Abstract
Background. Diabetes mellitus type 2 (DM) is known to be a major risk factor for the development of active tuberculosis, although its influence on latent Mycobacterium tuberculosis infection (hereafter, “latent infection”) remains poorly characterized.
Methods. We examined circulating plasma cytokine levels in individuals with latent infection with DM or pre-DM (ie, intermediate hyperglycemia) and compared them to levels in patients with latent infection and normal glycemic control.
Results. In persons with DM or pre-DM, latent infection is characterized by diminished circulating levels of type 1 (interferon γ, interleukin 2, and tumor necrosis factor α) and type 17 (interleukin 17F) cytokines. This was associated with decreased systemic levels of other proinflammatory cytokines (interleukin 1β and interleukin 18) and the antiinflammatory cytokine interleukin 10 but not with decreased systemic levels of type 2 cytokines. Moreover, latently infected individuals with DM had diminished levels of spontaneous and M. tuberculosis antigen–specific levels of type 1 and type 17 cytokines when antigen-stimulated whole blood was examined. Finally, there was no significant correlation between the levels of any of the cytokines measured (with the exception of interleukin 22) with hemoglobin A1c levels.
Conclusions. Our data reveal that latent infection in the presence of DM or pre-DM, is characterized by diminished production of cytokines, implicated in the control of M. tuberculosis activation, allowing for a potential immunological mechanism that could account for the increased risk of active tuberculosis in latently infected individuals with DM.
Keywords: bacterial, cytokines, diabetes, pre-diabetes, tuberculosis
The long-held association between tuberculosis and diabetes mellitus type 2 (DM) [1] has been given additional credence by a recent meta-analysis in which DM was shown to have a 3-fold increased risk for the development of active pulmonary tuberculosis [2]. In addition, DM is also known to be associated with a greater severity of tuberculosis, with a greater odds of cavitation, increased bacillary loads, delayed sputum conversion, treatment failure, relapse, and death [1, 3]. Despite the clinical and public health significance posed by the dual burden of tuberculosis and DM, very little is known about the immunological and biochemical mechanisms underlying this susceptibility.
Enhanced susceptibility to tuberculosis in patients with DM has been ascribed to several factors, including direct effects related to hyperglycemia and insulin resistance and indirect effects related to macrophage and lymphocyte function [1, 3, 4]. Pre-DM (ie, intermediate hyperglycemia) is a high-risk state for DM that is defined by levels of glycemic variables that are higher than normal but lower than thresholds for DM. Pre-DM is also associated with the presence of insulin resistance and B-cell dysfunction—abnormalities that start before changes in blood glucose levels become detectable [5]. While a number of studies have examined the interaction of DM with active tuberculosis [3, 4], very few studies have examined the interaction of DM (and its pre-DM precursor) with latent Mycobacterium tuberculosis infection (hereafter, “latent infection”). Whether diabetes hastens the progression to active tuberculosis by increasing the risk of latent infection in healthy individuals or by increasing the risk of progression to active tuberculosis in latently infected individuals is not known. Moreover, the impact of diabetes or pre-DM on the innate and adaptive immune responses to mycobacteria-derived antigens in latent infection is also not known.
Since type 1 and type 17 cytokines and the interleukin 1 (IL-1) family of cytokines are known to influence susceptibility to tuberculosis in both humans and in animal models [6, 7], we postulated that diabetes and/or pre-DM could perturb the normal homeostatic levels of these cytokines in latent infection. To this end, we compared levels of a panel of type 1, type 17, IL-1–family, and other relevant proinflammatory cytokines in individuals with latent infection and coincident DM or pre-DM to levels in individuals with latent infection but without DM. We also examined the influence of DM or pre-DM on M. tuberculosis antigen–specific cytokine production. Our data show both DM and pre-DM alter the levels of many proinflammatory cytokines at homeostasis and in response to M. tuberculosis antigens in latent infection and provides evidence for the potential contribution of impaired glucose tolerance to the increased risk for progression to active tuberculosis. Finally, our data also show that, among individuals with DM or pre-DM, those with latent infection exhibit significantly higher circulating levels of most proinflammatory cytokines, compared with those without latent infection.
MATERIALS AND METHODS
Study Population
We studied a group of 90 individuals with latent infection, 30 of whom had DM, 30 of whom had pre-DM, and 30 of whom had no DM. We also studied another 60 individuals without latent infection as controls (30 with DM and 30 without DM). Latent infection was diagnosed on the basis of positive results of the Mantoux skin test (induration diameter, >12 mm), using 2 tuberculin units of purified protein derivative (Statens Serum Institute), and positive results of the Quantiferon In-Tube Gold assay, with no chest symptoms of tuberculosis and normal chest radiograph findings [8]. DM and pre-DM were defined on the basis of glycated hemoglobin (HbA1c) percentages, using American Diabetes Association criteria (DM, > 6.5%; pre-DM, 5.7%–6.4%) [9]. All the individuals were human immunodeficiency virus negative. All individuals were naive to antituberculosis treatment. Anthropometric measurements, including height, weight, and waist circumference, and biochemical parameters, including plasma glucose level, lipid profiles, and HbA1c level, were obtained using standardized techniques as detailed elsewhere [10]. Hematologic analysis was performed on all individuals, using the Act-5 Diff hematology analyzer (Beckman Coulter). All individuals were examined as part of a clinical protocol approved by the institutional review boards of the National Institutes of Allergy and Infectious Diseases and the National Institute of Research in Tuberculosis, and informed written consent was obtained from all participants.
Plasma Enzyme-Linked Immunosorbent Assay (ELISA)
Plasma cytokine and chemokine levels were measured using a Bioplex multiplex cytokine assay system (Bio–Rad, Hercules, CA). The parameters analyzed were interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), interleukin 2 (IL-2), interleukin 17A (IL-17A), interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 13 (IL-13), interleukin 10 (IL-10), interleukin 6 (IL-6), interleukin 12p70 (IL-12p70), and granulocyte macrophage colony-stimulating factor (GM-CSF). Plasma levels of transforming growth factor β1 (TGF-β1; total level − acid-activated level), IL-1α, IL-1β, interleukin 18 (IL-18; all R&D Systems), IL-17F (Biolegend), and interleukin 22 (IL-22; ebioscience) were measured by ELISA. The lowest detection limits for the various cytokines were as follows: IL-1α, 2.4 pg/mL; IL-1β, 2.7 pg/mL; IL-2, 1.16 pg/mL; IL-4, 0.3 pg/mL; IL-5, 2.08 pg/mL; IL-6, 2.31 pg/mL; IL-10, 2.2 pg/mL; IL-12, 2.78 pg/mL; IL-13, 2.22 pg/mL; IL-17A, 2.57 pg/mL; IL-17F, 1.2 pg/mL; IL-22, 2.2 pg/mL; IL-18, 2.1 pg/mL; IFN-γ, 2.14 pg/mL; TNF-α, 4.89 pg/mL; and TGF-β, 7.8 pg/mL.
Quantiferon Supernatant ELISA
Whole blood specimens from a subset of latently infected with or without DM (22 per group) were incubated with no antigen, with M. tuberculosis antigens (ESAT-6, CFP-10, and TB 7.7), or with mitogen (phytohemagglutinin), using the Quantiferon In-Tube Gold kit. The unstimulated or M. tuberculosis antigen–stimulated whole-blood supernatants were then used to analyze the levels of IFN-γ, TNF-α, IL-17A, IL-10, and IL-1β, using the DuoSet ELISA kit (R&D systems).
Statistical Analysis
Geometric means were used for measurements of central tendency. Statistically significant differences between 3 groups were analyzed using the Kruskal–Wallis test with Dunn multiple comparisons, and those between 2 groups were analyzed using the nonparametric Mann–Whitney U test. Multiple comparisons were corrected using the Holm correction. Correlations were calculated using Spearman rank correlation. Multivariate linear models were built to test the association between the cytokine levels and the independent variables, including age, sex, lipid profile, and random blood glucose level. Analyses were performed using GraphPad Prism, version 5.01, or R, version 2.15.2.
RESULTS
Study Population Characteristics
The baseline characteristics, including demographic characteristics, clinical features, and biochemical features of the study population are shown in Table 1. As can be seen, compared with latently infected individuals without DM, individuals with latent infection and DM had higher fasting blood glucose, HbA1c, serum cholesterol, low density lipoprotein (LDL), and triglycerides levels but lower high-density lipoprotein (HDL) cholesterol levels. Similarly, compared with latently infected individuals without DM, latently infected individuals with pre-DM had higher HbA1c, serum cholesterol, LDL, and triglycerides levels but lower HDL cholesterol levels. Interestingly, the lipid profiles of latently infected individuals with DM and those with pre-DM did not differ significantly. The groups did not significantly differ in their baseline hematological parameters (data not shown) or in results of hepatic or renal function tests (Table 1). Finally, multivariate logistic regression analysis did not reveal any effect of age, sex, or serum lipid profile on the cytokine levels in the 3 groups (data not shown).
Table 1.
Demographic and Clinical Characteristics of the Study Population With or Without Latent Mycobacterium tuberculosis Infection
| Characteristic | Latent Infection, Plus |
No Latent Infection, Plus |
|||
|---|---|---|---|---|---|
| DM (n = 30) | Pre-DM (n = 30) | No DM (n = 30) | DM (n = 30) | No DM (n = 30) | |
| Male sex | 17 | 11 | 11 | 14 | 12 |
| Age, y, median (range) | 45.5 (28–65) | 43.3 (23–65) | 33.7 (19–60) | 45 (21–64) | 35.5 (20–54) |
| Height, m, median (range) | 1.565 (1.35–1.84) | 1.53 (1.37–1.76) | 1.535 (1.4–1.79) | 1.56 (1.35–1.79) | 1.52 (1.39–1.78) |
| Weight, kg, median (range) | 61 (50–99) | 58 (34–87) | 52.5 (28–92) | 58 (28–92) | 62 (34–87) |
| Body mass indexa | 25.89 (19.92–37.39) | 24.39 (15.50–31.11) | 22.55 (12.28–33.33) | 23.49 (12.28–30.85) | 26.14 (16.11–33.33) |
| Mantoux skin test, induration diameter, mmb | >12 | >12 | >12 | <12 | <12 |
| IFN-γ–release assay result | Positive | Positive | Positive | Negative | Negative |
| Random blood glucose level, mg/dL | 200 (71–537) | 101 (60–226) | 94 (64–139) | 144 (100–370) | 87 (71–128) |
| Glycated Hb level, % | 8.92 (6.53–13.13) | 6.2 (6.07–6.38) | 5.1 (4.57–5.36) | 7.90 (6.53–11.74) | 5.24 (4.63–5.42) |
| AST level, U/L | 24.4 (14–46) | 23.7 (12–58) | 22.1 (10–31) | 24.5 (13–50) | 21 (11–41) |
| ALT level, U/L | 23.7 (10–67) | 20.8 (10–77) | 15.8 (6–35) | 22 (10–67) | 15 (9–34) |
| Urea level, mg/dL | 22.7 (15–49) | 20.3 (12–35) | 19 (11–30) | 20.5 (11–38) | 20.5 (8–38) |
| Creatinine level, mg/dL | 0.83 (0.6–1.3) | 0.73 (0.5–1.1) | 0.77 (0.3–0.9) | 0.75 (0.6–1.2) | 0.8 (0.3–1.2) |
Data are geometric means (range), unless otherwise indicated.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; DM, diabetes mellitus type 2; Hb, hemoglobin; IFN-γ, interferon γ.
a Defined as the weight in kilograms divided the height in square meters.
b An induration diameter of >12 mm was considered positive.
Latently Infected Individuals With DM and Those With Pre-DM Exhibit Diminished Circulating Levels of Type 1, Type 17, and IL-1–Family Cytokines
Since type 1 and type 17 are known to be important in resistance to tuberculosis [7, 11], we determined the influence of DM or pre-DM on type 1 and type 17 cytokines in latent infection by measuring the circulating levels of IFN-γ, TNF-α, IL-2, IL-17A, IL-17F, and IL-22 in latently infected individuals with DM (n = 30), with pre-DM (n = 30), and with no DM (n = 30). The geometric mean systemic levels of all 3 type 1 cytokines were significantly lower in latently infected individuals with DM and those with pre-DM, compared with those without DM (for IFN-γ, 557.3 pg/mL and 589.1 pg/mL, respectively, compared with 963.8 pg/mL; for TNF-α, 377.7 pg/mL and 362 pg/mL, respectively, compared with 490 pg/mL; and for IL-2, 58.2 pg/mL and 64.4 pg/mL, respectively, compared with 90.2 pg/mL; Figure 1A). Similarly, geometric mean systemic levels of IL-17F in latently infected individuals with DM and those with pre-DM were also significantly lower than those in latently infected individuals without DM (26.2 pg/mL and 22.9 pg/mL, respectively, compared with 40.9 pg/mL). In contrast, geometric mean IL-22 levels were significantly higher in latently infected individuals with DM, compared with those with pre-DM and those without DM (273 pg/mL, compared with 33.6 pg/mL and 83.4, respectively). Thus, latent infection with diabetes or pre-DM is associated with diminished levels of type 1 and type 17 cytokines at homeostasis.
Figure 1.
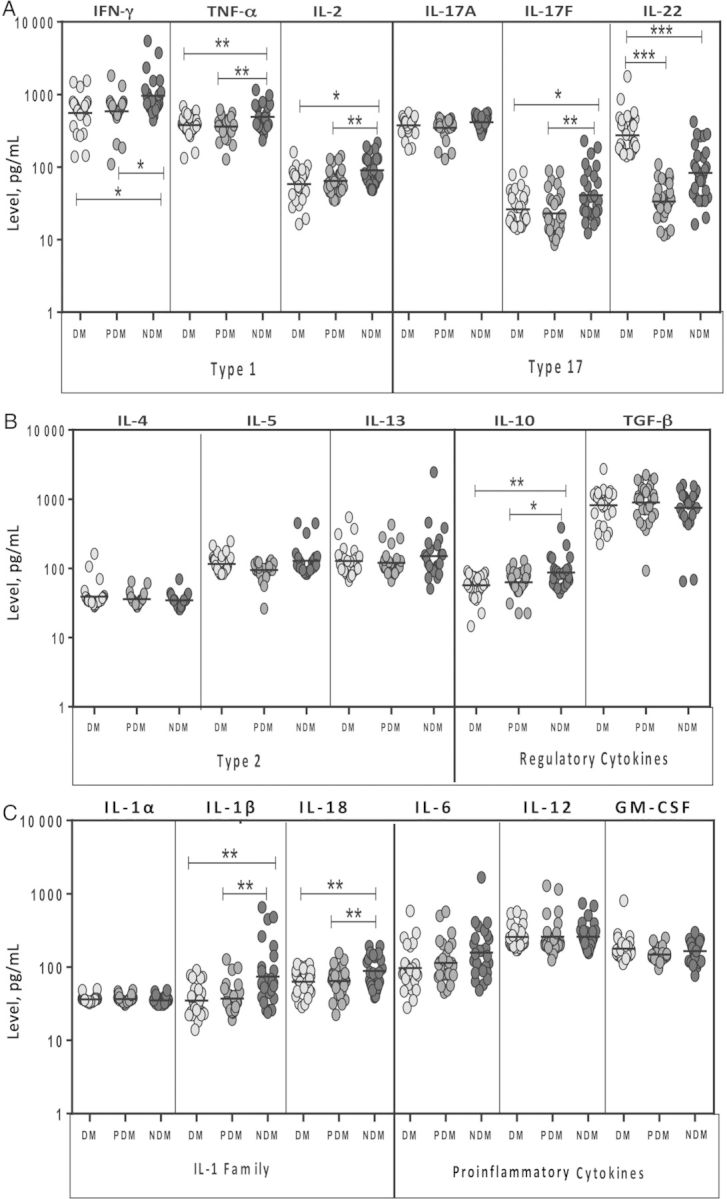
Systemic levels of type 1 (interferon γ [IFN-γ], tumor necrosis factor α [TNF-α], and interleukin 2 [IL-2]; A), type 17 (interleukin 17A [IL-17A], IL-17F, and interleukin 22 [IL-22]; A), type 2 (interleukin 4 [IL-4], interleukin 5 [IL-5], and interleukin 13 [IL-13]; B), regulatory (interleukin 10 [IL-10] and transforming growth factor β [TGF-β]; B), interleukin 1 (IL-1)–family (IL-1α, IL-1β, and interleukin 18 [IL-18]; C), and other proinflammatory (interleukin 6 [IL-6], interleukin 12 [IL-12], and granulocyte-macrophage colony-stimulating factor [GM-CSF]; C) cytokines in individuals with latent Mycobacterium tuberculosis infection concomitant with diabetes mellitus type 2 (DM; n = 30), concomitant with pre-DM (n = 30), or without DM (n = 30). Cytokine levels were measured by enzyme-linked immunosorbent assay. Each circle represents a single individual, and each bar represents the geometric mean. P values were calculated using the Kruskal–Wallis test with Dunn multiple comparisons. *P < .05, **P < .01, and ***P < .001.
Since type 2 and regulatory cytokines influence the progression to active tuberculosis [7], we sought to determine the influence of DM or pre-DM on type 2 and regulatory cytokines in latent infection and measured the plasma levels of IL-4, IL-5, IL-13, IL-10, and TGF-β in the 3 groups. As shown in Figure 1B, the geometric mean systemic levels of type 2 cytokines—IL-4, IL-5, and IL-13—were not significantly different between latently infected individuals with DM, those with pre-DM, and those without DM. However, the geometric mean systemic levels of the regulatory cytokine IL-10 were also significantly lower in latently infected individuals with DM and those with pre-DM, compared with levels in those without DM (56.9 pg/mL and 62.8 pg/mL, compared with 86.8 pg/mL). In contrast, the levels of the other potent regulatory cytokine, TGF-β, were not significantly different between the 3 groups.
Finally, since the IL-1 family of cytokines, as well as other proinflammatory cytokines, are known to be important for resistance to M. tuberculosis infection [7], we also measured circulating levels of these cytokines in the 3 groups. The geometric mean systemic levels of IL-1β and IL-18 but not IL-1α in latently infected individuals with DM and those with pre-DM were significantly lower than levels in those without DM (for IL-1β, 34.9 pg/mL and 37.1 pg/mL, respectively, compared with 74.3 pg/mL; and for IL-18, 62.8 pg/mL and 64.6 pg/mL, respectively, compared with 88.4 pg/mL; Figure 1C). No significant differences were found in the systemic levels of other proinflammatory cytokines examined, including IL-6, IL-12, and GM-CSF, between the 3 groups. Thus, latent infection with diabetes or pre-DM is associated with decreased levels of proinflammatory cytokines.
Individuals With Both Latent Infection and DM Exhibit Decreased M. tuberculosis Antigen–Stimulated Levels of Type 1 and Type 17 Cytokines
Since we observed alterations in the plasma levels of type 1 and type 17 cytokines, we sought to determine whether diabetes is associated with alterations in mycobacteria-specific production of these cytokines in latent infection. To this end, we measured levels of these cytokines following stimulation with a cocktail of M. tuberculosis–associated antigens (ESAT-6, CFP-10, and TB 7.7) in a subset of 22 diabetic individuals and 22 nondiabetic individuals with latent infection (Figure 2). As shown in Figure 2A, the spontaneously produced geometric mean levels of IFN-γ (2.4 pg/mL vs 7.3 pg/mL) and TNF-α (0.39 pg/mL vs 6.8 pg/mL) in latently infected diabetic individuals were significantly lower than those in latently infected nondiabetic individuals. Similarly, as shown in Figure 2B, the geometric mean M. tuberculosis antigen–stimulated (net cytokine) levels of IFN-γ (10.5 pg/mL vs 249.2 pg/mL), TNF-α (6.5 pg/mL vs 328.1 pg/mL), IL-17A (14.2 pg/mL vs 24.4 pg/mL), and IL-10 (95.6 pg/mL vs 220.6 pg/mL) in latently infected diabetic individuals were also significantly lower than those in latently infected nondiabetic individuals. Mitogen stimulation induced production of all cytokines, but the levels were not significantly different between the 2 groups (Figure 2C). Thus, latent infection with DM is associated with decreased levels of M. tuberculosis antigen–stimulated type 1 and type 17 cytokines.
Figure 2.
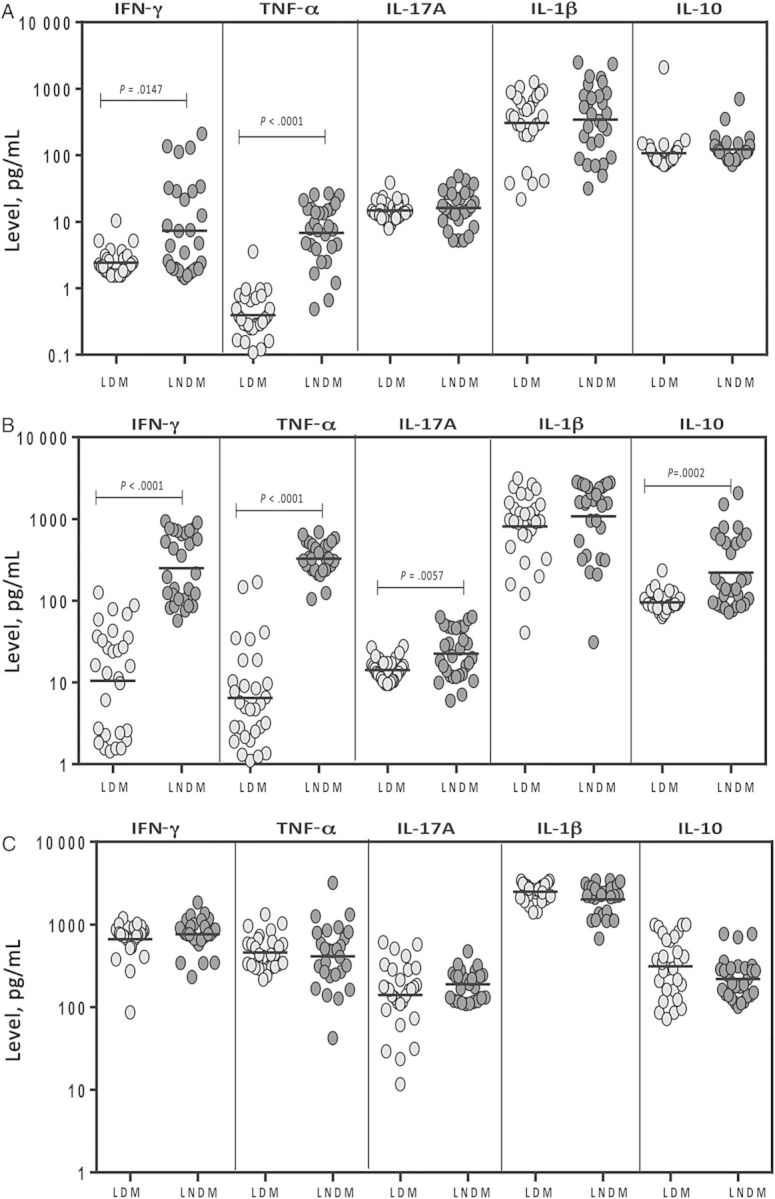
Mycobacterium tuberculosis antigen–stimulated and unstimulated levels of type 1 (interferon γ [IFN-γ] and tumor necrosis factor α [TNF-α]), type 17 (interleukin 17A [IL-17A]), and interleukin 1 (IL-1)–family (IL-1β) cytokines and interleukin 10 (IL-10) in whole-blood specimens from a subset of 22 individuals with latent M. tuberculosis infection concomitant with diabetes mellitus type 2 (LDM) and 22 with latent infection and no DM (LNDM). Cytokines were unstimulated (A), stimulated with M. tuberculosis antigen (B), or stimulated with mitogen (C), and levels were measured by enzyme-linked immunosorbent assay in. The M. tuberculosis antigen– or mitogen-stimulated cytokines are shown as net cytokine levels with the baseline level subtracted out. Each circle represents a single individual, and bars represent geometric means. P values were calculated using the Mann–Whitney test.
Relationship Between Systemic Cytokines and HbA1c Levels
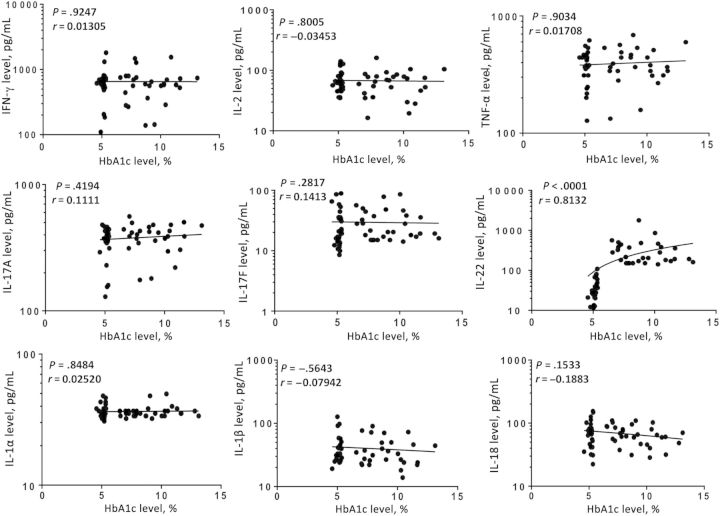
HbA1c is a very accurate indicator of the level of diabetic control, and increased values reflect poor control [9]. Since HbA1c levels have been previously shown to correlate with cytokine levels in individuals with active tuberculosis [12, 13], we wanted to examine whether this relationship exists in latent infection, as well. Thus, we assessed the association of type 1, type 17, IL-1–family cytokines, and type 1 IFNs with HbA1c levels in all latently infected individuals with DM or pre-DM in the study. As shown in Figure 3, the systemic levels of type 1 (IFN-γ, TNF-α, and IL-2), type 17 (IL-17A and IL-17F), and IL-1–family cytokines were not related to HbA1c levels in latent infection. In marked contrast, IL-22, the only cytokine present at significantly higher levels in individuals with latent infection and DM was also the only cytokine that exhibited a significantly positive relationship with HbA1c levels (P < .0001; r = 0.8132). This suggests that perturbations in the homeostatic levels of different cytokines in DM or pre-DM is not influenced by the glycemic status alone.
Figure 3.
Relationship between systemic levels of type 1 (interferon γ [IFN-γ], tumor necrosis factor α [TNF-α], and interleukin 2 [IL-2]), type 17 (interleukin 17A [IL-17A], IL-17F, and interleukin 22 [IL-22]), and interleukin 1 (IL-1)–family (IL-1α, IL-1β, and interleukin 18 [IL-18]) cytokines and hemoglobin A1c (HbA1c) levels in 60 individuals with latent Mycobacterium tuberculosis infection concomitant with diabetes mellitus type 2 (DM) or pre-DM. Each circle representing a single individual, and the bar represents the geometric mean. P values were calculated using the Spearman rank correlation.
Individuals With Both Latent Infection and DM Exhibit Elevated Circulating Levels of Type 1, Type 17 and IL-1 Family of Cytokines, Compared With Individuals Without Latent Infection
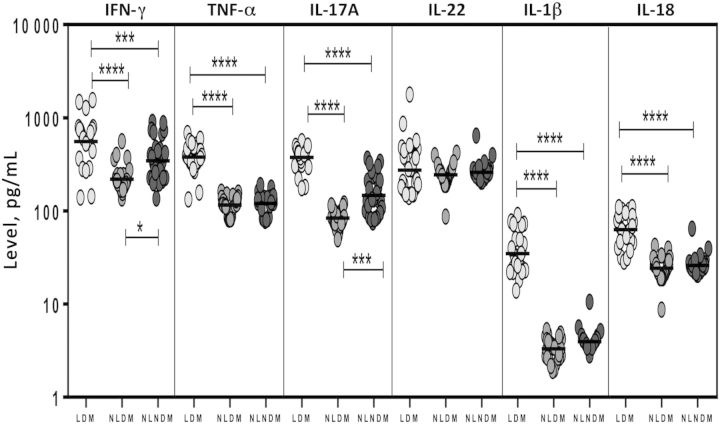
To determine the effect of latent infection status on the homeostatic levels of plasma cytokines, we measured the circulating levels of IFNγ, TNFα, IL-17A, IL-22, IL-1β and IL-18 in individuals with both latent infection and DM, individuals without latent infection and with DM, and individuals without both latent infection and DM. Comparison of individuals with both latent infection and DM to individuals without latent infection and with DM and individuals without both latent infection and DM revealed significantly higher geometric mean levels of IFN-γ (557.3 pg/mL, compared with 219.8 pg/mL and 345.8, respectively ), TNF-α (377.7 pg/mL, compared with 115.8 pg/mL and 120.5 pg/mL, respectively), IL-17A (376 pg/mL, compared with 84.1 pg/mL and 146.8 pg/mL, respectively), IL-1β (34.9 pg/mL, compared with 3.3 pg/mL and 4 pg/mL, respectively), and IL-18 (62.8 pg/mL, compared with 24.4 pg/mL and 26.1 pg/mL, respectively; Figure 4). Interestingly, latent infection had no effect on baseline IL-22 levels. Thus, latent infection is associated with significantly elevated levels of type 1, type 17, and IL-1–family cytokines, independent of DM status.
Figure 4.
Systemic levels of type 1 (interferon γ [IFN-γ] and tumor necrosis factor α [TNF-α]), type 17 (interleukin 17A [IL-17A] and interleukin 22 [IL-22]), and interleukin 1 (IL-1)–family (IL-1β and interleukin 18 [IL-18]) cytokines in 30 individuals with latent Mycobacterium tuberculosis infection concomitant with diabetes mellitus type 2 (LDM), 30 without latent infection and with DM (NLDM), and 30 without both latent infection and DM (NLNDM). Plasma levels were measured by enzyme-linked immunosorbent assay. Each circle representing a single individual, and each bar represents the geometric mean. P values were calculated using the Kruskal–Wallis test with Dunn multiple comparisons. *P < .05, **P < .01, ***P < .001, and ****P < .0001.
DISCUSSION
DM is associated with immune dysfunction with alterations in the components of the immune system, including altered levels of specific cytokines and chemokines, changes in the number and activation state of various immune cell subsets, and increased apoptosis and tissue fibrosis [14]. Pre-DM is characterized by the presence of blood glucose and HbA1c levels that are above normal limits [9]. Since glucose dysregulation and insulin resistance is common to both DM and pre-DM, the cumulative effects on the immune system overall are likely to be similar [9]. How these changes affect the immune response to infectious agents or third-party antigens remains unclear. More specifically, the underlying immunological basis for susceptibility to tuberculosis among those with DM is not well understood. One possible mechanism is that an impaired immune response in diabetic or prediabetic patients facilitates either primary infection with M. tuberculosis or reactivation of latent tuberculosis [3, 4]. Studies examining the innate and adaptive immune response to microbial antigens in diabetic patients suggest that these responses are compromised, particularly in those with chronic hyperglycemia [15–17].
Cytokines play a critical role in the host immune response against mycobacterial infections [11]. Of particular importance are IFN-γ and TNF-α, the functions of which have been well documented in both mouse models and human infections [7, 11]. Of lesser importance but playing a role nonetheless is IL-17A, the prototypical type 17 cytokine that is known to be necessary for mediating memory immune responses to M. tuberculosis in mice [18]. Finally, the roles of other type 17–associated cytokines, such as IL-17F and IL-22, have not been investigated in detail, although IL-22 has been to shown to contribute to the human antimycobacterial response [19]. Cytokines of the IL-1 family, especially IL-1α and IL-1β, are important for resistance to tuberculosis, with mice deficient in IL-1α, IL-1β, and IL-1 receptors showing greater susceptibility to tuberculosis [20, 21]. In addition, IL-18 [22] and IL-12 [23, 24] are both known to be crucial in immunity to M. tuberculosis infection. More recently, IL-6 has been shown to mediate inhibition of disease progression [25].
Our study on the homeostatic (or steady-state) levels of type 1 and type 17 cytokines reveals profoundly diminished plasma levels of most of these cytokines during DM or pre-DM with coincident latent infection. In conjunction with our analysis that other confounding variables, such as age, sex, or lipid profile, do not influence the levels of cytokines, our data imply a strong predilection for impaired cytokine production in diabetic individuals with coincident latent infection. While DM is generally thought to be associated with a compromised immune response [16], the exact nature of this immune compromise has not been defined previously and whether it occurs in prediabetics has not been examined to our knowledge. Our data suggest that it is poor glycemic control (or underlying mechanisms of insulin resistance) that may be associated with diminished systemic levels of type 1 and type 17 cytokines. Interestingly, the diminished production of type 1 and 17 cytokines is associated with significantly lower systemic levels of the IL-1 family of cytokines. Among the type 17 cytokines, IL-17F levels are significantly lower than IL-17A levels, although both share 55% homology. This could be due to the 2 different assays used for measurement. In addition, IL-22 deserves special mention in that it was the only cytokine measured that was present at higher levels in latently infected individuals with DM and that had levels that appeared to be positively correlated with HbA1c levels. In this context, it is interesting to note that IL-22 has been previously shown to be produced at higher levels in individuals with DM and to promote IL-β–driven inflammation in human adipose tissue [26]. To address the role of latent tuberculosis infection in influencing the homeostatic levels of these cytokines, we also compared latently infected individuals with 2 other control groups without latent infection (individuals with and individuals without DM) and demonstrated that latent infection, independent of DM status, is associated with increased baseline cytokine levels.
Although we feel that the steady-state levels of important cytokines reflects quite clearly the in vivo state, to address more specifically the M. tuberculosis–driven responses, we examined these responses in latent infection in the presence or absence of DM. Our data demonstrate that antigen-specific cytokine production during latent infection largely reflects what was seen in the systemic circulation at steady state, with diminished M. tuberculosis–stimulated production of most of the type 1, type 17, and other relevant cytokines and that a concomitant increase in regulatory or type 2 cytokines was not apparent in the present study. Thus, the down-modulation of systemic cytokine responses during pre-DM or DM is unlikely to be due to increased activity of regulatory cytokines.
Typically, DM is known to be associated with a chronic inflammatory milieu, with activation of the innate immune system and increased production of proinflammatory cytokines [16]. Indeed, therapeutic strategies targeting proinflammatory cytokines are in clinical trials for DM [27]. Moreover, we and others have previously shown that DM in the context of active pulmonary tuberculosis is associated with an exaggerated inflammatory response and with heightened levels of type 1, type 2, and other proinflammatory cytokines [12, 13, 28]. Thus, our data on the diminution in proinflammatory cytokine production both at steady state and following antigen stimulation in the context of latent infection potentially suggest a profound impact on comorbidity during latent infection concomitant with DM. Our study also examines the important modulatory role played by poor glucose control and/or nascent or full-blown insulin resistance in latent infection and offers important insights into the potential mechanism by which DM or pre-DM could influence the progression from latent infection to active tuberculosis. Our data support the growing body of evidence that suggests immunocompromise is an integral part of DM and that this compromise could have serious consequences in the face of intracellular pathogens that require type 1– and type 17–associated cytokines for resistance. While our study by design was cross-sectional and cannot delineate causal parameters, it provides an impetus to perform longitudinal studies examining the role of immunocompromise in the progression from latent infection to active tuberculosis and the role played by poor glucose control. In a broader context, the present study suggests that by maintaining good glucose control, restoration of immune competence (with its inherent improvement in antigen-specific responses) holds promise for mediating protection to intracellular pathogens and in the induction of the appropriate responses to exogenously delivered antigens though vaccination.
Notes
Acknowledgments. We thank the staff of the Department of Epidemiology, NIRT, Chennai, for valuable assistance in recruiting the patients for this study; and R. Anuradha, V. Gopinath, and Jovvian George of the NIH–ICER, for technical assistance.
T. B. N. and S. B. conceived and designed the study and wrote the manuscript. N. P. K., P. J. G., and S. B. performed the experiments and analyzed the data. P. P. and C. D. contributed materials and samples.
Financial support. This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9:737–46. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol. 2014;44:617–26. doi: 10.1002/eji.201344301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Restrepo BI, Schlesinger LS. Host-pathogen interactions in tuberculosis patients with type 2 diabetes mellitus. Tuberculosis (Edinb) 2013;93(suppl):S10–4. doi: 10.1016/S1472-9792(13)70004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2013;379:2279–90. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 8.Andrade BB, Pavan Kumar N, Mayer-Barber KD, et al. Plasma heme oxygenase-1 levels distinguish latent or successfully treated human tuberculosis from active disease. PLoS One. 2013;8:e62618. doi: 10.1371/journal.pone.0062618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Association AD. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aravindhan V, Mohan V, Surendar J, et al. Decreased prevalence of lymphatic filariasis among diabetic subjects associated with a diminished pro-inflammatory cytokine response (CURES 83) PLoS Negl Trop Dis. 2010;4:e707. doi: 10.1371/journal.pntd.0000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev. 2008;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar NP, Sridhar R, Banurekha VV, et al. Type 2 diabetes mellitus coincident with pulmonary tuberculosis is associated with heightened systemic type 1, type 17, and other proinflammatory cytokines. Ann Am Thorac Soc. 2013;10:441–9. doi: 10.1513/AnnalsATS.201305-112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Restrepo BI, Fisher-Hoch SP, Pino PA, et al. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis. 2008;47:634–41. doi: 10.1086/590565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 15.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–65. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 16.Nikolajczyk BS, Jagannathan-Bogdan M, Shin H, Gyurko R. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun. 2011;12:239–50. doi: 10.1038/gene.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–8. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 18.Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41:79–83. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scriba TJ, Kalsdorf B, Abrahams DA, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962–70. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer-Barber KD, Andrade BB, Barber DL, et al. Innate and adaptive interferons suppress IL-1alpha and IL-1beta production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 2011;35:1023–34. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugawara I, Yamada H, Hua S, Mizuno S. Role of interleukin (IL)-1 type 1 receptor in mycobacterial infection. Microbiol Immunol. 2001;45:743–50. doi: 10.1111/j.1348-0421.2001.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 22.Schneider BE, Korbel D, Hagens K, et al. A role for IL-18 in protective immunity against Mycobacterium tuberculosis. Eur J Immunol. 2010;40:396–405. doi: 10.1002/eji.200939583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altare F, Durandy A, Lammas D, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–5. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 24.Holscher C, Atkinson RA, Arendse B, et al. A protective and agonistic function of IL-12p40 in mycobacterial infection. J Immunol. 2001;167:6957–66. doi: 10.4049/jimmunol.167.12.6957. [DOI] [PubMed] [Google Scholar]
- 25.Martinez AN, Mehra S, Kaushal D. Role of interleukin 6 in innate immunity to Mycobacterium tuberculosis infection. J Infect Dis. 2013;207:1253–61. doi: 10.1093/infdis/jit037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalmas E, Venteclef N, Caer C, et al. T cell-derived IL-22 amplifies IL-1beta-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes. 2014;63:1966–77. doi: 10.2337/db13-1511. [DOI] [PubMed] [Google Scholar]
- 27.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–26. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 28.Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Nutman TB, Babu S. Expansion of pathogen-specific T-helper 1 and T-helper 17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus. J Infect Dis. 2013;208:739–48. doi: 10.1093/infdis/jit241. [DOI] [PMC free article] [PubMed] [Google Scholar]