w1160–70 [DOI] [PubMed] [Google Scholar]
- 24.Schwartz B, Mainous AG, III, Marcy SM. Why do physicians prescribe antibiotics for children with upper respiratory tract infections? J Am Med Assoc 1998;279:881–2 [DOI] [PubMed] [Google Scholar]
- 25.Coco A, Mainous AG. Relation of time spent in an encounter with the use of antibiotics in pediatric office visits for viral respiratory infections. Arch Pediatr Adolesc Med 2005;159:1145. [DOI] [PubMed] [Google Scholar]
- 26.McCaig LF, Besser RE, Hughes JM. Antimicrobial-drug prescription in ambulatory care settings, United States, 1992–2000. Emerg Infect Dis 2003;9:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCaig LF, Hughes JM. Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA 1995;273:214–19 [PubMed] [Google Scholar]
- 28.Hing E, Burt CW. Are there patient disparities when electronic health records are adopted? J Health Care Poor Underserved 2009;20:473–88 [DOI] [PubMed] [Google Scholar]
- 29.Butler MJ, Harootunian G, Johnson WG. Are low income patients receiving the benefits of electronic health records? A statewide survey. Health Informatics J 2013;19:91–100 [DOI] [PubMed] [Google Scholar]
- 30.Rogers EM. Diffusion of innovations. New York, NY: Free Press, 2003 [Google Scholar]
- 31.National Center for Health Statistics. Ambulatory Health Care Data. 2013. [cited 1 December 2013]; http://www.cdc.gov/nchs/ahcd.htm
- 32.National Center for Health Statistics. About the National Ambulatory Medical Care Survey. 2009. [cited 1 December 2013]; http://www.cdc.gov/nchs/ahcd/about_ahcd.htm
- 33.Mainous AG, III, Lambourne CA, Nietert PJ. Impact of a clinical decision support system on antibiotic prescribing for acute respiratory infections in primary care: quasi-experimental trial. J Am Med Inform Assoc 2013;20:317–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22 [Google Scholar]
- 35.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci 1986:54–75 [Google Scholar]
- 36.Belongia EA, Knobloch MJ, Kieke BA, Jret al. Impact of statewide program to promote appropriate antimicrobial drug use. Emerg Infect Dis 2005;11:912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranji SR, Steinman MA, Shojania KG, et al. Interventions to reduce unnecessary antibiotic prescribing: a systematic review and quantitative analysis. Med Care 2008;46:847–62 [DOI] [PubMed] [Google Scholar]
- 38.Christakis DA, Zimmerman FJ, Wright JA, et al. A randomized controlled trial of point-of-care evidence to improve the antibiotic prescribing practices for otitis media in children. Pediatrics 2001;107:e15. [DOI] [PubMed] [Google Scholar]
- 39.Samore MH, Bateman K, Alder SC, et al. Clinical decision support and appropriateness of antimicrobial prescribing. JAMA 2005;294:2305–14 [DOI] [PubMed] [Google Scholar]
- 40.Sittig DF, Singh H. A new sociotechnical model for studying health information technology in complex adaptive healthcare systems. Qual Saf Health Care 2010;19(Suppl 3):i68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suda KJ, Hicks LA, Roberts RM, et al. Trends and seasonal variation in outpatient antibiotic prescription rates in the United States, 2006–2010. Antimicrob Agents Chemother 2014;58:2763–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang CJ, Marken RS, Meili RC, et al. Functional characteristics of commercial ambulatory electronic prescribing systems: a field study. J Am Med Inform Assoc 2005;12:346–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crosson JC, Isaacson N, Lancaster D, et al. Variation in electronic prescribing implementation among twelve ambulatory practices. J Gen Intern Med 2008;23:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pevnick JM, Asch SM, Adams JL, et al. Adoption and use of stand-alone electronic prescribing in a health plan-sponsored initiative. Am J Manag Care 2010;16:182–9 [PMC free article] [PubMed] [Google Scholar]
- 45.Yusof MM, Kuljis J, Papazafeiropoulou A, et al. An evaluation framework for Health Information Systems: human, organization and technology-fit factors (HOT-fit). Int J Med Inform 2008;77:386–98 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.