The longer methicillin-resistant Staphylococcus aureus bacteremia (MRSAB) persists uncontrolled, the more pharmacotherapy is hindered. With rapid diagnostics, superior combination antimicrobial therapy, and the selective pressure consequences of prolonged in vivo persistence, MRSAB should clear within 3–4 days.
Keywords: MRSA bacteremia, vancomycin, daptomycin, rapid diagnostics, persistent bacteremia
Abstract
Persistent methicillin-resistant Staphylococcus aureus (MRSA) bacteremia (MRSAB) is associated with poor outcomes and serious complications. The MRSA guidelines define treatment failure and persistent bacteremia as lasting ≥7 days; however, this definition requires reevaluation. Aggressively reducing the bacterial inoculum promptly is critical because factors already in place before clinical presentation are driving resistance to the few antibiotics that are available to treat MRSAB. Alternative approaches to treat MRSAB should be considered within 3–4 days of persistent MRSAB. With rapid molecular diagnostics emerging in clinical microbiology laboratories and biomarkers as a potential for early patient risk stratification, a future shorter threshold may become possible.
Persistent methicillin-resistant Staphylococcus aureus (MRSA) bacteremia (MRSAB) is associated with serious complications, including prolonged hospitalization, increased morbidity, and high mortality [1]. Although the 2011 Infectious Diseases Society of America (IDSA) MRSA guidelines define persistent bacteremia and treatment failure as lasting ≥7 days [2], this definition is largely based on observational studies and expert opinion and warrants evaluation [3]. In fact, <1500 patients have been enrolled in randomized controlled trials specifically directed to investigate the treatment of MRSAB.
Recent scientific literature has described biologic mechanisms used by MRSA that emerge during prolonged bacteremia, allowing the organism to evade immunologic and antimicrobial killing. The emergence of immunologic evasion mechanisms and antimicrobial defenses affects MRSA killing by both pharmacotherapy and innate immunity, which may result in poor clinical outcomes [4]. In this article, we present evidence that the goal of MRSAB treatment should be clearance of the infection much sooner than the current suggested goal of 7 days. There are important consequences of waiting and watching prolonged MRSAB, even when patients seem clinically stable.
The objectives of this article are to (1) discuss the clinical significance of early clearance of MRSAB; (2) explain the biologic importance of early, effective treatment for MRSAB; and (3) identify novel and emerging treatment approaches, including rapid molecular diagnostics and biomarkers, to mitigate or prevent the emergence of these more resilient MRSA phenotypes.
PERSISTENT MRSAB POSES HIGH RISK OF COMPLICATIONS
Before examining the science behind the dangers of persistent MRSAB, it is important to review existing clinical evidence for documented risks of persistent MRSAB, which encompasses both relapse and persistently positive blood cultures. As the IDSA guidelines support, there are clear data indicating that persistent MRSAB past the threshold of 7 days is associated with poor clinical outcomes [2]. The probability of a metastatic infection increases with longer durations of bacteremia, to approximately 45% after ≥10 days of MRSAB [5]. Hawkins et al [1] showed that mortality rates for persistent MRSAB (defined as ≥7 days) were significantly higher than those of nonpersistent controls (54.8% and 31.4%, respectively; P < .01). In another investigation, the 30-day crude mortality rate of patients with persistent MRSAB (defined as ≥7 days) was more than 3-fold higher than for patients with nonpersistent MRSAB (58.1% vs 16.7%, respectively; P < .001), and the 30-day cumulative survival was significantly lower for patients with persistent MRSAB (41.9%) than for those with nonpersistent MRSAB (83.3%) [6]. Persistent MRSAB is highly associated with relapse, defined as return of MRSAB 2 weeks after negative blood cultures (odds ratio [OR] 10.1; 95% confidence interval [CI], 2.0–49.6) [7]; molecular typing of isolates demonstrated that recurrent isolates were identical to the primary bloodstream isolates in 91% of the patients [7].
However, MRSAB persisting for as few as 3 days on therapy has been associated with poor outcomes. Khatib et al [5] conducted a prospective observational study among 245 cases of S. aureus bacteremia (125 MRSA) in 234 patients. Persistence (defined as bacteremia ≥3 days) was identified in 49 of 125 MRSA cases (39.2%). Metastatic foci and complications were uncommon in patients with bacteremia for 1–2 days. However, they were significantly more common in those with bacteremia for 3 days and increased even more in patients with longer durations of bacteremia. Factors associated with duration of bacteremia included an endovascular source, vancomycin treatment, and metastatic infection. Another small retrospective study that also examined patient immunologic markers determined that ≥4 days of S. aureus bacteremia was associated with increased mortality [8]. Therefore, given the adverse clinical events documented in several independent studies, shortening the definition of persistent MRSAB to 3–4 days is a more sensitive breakpoint in the early detection of high-risk patients and providing alternative therapy.
THE INGREDIENTS OF ESTABLISHING THE “TREATMENT FAILURE PERFECT STORM” IN MRSAB
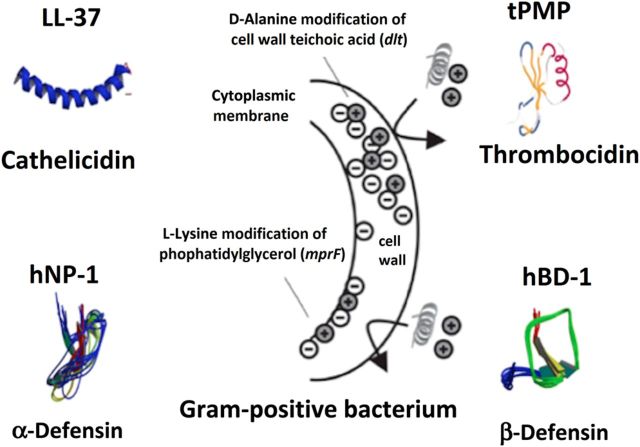
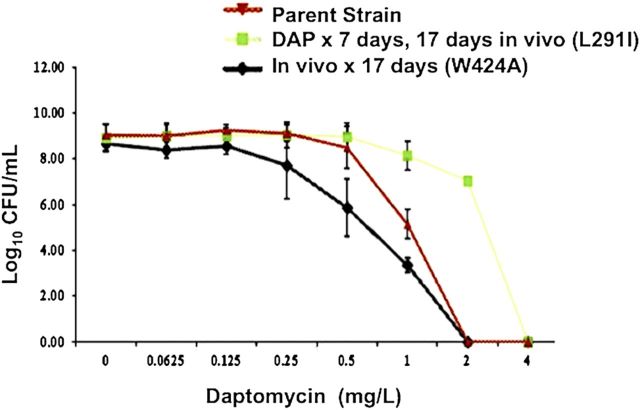
The first step in understanding the biologic importance of prompt treatment for MRSAB is appreciating that microorganisms and humans share the ability to produce antimicrobial molecules. The principle human antimicrobial molecules, cationic host defense peptides (HDPs), such as cathelicidins and thrombocidins, are fundamental components of the innate immune system [9]. In general, HDPs are electrostatically designed to attract to, bind to, and insert into bacterial surfaces that are relatively negatively charged, causing membrane disruption and subsequent bacterial death. Staphylococcus aureus has developed mechanisms of resistance to HDPs by making their surface more positively charged and changing membrane fluidity (Figure 1) [9, 10]. A noteworthy study of a rabbit model of osteomyelitis by Azmi et al [11] has shown the in vivo selection of daptomycin nonsusceptible MRSA without administration of any antibiotics (Figure 2). A key aspect of this study is that antimicrobial resistance without antibiotic exposure was a result of prolonged infection in the animals. It is believed that the antimicrobial peptides produced by the innate immune system of the animal triggered changes in membrane fluidity and charge, resulting in significant resistance to killing by daptomycin. The relationship between HDP resistance and antimicrobial defenses is not only limited to daptomycin. Vancomycin selective pressure in vitro and in vivo independently selects for resistance to HDP [4]. Thus, a patient receiving vancomycin during persistent uneradicated high-inoculum infection is anticipated to have the selective pressure imposed by HDPs compounded further. Experts on the MRSA treatment guidelines recognize this concern and recommend high-dose daptomycin therapy (10 mg/kg/d) in vancomycin treatment failure settings, possibly with combination therapy [2].
Figure 1.
Examples of cationic antimicrobial host defense peptides. Abbreviations: hBD-1, human beta-defensin-1; hNP-1, human neutrophil peptide-1; mprF, multiple peptide resistance factor; tPMP, thrombin-induced platelet microbicidal protein.
Figure 2.
Daptomycin population analysis of methicillin-resistant Staphylococcus aureus harvested from rabbit model of prosthetic joint infection with or without daptomycin therapy, with accompanying mprF mutations, compared with the baseline isolate. Abbreviations: CFU, colony forming units; DAP, daptomycin; mprF, multiple peptide resistance factor.
EMPLOYING RAPID DIAGNOSTICS AND BIOMARKERS TO SHORTEN MRSAB DURATION GOALS
Various investigators have identified delay in the initiation of appropriate antimicrobial therapy as an integral determinant of clinical outcomes for severe diseases, including MRSAB [12, 13]. A key hurdle to overcome in management of these serious infections is the inherent lapse of time required for growth and workup of bacteria from patient samples in the clinical microbiology laboratory. For example, in patients with MRSAB, there is a 48-hour minimum from the time a blood sample is obtained for culture to pathogen identification without use of recently available molecular-based testing. In a patient with established MRSAB, confirmation of blood sterilization may take 3–5 days. The importance of rapidly identifying an organism and treating the patient appropriately has been highlighted with the increased development and usage of rapid diagnostic tests, such as rapid polymerase chain reaction (rPCR) and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF). Bauer et al [14] evaluated the clinical outcomes of rPCR MRSA/S. aureus blood culture test combined with an Antimicrobial Stewardship Program (ASP) at The Ohio State University Wexner Medical Center. In the post-rPCR group, the mean delay in switching to the most effective therapy (from vancomycin to daptomycin) was 4.5 days, versus 10 days in the pre-rPCR MRSA group. Likewise, Huang et al [15] evaluated the impact of MALDI-TOF with an ASP in patients with bacteremia and candidemia. Overall, this intervention decreased the time to organism identification (84.0 vs 55.9 hours; P < .001), effective antibiotic therapy (30.1 vs 20.4 hours; P = .02), and optimal antibiotic therapy (90.3 vs 47.3 hours; P < .001), leading to decreased mortality rates (20.3% vs 14.5%; P = .02) and recurrent bacteremia (5.9% vs 2.0%; P < .001). At this time, MALDI-TOF is still limited to identification after blood culture bottle growth. This may explain why the delay to identification and appropriate antibiotic therapy, although improved from conventional methods, still often exceeds 24 hours on average.
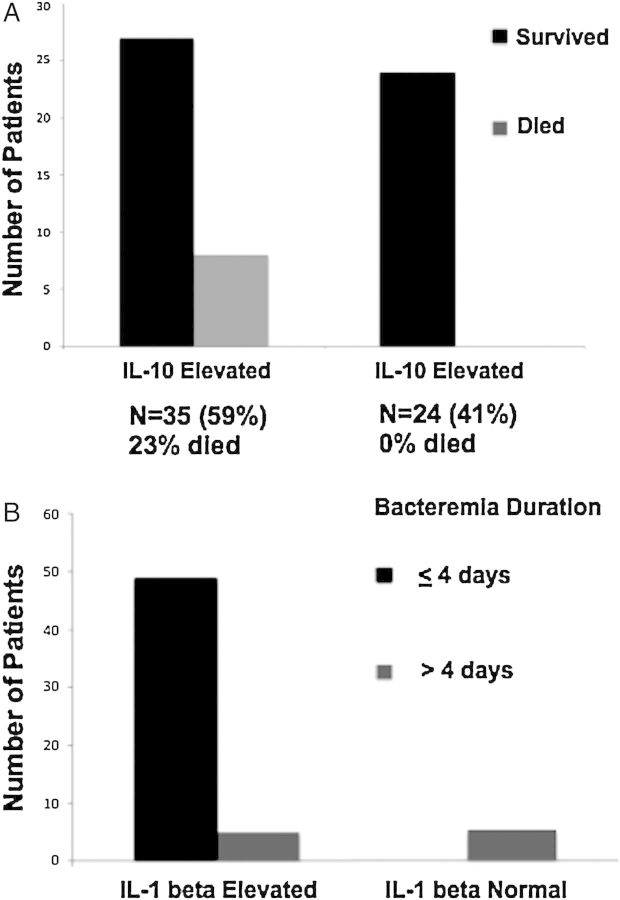
Despite rapid molecular methods to speciate and perform susceptibility testing, no method exists that risk-stratifies patients at the time of clinical presentation. However, recent data suggest that this is possible using biomarker cytokines produced by the patient innate immune system. In a study by Rose et al [8], elevated serum concentrations of the anti-inflammatory cytokine interleukin-10 measured by commercially available enzyme-linked immunosorbent assays identified all patients who died of S. aureus bacteremia. The mortality rate was 0% among 24 patients with normal interleukin 10 (IL-10) concentrations but 23% among 35 patients with elevated IL-10 concentrations on the day of admission (Figure 3A). In the same study, elevated interleukin 1β levels were associated with rapid bacteremia clearance (<4 days), and these levels were not elevated in patients with prolonged bacteremia (Figure 3B). Although the serum concentrations of interleukin 1β, IL-10, and other cytokines can currently be ordered by most clinicians through send-out reference laboratories, their incorporation into mainstream clinical practice would require these assays to be brought “in house” to hospital clinical laboratories, as is slowly being done with procalcitonin assays.
Figure 3.
A, Elevated serum interleukin 10 (IL-10) levels at time of presentation predict mortality rates in Staphylococcus aureus bacteremia. The mortality rate was 23% among 35 patients with elevated IL-10 levels versus 0% among 24 with normal levels (adapted from Rose et al [8]). B, Serum interleukin 1β (IL-1β) concentrations and S. aureus bacteremia duration (adapted from Rose et al [8]).
IMPORTANCE OF SOURCE CONTROL AND OPTIMIZATION OF PHARMACOTHERAPY FOR MRSAB
Before discussing pharmacotherapy, it is important to mention the crucial role of source control in the management of MRSAB. The failure to remove infected prosthetic devices and intravascular material has been highly correlated with recurrence. Fowler et al [16] revealed that among 244 patients with S. aureus bacteremia, 56% of the 23 patients from whom the infected devices were not removed experienced relapse of infection or death, compared with 16% of the 221 patients whose devices were removed or who did not have a device (P < .01). Yoon et al [17] showed that retention of implicated medical devices was an independent predictor of MRSAB persistence (OR, 10.35; 95% CI, 1.03–104.55). Accordingly, the IDSA MRSA guidelines recommend that clinicians identify the source and extent of infection and eliminate and/or debride other infection sites [2].
Therapeutic Options Superior to Vancomycin
Although vancomycin has been considered the standard therapy for MRSAB, it has also been associated with persistent MRSAB, including relapse. The slow clinical response observed with vancomycin was originally evaluated by Levine et al [3] more than 2 decades ago; 44 patients with MRSA infective endocarditis were randomly assigned to receive either vancomycin or vancomycin plus rifampin for 28 days. The median duration of bacteremia was 9 days (7 days for the vancomycin group and 9 days for the vancomycin plus rifampin group), and the median duration of fever was 7 days. Alternative therapies have been shown to be more beneficial clinically than vancomycin for MRSAB. The studies described below provide data in real-world clinical scenarios, showing that the comparison agent led to better clinical outcomes than vancomycin. Most of the studies compared vancymycin with daptomycin, the agent most commonly used after vancomycin therapy and recommended in the IDSA MRSA guidelines [2].
Murray et al [18] conducted a matched, retrospective study in 170 patients, comparing the clinical effectiveness of daptomycin versus vancomycin for MRSAB with a vancomycin minimum inhibitory concentration (MIC) >1 mg/L. The primary outcome was clinical failure, defined as 30-day mortality or bacteremia persisting for ≥7 days. Clinical failure at 30 days was significantly lower in the daptomycin than in the vancomycin arm (20.0% vs 48.2%, respectively; P < .001). Furthermore, both the 30-day mortality and persistent bacteremia rates were significantly lower in the daptomycin group than in the vancomycin group (mortality, 3.5% vs 12.9% [P = .047]; persistent bacteremia, 18.8% vs 42.4% [P = .001]). Logistic regression analysis confirmed the association between vancomycin treatment and the increased risk of clinical failure (adjusted OR, 4.5; 95% CI, 2.1–9.8).
Kullar et al [19] conducted a 2-phase quasi-experimental study in 170 patients with MRSAB susceptible to vancomycin; phase I included 70 patients with initial blood MRSA isolates exhibiting vancomycin MICs >1 mg/L and treated with vancomycin, and phase II included 100 patients who were switched to daptomycin after initial vancomycin therapy, according to the institutional treatment pathway. The clinical success rate was 35% higher in phase II (75.0% vs 41.4% for phase I; P < .001). The most frequent component of clinical failure in both phases was ≥ 7 days of bacteremia, however rates of persistent bacteremia were significantly higher in patients in phase I vs phase II (44.3% vs 21%; P < .001). Treatment during phase I was independently associated with failure (adjusted OR, 4.37; 95% CI, 1.68–6.76; P < .001), and hospital readmission rates were significantly higher (33% vs 21% for phase II; P = .08). Moore et al [20] conducted a similar study and found that vancomycin treatment was independently associated with clinical failure (OR, 3.13; 95%, CI, 1.00–9.76). A comparison of 60-day mortality between vancomycin- and daptomycin-treated patients revealed a higher probability of survival in the daptomycin-treated group (P = .02). McDaneld et al [21] conducted a meta-analysis of 7 retrospective clinical studies evaluating the clinical outcomes of daptomycin used as first-line or salvage therapy for MRSA infections, compared with vancomycin. Daptomycin therapy in bacteremic patients led to lower 60-day (8% vs 20%; P = .046) and 30-day (3.5% vs 12.9%; P = .047) mortality rates and increased treatment success (68.6% vs 43.1% [P = .008]).
Jang et al [22] evaluated linezolid salvage therapy with or without ertapenem versus salvage therapy with vancomycin plus gentamicin or rifampin in 35 patients with persistent MRSAB. The early microbiologic response (ie, negative blood cultures within 72 hours) was significantly increased in the linezolid-based salvage therapy compared with the vancomycin group (75% vs 17%, respectively; P = .006). Notably, the S. aureus–related mortality rate was lower for patients treated with linezolid salvage regimens than for those continually treated with vancomycin-based regimens (13% vs 53%; P = .03).
Combination Therapy
Data on the use of vancomycin in combination with other antimicrobials were recently reviewed [23]. Although combination therapy with vancomycin is common, the published data supporting the addition of gentamicin, rifampin, or other agents to vancomycin are limited, and such combinations may cause harm and are not currently recommended. Evidence from several in vitro pharmacokinetic/pharmacodynamic models has shown the benefit of combining daptomycin with other antimicrobials to prevent emergence of resistance and improve killing [24]. Three independent animal models of soft tissue infection, endocarditis, and osteomyelitis have demonstrated that daptomycin combined with rifampin improved S. aureus clearance and prevented daptomycin resistance compared with daptomycin monotherapy [25–27]. Moreover, high-dose daptomycin therapy (human equivalent, 10 mg/kg/d) has shown promise in rabbit endocarditis models for the prevention and treatment of daptomycin-resistant infections [28] and is recommended in the MRSA treatment guidelines for patients in whom vancomycin therapy has failed [2].
One novel approach to treating persistent MRSAB has entailed the combination of daptomycin with β-lactams [29]. In vitro analyses have shown that β-lactam antibiotics affect the surface charge of MRSA, yielding better daptomycin binding, and results in synergistic killing [29]. β-Lactams with penicillin-binding protein 1 enhance daptomycin anti-MRSA activity the most [30]. Data from the Cubicin Outcomes Registry and Experience have also suggested that some patients treated with daptomycin in combination with a β-lactam show a trend toward improved outcomes, compared with those receiving daptomycin monotherapy, particularly for bacteremia involving presumed or confirmed endocarditis and osteomyelitis, with a lack of benefit in soft-tissue infections [31]. It is becoming increasingly apparent that β-lactam antibiotics increase the vulnerability of MRSA to killing not just by daptomycin but also by the human innate immune system. Daptomycin and vancomycin do not share this property [32], which may explain the historical difference in bacteremia duration for MRSA (8–9 days) versus methicillin-susceptible S. aureus bacteremia (3–4 days) [33]. Thus, the combination of β-lactams with daptomycin or vancomycin warrants further investigation in clinical trials.
Ceftaroline
Among β-lactam antibiotics, ceftaroline is the only available agent with in vitro and in vivo MRSA activity [34]. Casapao et al [35] evaluated the effectiveness and safety of ceftaroline in patients, including those with MRSAB. A total of 527 patients were included in the retrospective study; 67% were treated for off-label indications, and 148 (28%) had bacteremia. Most patients (80%) were switched to ceftaroline as salvage therapy, and clinical success was achieved in 88% (426 of 484 patients). The lack of multivariable analysis for patients with MRSAB makes it difficult to interpret these findings. Further research on ceftaroline monotherapy for MRSAB is needed, because clinical uses for this treatment are increasing.
Ceftaroline has a fairly short half-life, and a higher dosage of 600 mg intravenously every 8 hours may be required to achieve sufficient percentage of time above MIC (%T > MIC) for difficult infections [36]. In a review of ceftaroline treatment for MRSA infective endocarditis and deep-seated infections [37], ceftaroline was given nearly exclusively at the higher dose of 600 mg intravenously every 8 hours. In a larger review by Casapao et al [35], most patients (86%) were given the approved dose of 600 mg intravenously every 12 hours. There are limited safety data on the use of ceftaroline at 600 mg intravenously every 8 hours, but pharmacokinetic and limited clinical experience suggests that this may be an appropriate approach for complicated MRSAB.
Ceftaroline has also shown positive results in combination with daptomycin for MRSAB. Rose et al [38] showed that daptomycin plus ceftaroline as initial combination therapy for MRSAB resulted in rapid and sustained bactericidal activity and prevented daptomycin resistance. In the largest clinical study evaluating the use of daptomycin plus ceftaroline [39], 26 cases of sustained staphylococcal bacteremia (20 MRSA, 2 vancomycin-intermediate S. aureus, 2 methicillin-susceptible S. aureus, 2 methicillin-resistant Staphylococcus epidermidis) were treated successfully with the combination therapy. In vitro analyses of select isolates from these patients demonstrated ceftaroline-daptomycin synergy, accompanied by increased daptomycin surface binding and increased vulnerability to innate immunity killing of MRSA induced by ceftaroline. Bacteremia cleared in a median of 2 days after daptomycin plus ceftaroline was started, after persisting a median of 10 days before initiation of this salvage regimen. Despite clinical data limited to the above findings, the current Sanford Guide recommends using daptomycin plus ceftaroline for the treatment of refractory MRSAB, including cases due to vancomycin-intermediate S. aureus.
CONCLUSIONS
We have reviewed the strands of clinical and basic science evidence in the literature pointing to a potentially catastrophic microbiologic situation that unfolds in high-inoculum endovascular MRSA infections. The longer MRSAB persists uncontrolled, the greater the more pharmacotherapy is hindered. In recent years, because of the emerging threat of multidrug-resistant pathogens in the setting of dwindling novel antibiotic resources, ASP has taken center stage as a way physicians and pharmacists can work together to minimize patient antibiotic exposure, streamline antimicrobial therapy, improve patient outcomes, and reduce the emergence of antibiotic resistance. Given that the in vivo environment is not an antibiotic-free world but rather an environment in which HDPs produced by the innate immune system are produced and select bacterial fitness, prompt eradication of infection and reduced exposure of MRSA to HDP-driven antibiotic resistance is critical to ASPs.
Although clinical data are lacking, we recommend that the 7-day threshold to seeking alternative combination antibiotic therapy be shortened to 3–4 days. Aggressive source control is vital in this approach. With molecular diagnostics slowly emerging in clinical microbiology laboratories and biomarkers showing potential for early patient risk stratification, an even shorter threshold may be possible. Clinical outcomes studies evaluating these measures are warranted.
Note
Potential conflicts of interest. R. K. is employed by Cubist Pharmaceuticals and owns Cubist Pharmaceuticals stock; J. A. M has received research grant support to his institution from Pfizer and Cubist; and G. S. has received speaking honoraria from Cubist, Forest, and Novartis Pharmaceuticals, consulting fees from Cubist and Forest Pharmaceuticals, and research grant support from Forest Pharmaceuticals.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hawkins C, Huang J, Jin N, et al. Persistent Staphylococcus aureus bacteremia: an analysis of risk factors and outcomes. Arch Intern Med. 2007;167:1861–7. doi: 10.1001/archinte.167.17.1861. [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285–92. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 3.Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115:674–80. doi: 10.7326/0003-4819-115-9-674. [DOI] [PubMed] [Google Scholar]
- 4.Sakoulas G, Eliopoulos GM, Fowler VG, Jr, et al. Reduced susceptibility of Staphylococcus aureus to vancomycin and platelet microbicidal protein correlates with defective autolysis and loss of accessory gene regulator (agr) function. Antimicrob Agents Chemother. 2005;49:2687–92. doi: 10.1128/AAC.49.7.2687-2692.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khatib R, Johnson LB, Fakih MG, et al. Persistence in Staphylococcus aureus bacteremia: incidence, characteristics of patients and outcome. Scand J Infect Dis. 2006;38:7–14. doi: 10.1080/00365540500372846. [DOI] [PubMed] [Google Scholar]
- 6.Ok HS, Lee HS, Park MJ, et al. Predictors and clinical outcomes of persistent methicillin-resistant Staphylococcus aureus bacteremia: a prospective observational study. Korean J Intern Med. 2013;28:678–86. doi: 10.3904/kjim.2013.28.6.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsh KJ, Skrobarcek KA, Abbott AN, et al. Predictors of relapse of methicillin-resistant Staphylococcus aureus bacteremia after treatment with vancomycin. J Clin Microbiol. 2011;49:3669–72. doi: 10.1128/JCM.05287-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose WE, Eickhoff JC, Shukla SK, et al. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis. 2012;206:1604–11. doi: 10.1093/infdis/jis552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nizet V. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr Issues Mol Biol. 2006;8:11–26. [PubMed] [Google Scholar]
- 10.Mishra NN, McKinnell J, Yeaman MR, et al. In vitro cross-resistance to daptomycin and host defense cationic antimicrobial peptides in clinical methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2011;55:4012–8. doi: 10.1128/AAC.00223-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azmi S, Srivastava S, Mishra NN, et al. Characterization of antimicrobial, cytotoxic, and antiendotoxin properties of short peptides with different hydrophobic amino acids at “a” and “d” positions of a heptad repeat sequence. J Med Chem. 2013;56:924–39. doi: 10.1021/jm301407k. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 13.Lodise TP, McKinnon PS, Swiderski L, et al. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis. 2003;36:1418–23. doi: 10.1086/375057. [DOI] [PubMed] [Google Scholar]
- 14.Bauer KA, West JE, Balada-Llasat JM, et al. An antimicrobial stewardship program's impact with rapid polymerase chain reaction methicillin-resistant Staphylococcus aureus/S. aureus blood culture test in patients with S. aureus bacteremia. Clin Infect Dis. 2010;51:1074–80. doi: 10.1086/656623. [DOI] [PubMed] [Google Scholar]
- 15.Huang AM, Newton D, Kunapuli A, et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis. 2013;57:1237–45. doi: 10.1093/cid/cit498. [DOI] [PubMed] [Google Scholar]
- 16.Fowler VG, Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis. 1998;27:478–86. doi: 10.1086/514686. [DOI] [PubMed] [Google Scholar]
- 17.Yoon YK, Kim JY, Park DW, et al. Predictors of persistent methicillin-resistant Staphylococcus aureus bacteraemia in patients treated with vancomycin. J Antimicrob Chemother. 2010;65:1015–8. doi: 10.1093/jac/dkq050. [DOI] [PubMed] [Google Scholar]
- 18.Murray KP, Zhao JJ, Davis SL, et al. Early use of daptomycin versus vancomycin for methicillin-resistant Staphylococcus aureus bacteremia with vancomycin minimum inhibitory concentration >1 mg/L: a matched cohort study. Clin Infect Dis. 2013;56:1562–9. doi: 10.1093/cid/cit112. [DOI] [PubMed] [Google Scholar]
- 19.Kullar R, Davis SL, Kaye KS, et al. Implementation of an antimicrobial stewardship pathway with daptomycin for optimal treatment of methicillin-resistant Staphylococcus aureus bacteremia. Pharmacotherapy. 2013;33:3–10. doi: 10.1002/phar.1220. [DOI] [PubMed] [Google Scholar]
- 20.Moore CL, Osaki-Kiyan P, Haque NZ, et al. Daptomycin versus vancomycin for bloodstream infections due to methicillin-resistant Staphylococcus aureus with a high vancomycin minimum inhibitory concentration: a case-control study. Clin Infect Dis. 2012;54:51–8. doi: 10.1093/cid/cir764. [DOI] [PubMed] [Google Scholar]
- 21.McDaneld PM, Spooner LM, Mohr JF, et al. Use of daptomycin to treat infections with methicillin-resistant Staphylococcus aureus isolates having vancomycin minimum inhibitory concentrations of 1.5 to 2 μg/mL. Ann Pharmacother. 2013;47:1654–65. doi: 10.1177/1060028013508272. [DOI] [PubMed] [Google Scholar]
- 22.Jang HC, Kim SH, Kim KH, et al. Salvage treatment for persistent methicillin-resistant Staphylococcus aureus bacteremia: efficacy of linezolid with or without carbapenem. Clin Infect Dis. 2009;49:395–401. doi: 10.1086/600295. [DOI] [PubMed] [Google Scholar]
- 23.Deresinski S. Vancomycin in combination with other antibiotics for the treatment of serious methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2009;49:1072–9. doi: 10.1086/605572. [DOI] [PubMed] [Google Scholar]
- 24.Bayer AS, Schneider T, Sahl HG. Mechanisms of daptomycin resistance in Staphylococcus aureus: role of the cell membrane and cell wall. Ann N Y Acad Sci. 2013;1277:139–58. doi: 10.1111/j.1749-6632.2012.06819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saleh-Mghir A, Muller-Serieys C, Dinh A, et al. Adjunctive rifampin is crucial to optimizing daptomycin efficacy against rabbit prosthetic joint infection due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55:4589–93. doi: 10.1128/AAC.00675-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cirioni O, Mocchegiani F, Ghiselli R, et al. Daptomycin and rifampin alone and in combination prevent vascular graft biofilm formation and emergence of antibiotic resistance in a subcutaneous rat pouch model of staphylococcal infection. Eur J Vasc Endovasc Surg. 2010;40:817–22. doi: 10.1016/j.ejvs.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Lefebvre M, Jacqueline C, Amador G, et al. Efficacy of daptomycin combined with rifampicin for the treatment of experimental methicillin-resistant Staphylococcus aureus (MRSA) acute osteomyelitis. Int J Antimicrob Agents. 2010;36:542–4. doi: 10.1016/j.ijantimicag.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Sakoulas G, Eliopoulos GM, Alder J, et al. Efficacy of daptomycin in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:1714–8. doi: 10.1128/AAC.47.5.1714-1718.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhand A, Bayer AS, Pogliano J, et al. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin Infect Dis. 2011;53:158–63. doi: 10.1093/cid/cir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berti AD, Sakoulas G, Nizet V, et al. beta-Lactam antibiotics targeting PBP1 selectively enhance daptomycin activity against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2013;57:5005–12. doi: 10.1128/AAC.00594-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moise PA, Amodio-Groton M, Rashid M, et al. Multicenter evaluation of the clinical outcomes of daptomycin with and without concomitant beta-lactams in patients with Staphylococcus aureus bacteremia and mild to moderate renal impairment. Antimicrob Agents Chemother. 2013;57:1192–200. doi: 10.1128/AAC.02192-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakoulas G, Okumura CY, Thienphrapa W, et al. Nafcillin enhances innate immune-mediated killing of methicillin-resistant Staphylococcus aureus. J Mol Med (Berl) 2014;92:139–49. doi: 10.1007/s00109-013-1100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fowler VG, Jr, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355:653–65. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 34.Friedland HD, O'Neal T, Biek D, et al. CANVAS 1 and 2: analysis of clinical response at day 3 in two phase 3 trials of ceftaroline fosamil versus vancomycin plus aztreonam in treatment of acute bacterial skin and skin structure infections. Antimicrob Agents Chemother. 2012;56:2231–6. doi: 10.1128/AAC.05738-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casapao AM, Davis SL, Barr VO, et al. A large retrospective evaluation of the effectiveness and safety of ceftaroline fosamil therapy. Antimicrob Agents Chemother. 2014;58:2541–6. doi: 10.1128/AAC.02371-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steed ME, Rybak MJ. Ceftaroline: a new cephalosporin with activity against resistant gram-positive pathogens. Pharmacotherapy. 2010;30:375–89. doi: 10.1592/phco.30.4.375. [DOI] [PubMed] [Google Scholar]
- 37.Lin JC, Aung G, Thomas A, et al. The use of ceftaroline fosamil in methicillin-resistant Staphylococcus aureus endocarditis and deep-seated MRSA infections: a retrospective case series of 10 patients. J Infect Chemother. 2013;19:42–9. doi: 10.1007/s10156-012-0449-9. [DOI] [PubMed] [Google Scholar]
- 38.Rose WE, Schulz LT, Andes D, et al. Addition of ceftaroline to daptomycin after emergence of daptomycin-nonsusceptible Staphylococcus aureus during therapy improves antibacterial activity. Antimicrob Agents Chemother. 2012;56:5296–302. doi: 10.1128/AAC.00797-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakoulas G, Moise PA, Casapao AM, et al. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin Ther. 2014 doi: 10.1016/j.clinthera.2014.05.061. doi:10.1016/j.clinthera.2014.05.061. [DOI] [PubMed] [Google Scholar]