Abstract
Background. The divergent epidemiological behavior of Streptococcus pneumoniae serotypes suggests that serotype-specific features such as capsule O-acetylation influence the propensity of a strain to cause invasive pneumococcal disease (IPD). We hypothesize that innate host factors mediate the observed negative association between IPD and the serotype 11A (ST11A) capsule O-acetyltransferase gene, wcjE.
Methods. We evaluated the ability of ficolin-2, an initiator of the lectin complement pathway that was previously shown to bind ST11A pneumococci, to recognize and mediate complement-dependent opsonophagocytosis of different pneumococcal serotypes. We supplemented findings with an epidemiological meta-analysis comparing invasiveness of the 30 most prevalent pneumococcal serotypes.
Results. Ficolin-2 bound ST11A capsule polysaccharide and other wcjE-containing pneumococcal serotypes, except ST9V and ST20B. Ficolin-2 did not bind wcjE-null serotypes, including the wcjE-null variant of ST11A, ST11E. We observed C1q-independent complement deposition and phagocytic killing of pneumococci expressing ST11A but not those expressing ST11E. Inhibition of ficolin-2 binding abrogated ST11A-associated complement deposition and phagocytosis. In children, invasiveness of ST11A was the lowest among serotypes tested in our meta-analysis, while ST9V was among the highest.
Conclusions. Ficolin-2 mediates serum protection by recognizing specific O-acetylated epitopes of pneumococcal capsule polysaccharides, exemplifying a novel host-microbe interaction that innately offers serotype-specific immunity to IPD.
Keywords: Streptococcus pneumoniae, complement, ficolin-2, serotype 11A, invasiveness
Streptococcus pneumoniae (ie, pneumococcus) is a ubiquitous colonizer of the nasopharynx whose incidental invasion of the blood stream results in invasive pneumococcal disease (IPD). A prerequisite for invasion is expression of a polysaccharide (PS) capsule, of which >90 capsule serotypes have been identified [1]. The propensity of a colonizing pneumococcal strain to cause IPD (ie, its invasiveness) can vary greatly according to which serotype is expressed [2–4]. Accordingly, some prevalent serotypes, such as serotype 11A (ST11A), appear to be less invasive than others [5, 6]. The causal link between pneumococcal serotype and invasiveness in humans remains elusive.
Recent discovery and characterization of ST11E, which was previously mistyped as ST11A [7], suggest that minor changes in capsule structure affect pneumococcal survival in different host tissues. ST11A and ST11E PS structures are identical except for the presence of O-acetylation (OAc) of carbon-6 of β-galactose (βGal6-OAc) on ST11A PS [8]. This OAc is dependent on the O-acetyltransferase gene wcjE encoded in the ST11A capsule synthesis (cps) locus [7]. Peculiarly, each ST11E strain contains a different inactivating mutation to wcjE [7, 9]. Furthermore, ST11E strains are rarely isolated from the nasopharynx but can account for up to 50% of IPD isolates typed as ST11A by the Quellung reaction [9]. This unique genetic heterogeneity and epidemiological behavior suggests that ST11E strains are not transmitted between hosts but instead independently evolve from colonizing ST11A strains during host invasion. Thus, loss of wcjE-dependent capsule OAc may benefit survival in blood. We hypothesize that a protective serum factor specifically recognizes ST11A but not ST11E, resulting in a decreased ability of ST11A pneumococci to cause IPD.
Ficolin-1, -2, and -3 are serum-associated pattern-recognition molecules in humans [10, 11]. Functionally and structurally similar to mannose-binding lectin (MBL), ficolins form complexes with MBL-associated serine proteases and, upon binding, initiate the lectin complement pathway and direct opsonophagocytosis [12, 13]. Reportedly, binding of human ficolin-2 (L-ficolin) to pneumococci initiates the complement cascade in vitro [14, 15], and mice deficient in expression of ficolin-A, a putative ficolin-2 ortholog, display increased susceptibility to experimental IPD [15, 16]. However, genetic polymorphisms putatively decreasing ficolin-2 function [17, 18] are not associated with increased susceptibility to IPD [19], and reports of ficolin-2 ligand specificity and complement deposition on pneumococci are inconsistent [14, 15, 20]. Here, we evaluated the relationship between ficolin-2 and the clinical behavior of pneumococcal serotypes.
METHODS
Reagents and Buffers
Reagents were purchased from Sigma Aldrich or Fisher Scientific unless otherwise specified. Ficolin-binding buffer (FBB) contained 1 × Hanks’ buffered saline solution (Gibco, Life Technologies) with 2.2 mM CaCl2 and 0.5% bovine serum albumin (BSA). Gelatin veronal buffer (GVB) contained 0.15 mM CaCl2, 141 mM NaCl, 0.5 mM MgCl2, 0.1% gelatin, and 5 mM barbital sodium C-IV at pH 7.3. Normal human serum (NHS) was obtained from a consented healthy adult volunteer donor according a protocol approved by the University of Alabama at Birmingham Institutional Review Board. NHS contained low levels of antibodies to ST11A and ST11E (data not shown).
Strains and Culture Conditions
ST11A and ST11E clinical isolates are described elsewhere [7]. The ST20A isolate ATCC6320 was obtained from the American Type Culture Collection (ATCC, Manassas, VA). All other strains were obtained from Statens Serum Institut (SSI, Copenhagen, Denmark). To exclude effects of other genetic factors, isogenic ST11A, ST11E, and nonencapsulated strains (JC03, JC04, and AMB03, respectively) were generated in the same genetic background as described previously [21]. Bacteria were cultured at 37°C and 5% CO2 on blood agar plates (BD Biosciences, San Jose, CA) or in Todd Hewitt broth (BD Biosciences, San Jose, CA) with 0.5% yeast extract (THY).
Generation of Isogenic Recombinant Strains
The wcjE-null strains AMB13 (ST20Ax) and JC05 (ST11Dx) were generated from ATCC6320 (ST20A) and 70/86 (ST11D, SSI) following protocols previously described [21]. Absence of WcjE-OAc on ST20Ax and ST11Dx was confirmed by loss of reactivity with factor serum 20b (SSI) [22] or Hyp11AG2 murine monoclonal antibody (mAb) [8], respectively. Isogenic ST9V and ST9A strains AMB15 and AMB16 were generated by recombinatorial replacement of the cps locus in strain JC03 with cps loci from strains 980/63 and Wilder (SSI), respectively [23, 24]. ST9V and ST9A expression was confirmed by factor serum 9g and 9d (SSI) reactivity.
Flow Cytometry and Detection Antibodies
Flow cytometry data for bacteria and beads was obtained using a FACSCalibur or FACSArray Bioanalyzer (BD Biosciences, San Jose, CA), respectively, and analyzed using FCS Express V3 (De Novo Software, Los Angeles, CA). Capsule-specific mAbs Hyp11AM1 and Hyp11AG2 are described elsewhere [21, 25]. Surface-associated ficolin-2 was detected using GN5 mAb (Hycult, catalog no. HM2091). Complement was detected using anti-C3 (Pierce, catalog no. LF-MA0132) and fluorescein isothiocyanate (FITC)–labeled anti-C4b/C4c (Pierce, catalog no. PA1-28407) antibodies. Nonlabeled antibodies were detected with FITC-labeled (catalog no. 1021-02) or phycoerythrin-labeled (catalog no. 1030-09) secondary antibodies (Southern Biotech, Birmingham, AL). Antibody staining of bacteria and latex beads was performed for 30 minutes at 4°C.
Polysaccharide Purification and Conjugation to Latex Beads
Purification of ST11A and ST11E PS from strains MNZ272 and MNZ264, respectively, is described elsewhere [8, 26]. ST15B PS was obtained from ATCC. PS conjugation to latex beads is described elsewhere [25].
Ficolin-2 Binding and Inhibition Assays
A total of 5 × 105 colony-forming units (CFUs) were incubated in 100 µL of FBB containing 2.5% NHS for 1 hour at 4°C. After washing with FBB, surface-associated ficolin-2 was detected by flow cytometry. For ficolin-2 binding to PS-conjugated beads, Tris-buffered saline with 0.05% Tween-20 and 0.34% NHS was used. In inhibition experiments, 10% NHS was mixed at a ratio of 1 to 1 with buffer control or with serially diluted inhibitor consisting of the following components: BSA, acetylated BSA (acBSA), N-acetylglucosamine (GlcNAc), glucose (Glc), purified pneumococcal lipoteichoic acid (LTA) [27], TA (from SSI) containing 1 or 2 phosphocholine (PC) molecules per repeating unit, purified ST11A PS, or purified ST11E PS. Inhibitor/serum mixtures were preincubated for 10 minutes on ice and then mixed 1:1 with 50 µL of FBB containing 5 × 105 CFUs.
Complement Deposition Assays
A total of 50 µL of GVB containing 10%, 20%, or 30% NHS (5%, 10%, or 15% of the final concentration) was added to wells containing 5 × 105 CFUs in 50 µL of FBB at indicated intervals. Reactions were simultaneously stopped by placing wells on ice. After washing with ice-cold GVB, C3 and C4b/C4c was detected by flow cytometry. In inhibition experiments, NHS was preincubated for 10 minutes on ice with the following inhibitors: 10 mg/L acBSA, 10 mg/L BSA, 1 mM PC, 1 mg/L TA (CPS-multi, SSI), 50 mM GlcNAc, 50 mM Glc, or a 1-to-100 dilution of silica clot activator (SCA; as described elsewhere [28]). In assays using C1q-depleted serum (Millipore, catalog no. 234404), which was codepleted of ficolin-2 as determined by enzyme-linked immunosorbent assay and flow cytometry analysis (data not shown), 5 × 105 CFUs were preincubated in 100 µL of GVB containing 45% recombinant ficolin-2 (rFicolin-2) supernatant with or without inhibitors for 1 hour at 4°C, washed with GVB, and then incubated in 100 µL of GVB containing 5% C1q-depleted serum and 40% rFicolin-2 supernatant with or without inhibitors. The supernatant contained 1.2 mg/L of rFicolin-2. A detailed description of rFicolin-2 production is described in the Supplementary Materials.
Opsonophagocytic Killing (OPK) Assay
A total of 10 µL of GVB containing 15% NHS or heat-inactivated NHS and 10 µL GVB with or without 30 mg/L acBSA, 30 mg/L BSA, 3 mM PC, or SCA was mixed in wells and preincubated on ice for 10 minutes. Control wells received 20 µL GVB containing 10% of a 1:80 dilution of a pool of heat-inactivated sera from vaccinated adults [29], in NHS or heat-inactivated NHS. All wells then received 10 µL GVB containing 500–1000 CFUs and were incubated for 15 minutes with shaking at 37°C. Wells then received 50 µL of GVB alone or GVB containing 4 × 105 HL60 cells (ATCC [CCL-240]), incubated for 30 minutes with shaking at 37°C, and finally placed on ice for 20 minutes. Cytochalasin-B–treated HL60 cells were pretreated with dimethyl sulfoxide (DMSO) plus 50 µM cytochalasin-B or with DMSO alone. CFUs were cultured on THY agar plates and enumerated using ProtoCOL colony-counting software (Synbiosis, UK), in accordance with the well-characterized UAB OPK protocol [29] (described in detail at: http://www.vaccine.uab.edu. Accessed 8 April 2014).
Meta-analysis Design
We performed a meta-analysis using pediatric carriage and IPD prevalence data from 18 studies before widespread use of vaccination. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using the Cochran-Mantel-Haenszel procedure from Proc Freq in SAS, version 9.2 (Cary, NC). More-detailed methods are provided in the Supplementary Materials.
RESULTS
Ficolin-2 Specifically Binds Serotype 11A but Not Serotype 11E Capsular Polysaccharide
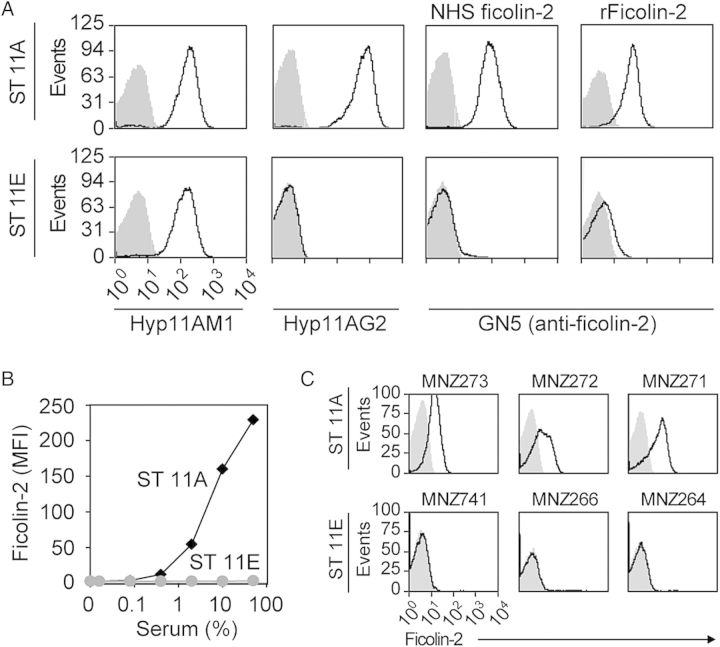
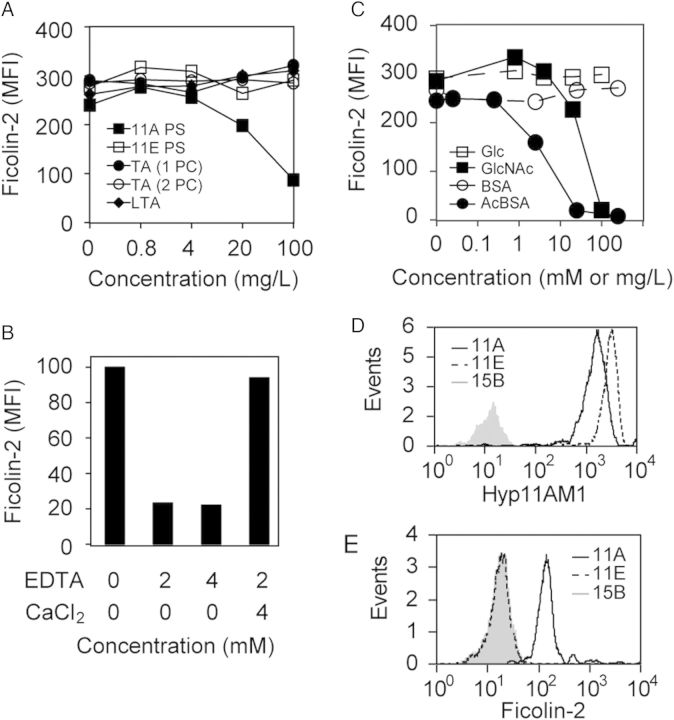
The isogenic strains JC03 and JC04 expressed similar amounts of ST11A and ST11E capsule, respectively (Figure 1A ); however, surface-associated ficolin-2 was detected on JC03 (11A) incubated in NHS and FBB containing rFicolin-2 but was not detected on JC04 (11E), even after incubation in up to 50% NHS (Figure 1A and 1B). Accordingly, NHS ficolin-2 bound ST11A but not ST11E clinical isolates (Figure 1C). While screening for potential ligands bound by ficolin-2, we observed dose-dependent inhibition of ficolin-2 binding ST11A bacteria with purified ST11A PS but not with purified pneumococcal LTA, TA with 1 or 2 phosphocholines (1PC or 2PC) per repeat unit, or purified ST11E PS (Figure 2A). In support of the hypothesis that ficolin-2 specifically binds ST11A PS, ficolin-2 bound to latex beads coated with purified ST11A PS but not with ST11E PS (Figure 2D–E).
Figure 1.
Flow-cytometric detection of ficolin-2 binding to ST11A pneumococci. Data are representative of at least 3 experiments. A, Flow-cytometric histograms depict Hyp11AM1 (first column), Hyp11AG2 (second column), serum ficolin-2 (third column), and recombinant ficolin-2 (fourth column) binding to the ST11A strain JC03 (top panels) and ST11E strain JC04 (bottom panels). Hyp11AM1 and Hyp11AG2 are murine monoclonal antibodies that bind to the polysaccharide (PS) of both ST11A and ST11E or only ST11A, respectively. Gray-shaded curves depict bacteria incubated with isotype control antibodies and represent negative controls. B, Ficolin-2 bound to JC03 (11A; black diamonds) but not to JC04 (11E; grey circles) after incubation in serial dilutions of serum, quantified as a mean fluorescence intensity (MFI). C, Flow-cytometric histograms depict ficolin-2 binding to ST11A clinical isolates (MNZ273, MNZ272, and MNZ271) but not to ST11E clinical isolates (MNZ741, MNZ266, and MNZ264).
Figure 2.
Specificity of ficolin-2 binding to ST11A bacteria was examined by flow cytometry and expressed as mean fluorescence intensity (MFI). All graphs are representative of at least 3 experiments. A, Serum ficolin-2 binding to ST11A pneumococci in the presence of serially diluted purified polysaccharide (PS) preparations: ST11A PS (solid squares), ST11E PS (open squares), teichoic acid with 1 phosphocholine per repeat unit (TA 1PC; solid circles), teichoic acid with 2 PCs per repeat unit (TA 2PC; open circles), or lipoteichoic acid (LTA; solid diamonds). B, Serum ficolin-2 binding to JC03 incubated in 2.5% serum in the presence of specified concentrations (mM) of ethylenediaminetetraacetic acid (EDTA) and CaCl2. C, Serum ficolin-2 binding to ST11A pneumococci in the presence of inhibitors: glucose (Glc; open squares), bovine serum albumin (BSA; open circles), N-acetylglucosamine (GlcNAc; solid squares), or acetylated-BSA (acBSA; solid circles). Inhibitor concentrations are mM for Glc and GlcNAc and mg/L for BSA and acBSA. D–E, Flow cytometric detection of ficolin-2 binding to ST11A polysaccharide (PS)–coated latex beads. Data are representative of at least 3 experiments. D, Latex beads were conjugated with purified ST11A PS (solid black curves), ST11E PS (dotted black curves), or 15B PS (gray curves) and were examined for ST11A/11E PS, using Hyp11AM1 monoclonal antibody. E, The same PS-conjugated latex beads used in panel D were incubated in 0.17% normal human serum and examined for ficolin-2 binding, using anti-ficolin-2 mAb GN5.
Ficolin-2 specifically binds acetylated compounds in a calcium-dependent manner through its fibrinogen-like domain [30, 31]. In support of the hypothesis that ficolin-2 also binds ST11A capsule PS through its fibrinogen-like domain, ficolin-2 binding to ST11A bacteria was inhibited by ethylenediaminetetraacetic acid chelation and restored with excess calcium (Figure 2B). Furthermore, acBSA and GlcNAc but not BSA or Glc mediated dose-dependent inhibition of ficolin-2 binding (Figure 2C).
Ficolin-2 Recognizes Pneumococcal Serotypes Associated With wcjE-Dependent O-acetylation
Most reported ficolin-2 ligands are N-acetylated molecules [13, 31, 32]. In contrast, ST11A PS lacks N-acetylation [8]. We examined ficolin-2 recognition of other serogroup 11 pneumococci, whose capsules vary in O- and N-acetylation [8], and detected surface-associated ficolin-2 on pneumococci expressing ST11A, ST11D, or ST11F but not ST11E, ST11B, or ST11C (Figure 3A). As only the former 3 capsules contain wcjE-dependent βGal6-OAc [8, 33] but ST11B and ST11C PS contain GlcNAc [8], we concluded capsule O-acetylation, not N-acetylation, determined ficolin-2 recognition.
Figure 3.
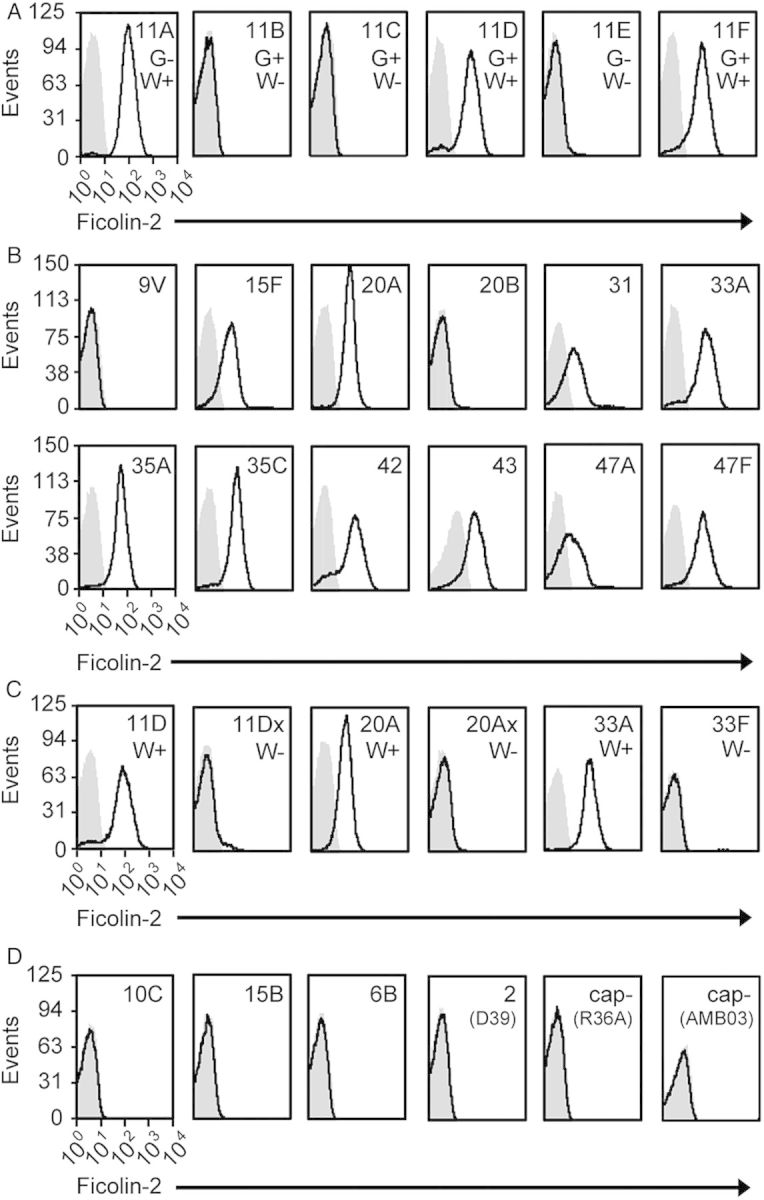
Ficolin-2 binding to various pneumococci was examined by flow cytometry. Bacteria were incubated in 2.5% normal human serum and stained with GN5 (anti-ficolin-2) monoclonal antibody (Ab; black curves) or an isotype control Ab (grey curves). All histograms are representative of at least 3 experiments. A strain's serotype or name is listed in the top right corner of each histogram. A, Ficolin-2 binding to pneumococci expressing serogroup 11 serotypes. The presence or absence of GlcNAc (G+ or G−) or WcjE-mediated βGal6-OAc (W+ or W−) on capsule polysaccharide is noted for each serotype. B, Ficolin-2 binding to serotypes whose cps loci encode an intact copy of wcjE. C, Comparison of ficolin-2 binding between serotypes encoding wcjE (W+; ST11D, ST20A, ST33A) and corresponding wcjE-null (W−) recombinant strains (11D× and 20A×) or clinical isolate (ST33F). D, Ficolin-2 binding to pneumococci expressing capsules containing non-wcjE O-acetylation (10C and 15B), capsules without O-acetylation (6B and D39), or nonencapsulated (cap-) strains (R36A and AMB03). Strains R36A and AMB03 are derivatives of D39 (ST2) and JC03 (ST11A), respectively.
Indeed, ficolin-2 bound most serotypes whose cps loci encode wcjE (ie, ST11A, ST11D, ST11F, ST15F, ST20A, ST31, ST33A, ST35A, ST35C, ST42, ST43, ST47A, and ST47F) (Figure 3B and Table 1) [34] but did not bind any corresponding wcjE-null clinical isolates (ST11E and ST33F) or recombinant strains (ST11Dx and ST20Ax; Figure 3C). Ficolin-2 also did not bind any other serotype (data not shown) or the nonencapsulated strains R36A and AMB03 (Figure 3D). Notably, ficolin-2 did not bind ST9V or ST20B pneumococci, despite the presence of WcjE-OAc on their capsules (Figure 3B) [1, 22, 36], or to pneumococci producing wcjE-independent O-acetylated capsules (eg, ST10C and ST15B; Figure 3D) [34, 37, 38]. Thus, wcjE-dependent OAc was required, although not sufficient, for ficolin-2 recognition of pneumococci.
Table 1.
Summary of WcjE-Mediated O-acetylation and Ficolin-2 Binding
Ficolin-2 Binding Is Associated With Increased Rate and Quantity of Complement Deposition on Serotype 11A Pneumococci
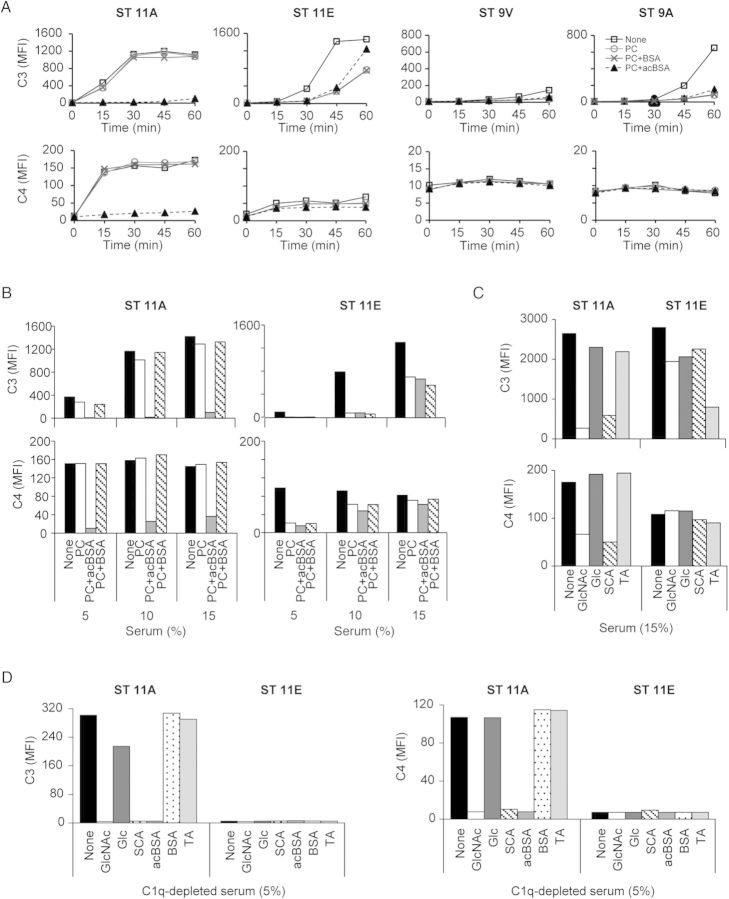
We evaluated NHS ficolin-2–mediated complement deposition on isogenic strains expressing ST11A, ST11E, ST9V, and ST9A (Figure 4A). These serotypes were chosen because, similar to ST11A and ST11E, ST9V PS contains wcjE-dependent OAc, which the homologous ST9A PS lacks [36, 39]. ST11A pneumococci incubated in 5% NHS were fully coated with C4 and C3 within 15 and 30 minutes, respectively (Figure 4A). C3 deposition was noticeably delayed on ST11E and ST9A bacteria and nearly absent on ST9V. Reduced C4 deposition was detected on ST11E as compared to ST11A bacteria, while no obvious deposition was detected on ST9V and ST9A bacteria (Figure 4A). Notably, surface-associated C3 on ST11A and ST11E bacteria was invariably associated with surface-associated C4 (Supplementary Materials). C3 deposition on ST11A and ST11E bacteria increased in a NHS concentration–dependent fashion, and near-maximal levels were reached after 15-minute incubation in 15% NHS (Figure 4B). As C4 deposition remained unaffected (Figure 4B), C3-related changes likely resulted from the amplification by the alternative pathway at higher NHS concentrations [40].
Figure 4.
Complement deposition on isogenic strains expressing ST11A, ST11E, ST9V, and ST9A polysaccharide was examined by flow cytometry and expressed as mean fluorescence intensity (MFI). All data are representative of at least 3 experiments performed in triplicate. A, Deposition of C3 (top panels) and C4 (bottom panels) over time on ST11A (JC03; column 1), ST11E (JC04; column 2), ST9V (AMB15; column 3), and ST9A (AMB16; column 4) bacteria in the presence of 5% normal human serum (NHS) only (open squares), 5% NHS plus 1 mM phosphocholine (PC; open circles), 5% NHS plus 1 mM PC and 10 mg/L bovine serum albumin (BSA; Xs), or 5% NHS plus 1 mM PC and 10 mg/L acetylated BSA (acBSA; closed triangles). B, Deposition of C3 (top panel) and C4 (bottom panel) on ST11A (JC03) and ST11E (JC04) bacteria after 15-minute incubation with 5%, 10%, or 15% NHS with no inhibitor (none), 1 mM PC, 1 mM PC plus 10 mg/L acBSA (PC + acBSA), or 1 mM PC plus 10 mg/L BSA (PC + BSA). C, Deposition of C3 (top panel) and C4 (bottom panel) on ST11A (JC03) and ST11E (JC04) bacteria after 15-minute incubation with 15% NHS with no inhibitor (none), 50 mM N-acetylglucosamine (GlcNAc), 50 mM glucose (Glc), silica clot activator (SCA), or 1 mg/L pneumococcal teichoic acid (TA). D, Deposition of C3 (left panel) and C4 (right panel) on ST11A (JC03) and ST11E (JC04) bacteria after 1-hour incubation in 5% C1q-depleted serum with no inhibitor (none), 50 mM GlcNAc, 50 mM Glc, SCA, 10 mg/L acBSA, 10 mg/L BSA, or 1 mg/L TA.
We used various inhibitors to isolate the molecules that were responsible for complement deposition on these serotypes. PC is a major surface antigen present on LTA and TA of pneumococci. C-reactive protein and antibodies against PC are ubiquitous in human serum, opsonize pneumococci, and mediate complement deposition [41, 42]. Therefore, we controlled for their effects by using PC as an inhibitor. Co-incubation with PC plus acBSA but not with BSA or PC alone suppressed C3 and C4 deposition on ST11A bacteria (Figure 4A), even when incubated in 15% NHS (Figure 4B). Deposition on ST11A was also inhibited by GlcNAc and SCA, inhibitors of ficolin-2 binding to ST11A [28], but not by Glc or pneumococcal TA (Figure 4C). Delayed C3 deposition on ST11E and ST9A bacteria, however, was inhibited by PC alone, with no added effect by BSA or acBSA (Figure 4A). PC also inhibited small C4 deposition on ST11E, although at higher serum concentrations the inhibitory effect was decreased as the inhibitor concentration remained unchanged (Figure 4B). Pneumococcal TA also reduced C3 deposition on ST11E bacteria (Figure 4C). Complement deposition on ST9V pneumococci was unaffected by inhibitors. In summary, competitive inhibition of ficolin-2 binding specifically decreased NHS-mediated complement deposition on ST11A pneumococci.
Ficolin-2 Mediates Complement Deposition on Serotype 11A Bacteria in C1q-Depleted Serum
We examined the role of the classical pathway, using C1q-depleted serum supplemented with rFicolin-2. C1q-depleted serum was supplemented with rFicolin-2 because it lacked detectable ficolin-2 (see Methods). In absence of rFicolin-2, C1q-depleted serum did not mediate complement deposition on either serotype (data not shown). With the rFicolin-2–supplemented serum, we detected C3 and C4 deposition on ST11A bacteria, but not ST11E bacteria, that was inhibited by GlcNAc, SCA, and acBSA but not by Glc, BSA, or pneumococcal TA (Figure 4B). We concluded that ficolin-2 binding to ST11A activates complement independent of C1q. However, complement activation on ST11E requires C1q.
Ficolin-2 Binding Is Associated With Increased OPK of Serotype 11A Pneumococci
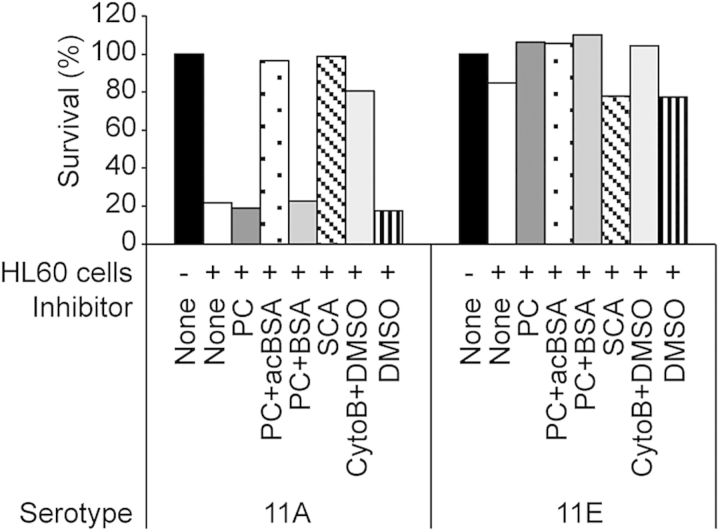
We examined the contribution of ficolin-2 to the OPK of ST11A and ST11E pneumococci incubated in 5% NHS for 15 minutes (Figure 5). Paralleling findings above, we observed phagocyte (HL60)–dependent killing of ST11A, which was inhibited by preincubating NHS with acBSA and SCA but not with BSA or PC alone. Heat inactivation of NHS (data not shown) and pretreatment of HL60 cells with cytochalasin-B (Figure 5) restored survival, indicating that bacterial killing depended on functional complement and phagocytosis, respectively. We observed no OPK of ST11E pneumococci incubated in NHS alone (Figure 5), but supplementation of NHS with heat-inactivated sera of individuals immunized with ST11A PS resulted in indistinguishable OPK of both ST11A and ST11E (data not shown). We concluded that binding by ficolin-2 in NHS can mediate OPK of pneumococci expressing ST11A but not those expressing ST11E.
Figure 5.
Opsonophagocytic killing of ST11A (JC03) and ST11E (JC04). Bacteria were incubated in the presence of normal human serum (NHS) with no inhibitor (None), 1 mM phosphocholine (PC), 1 mM PC plus 10mg/L acetylated bovine serum albumin (PC + acBSA), 1 mM PC plus 10 mg/L bovine serum albumin (PC + BSA), silica clot activator (SCA), 50 µM cytochalasin-B in dimethyl sulfoxide (CytoB + DMSO), or dimethyl sulfoxide alone (DMSO). Percentage survival is calculated as the ratio of colony-forming units (CFUs) in the presence of NHS versus CFUs in the presence of heat-inactivated NHS.
Serotype 11A Displays the Least Invasive Potential of Major Pneumococcal Serotypes
Theoretically, ficolin-2 recognition and clearance of ST11A from the blood stream reduces its ability to cause IPD. To assess this hypothesis, we performed a meta-analysis of 18 epidemiological studies reporting the prevalence of pneumococcal serotypes among 8917 carriage and 4965 IPD isolates (Supplementary Materials). We specifically analyzed the 30 most prevalent serotypes/serogroups, composing 73% of carriage isolates (6479) and 91% of IPD isolates (4508).
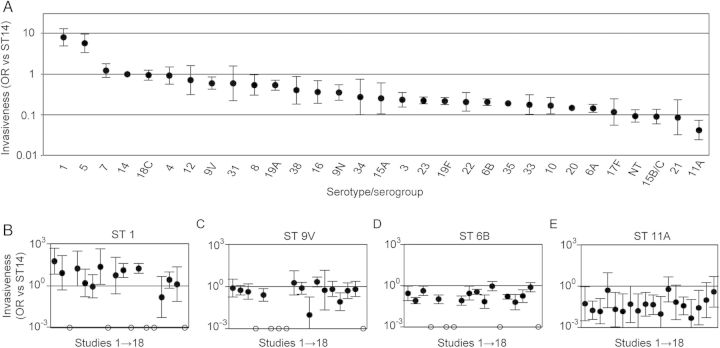
As in a prior meta-analysis [2], serotype invasiveness was determined by comparing the ratio of the number of invasive to carriage isolates expressing a serotype to the corresponding ratio expressing ST14. Pooled ORs varied >100-fold across serotypes/serogroups (Figure 6A) but generally corresponded well with the ORs in individual studies (Figure 6B–D). Remarkably, ST11A appeared to be the least invasive serotype (Figure 6A). In fact, a 10-fold reduction in invasiveness was observed when comparing ST11A (OR, 0.04 [95% CI, .02–.07]) to serotype ST9V (OR, 0.60 [95% CI, .43–.85]), which is not recognized by ficolin-2 (Figure 3B). This separation in invasiveness was observed in individual studies (Figure 6C and 6E). In summary, ST11A displayed a stably and remarkably low propensity to cause IPD in nonimmunized pediatric populations.
Figure 6.
Meta-analysis of 18 studies for pneumococcal serotype invasiveness in children before the widespread use of pediatric pneumococcal vaccines. Odds ratios (ORs) were determined by comparing the ratio of the number of invasive to carriage isolates expressing a serotype to the corresponding ratio expressing serotype 14 in each study (Supplementary Materials). A, The pooled odds ratios (ORs) with 95% confidence intervals (CIs) for the 30 most prevalent serotypes or serogroups identified in the meta-analysis, listed from highest to lowest ORs. B–E, Study-specific ORs and 95% CIs for serotypes representative of high (ST1; B), medium (ST9V [C]; ST6B [D]), and low (ST11A; E) invasiveness. Data are displayed according to the numbering of respective studies (1–18) in the table in the Supplementary Materials. Open circles represent studies with no data for that serotype.
DISCUSSION
Although ficolin-2 reportedly recognizes various microorganisms, only a few microbial ligands are molecularly defined [13, 43, 44]. We demonstrated that ficolin-2 binds pneumococcal capsule but does not bind other major surface carbohydrates. Furthermore, consistent with reports that ficolin-2 binds a subset of encapsulated pneumococci [14, 20], ficolin-2 specifically recognized all wcjE-containing serotypes except ST9V and ST20B. Putative wcjE-dependent capsule features included βGal6-OAc in serogroup 11; β-galactofuranose-5,6-OAc in ST20A, ST20B, ST33A, and ST35A; and β-galactofuranose-3,5-OAc in ST47A (Table 1) [1, 8, 22, 35]. Therefore, in addition to microbial ligands containing N-acetylated sugars (eg, GlcNAc and sialic acid) [13, 43, 44], ficolin-2 binds O-acetylated carbohydrates, independent of N-acetylation. It is unclear why ficolin-2 does not bind ST9V PS, which contains wcjE-dependent β-N-acetyl-mannosamine-6-OAc [36], or ST20B, which contains glucosylation absent in ST20A PS [1]. Clearly, however, WcjE-OAc alone is insufficient for ficolin-2 binding.
It is unclear whether the lectin complement pathway contributes to protection against pneumococcal disease in humans. We report that ficolin-2 mediated complement deposition on ST11A pneumococci, even at low serum levels and in the absence of C1q (ie, conditions that minimize the contributions of the alternative and classical pathways, respectively), and this complement deposition was associated with ficolin-2–dependent phagocytic killing of ST11A bacteria. We did not directly evaluate whether ficolin-2–associated complement deposition proceeded through a C4/C2 bypass pathway, as described by others [15]. However, given that C4 deposition preceded C3 deposition here, we propose that ficolin-2–dependent opsonization of certain pneumococcal serotypes is mediated dominantly by the conventional, C4-dependent lectin pathway, as reported for other streptococci [44].
No epidemiological study has demonstrated an association between human ficolin-2 deficiency and increased susceptibility to pathogens demonstrated to be targeted by ficolin-2 in vitro. Thus, it remains unclear whether ficolin-2 imparts innate immunity against infections. In our meta-analysis, ST11A displayed the lowest relative odds of invasiveness, although these odds are actually lower, considering that up to 50% of IPD isolates typed as ST11A express ST11E [9]. This behavior was conserved across individual studies, reflecting a strong innate resistance to ST11A IPD in humans. We propose that ficolin-2 readily clears ST11A strains from blood, inhibits their ability to cause IPD in healthy individuals, and selects for ST11E evolution during invasion. ST11E was not included in our analysis because of the inability to identify it by use of conventional serotyping assays [7], although we hypothesize that ST11E would have a high invasive potential, compared with other serotypes, because of its rarity in carriage [9]. Several serogroups that contain serotypes recognized by ficolin-2 (ie, ST33A, ST35A, and ST20A) also displayed low invasiveness (Figure 6A). However, ST31, despite ficolin-2 binding, demonstrated high invasiveness. ST31 was the least common among the 30 serotypes studied here; thus, its invasiveness was determined with a few strains. Moreover, ST31 has not been studied for subtypes that do not bind ficolin-2, such as 20B. Another explanation could be that ST31 may interact with other innate immune molecules, such as factor H, as shown in a recent study [45].
Although requiring clinical and epidemiological confirmation, our model of ficolin-2–mediated immunity is supported by some initial observations. For example, ficolin-2 serum concentrations appear to decrease with age [46], and we have observed an increase in the occurrence of IPD by ficolin-2–targeted serotypes in older adults, compared with pediatric populations (data not shown). Furthermore, a deficiency in the lectin complement pathway may explain why IPD due to ST11A is associated with poor clinical outcomes [47–49] and immunocompromised individuals with malignancy [4]. By further linking lectins to serotype-specific clinical behaviors, it may be feasible to use the serotypes of patient isolates as clinical markers for immunodeficiencies. Similarly, one can envision using pneumococcus, which expresses a large diversity of capsular polysaccharides, as a naturally available so-called glyco-array for identifying novel innate immune molecules.
Interestingly, we observed inhibition of C3 deposition by PC and pneumococcal TA on ST11E and ST9A, while C3 deposition on ST11A and ST9V was unaffected. Perhaps WcjE-OAc alters pneumococcal surface properties beyond capsule glyco-epitope modification. Indeed, greater quantity of anti-PC antibodies bind to ST11E bacteria than to ST11A bacteria (unpublished data), suggesting that WcjE-OAc may reduce access to LTA, TA, and other structures located within the cell wall on ST11A. These observations can be reconciled with the following model: WcjE-OAc enhances interaction among PS chains and produces capsule with improved shielding capacity. This model can explain why the cell wall is more accessible in ST11E than in ST11A. Furthermore, wcjE-dependent alteration of surface properties may be protective in certain niches such as nasopharynx and represent the evolutionary benefit to conserving wcjE in multiple serotypes.
Full understanding of the impact of WcjE-mediated capsule features has special implications in the design of next-generation pneumococcal vaccines. The current paradigm in pneumococcal vaccine design holds that all serotypes should be equally targeted because their absolute carriage and disease rates increase. However, if certain serotypes are deficient in causing disease yet are able to outcompete potentially harmful serotypes in the nasopharynx, it may be beneficial to allow certain serotypes to become prevalent. Indeed, replacement of vaccine-targeted serotypes by low-invasive serotypes such as ST11A and ST15B/ST15C may contribute to lower-than-expected levels of pneumococcal disease in areas where carriage rates have returned to prevaccination levels [50]. Entertaining the concept of so-called serotype allowance requires full understanding of the mechanisms of serotype-associated virulence. Therefore, further host-pneumococcal interactions, such as this novel link between ficolin-2 recognition and the low invasiveness of ST11A, must be identified and explored.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank W. H. Benjamin Jr, D. E. Briles, S. Michalek, J. D. Mountz, M. C. McEllistrem, and J. P. Atkinson, for critical reading of the manuscript.
A. M. B. and J. J. C. contributed to the project equally. A. M. B., J. J. C., and M. H. N. designed the experiments and wrote the manuscript. A. M. B., J. J. C., K. A. G., and J. Y. performed all laboratory experiments. J. J. C. and G. R. C. performed the meta-analysis. G. R. C. reviewed the manuscript.
Financial support. This work was supported by the National Institutes of Health (grants T32 AI007051 [to A. M. B.], R0-1 AI31473 and R56 AI31473 [to M. H. N.], and F31 A1093103 [to J. J. C.]).
Potential conflicts of interest. The University of Alabama at Birmingham (UAB) has intellectual property rights on some reagents used in the study, and all authors are UAB employees.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Calix JJ, Porambo RJ, Brady AM, et al. Biochemical, genetic and serological characterization of two capsule subtypes among Streptococcus pneumoniae serotype 20 strains: discovery of a new pneumococcal serotype. J Biol Chem. 2012;287:27885–94. doi: 10.1074/jbc.M112.380451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brueggemann AB, Peto TE, Crook DW, Butler JC, Kristinsson KG, Spratt BG. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J Infect Dis. 2004;190:1203–11. doi: 10.1086/423820. [DOI] [PubMed] [Google Scholar]
- 3.Sleeman KL, Griffiths D, Shackley F, et al. Capsular serotype-specific attack rates and duration of carriage of Streptococcus pneumoniae in a population of children. J Infect Dis. 2006;194:682–8. doi: 10.1086/505710. [DOI] [PubMed] [Google Scholar]
- 4.Lujan M, Burgos J, Gallego M, et al. Effects of immunocompromise and comorbidities on pneumococcal serotypes causing invasive respiratory infection in adults: implications for vaccine strategies. Clin Infect Dis. 2013;57:1722–30. doi: 10.1093/cid/cit640. [DOI] [PubMed] [Google Scholar]
- 5.Sa-Leao R, Pinto F, Aguiar S, et al. Analysis of invasiveness of pneumococcal serotypes and clones circulating in Portugal before widespread use of conjugate vaccines reveals heterogeneous behavior of clones expressing the same serotype. J Clin Microbiol. 2011;49:1369–75. doi: 10.1128/JCM.01763-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanage WP, Kaijalainen TH, Syrjanen RK, et al. Invasiveness of serotypes and clones of Streptococcus pneumoniae among children in Finland. Infect Immun. 2005;73:431–5. doi: 10.1128/IAI.73.1.431-435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calix JJ, Nahm MH. A new pneumococcal serotype, 11E, has variably inactivated wcjE gene. J Infect Dis. 2010;202:29–38. doi: 10.1086/653123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calix JJ, Nahm MH, Zartler ER. Elucidation of structural and antigenic properties of pneumococcal serotype 11A, 11B, 11C, and 11F polysaccharide capsules. J Bacteriol. 2011;193:5271–8. doi: 10.1128/JB.05034-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calix JJ, Dagan R, Pelton SI, Porat N, Nahm MH. Differential occurrence of Streptococcus pneumoniae serotype 11E between asymptomatic carriage and invasive pneumococcal disease isolates reflects a unique model of pathogen microevolution. Clin Infect Dis. 2012;54:794–9. doi: 10.1093/cid/cir953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsushita M, Endo Y, Taira S, et al. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J Biol Chem. 1996;271:2448–54. doi: 10.1074/jbc.271.5.2448. [DOI] [PubMed] [Google Scholar]
- 11.Thiel S, Vorup-Jensen T, Stover CM, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–10. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita M. Ficolins: complement-activating lectins involved in innate immunity. J Innate Immun. 2010;2:24–32. doi: 10.1159/000228160. [DOI] [PubMed] [Google Scholar]
- 13.Aoyagi Y, Adderson EE, Rubens CE, et al. L-Ficolin/mannose-binding lectin-associated serine protease complexes bind to group B streptococci primarily through N-acetylneuraminic acid of capsular polysaccharide and activate the complement pathway. Infect Immun. 2008;76:179–88. doi: 10.1128/IAI.00837-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krarup A, Sorensen UB, Matsushita M, Jensenius JC, Thiel S. Effect of capsulation of opportunistic pathogenic bacteria on binding of the pattern recognition molecules mannan-binding lectin, L-ficolin, and H-ficolin. Infect Immun. 2005;73:1052–60. doi: 10.1128/IAI.73.2.1052-1060.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali YM, Lynch NJ, Haleem KS, et al. The lectin pathway of complement activation is a critical component of the innate immune response to pneumococcal infection. PLoS Pathog. 2012;8:e1002793. doi: 10.1371/journal.ppat.1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endo Y, Takahashi M, Iwaki D, et al. Mice deficient in ficolin, a lectin complement pathway recognition molecule, are susceptible to Streptococcus pneumoniae infection. J Immunol. 2012;189:5860–6. doi: 10.4049/jimmunol.1200836. [DOI] [PubMed] [Google Scholar]
- 17.Hummelshoj T, Munthe-Fog L, Madsen HO, Fujita T, Matsushita M, Garred P. Polymorphisms in the FCN2 gene determine serum variation and function of Ficolin-2. Hum Mol Genet. 2005;14:1651–8. doi: 10.1093/hmg/ddi173. [DOI] [PubMed] [Google Scholar]
- 18.Herpers BL, Immink MM, de Jong BA, van Velzen-Blad H, de Jongh BM, van Hannen EJ. Coding and non-coding polymorphisms in the lectin pathway activator L-ficolin gene in 188 Dutch blood bank donors. Mol Immunol. 2006;43:851–5. doi: 10.1016/j.molimm.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 19.Chapman SJ, Vannberg FO, Khor CC, et al. Functional polymorphisms in the FCN2 gene are not associated with invasive pneumococcal disease. Mol Immunol. 2007;44:3267–70. doi: 10.1016/j.molimm.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Kjaer TR, Hansen AG, Sorensen UB, et al. M-ficolin binds selectively to the capsular polysaccharides of Streptococcus pneumoniae serotypes 19B and 19C and of a Streptococcus mitis strain. Infect Immun. 2013;81:452–9. doi: 10.1128/IAI.01148-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calix JJ, Brady AM, Du VY, Saad JS, Nahm MH. Spectrum of pneumococcal serotype 11A variants results from incomplete loss of capsule O-acetylation. J Clin Microbiol. 2014;52:758–65. doi: 10.1128/JCM.02695-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin FL, Vinogradov E, Deng C, et al. Identification of the common antigenic determinant shared by Streptococcus pneumoniae serotypes 33A, 35A, and 20 capsular polysaccharides. Carbohydr Res. 2013;380C:101–7. doi: 10.1016/j.carres.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Sung CK, Li H, Claverys JP, Morrison DA. An rpsL cassette, janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl Environ Microbiol. 2001;67:5190–6. doi: 10.1128/AEM.67.11.5190-5196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyams C, Yuste J, Bax K, Camberlein E, Weiser JN, Brown JS. Streptococcus pneumoniae resistance to complement-mediated immunity is dependent on the capsular serotype. Infect Immun. 2010;78:716–25. doi: 10.1128/IAI.01056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu J, Carvalho Mda G, Beall B, Nahm MH. A rapid pneumococcal serotyping system based on monoclonal antibodies and PCR. J Med Microbiol. 2008;57:171–8. doi: 10.1099/jmm.0.47549-0. [DOI] [PubMed] [Google Scholar]
- 26.Zartler ER, Porambo RJ, Anderson CL, Chen LH, Yu J, Nahm MH. Structure of the capsular polysaccharide of pneumococcal serotype 11A reveals a novel acetylglycerol that is the structural basis for 11A subtypes. J Biol Chem. 2009;284:7318–29. doi: 10.1074/jbc.M807952200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo HS, Cartee RT, Pritchard DG, Nahm MH. A new model of pneumococcal lipoteichoic acid structure resolves biochemical, biosynthetic, and serologic inconsistencies of the current model. J Bacteriol. 2008;190:2379–87. doi: 10.1128/JB.01795-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brady AM, Spencer BL, Falsey AR, Nahm MH. Blood collection tubes influence serum ficolin-1 and ficolin-2 levels. Clin Vaccine Immunol. 2014;21:51–5. doi: 10.1128/CVI.00607-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol. 2006;13:1004–9. doi: 10.1128/CVI.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garlatti V, Martin L, Lacroix M, et al. Structural insights into the recognition properties of human ficolins. J Innate Immun. 2009;2:17–23. doi: 10.1159/000233475. [DOI] [PubMed] [Google Scholar]
- 31.Krarup A, Mitchell DA, Sim RB. Recognition of acetylated oligosaccharides by human L-ficolin. Immunol Lett. 2008;118:152–6. doi: 10.1016/j.imlet.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krarup A, Thiel S, Hansen A, Fujita T, Jensenius JC. L-ficolin is a pattern recognition molecule specific for acetyl groups. J Biol Chem. 2004;279:47513–9. doi: 10.1074/jbc.M407161200. [DOI] [PubMed] [Google Scholar]
- 33.Oliver MB, Jones C, Larson TR, et al. Streptococcus pneumoniae serotype 11D has a bispecific glycosyltransferase and expresses two different capsular polysaccharide repeating units. J Biol Chem. 2013;288:21945–54. doi: 10.1074/jbc.M113.488528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bentley SD, Aanensen DM, Mavroidi A, et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2006;2:e31. doi: 10.1371/journal.pgen.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen BO, Hindsgaul O, Paulsen BS, Redondo AR, Skovsted IC. Structural elucidation of the capsular polysaccharide from Streptococcus pneumoniae serotype 47A by NMR spectroscopy. Carbohydr Res. 2014 doi: 10.1016/j.carres.2013.11.013. 386:62–7. [DOI] [PubMed] [Google Scholar]
- 36.Calix JJ, Saad JS, Brady AM, Nahm MH. Structural characterization of Streptococcus pneumoniae serotype 9A capsule polysaccharide reveals role of glycosyl 6-O-acetyltransferase wcjE in serotype 9V capsule biosynthesis and immunogenicity. J Biol Chem. 2012;287:13996–4003. doi: 10.1074/jbc.M112.346924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones C, Lemercinier X. Full NMR assignment and revised structure for the capsular polysaccharide from Streptococcus pneumoniae type 15B. Carbohydr Res. 2005;340:403–9. doi: 10.1016/j.carres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Nahm MH, Bush CA, Cisar JO. Comparative structural and molecular characterization of Streptococcus pneumoniae capsular polysaccharide serogroup 10. J Biol Chem. 2011;286:35813–22. doi: 10.1074/jbc.M111.255422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calix JJ, Oliver MB, Sherwood LK, Beall BW, Hollingshead SK, Nahm MH. Streptococcus pneumoniae serotype 9A isolates contain diverse mutations to wcjE that result in variable expression of serotype 9V-specific epitope. J Infect Dis. 2011;204:1585–95. doi: 10.1093/infdis/jir593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moller-Kristensen M, Thiel S, Sjoholm A, Matsushita M, Jensenius JC. Cooperation between MASP-1 and MASP-2 in the generation of C3 convertase through the MBL pathway. Int Immunol. 2007;19:141–9. doi: 10.1093/intimm/dxl131. [DOI] [PubMed] [Google Scholar]
- 41.Mold C, Rodic-Polic B, Du Clos TW. Protection from Streptococcus pneumoniae infection by C-reactive protein and natural antibody requires complement but not Fc gamma receptors. J Immunol. 2002;168:6375–81. doi: 10.4049/jimmunol.168.12.6375. [DOI] [PubMed] [Google Scholar]
- 42.Kaplan MH, Volanakis JE. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974;112:2135–47. [PubMed] [Google Scholar]
- 43.Lynch NJ, Roscher S, Hartung T, et al. L-ficolin specifically binds to lipoteichoic acid, a cell wall constituent of Gram-positive bacteria, and activates the lectin pathway of complement. J Immunol. 2004;172:1198–202. doi: 10.4049/jimmunol.172.2.1198. [DOI] [PubMed] [Google Scholar]
- 44.Aoyagi Y, Adderson EE, Min JG, et al. Role of L-ficolin/mannose-binding lectin-associated serine protease complexes in the opsonophagocytosis of type III group B streptococci. J Immunol. 2005;174:418–25. doi: 10.4049/jimmunol.174.1.418. [DOI] [PubMed] [Google Scholar]
- 45.Hyams C, Trzcinski K, Camberlein E, et al. Streptococcus pneumoniae capsular serotype invasiveness correlates with the degree of factor H binding and opsonisation with C3b/iC3b. Infect Immun. 2013;81:354–63. doi: 10.1128/IAI.00862-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sallenbach S, Thiel S, Aebi C, et al. Serum concentrations of lectin-pathway components in healthy neonates, children and adults: mannan-binding lectin (MBL), M-, L-, and H-ficolin, and MBL-associated serine protease-2 (MASP-2) Pediatr Allergy Immunol. 2011;22:424–30. doi: 10.1111/j.1399-3038.2010.01104.x. [DOI] [PubMed] [Google Scholar]
- 47.Harboe ZB, Thomsen RW, Riis A, et al. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med. 2009;6:e1000081. doi: 10.1371/journal.pmed.1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inverarity D, Lamb K, Diggle M, et al. Death or survival from invasive pneumococcal disease in Scotland: associations with serogroups and multilocus sequence types. J Med Microbiol. 2011;60:793–802. doi: 10.1099/jmm.0.028803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Hoek AJ, Andrews N, Waight PA, George R, Miller E. Effect of serotype on focus and mortality of invasive pneumococcal disease: coverage of different vaccines and insight into non-vaccine serotypes. PLoS One. 2012;7:e39150. doi: 10.1371/journal.pone.0039150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378:1962–73. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.