Abstract
Objective
To examine whether there is a common sequence of adoption of electronic health record (EHR) functions among US hospitals, identify differences by hospital type, and assess the impact of meaningful use.
Materials and methods
Using 2008 American Hospital Association (AHA) Information Technology (IT) Supplement data, we calculate adoption rates of individual EHR functions, along with Loevinger homogeneity (H) coefficients, to assess the sequence of EHR adoption across hospitals. We compare adoption rates and Loevinger H coefficients for hospitals of different types to assess variation in sequencing. We qualitatively assess whether stage 1 meaningful use functions are those adopted early in the sequence.
Results
There is a common sequence of EHR adoption across hospitals, with moderate-to-strong homogeneity. Patient demographic and ancillary results functions are consistently adopted first, while physician notes, clinical reminders, and guidelines are adopted last. Small hospitals exhibited greater homogeneity than larger hospitals. Rural hospitals and non-teaching hospitals exhibited greater homogeneity than urban and teaching hospitals. EHR functions emphasized in stage 1 meaningful use are spread throughout the scale.
Discussion
Stronger homogeneity among small, rural, and non-teaching hospitals may be driven by greater reliance on vendors and less variation in the types of care they deliver. Stage 1 meaningful use is likely changing how hospitals sequence EHR adoption—in particular, by moving clinical guidelines and medication computerized provider order entry ahead in sequence.
Conclusions
While there is a common sequence underlying adoption of EHR functions, the degree of adherence to the sequence varies by key hospital characteristics. Stage 1 meaningful use likely alters the sequence.
Keywords: Electronic Health Records, Hospitals, Adoption, Meaningful Use
Background and significance
In 2009, the federal government passed the Health Information Technology for Economic and Clinical Health (HITECH) Act to spur widespread adoption of health information technology. The centerpiece of HITECH is a financial incentive for doctors and hospitals to implement electronic health records (EHRs) and use them in ways expected to improve the safety, effectiveness, and efficiency of care—known as the meaningful use criteria.
The incentives are structured to encourage healthcare providers to start early and move quickly on adoption of a specific set of EHR functions, without explicit attention to their relationship to other electronic functions. This is potentially problematic because EHR adoption in the hospital setting is a complex process of implementing inter-related functions.1 These include ancillary clinical systems (eg, laboratory, radiology) for results management, various types of computerized provider order entry (CPOE), clinical documentation (eg, physician and nursing notes), clinical decision support, and barcoding (eg, for closed-loop medication administration). Each of these functions must work with others to create a functional system, and the interdependencies between them require complex decisions regarding which functions to adopt, and in what order. For example, drug–drug alerts, a type of clinical decision support, can only be implemented if patient medications are tracked electronically, either at the point of entry (via CPOE) or in a medication list. In other cases, there may not be a logically dependent sequence, but one that carries certain advantages. For example, while electronic reporting of radiology results is not a prerequisite for radiology CPOE, implementing reporting functionality first means that adding CPOE creates an electronic loop for processing radiological tests.
Given that meaningful use requires adoption of certain EHR functions, a better understanding of how hospitals typically sequence their adoption of EHR functions may reveal how the incentive program will alter this approach, as well as potential unintended consequences. There is surprisingly little empirical evidence that examines the sequence of adoption of EHR functions. There are popular industry models, including the HIMSS electronic medical record adoption model (EMRAM), which depict different stages of adoption, but they are not based on systematic, empirical data.2 Further, these models do not take into account differences in EHR adoption based on key hospital characteristics.3 For example, a recent study found that larger hospitals had more organizational capacity to implement bar-coded medication administration soon after CPOE, whereas smaller hospitals typically did not adopt bar-coded medication administration until much later in their EHR adoption trajectory.4 In addition, existing industry models do not address meaningful use and the various functions that hospitals must have in place to achieve the criteria.2 Stage 1 meaningful use requires structured clinical data entry, CPOE for medications, as well as some clinical decision support, but does not require other types of CPOE, clinical notes, or barcoding.5 Whether this requirement conforms to or diverges from hospitals’ prioritization of EHR adoption is not yet known.
Objective
In this paper, we use national data to assess the sequence of EHR adoption in hospitals, whether the sequence differs based on key hospital characteristics, and whether the sequence is consistent with the emphasis of stage 1 meaningful use. Specifically, we sought to answer the following questions1: Is there a common sequence in which hospitals adopt EHR functions?2 Does this sequence differ based on the size, rural/urban location, and teaching status of the hospital?3 Are the functions required for stage 1 meaningful use those that are likely to be implemented early? We answer these questions using data from the 2008 American Hospital Association (AHA) Information Technology (IT) Supplement survey that captures the specific EHR functions implemented by hospitals. Our results offer the first national empirical data on sequencing of EHR adoption in different types of hospitals and highlight the ways in which meaningful use may shape hospitals’ approach to EHR adoption. This information is critically important to policymakers as they craft future stages of meaningful use as well as to the many hospitals that are still in the midst of deciding how to approach EHR adoption.
Materials and methods
Data
We used national data from the IT supplement to the annual AHA survey, which was administered between March and September 2008 to all acute-care hospitals.1 For each of 28 electronic functions, respondents reported whether their hospital had fully implemented it in all major clinical units, had fully implemented it in one or more (but not all) major clinical units, or had not yet fully implemented it in any unit of the hospital. While more recent IT supplement data are available, we chose the 2008 data because they captured hospital EHR adoption prior to HITECH, and therefore allow us to assess the approach to adoption before hospitals knew of the functions included in meaningful use.
Sample
Our sample for analysis was limited to the 2794 general, acute-care, non-federal hospitals located in the 50 states and the District of Columbia that responded to at least half of the 28 function questions on the IT supplement survey. Of the 3441 hospitals that responded to the survey, 13 were excluded because they were located outside the 50 states or DC, 109 because they were federally owned, 517 because they were not general hospitals, and 9 because they did not respond to at least half of the 28 EHR function questions. In our sample, we imputed missing data under the assumption that missing data represented functions that were not implemented.
We merged the IT supplement data with information on hospital characteristics from the 2008 AHA annual survey in order to describe our sample (table 1) as well as compare IT supplement respondents to non-respondents (see online supplementary appendix table A1). The majority of sample hospitals were private and non-profit (64%). The sample was almost evenly split between hospitals that were members of a system (53%) and those that were not (47%). The majority were located in urban areas (57%) and were not teaching hospitals (81%). There were modest differences between respondents and non-respondents to the AHA IT supplement across these key characteristics.
Table 1.
Hospital sample characteristics
| N=2794 | % | |
|---|---|---|
| Size (total beds) | ||
| 0–99 | 1278 | 46 |
| 100–399 | 1206 | 43 |
| 400+ | 310 | 11 |
| Ownership | ||
| Public | 675 | 24 |
| Private, non-profit | 1772 | 64 |
| Private, for-profit | 347 | 12 |
| Member of a system | ||
| Yes | 1489 | 53 |
| No | 1305 | 47 |
| Member of a network | ||
| Yes | 999 | 36 |
| No | 1795 | 64 |
| Location | ||
| Urban | 1586 | 57 |
| Non-urban | 1208 | 43 |
| Teaching | ||
| Major or minor | 525 | 19 |
| Non-teaching | 2269 | 81 |
Loevinger homogeneity coefficients from Mokken scale analysis
We assessed the extent to which the 28 functions adhere to a homogeneous sequence using Loevinger homogeneity (H) coefficients from Mokken scale analysis (MSA). Loevinger H coefficients measure the extent to which a set of binary items adheres to an idealized sequence, known as a Guttmann scale. Items on the scale are arranged by frequency of agreement. To conform to a perfect Guttmann scale, a subject who agrees with a particular item must also agree with all items that are more frequently agreed to by all subjects.6 In our analysis, this would mean that less commonly adopted EHR functions, which are assumed to be more advanced, are implemented only if all more commonly adopted functions are adopted. Since it is rare to find a perfect Guttmann scale, Loevinger H coefficients assess the degree to which items adhere to the Guttmann scale, with closer adherence indicating a more homogeneous scale.7
Loevinger H coefficients reflect the number of violations of the Guttmann scale (errors) observed in the data and calculate the number of expected errors from marginal probabilities under the assumption of independence. The number of expected errors is dependent on the frequency of positive responses to each survey question, so that the likelihood that items that are more frequently positively responded to will also have more observed errors is accounted for in the calculation of the final coefficient. The Loevinger H coefficient, Hi, is created for each item i by dividing the number of observed Guttmann errors by the number of errors expected, and subtracting the quotient from 1:
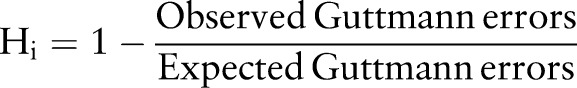 |
Hi varies between 0 and 1, and a higher Hi indicates that an item better adheres to the perfect Guttmann scale and less frequently violates its expected order. The H coefficient of the entire scale is similarly created by summing the observed errors of all items, dividing the sum by the total number of expected Guttmann errors, and subtracting the quotient from 1. The rule of thumb in interpreting these homogeneity coefficients is that a coefficient exceeding 0.3 indicates acceptable homogeneity, a 0.4 or above indicates moderate homogeneity, and a 0.5 or above indicates strong homogeneity.
While conducting MSA to create a valid Mokken scale requires that four assumptions are met (unidimensionality, monotonicity, local stochastic independence, and item invariant ordering), the particular statistic from MSA that we use—the Loevinger H coefficient—does not rest on any assumptions about the structure of the data or the relationship between items.8 9 Loevinger H is simply a descriptive statistic that is generated as part of MSA to capture the degree of homogeneity in item sequencing.
Our analyses do, however, rest on the assumption that there is a dominant latent factor driving sequencing of hospital EHR adoption, which in the context of our study could be conceived of as hospital capability to implement an EHR system. To confirm that our data met this assumption, we performed an exploratory factor analysis to ensure that there was a dominant latent factor and used the rest-score method to assess whether there were any violations in monotonicity (ie, a monotonic increase in the probability of positively responding to an item with an increase in the number of other positive item responses).6 7 9 Results of the exploratory factor analysis showed an eigenvalue of the first factor (9.34) that was more than three times that of the second factor (3.09), and there were no violations of the monotonicity assumption.
Analytic approach
To address our first research question, we assessed the existence of a common sequence of EHR adoption by calculating Loevinger H coefficients for individual EHR functions as well as across all functions. To facilitate the interpretation of the homogeneity scores, we also calculated the adoption rate (ie, the percentage of sample hospitals that have implemented that function) and ‘rank’ (the order from most adopted to least adopted functions) for each function.
Because this approach generates 84 results (ie, three results for each of the 28 functions), we sought to ease interpretation by averaging the item homogeneity, adoption rate, and rank of EHR functions within each of the five categories of EHR functions included on the IT supplement: Clinical Documentation (including Patient Demographics, Medication Lists, Discharge Summaries, Nursing Assessments, Advanced Directives, Problem Lists, and Physician Notes), Results Management (including Radiology Reports, Lab Reports, Radiology Images, Diagnostic Test Results, Consultant Reports, and Diagnostic Test Images), CPOE (including Radiology Test, Laboratory Test, Nursing Orders, Medications, and Consultation Request CPOE), Barcode (including Patient ID, Tracking Pharmaceuticals, Pharmaceutical Administration, and Laboratory Specimen Barcoding) and Decision Support (including Drug–Drug, Drug–Allergy, Drug–Lab Interaction checking, Drug Dosing Support, Clinical Guidelines, and Clinical Reminders).
To address our second research question, we recalculated homogeneity coefficients, adoption rates, and rank, stratified by three hospital characteristics: size (small<100 beds, medium 100–399 beds, large 400+ beds), location (urban vs non-urban), and teaching status (teaching vs non-teaching). We focused on these three characteristics because they predict hospital EHR adoption10 and because they are proxies for the factors that may influence how hospitals sequence EHR adoption: internal and external resources available to implement new technology, interest in pursuing new technology, and complexity of care delivered.11 Results were summarized by each of the five function categories in order to facilitate comparison by hospital type, with function-level results reported in the online supplementary appendix. In order to assess whether the homogeneity score across functions statistically differed based on hospital characteristics, we calculated standard errors (SEs) for the homogeneity scores using the mokken statistical package available in R.12 13
Finally, we qualitatively assessed whether the functions required for stage 1 meaningful use ‘core’ criteria are those that are adopted early by hospitals. For the assessment, we identified a subset of 11 functions that are emphasized in the stage 1 criteria (see online supplementary appendix table A2). We expected that meaningful use would be unlikely to have a substantial impact on how hospitals approach functions that are highly adopted with high homogeneity scores. In contrast, functions that have low or moderate adoption rates and low homogeneity may be the most affected by meaningful use, since there is apparent variation in when hospitals choose to or are able to implement the function. Functions that have low adoption rates and high homogeneity, on the other hand, are likely to be the most challenging for hospitals to implement early in response to meaningful use, because successful implementation of those functions may depend on the presence of other, more fundamental EHR functions.
Results
Sequencing of EHR adoption
The overall homogeneity score across all functions in our sample of hospitals was 0.48, suggesting moderate-to-strong evidence of a common sequence in which hospitals adopt EHR functions (table 2). Certain EHR functions reflected much stronger homogeneity than others. For example, patient demographics, radiology reports, and laboratory reports had homogeneity scores of at least 0.7 (table 2). These functions were also most widely adopted, indicating a strong tendency of hospitals to adopt these functions first.
Table 2.
Sequencing of electronic health record function adoption and relationship to meaningful use functions (in bold) (N=2794)
| Function category | Specific function | Item homogeneity | Adoption rate | Rank |
|---|---|---|---|---|
| Clinical documentation | Patient demographics | 0.71 | 0.85 | 1 |
| Results | Radiology reports | 0.78 | 0.85 | 2 |
| Results | Laboratory reports | 0.77 | 0.84 | 3 |
| Results | Radiology images | 0.56 | 0.80 | 4 |
| Clinical documentation | Medication lists | 0.56 | 0.62 | 5 |
| Decision support | Drug-drug interaction alerts | 0.55 | 0.62 | 6 |
| Decision support | Drug–allergy alerts | 0.56 | 0.62 | 7 |
| Results | Diagnostic test results | 0.49 | 0.61 | 8 |
| Clinical documentation | Discharge summaries | 0.51 | 0.60 | 9 |
| Results | Consultant reports | 0.45 | 0.58 | 10 |
| Clinical documentation | Nursing assessments | 0.52 | 0.58 | 11 |
| Barcode | Laboratory specimens barcode | 0.33 | 0.57 | 12 |
| Barcode | Patient ID barcode | 0.38 | 0.50 | 13 |
| Decision support | Drug–laboratory interaction alerts | 0.48 | 0.49 | 14 |
| Results | Diagnostic test images | 0.40 | 0.48 | 15 |
| Decision support | Advanced directives | 0.43 | 0.46 | 16 |
| Clinical documentation | Drug dosing support | 0.47 | 0.45 | 17 |
| Clinical documentation | Problem lists | 0.47 | 0.44 | 18 |
| Barcode | Tracking pharmaceuticals barcode | 0.36 | 0.34 | 19 |
| Decision support | Clinical reminders | 0.49 | 0.34 | 20 |
| Barcode | Pharmaceutical administration barcode | 0.37 | 0.32 | 21 |
| CPOE | Radiology tests CPOE | 0.41 | 0.32 | 22 |
| CPOE | Laboratory tests CPOE | 0.41 | 0.31 | 23 |
| CPOE | Nursing orders CPOE | 0.47 | 0.30 | 24 |
| CPOE | Medications CPOE | 0.47 | 0.27 | 25 |
| Clinical documentation | Physician notes | 0.40 | 0.27 | 26 |
| Decision support | Clinical guidelines | 0.50 | 0.26 | 27 |
| CPOE | Consultation requests CPOE | 0.54 | 0.23 | 28 |
| All | 0.48 |
CPOE, computerized provider order entry; EHR, electronic health record.
Other items with strong homogeneity (0.50 or above) included radiology images, medication lists, drug–allergy alerts, drug–drug interactions, discharge summaries, nursing assessments, clinical guidelines, and CPOE for consultation requests. The adoption rates of these functions varied. For example, while 80% of hospitals had adopted radiology images, only 26% of hospitals had clinical guidelines implemented. Thus, radiology images were commonly adopted early on by hospitals, while clinical guidelines were commonly adopted late in the sequence.
Several functions, including many of the barcode functions, had only acceptable homogeneity. While on average these functions tended to be adopted in the middle of the sequence, the low homogeneity reflects variation in the sequence of adoption, such that some hospitals adopted these functions early in the sequence and others adopted them late.
These patterns were also reflected in the summarized homogeneity scores, adoption rates, and ranks (table 3). Results management, clinical documentation, and decision support function categories had the strongest average homogeneity (0.57, 0.51, and 0.51, respectively) and tended to be implemented in the early to middle part of the sequence (average rank of 7, 12, and 15, respectively, on a 1–28 scale). The homogeneity of CPOE functions was also moderate-to-strong (0.46), but CPOE functions were implemented on average late in the sequence (average rank of 24). Finally, for barcode functions, the average homogeneity was adequate (0.36), suggesting that while on average these functions appear in the middle of the sequence (average rank of 16), some hospitals implement early and others implement late.
Table 3.
Average homogeneity, adoption rate, and rank by function category
| Averages | |||
|---|---|---|---|
| Homogeneity | Adoption rate | Rank | |
| Clinical documentation | 0.51 | 0.55 | 12 |
| Results | 0.57 | 0.69 | 7 |
| CPOE | 0.46 | 0.29 | 24 |
| Barcode | 0.36 | 0.43 | 16 |
| Decision support | 0.51 | 0.46 | 15 |
| All | 0.48 | – | – |
CPOE, computerized provider order entry.
Stratified results
The stratified analyses showed key differences based on hospital type. Small hospitals had a significantly greater scale homogeneity than medium/large hospitals (0.50 vs 0.38; p<0.0001), revealing that small hospitals more closely adhered to a common sequence of EHR adoption than medium and large hospitals (table 4). There were also substantial differences in average homogeneity by function category. The most substantial difference in average homogeneity was for barcode functions, with more consistency in sequencing among small hospitals compared to medium and large hospitals (0.40 vs 0.24). A similar, though weaker, pattern held for clinical documentation (0.54 vs 0.41) and results management (0.56 compared to 0.46). For the other function categories, differences in homogeneity were smaller. (Full results by size category are shown in online supplementary appendix table A3.) Average rank also reflected differences between small and medium/large hospitals in the sequence of EHR adoption. Small hospitals tended to adopt results management functions slightly later than medium/large hospitals, although still early on in the overall sequence (average rank of 8 compared to 6). In contrast, small hospitals tended to adopt CPOE functions slightly earlier than medium/large hospitals, although all hospitals adopted CPOE functions late in the sequence (average rank of 23 compared to 25). There were minimal differences in average rank for clinical documentation, barcode, and decision support functions.
Table 4.
Average homogeneity, adoption rate, and rank by function category: differences by hospital size
| Average homogeneity | Percent differences in average homogeneity | ||
|---|---|---|---|
| Small | Medium/large | ||
| Clinical documentation | 0.54 | 0.41 | −31.7 |
| Results | 0.56 | 0.46 | −21.7 |
| CPOE | 0.46 | 0.42 | −8.7 |
| Barcode | 0.40 | 0.24 | −66.7 |
| Decision support | 0.53 | 0.43 | −23.3 |
| All (SE) | 0.50 (0.011) | 0.38 (0.011) | −31.5 (p<0.0001) |
| Average adoption rate | Percent differences in average adoption rate | ||
| Clinical documentation | 0.43 | 0.65 | 51.2 |
| Results | 0.54 | 0.82 | 51.8 |
| CPOE | 0.23 | 0.33 | 43.5 |
| Barcode | 0.32 | 0.53 | 65.6 |
| Decision support | 0.35 | 0.61 | 74.3 |
| All (SE) | 0.38 (0.0079) | 0.59 (0.0061) | 55.3 (p<0.0001) |
| Average rank | Differences in average rank | ||
| Clinical documentation | 12 | 13 | 1 |
| Results | 8 | 6 | −2 |
| CPOE | 23 | 25 | 2 |
| Barcode | 17 | 17 | 0 |
| Decision support | 15 | 14 | −1 |
CPOE, computerized provider order entry.
Scale homogeneity was significantly higher in rural hospitals compared to urban hospitals (0.51 vs 0.42; p<0.0001) (table 5). By category, differences in average homogeneity were of approximately similar magnitude, except for the barcode functions, for which rural hospitals exhibited higher homogeneity than urban hospitals. For example, the mean homogeneity for clinical documentation was 0.54 among rural hospitals compared to 0.46 among urban hospitals, while for barcode functions, mean homogeneity was 0.41 among rural hospitals compared to 0.29 among urban hospitals. Results management functions tended to be adopted slightly earlier in urban hospitals (average rank of 6 compared to 8).
Table 5.
Average homogeneity, adoption rate, and rank by function category: differences by hospital location
| Average homogeneity | Percent differences in average homogeneity | ||
|---|---|---|---|
| Rural | Urban | ||
| Clinical documentation | 0.54 | 0.46 | −14.8 |
| Results | 0.58 | 0.52 | −10.3 |
| CPOE | 0.46 | 0.43 | −6.4 |
| Barcode | 0.41 | 0.29 | −29.3 |
| Decision support | 0.55 | 0.46 | −16.4 |
| All (SE) | 0.51 (0.012) | 0.42 (0.011) | −17.6 (p<0.0001) |
| Average adoption rate | Percent differences in average adoption rate | ||
| Clinical documentation | 0.48 | 0.61 | 27.0 |
| Results | 0.58 | 0.79 | 36.2 |
| CPOE | 0.22 | 0.34 | 54.5 |
| Barcode | 0.36 | 0.49 | 36.1 |
| Decision support | 0.39 | 0.52 | 36.6 |
| All (SE) | 0.41 (0.0079) | 0.56 (0.0061) | 233.3 (p<0.0001) |
| Average rank | Differences in average rank | ||
| Clinical documentation | 12 | 13 | 1 |
| Results | 8 | 6 | −2 |
| CPOE | 24 | 24 | 0 |
| Barcode | 16 | 17 | 1 |
| Decision support | 15 | 16 | 1 |
CPOE, computerized provider order entry.
Scale homogeneity was higher in non-teaching hospital than teaching hospitals (0.49 vs 0.35; p<0.0001). This difference was most pronounced for barcoding, results management, and clinical documentation functions, and least pronounced for CPOE and decision support functions (table 6). Non-teaching hospitals tended to adopt results management (8 compared to 5) and CPOE (25 compared to 23) functions later, and barcoding functions earlier (16 compared to 19) in the sequence than teaching hospitals.
Table 6.
Average homogeneity, adoption rate, and rank by function category: differences by hospital teaching status
| Average homogeneity | Percent differences in average homogeneity | ||
|---|---|---|---|
| Teaching | Non-teaching | ||
| Clinical documentation | 0.37 | 0.53 | 43.2 |
| Results | 0.40 | 0.57 | 42.5 |
| CPOE | 0.42 | 0.45 | 7.1 |
| Barcode | 0.22 | 0.38 | 72.7 |
| Decision support | 0.41 | 0.52 | 26.8 |
| All (SE) | 0.35 (0.018) | 0.49 (0.009) | 40.0 (p<0.0001) |
| Average adoption rate | Percent differences in average adoption rate | ||
| Clinical documentation | 0.69 | 0.52 | −32.7 |
| Results | 0.87 | 0.65 | −33.8 |
| CPOE | 0.43 | 0.25 | −72.0 |
| Barcode | 0.52 | 0.41 | −26.8 |
| Decision support | 0.59 | 0.43 | −37.2 |
| All (SE) | 0.63 (0.0097) | 0.47 (0.0056) | −34.0 (p<0.0001) |
| Average rank | Differences in average rank | ||
| Clinical documentation | 12 | 12 | 0 |
| Results | 5 | 8 | 3 |
| CPOE | 23 | 25 | 2 |
| Barcode | 19 | 16 | −3 |
| Decision support | 16 | 15 | −1 |
CPOE, computerized provider order entry.
Impact of meaningful use
When we assessed the extent to which the functions that hospitals typically adopted early in the sequence—prior to meaningful use—are those prioritized by meaningful use, we found mixed results. Some functions, such as patient demographics, medication lists, drug–allergy alerts, drug–drug interactions, and discharge summaries, were homogeneously adopted by hospitals early in their sequence (table 2). In contrast, clinical guidelines and medication CPOE as well as other decision support and clinical documentation functions were homogeneously adopted late in the sequence. This suggests that meaningful use may cause hospitals to change their planned order of EHR function adoption, and move these functions ahead in sequence.
The extent to which meaningful use may impact how hospitals approach EHR adoption varied based on key hospital characteristics. For example, in small hospitals compared to medium/large hospitals, clinical reminders and clinical guidelines (two functions that are prioritized in stage 1 meaningful use) were all homogeneously adopted later and less frequently (see online supplementary appendix table A4). Meaningful use may therefore result in small hospitals increasing their focus on clinical decision support adoption to a greater extent than larger hospitals. Comparing rural to urban hospitals, drug–allergy alerts, another stage 1 meaningful use priority, were homogeneously adopted later, suggesting that rural hospitals may focus on increasing adoption of this function to a greater extent than urban hospitals (see online supplementary appendix table A5). Finally, medication CPOE was moderately homogeneously adopted later and less frequently in non-teaching hospitals than in teaching hospitals (see online supplementary appendix table A6). Thus, non-teaching hospitals are likely to place more emphasis on increasing adoption of this function compared to their teaching counterparts.
Discussion
Both policymakers and healthcare providers are devoting substantial attention and resources to increase adoption of EHRs in order to improve the quality and reduce the cost of care. For hospitals, EHR adoption is a complex process, typically occurring incrementally with functions added in a carefully planned sequence over time. While national data on the overall level of hospital EHR adoption have been widely reported, this is the first paper to explicitly examine the sequence of adoption of EHR functions in US hospitals. We find moderate-to-strong evidence that a common sequence is followed. However, the extent of adherence to a common sequence varies based on key hospital characteristics, with small, rural, and non-teaching hospitals exhibiting stronger homogeneity compared to their larger, urban, teaching counterparts. We also find that many functions emphasized in stage 1 meaningful use are typically adopted later in the sequence, in particular clinical guidelines and medication CPOE, suggesting that meaningful use may change the planned prioritization of EHR function adoption. These findings will help policymakers anticipate the impact of meaningful use, and how it may vary for different types of hospitals. For hospitals working to increasingly implement EHR functions, we offer the first empirical data on sequencing to serve as a guide.
The differences in homogeneity in EHR adoption sequence by hospital type largely adhered to our expectations. For example, it is not surprising that small hospitals have a more homogeneous sequence than larger hospitals. We suspect that this is primarily driven by two factors. First, small hospitals are likely to rely more heavily on vendors, who have standard approaches to EHR adoption, while larger hospitals may use multiple vendors or have home-grown systems, and therefore make internal decisions about sequencing. Second, larger hospitals are more complex and deal with a more diverse group of stakeholders, which likely introduce varied considerations into the selected sequence of EHR adoption. We expected that rural hospitals would have a more homogeneous sequence compared to urban hospitals for similar reasons. Rural hospitals may have trouble attracting advanced IT staff and therefore rely more heavily on vendors’ standard approaches to guide their sequencing. Finally, teaching hospitals likely have more heterogeneity compared to non-teaching hospitals both because they typically deliver more complex care and because they are early EHR adopters, often as a result of developing customized home-grown systems.
Stage 1 meaningful use was designed to focus on basic EHR tasks, such as structured entry of clinical data. However, certain functions thought to be more advanced—clinical decision support and CPOE for medications—were included because of the evidence for their impact on improving care quality.14 15 Our results confirm that hospitals would typically have adopted these functions later in the sequence, likely because they are more advanced. Nonetheless, our results raise the question of the potential unintended consequences of creating incentives that may result in hospitals moving up certain functions in the sequence. For example, nursing assessments—important for patient handoffs and care continuity—are typically adopted in the middle of the sequence, but ahead of clinical reminders and medication CPOE. Since such assessments are not part of stage 1 meaningful use, hospitals may deprioritize their implementation, dampening their effort to improve the quality of care coordination. While the evidence is not yet mature enough to support an assessment of the comparative effectiveness of different functions, it is important to assess which valuable functions may be deferred as a result of meaningful use and the potential consequences.
Our work refines and extends industry models of EHR adoption by being the first to empirically examine sequencing of EHR adoption across US hospitals. The most widely known industry model, the seven-stage HIMSS EMRAM, is largely consistent with our empirically derived results.2 However, while the EMRAM places all CPOE functions within stage 4, our results reveal which types of CPOE are typically adopted early (laboratory and radiology) and which are adopted late (consultation requests), detail which is likely helpful for hospitals. There is also literature that explores questions related to sequencing. One study looked at hospital adoption of various functions to support medication safety and found that the most widely adopted was automated dispensing machines and the least adopted were bar-coded medication administration and robot for medication dispensing.3 While this reflects heterogeneity in function-level adoption rates, it does not specifically address the question of sequencing. A more recent article, based on semi-structured interviews with hospital chief information officers regarding the impact of the meaningful use program, found that the program has accelerated the implementation of some key functions, in particular CPOE, with hospitals reporting that ‘the inclusion of CPOE in the meaningful use requirements led them to pursue its adoption sooner than they otherwise would have.’4 Our results support this conclusion quantitatively using national data and reveal other functions whose adoption may be similarly accelerated by stage 1 meaningful use.
Our work has several limitations. First, our analysis assesses homogeneity in EHR function adoption in cross-sectional data, and does not track the sequence of adoption longitudinally. The underlying assumption is that in any given cross-section that captures hospital EHR adoption, each hospital is at a different point in their adoption sequence, such that cumulatively we are able to detect the presence of an underlying sequence. As a result, we cannot discuss the time horizon over which the sequence that we observe typically takes place, nor can we assess whether results would differ if we tracked hospital EHR function adoption longitudinally. Second, certain EHR functions have a logical dependence. For instance, drug interaction checking is likely impossible without documentation of medications on a medication list or the electronic ordering of medications. While this does not bias the analysis, it reflects the fact that in our empirical context, not all conceptually possible sequences are possible in reality. Finally, our results do not address the causal mechanism that drives hospitals’ decisions about sequencing. Early versus late adoption of specific functions may reflect how hospital leadership assesses the costs and benefits of each function, or mimetic processes in which hospitals follow the industry norms. Our analytic approach does not allow us to distinguish between these patterns.
Conclusion
We examine data on hospital adoption of EHR functions and find that there is a homogeneous sequence of adoption. This represents the first national empirical data on sequencing of hospital EHR adoption. We find stronger homogeneity among small, rural, and non-teaching hospitals, which is likely driven by greater reliance on vendors and less variation in the types of care that they deliver compared to larger, urban, teaching hospitals. Perhaps most importantly, we find that stage 1 meaningful use may change how hospitals sequence EHR adoption. In particular, clinical guidelines and medication CPOE are homogeneously adopted late in the sequence, but because they are priorities in stage 1 meaningful use, hospitals may move these functions ahead in sequence. It will be important to assess the impact of such re-ordering on patient care and the cost of EHR adoption in hospitals.
Supplementary Material
Footnotes
Contributors: JAM and JE: designed the study, acquired the data, interpreted the analyses, and drafted and revised the manuscript; SYL: designed the study, acquired the data, interpreted the analyses, and revised the manuscript. All authors take responsibility for the work and ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This work was supported by the University of Michigan's MCubed funding program and a grant from the Blue Cross Blue Shield of Michigan Foundation.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Jha AK, DesRoches CM, Campbell EG, et al. Use of electronic health records in U.S. hospitals. N Engl J Med 2009;360:1628–38 [DOI] [PubMed] [Google Scholar]
- 2.Health Information Management and Systems Society. Electronic Medical Record Adoption Model (EMRAM)SM. 2014. http://www.himssanalytics.org/emram/emram.aspx with original model described in: http://www.providersedge.com/ehdocs/ehr_articles/himss_emr_definition_model_v1-0.pdf
- 3.Furukawa MF, Raghu TS, Spaulding TJ, et al. Adoption of health information technology for medication safety in U.S. hospitals, 2006. Health Aff 2008;27:865–75 [DOI] [PubMed] [Google Scholar]
- 4.Botta M, Cutler D. Meaningful use: floor or ceiling? Healthcare 2014;2:48–52 [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal D, Tavenner M. The “Meaningful Use” regulation for electronic health records. N Engl J Med 2010;363:501–4 [DOI] [PubMed] [Google Scholar]
- 6.van Schuur WH. Mokken scale analysis: between the Guttman scale and parametric item response theory. Political Anal 2003;11:139–63 [Google Scholar]
- 7.Mokken RJ. A theory and procedure of scale analysis: with applications in political research. Walter de Gruyter, 1971 [Google Scholar]
- 8.Loevinger J. The technic of homogeneous tests compared with some aspects of “scale analysis” and factor analysis. Psychol Bull 1948;45:507–29 [DOI] [PubMed] [Google Scholar]
- 9.Meijer RR, Sijtsma K, Smid NG. Theoretical and empirical comparison of the Mokken and the Rasch approach to IRT. Appl Psychol Meas 1990;14:283–98 [Google Scholar]
- 10.DesRoches CM, Charles D, Furukawa MF, et al. Adoption of electronic health records grows rapidly, but fewer than half of US hospitals had at least a basic system in 2012. Health Aff 2013;32:1478–85 [DOI] [PubMed] [Google Scholar]
- 11.Dranove D, Forman C, Goldfarb A, et al. The trillion dollar conundrum: complementarities and health information technology. National Bureau of Economic Research, 2012 [Google Scholar]
- 12.van der Ark LA. Mokken scale analysis in R. J Stat Software 2007;20:1–19 [Google Scholar]
- 13.Kuijpers RE, Van der Ark LA, Croon MA. Standard errors and confidence intervals for scalability coefficients in Mokken scale analysis using marginal models. Sociol Methodol 2013;43:42–69 [Google Scholar]
- 14.Garg AX, Adhikari NKJ, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA 2005;293:1223–38 [DOI] [PubMed] [Google Scholar]
- 15.Chaudhry B, Jerome W, Shinyi W, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med 2006;144:E12–W8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


