Abstract
Delivering useful clinical decision support to providers who are ordering high risk drugs for high risk patients is imperative for safe pharmacotherapy. This paper presents a focused electronic clinical decision support intervention designed to decrease the risk of corrected QT interval (QTc) related adverse drug events in a high risk patient population. Results showed that a customized alert can both decrease the number of alerts sent to providers while still improving the safety of prescribing practices for intravenous haloperidol. The alert leveraged components of the electronic health record to significantly decrease the rate of inappropriate prescription of intravenous haloperidol in patients with QTc >500 ms from 50% to 14%. The results also suggest providers may abandon the appropriate prescription of a medication in response to an alert. The findings support the necessity of careful targeting of electronic alerts and monitoring for unintended consequences when implementing these types of electronic alerts.
Background and significance
Off-label use of intravenous (IV) haloperidol for prevention and treatment of delirium in hospitalized patients is becoming increasingly common.1–4 A warning recommending ECG monitoring was added to the product labeling for IV haloperidol in September 2007 due to case reports of QT interval prolongation (QTP), torsades de points (TdP), and sudden death associated with its use.5 Data from case reports and small studies indicate that a heart rate corrected QT interval (QTc) >500 ms is associated with an increased risk of TdP.6 While most healthcare professionals are familiar with antiarrhythmic drugs being associated with QTP and TdP, many are not familiar with the large number of non-antiarrhythmic drugs, including antipsychotics, that can also cause these cardiac conduction disturbances and life threatening arrhythmias.6 Even when the association is known, ECG monitoring may not be performed prior to or during the administration of QTc prolonging agents. Our prior work found one in six patients prescribed IV haloperidol in the hospital had pre-existing QTc prolongation of >500 ms.7
As hospitals across the country adopt electronic health records (EHR) and computerized provider order entry (CPOE) systems, the ability to electronically alert prescribing providers of these types of drug–disease interactions is becoming widespread. Unfortunately, poorly targeted alerts can lead to ‘alert fatigue’, and result in alerts which are bypassed or ignored. Minimizing inappropriate firing of these alerts continues to be a challenge and a barrier to achieving clinically meaningful results from their implementation.8–10 Recent studies have shown that prescriber awareness of drug induced QTP can be improved with the use of computer alert systems which notify providers of patients with a prolonged QTc and help implement directed protocols around IV haloperidol administration.11 12
Objective
We describe a system wide quality improvement initiative using a focused computerized alert at the time of medication order entry, triggered only for patients with prolonged QTc. The primary objective was to decrease unsafe use of IV haloperidol in this high risk patient population.
Methods
Setting
The intervention was performed at the University of Colorado Hospital, which is a 412 bed academic tertiary care hospital in western USA. Retrospective cohort analysis was limited to hospital inpatients or patients undergoing surgical procedures in the hospital operating rooms. This quality improvement project was approved by the Colorado Multiple Institutional Review Board. Providers were attending physicians, residents, fellows, advance practice nurses, or physician assistants, but the majority of medication orders are placed by residents and fellows.
Materials and methods
The study hospital uses an integrated EHR (V.2010; Epic Systems, Verona, Wisconsin, USA) including CPOE and an integrated pharmacy module for reconciling and dispensing hospital medications. The electronic alerting system used was an Epic functionality called ‘best practice advisories’ (BPAs).
Electronic alert specifications
A BPA was designed to fire at the initiation of an order for IV haloperidol if a patient's last ECG demonstrated a QTc interval that was >500 ms, as determined by the automated ECG machine analysis. The retrospective ECG review period was 14 days for QTc determination, and the alert did not fire if no ECG was available in that time frame. Figure 1 demonstrates a representative IV haloperidol BPA.
Figure 1.
The ‘best practice advisory’ window of the application. ©2014 Epic Systems Corporation. Used with permission.
Definitions
IV haloperidol use in patients with a QTc >500 ms was defined as clinically appropriate in three scenarios. First, if the patient was receiving end of life care, then the benefit was thought to outweigh the risk. Second, if the benefit of the IV haloperidol administration outweighed the risk of arrhythmia, as documented by the ordering provider in the medical record. Third, if the patient had a falsely prolonged QTc due to prolonged QRS duration as the patient may have a normal corrected QTc. For the alert, we were unable to correct QTc for QRS duration, and so we instead gave providers the option to select ‘falsely prolonged QTc’ as an appropriate reason for continuing with their order. All other use of IV haloperidol in patients with last QTc >500 ms were recorded as inappropriate prescribing. The rate of inappropriate haloperidol administration was calculated by dividing the doses of IV haloperidol given to patients without end of life or prolonged QRS by the total number of alerts that fired.
Pre-intervention evaluation
During the pre-intervention period, the BPA sent a message to the investigators each time the alert would have fired, but it was initially blinded from the ordering provider. This 8 month run-in period from December 2011 to July 2012 provided a baseline of how often IV haloperidol was being ordered in patients with last recorded QTc >500 ms.
Post-intervention evaluation
The BPA was opened up to alert prescribing providers in August 2012. The 8 month post-intervention period lasted from September 2012 to April 2013. The provider alert fired at initiation of the electronic order for IV haloperidol if the patient's last QTc was >500 ms. This alert informed the provider that IV haloperidol was contraindicated in a patient with a QTc >500 ms, listed the patient's last QTc and QRS duration, and then gave them options to continue to order the medication for specified reasons or to cancel the order (figure 1). Regardless of what the providers chose within the alert, the investigators performed chart abstraction on all alerts to verify if haloperidol was actually ordered and if the aforementioned appropriateness criteria were met. There was also no hard stop in the alert, so providers were allowed to cancel the BPA and continue ordering IV haloperidol without choosing an option within the alert.
Analysis
Monthly rates of IV haloperidol prescription in patients with QTc >500 ms were calculated to compare the pre-intervention with the post-intervention period. The overall rate of IV haloperidol prescription was also recorded during the pre-intervention period to determine how often a non-customized alert would have fired. Run chart analysis was used to evaluate for shift with the premise that six consecutive data points below the baseline median would represent a non-random pattern associated with the intervention with a statistical probability of <0.05. The Student's t test was also used to compare the mean rate of monthly inappropriate IV haloperidol prescription prior to and after the intervention. For the Student's t test, statistical analysis was performed using SAS, V.9.2 (SAS Institute Inc, Cary, North Carolina, USA).
Results
Pre-intervention
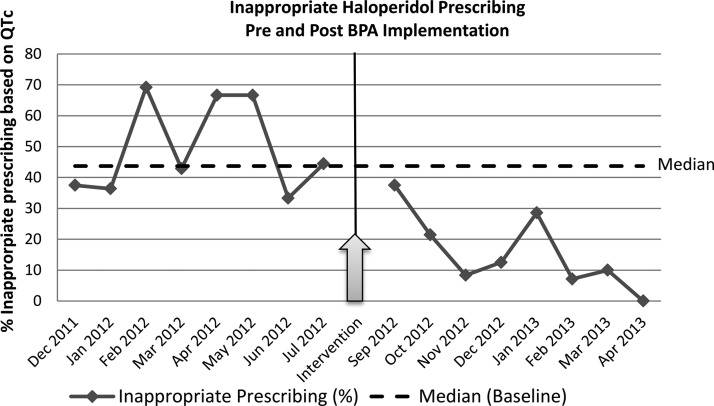
During the 8 month run-in period, there were 66 orders for IV haloperidol in patients with a QTc >500 ms, representing an average of 8.3/month (SD=2.6, range=6–13). Of the 66 alerts, none was associated with charted documentation of benefit outweighing risk. Sixteen (24.2%) were in patients receiving end of life care and 17 (25.8%) were in patients who had a prolonged QRS. The remaining 33 (50.0%) were inappropriate prescribing, as defined above. During the baseline period, the median percentage of inappropriate prescribing of IV haloperidol was 43.7%. A run chart of monthly inappropriate prescribing rate is shown in figure 2. The total number of IV haloperidol prescriptions in all patients across the study institution was 1124 during the 8 months pre-intervention period.
Figure 2.
Rate of inappropriate intravenous haloperidol ordered/administered. BPA, best practice advisory.
Post-intervention
During the 8 month post-intervention period, 87 alerts were triggered by providers ordering IV haloperidol in patients with a QTc >500 ms, with an average of 10.9/month (SD=3.0, range=7–14). Of these 87 alerts, 24 (27.6%) were in patients receiving end of life care and 34 (39.1%) were in patients who had a prolonged QRS. The 29 (33.3%) remaining were in patients with no clear documentation that the benefits of IV haloperidol outweighed the risk factor of prolonged QTc. The IV haloperidol order was abandoned in 40 (46.0%) patients with a QTc >500 ms on whom the alert fired. Of these 40, 18 (45.0%) would have been inappropriate prescribing and 22 (55.0%) were in patients with prolonged QRS or end of life care. During the intervention period, 12 patients were prescribed what was considered inappropriate IV haloperidol despite the alert. A decrease in the rate of completed inappropriate haloperidol prescription was observed from an average of 4.1/month pre-intervention to 1.5/month post-intervention (p=0.00025). The proportion of patients administered inappropriate haloperidol dropped from 50% (SD 15%) to 14% (SD 12%) (figure 3). During the post-intervention period, the median percentage of inappropriate prescribing of IV haloperidol was 10.0%. Comparing the pre-intervention with the post-intervention period, our run chart analysis demonstrates a shift with 8 consecutive months of inappropriate IV haloperidol prescriptions below the baseline median. This represents a non-random pattern associated with the intervention initiation (p<0.05) (figure 2).
Figure 3.
Rate of inappropriate intravenous haloperidol ordered/administered pre- and post-intervention.
Discussion
Development of a targeted CPOE alert significantly decreased the rate of inappropriate prescription of IV haloperidol in patients with QTc >500 ms from 50% to 14%. Educational interventions alone to improve safe medication prescription are subject to a waning effect over time whereas non-specific warnings are prone to result in alert fatigue and may be bypassed or ignored. This specific alert only fired in patients with QTc >500 ms compared with how often a non-specific alert would have fired (8.3/month vs 140.5/month). The information contained in the alert also raised awareness of contraindications of IV haloperidol based on prolonged QTc.
As a result of the intervention, 63.6% of all inappropriate orders for IV haloperidol were abandoned prior to completion of the order. Our evaluation demonstrated that a customized drug–disease interaction based alert can decrease the prescription of dangerous medications in high risk patients when carefully designed and implemented. Previous studies have demonstrated that electronic drug–drug interaction alerts can decrease the rate of adverse drug events, but they often do not report on the rate of inappropriate alert firing and how often providers ignore the alerts.13 Our study maximized the usefulness of the alert in four particular ways: we customized the alert to a particular at risk patient population, educated the provider through the alert, supplied clinically relevant patient specific information in the alert, and gave providers the ability to quickly opt out of the suggested clinical path. This is in line with Osheroff's five rights of electronic clinical decision support which are to deliver the right information, to the right person, in the right format, through the right channel, at the right time.14 Our intervention utilized only a single value, last QTc, to improve the effectiveness of this alert, but there is a wealth of clinical coded data in the EHR that can and should be leveraged to improve the usefulness of alerts. We also recognize that this was a small quality improvement study implemented in a single academic hospital with a particular EHR, and so its generalizability is limited.
Our analysis revealed the occasional discontinuation of IV haloperidol use in end of life care patients or in patients with a prolonged QRS in whom the medication may have been clinically appropriate. This finding raises concerns that even targeted alerts could have the unintended consequence of reducing appropriate use of high risk medications. We tried to mitigate this risk by including reasons within the alert as to why providers may want to continue with their appropriate IV haloperidol order, but our results demonstrated that additional strategies may need to be developed. As with all electronic alerts, we run the risk of providers getting used to these types of alerts and not looking at a patient's ECG prior to writing for IV haloperidol. This study was not powered to detect rare but catastrophic complications of prescribing IV haloperidol, such as TdP. Therefore, we cannot draw conclusions about whether reduction in prescriptions in IV haloperidol would reduce adverse patient outcomes, although this is a reasonable presumption based on the Food and Drug Administration warning.
Limitations
The limitations of this study include the inability to know how exactly the ordering clinician was weighing risks and benefits of the IV haloperidol order. The baseline rate of prescribing IV haloperidol in patients with QTc >500 ms was higher in the post-intervention period compared with the pre-intervention period (8.25/month with SD=2.6 vs 10.9/month with STD=3.0) for unclear reasons. Despite this baseline increase, there remained a decrease in the percentage of inappropriate haloperidol order initiation from the run-in period to the follow-up period (50% with SD=15 vs 33% with SD=11). Our alert did not capture patients with QTc <500 ms who may still be at risk for haloperidol associated TdP,15 and further efforts need to be made to maximize the sensitivity and specificity of these types of alerts by evaluating alternative QTc thresholds other than TdP risk factors.
Conclusion
Implementation of a customized alert significantly decreased the rate of inappropriate prescription of a high risk medication in a vulnerable patient population. Given the success of this study, out next steps will be to extend the alert to cover other high risk QTc prolonging medications, as classified by the Arizona Center for Education and Research on Therapeutics (AzCERT). The results also highlight the necessity of careful analysis of balancing measures in CPOE quality improvement measures to detect unintended consequences. To improve medication safety in healthcare, we must move beyond educational initiatives or swamping providers at the front lines of medicine with poorly targeted alarms. Although leaders in the health information technology field continue to assert that high reliability healthcare systems demand thoughtful design of CPOE alerts, more of this theory needs to be put into practice.16–18
Footnotes
Contributors: JMP was the main coordinator for the project. JMP worked on the design of the project, primary data collection for the post-intervention period, and data analysis for the entire project. He was the primary author for the manuscript, particularly for the initial writing of the methods, results, and discussion sections of the paper, as well as critical review and editing of the entire manuscript. He built or assisted in the build of all figures. DC was a contributing author of the manuscript and assisted in the coordination of the project. Because of her experience with similar work, DC helped with the design of the project, primary data collection for the pre-intervention period, and assisted with data analysis, with primary work on the run chart analysis for the project. DC helped in authoring the manuscript, particularly for the initial writing of the background and significance section, as well as critical review and editing of the entire manuscript. MAJ was the main technical coordinator for the intervention for the project. He worked on the concept, design, customization, and evaluation of the electronic alert used in the project. MAJ assisted in delivering the primary data to the rest of the team in an organized format, and assisted with data analysis for the entire project. He was the primary author of the technical portion of the methods section of the manuscript, and he critically reviewed and edited the entire manuscript. EC was the driving force behind the conceptualization of the project. Because of his experience with the subject matter, EC helped with the design of the project and assisted with data analysis and data display for the manuscript. He was involved in all steps of critical review and editing of the entire manuscript and revisions. All authors give approval for the final version of the manuscript to be published and agree to be accountable for all aspects of the work ensuring that questions related to the accuracy and/or integrity of any part of the work are appropriately investigated and resolved.
Competing interests: None.
Ethics approval: The study was approved by the Colorado Multiple Institutions Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.[No authors listed]. American Psychiatric Association: practice guidelines for the treatment of patients with delirium. Am J Psychiatry 1999;156(5 Suppl):1–20, updated 2004 [PubMed] [Google Scholar]
- 2.Lonergan E, Britton AM, Luxenberg J. Antipsychotics for delirium. Cochrane Database Syst Rev 2007;2:CD005594. [DOI] [PubMed] [Google Scholar]
- 3.van den Boogaard M, Schoonhoven L, van Achterberg T, et al. Haloperidol prophylaxis in critically ill patients with a high risk for delirium. Crit Care 2013; 17:R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirota T, Kishi T. Prophylactic antipsychotic use for postoperative delirium: a systematic review and meta-analysis. J Clin Psychiatry 2013;74:e1136–44 [DOI] [PubMed] [Google Scholar]
- 5.Information for Healthcare Professionals: Haloperidol. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm085203.htm (accessed 22 Jun 2013).
- 6.Drew BJ, Ackerman MJ, Funk M, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation 2010;121: 1047–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung D, Wolfe B, Wald H, et al. Unsafe use of intravenous haloperidol: evaluation of recommendation-concordant care among hospitalized elderly. J Am Geriatr Soc 2013;61:160–1 [DOI] [PubMed] [Google Scholar]
- 8.Nanji KC, Slight SP, Seger DL, et al. Overrides of medication-related clinical decision support alerts in outpatients. J Am Med Inform Assoc 2014;21:487–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiner JP, Kfuri T, Chan K, et al. e-Iatrogenesis: the most critical unintended consequence of CPOE and other HIT. J Am Med Inform Assoc 2007;14: 387–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kesselheim A, Cresswell K, Phansalkar S, et al. Clinical decision support systems could be modified to reduce ‘alert fatigue’ while still minimizing the risk of litigation. Health Affairs 2011;30:2310–17 [DOI] [PubMed] [Google Scholar]
- 11.Haugaa KH, Bos M, Tareell RF, et al. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc 2013;88:315–25 [DOI] [PubMed] [Google Scholar]
- 12.Muzyk AJ, Rivelli SK, Jiang W, et al. A computerized physician order entry set designed to improve safety of intravenous haloperidol utilization. Drug Saf 2012;35:725–31 [DOI] [PubMed] [Google Scholar]
- 13.Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA 1998;280:1311–16 [DOI] [PubMed] [Google Scholar]
- 14.Osheroff JA, Teich JA, Levick D. et al . Improving outcomes with clinical decision support: an implementer's guide. 2nd edn. Chicago, IL: HIMSS, 2012:15 [Google Scholar]
- 15.Meyer-Massetti C, Cheng CM, Sharpe BA, et al. The FDA extended warning for intravenous haloperidol and torsades de pointes: how should institutions respond? J Hosp Med 2010;5:E8–16 [DOI] [PubMed] [Google Scholar]
- 16.Karch BT. Clinical practice improvement and redesign: how change in workflow can be supported by clinical decision support. AHRQ Publication No. 09-0054-EF. Rockville, Maryland: Agency for Healthcare Research and Quality, 2009 [Google Scholar]
- 17.Phansalkar S, Wright A, Kuperman G, et al. Towards meaningful medication-related clinical decision support: recommendations for an initial implementation. Appl Clin Inf 2011;2:50–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bates DW, Cohen M, Leape LL, et al. Reducing the frequency of errors in medicine using information technology. J Am Med Inform Assoc 2001;8:299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]