Abstract
Background. Although virus-specific CD4+ T lymphocytes emerge rapidly during primary cytomegalovirus (CMV) infection in humans, they exhibit a state of prolonged functional exhaustion of unknown etiology. To investigate the suitability of rhesus macaques as a model of primary human CMV infection, we examined the virologic and immunologic features of naturally acquired primary CMV infection in rhesus macaques.
Methods. CMV-specific CD4+ T lymphocytes and CMV load in blood, saliva, and urine were evaluated in a cohort of simian immunodeficiency virus (SIV)–negative rhesus macaques stratified by age into infant, juvenile, and adult groups.
Results. CMV infection was detected in juvenile and adult monkeys but not in infant monkeys. CMV loads and shedding frequency in urine and saliva were significantly higher in the 2–3-year old juvenile monkeys, compared with the adult monkeys. The increased CMV load in juvenile monkeys was associated with lower polyfunctionality, impaired proliferation, and increased expression of the inhibitory receptor PD-1 in CMV-specific CD4+ T lymphocytes. The proliferative defect was partially reversible by exogenous PD-1 blockade or addition of interleukin 2.
Conclusions. Postnatal acquisition of primary CMV infection in rhesus macaques results in prolonged virus excretion and impaired CMV-specific CD4+ T-lymphocyte function, findings that recapitulate key features of primary CMV infection in humans.
Keywords: cytomegalovirus, primary cytomegalovirus infection, rhesus macaque, viral excretion, CD4+ T lymphocyte, functional exhaustion, PD-1, IL-2, IFN-γ, TNF-α
Cytomegalovirus (CMV) is a herpesvirus establishing life-long persistence after primary infection. CMV infection can cause severe manifestations in immunocompromised subjects and fetuses but is usually asymptomatic in immunocompetent hosts. Data from several studies support an important role for CD4+ T lymphocytes in the control of human CMV infection. Early appearance of functional CMV-specific CD4+ T cells is associated with asymptomatic infections after kidney transplantation [1] and with a lower risk of viremia after hematopoietic stem cell transplantation [2]. In chronic infection, CMV-specific CD4+ T cells acquire direct antiviral functions and are pivotal for optimal B and CD8+ T-lymphocyte responses [3]. CMV-specific CD4+ T cells produce multiple cytokines, including interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), and interleukin 2 (IL-2), have potent proliferative capacity and can display major histocompatibility complex class II–restricted cytotoxicity [4, 5].
Asymptomatic primary CMV infection in immunocompetent hosts, particularly children, often leads to virus shedding in bodily secretions for a prolonged duration, suggesting incomplete immune control [6, 7]. Following primary infection, CMV-specific CD4+ T cells emerge rapidly but acquire the capacity to proliferate in vitro in response to viral antigens only several months after seroconversion [1, 7, 8]. While the delayed acquisition of functional CD4+ T-lymphocyte responses in primary infection is likely to contribute to the incomplete control of CMV replication, the etiology of functional CD4+ T-lymphocyte exhaustion remains uncertain. We recently observed that the slow acquisition of proliferative responses in primary CMV infection is associated with a reduced capacity to produce cytokines, including IL-2, IFN-γ, and TNF-α [9]. Clinical studies suggest that this functional impairment is a risk factor for mother-to-fetus transmission of CMV and for CMV retinitis in human immunodeficiency virus (HIV)–infected patients [10–13]. Thus, development of therapeutic strategies to prevent the clinical complications associated with primary CMV infection will require a better understanding of the mechanisms compromising its immune control.
Rhesus macaques are currently the best available animal model of human CMV infection [14]. CMV infection is widely prevalent among captive group-housed rhesus macaques, and rhesus CMV shares close genetic similarity to human CMV [15–17]. Similar to humans, rhesus CMV infection is asymptomatic in immunocompetent macaques, and CMV reactivation-associated disease manifests only in the setting of immunosuppression [18–20]. In contrast to chronic infection, there are limited data on the virologic and immunologic events in naturally acquired primary rhesus CMV infection. We and others have shown that maternal antibodies to CMV wane around 1 year of age and that seroconversion following natural infection occurs soon thereafter [15, 18]. Furthermore, hand-reared newborn macaques separated at birth from their mothers remain CMV seronegative after loss of maternal CMV antibodies [18], suggesting that natural CMV acquisition occurs in the postnatal period. In this study, we investigated the relationship between viral load and CMV-specific CD4+ T-lymphocyte responses at different time points following natural CMV acquisition in rhesus macaques.
MATERIAL AND METHODS
Study Groups
In a cross-sectional study, 51 group-housed Indian rhesus macaques free of simian type D retrovirus, simian T-lymphotropic virus type 1, simian immunodeficiency virus, and herpes B virus infections in the specific-pathogen-free colony of the New England Primate Research Center (NEPRC) were enrolled and stratified into 4 age-based groups; 12–15-month-old infants (n = 8) designated as 1 year old; 25–33-month-old monkeys (n = 16), designated as 2 years old; 35–43-month-old monkeys (n = 9), designated as 3 years old; and >5-year-old monkeys (n = 18), designated as adults. Heparinized blood was collected from all monkeys. For 41, urine (clean-catch) and saliva (mouthwash with phosphate-buffered saline) specimens were also collected. Appropriate guidelines were followed for animal experimentation studies, and all animals were maintained in accordance with institutional and federal guidelines for animal care [21].
Measurement of CMV DNA by Real-Time Polymerase Chain Reaction (PCR)
Urine and mouthwash specimens were concentrated using Ultracel YM-30 filters (Amicon) and frozen for subsequent DNA extraction. DNA was extracted from urine by using the QIAmp RNA mini kit (Qiagen, Valencia, CA) and from saliva and plasma by using the QIAmp DNA mini kit. CMV DNA was quantitated by real-time PCR as previously described [19], and data are expressed as copies per milliliters of plasma or copies per microgram of input DNA in saliva and urine.
Intracellular Cytokine Staining Assay
Before intracellular cytokine staining, cryopreserved peripheral blood mononuclear cells (PBMCs) were stimulated with whole-virus rhesus CMV lysate antigen or pools of 15-mer peptides spanning the rhesus CMV pp65, IE1, and IE2 proteins, as previously described [19, 22]. Cells were stimulated for 6 hours at 37°C and 5% CO2, and brefeldin A (GolgiPlug, BD Bioscience) was added for the last 4 hours of stimulation. Cells were stained using the following antihuman antibodies: CD3 Pacific Blue, CD4 PerCP-Cy5.5, CD95 FITC, CD28 ECD, PD-1 APC, Tim-3 PE, IFN-γ PE-Cy7, IL-2 PE, TNF-α APC, and CD69 APC-Cy7 (all from BD Bioscience, except CD28, which is from Beckman Coulter, PD-1 clone EH12.2H7, which is from Biolegend, and Tim-3 clone 344 823 which is from R&D). Cytokines and CD69 were stained intracellularly after cell fixation and permeabilization, using the CALTAG Fix & Perm Kit (CALTAG) according to the manufacturer's instructions.
Assessment of Proliferative Responses
Freshly isolated PBMCs were cultured in Roswell Park Memorial Institute 1640 medium containing 10% fetal calf serum, Pen/Strep, and glutamine in 96-well plates. Cells were stimulated for 6 days with lysates of CMV-infected or uninfected fibroblasts, pulsed with BrdU for the last 18 hours of stimulation, and stained according to manufacturer's instructions with CD3 Pacific Blue, CD4 PerCP-Cy5.5, Ki67 FITC, and BrdU APC (all from BD Bioscience). For functional experiments, exogenous IL-2 or antibodies directed against PD-1 (5 µg/mL) and Tim-3 (5 µg/mL) were added at initiation of the stimulation period.
Statistical Analyses
Flow cytometry data were acquired on a BD LSRII cytometer (BD Bioscience) and analyzed using FlowJo software, version 8.8.6 (TreeStar). Unpaired and paired comparisons of 2 groups were performed by the nonparametric Mann–Whitney and Wilcoxon signed rank tests, respectively. The Kruskal–Wallis test and the Dunn post hoc test were used for multiple comparisons. The analysis of CD4+ T-cell polyfunctionality was performed using SPICE software, version 5.2 (Exon, National Institutes of Allergy and Infectious Diseases, National Institutes of Health). The polyfunctionality index was computed as described by Larsen et al [23].
RESULTS
Viral Excretion Varies With Age in Naturally Infected Rhesus Macaques
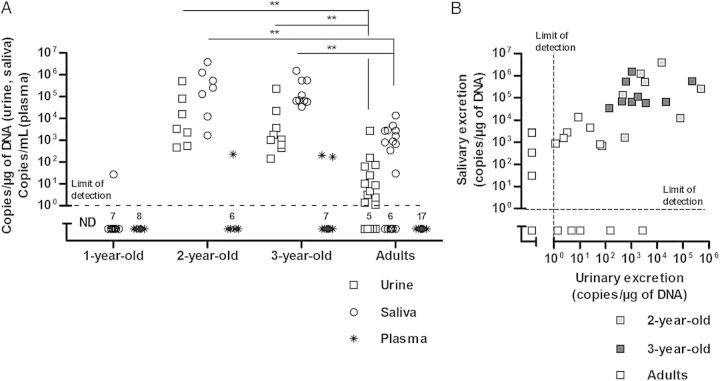
To characterize the kinetics of natural CMV infection in rhesus macaques, quantification of CMV replication was performed in urine, saliva, and plasma specimens from eight 1-year-old, seven 2-year-old, nine 3-year-old, and 17 adult macaques (Figure 1A). Only a single 1-year-old monkey (12%) excreted virus in saliva, whereas all 2- and 3-year-old monkeys excreted CMV in their urine and saliva (Figure 1A). Interestingly, even in the presence of viral shedding in all 2- and 3-year-old monkeys, CMV was detected in plasma specimens from only 3 of 16 animals. CMV excretion was also detected in 71% of urine samples and 65% of saliva samples in adults but was of significantly lower magnitude, compared with observations for the 2- and 3-year-old groups (Figure 1A). Because of the similar viral excretion patterns, the 2- and 3-year-old monkeys were henceforth grouped together as juveniles. While excretion in both saliva and urine was the rule in juvenile animals, only 41% of adult animals showed a similar pattern (Figure 1B). Excretion was restricted to saliva or urine in 24% and 29% of adult animals, respectively, and 1 animal (6%) showed no excretion (Figure 1B). Together, these results indicate that monkeys in the juvenile age group display intense viral replication and shedding following natural rhesus CMV infection.
Figure 1.
A, Viral loads in bodily fluids and plasma according to age. Cytomegalovirus (CMV) DNA was detected in urine, saliva, and plasma specimens from 1-year-old, 2-year-old, 3-year-old, and adult group-housed rhesus macaques. Individual data are represented. Two-year-old, 3-year-old, and adult shedders were compared using the Kruskal–Wallis test (P < .001 for excretion in urine and saliva specimens). The Dunn posttest was used to compare adult excretors to 2-year-old and 3-year-old monkeys, by pairs (P < .01, for all combinations). B, Viral excretion in urine and saliva in 2-year-old, 3-year-old, and adult rhesus macaques. Abbreviation: ND, not done.
Reduced Polyfunctionality of CMV-Specific CD4+ T Lymphocytes in Juvenile Rhesus Macaques
CMV-specific CD4+ T lymphocytes were detected after intracellular cytokine staining for IFN-γ, IL-2, and TNF-α following stimulation with rhesus CMV antigens. Because of change in the size of the naive and memory CD4+ T-cell compartment with age [24], the frequency of CMV-specific responder cells was expressed as a fraction of the CD95+ memory CD4+ T-lymphocyte compartment. CMV-specific CD4+ T cells were defined as cells coexpressing the activation marker CD69 and at least 1 of the 3 cytokines on stimulation with the rhesus CMV antigens. The total frequency of cytokine-producing cells was determined by combining the 3 cytokine-specific gates with an ‘or’ conjunction, using FlowJo software.
CMV-specific CD4+ T-cell responses against the rhesus CMV lysate were detectable in juvenile and adult but not infant rhesus macaques. The magnitude of the individual and total cytokine response to CMV lysate stimulation was significantly lower in juvenile monkeys, as compared to adult monkeys (Figure 2A). A similar trend was observed for responses to rhesus CMV peptide pools (Figure 2B). To further investigate the quality of the CMV-specific CD4+ T-lymphocyte response, all possible combinations of simultaneous production of IFN-γ, IL-2, and TNF-α were analyzed using the SPICE software (Figure 3). The dominant CMV-specific CD4+ T-lymphocyte response was distributed among 3 major subsets: single TNF-α+, double IFN-γ+/TNF-α+, and triple IFN-γ+/IL-2+/TNF-α+. While the frequency of single-positive cells was comparable in juvenile and adults monkeys, frequencies of double- and especially triple-positive cells were significantly reduced in juveniles (Figure 3A). Thus, the CMV-specific CD4+ T-cell pool in juveniles contained a smaller fraction of polyfunctional cells, compared with adult macaques (Figure 3B). This was confirmed calculating the polyfunctionality index developed by Larsen et al [23], which was significantly lower in juvenile as compared to adult macaques (Figure 3B).
Figure 2.
Frequency of cytokine-producing CD4+ T lymphocytes. Cytokine-producing cells among memory CD4+ T lymphocytes were measured by intracellular cytokine staining following stimulation with lysate of rhesus cytomegalovirus (CMV) whole-virus antigen (A) and rhesus CMV peptide pools (B). Individual data and median values are shown. Juvenile and adult rhesus macaques were compared using the Mann–Whitney test (P > .05 for pp65 [total] and IE2 [total]; P < .05 for CMV [total], CMV [interferon γ {IFN-γ}], CMV [tumor necrosis factor α {TNF-α}], and IE1 [total]; P < .01 for CMV [interleukin 2 {IL-2}]).
Figure 3.
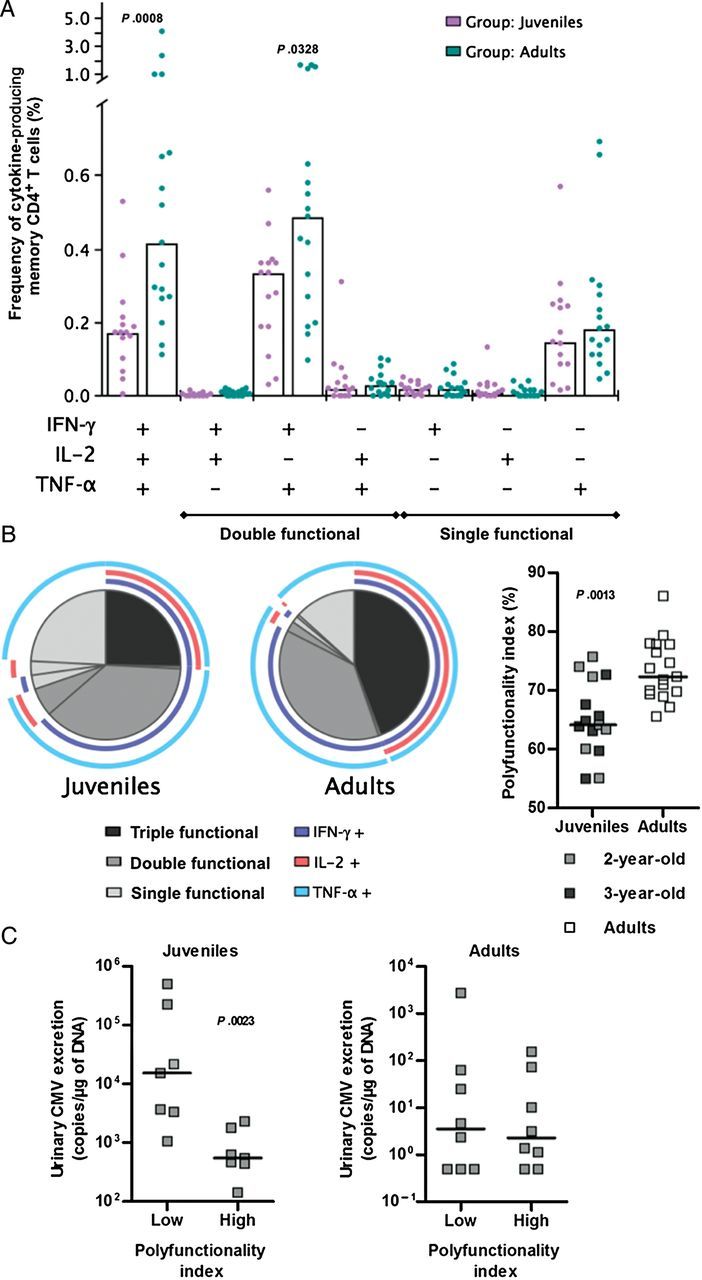
A, Distribution of CMV-specific CD4+ T cells among functional subsets. CD4+ T lymphocytes responding to the whole cytomegalovirus (CMV) lysate are distributed in functional subsets according to the production of interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), and interleukin 2 (IL-2). Individual data after subtraction of background and median values are shown. The Mann–Whitney test was used for group comparison (P < .05 for IFN-γ+ IL-2− TNF-α+ CD4+ T cells, P < .001 for triple-positive CD4+ T cells). B, Polyfunctionality of CMV-specific CD4+ T cells. The pie charts in the left panel illustrate the proportion of cells producing 1 (pale grey), 2 (medium grey), and 3 (dark grey) cytokines among total responding cells in juvenile and adult rhesus macaques. Colored arcs show individual cytokines. In the right panel, the polyfunctionality index was calculated for juvenile and adult macaques in response to the whole CMV lysate, and values were compared using the Mann–Whitney test (individual data and medians are shown; P < .01). C, Relationship between urinary CMV excretion and CD4+ T-cell polyfunctionality. Animals have been stratified according to the polyfunctionality index of CMV-specific CD4+ T cells (low: index below median; high: index above median). Viral excretion in urine specimens was compared between these subgroups, using the Mann–Whitney test. Individual data and median values are shown (P < .01 for juveniles, P > .05 for adults).
The function of CMV-specific CD4+ T cells in juvenile and adult rhesus macaques could be modulated both by age and viral replication. To eliminate age-related bias, the relationship between the polyfunctionality index of CMV-specific CD4+ T cells and viral excretion in urine or saliva was studied separately in juvenile and adult monkeys. While the magnitude of viral excretion in adult monkeys was unaffected by the polyfunctionality index, juvenile macaques with a low index showed significantly higher levels of urinary excretion (Figure 3C). Of note, this difference was not observed for salivary CMV excretion (data not shown).
Decreased Proliferative Capacity of CMV-Specific CD4+ T Lymphocytes in Juvenile Rhesus Macaques
Low proliferative responses are the hallmark of CD4+ T-cell responses in primary human CMV infection [7, 8]. To evaluate the proliferative ability of CMV-specific CD4+ T cells, we measured CD4+ T-cell proliferation in response to rhesus CMV lysate stimulation, using the BrdU incorporation assay. To avoid underestimation of the proliferative ability due to a higher proportion of naive cells in juvenile monkeys, the proliferation rate was expressed as follows: [Ki67pos BrdUpos among CD4+ T cells]/[proportion of memory cells among CD4+ T cells]. CD4+ T-lymphocyte proliferation in response to CMV stimulation was significantly lower in juvenile as compared to adult macaques (Figure 4A). This proliferative difference was not due to the lower frequency of CMV-specific CD4+ T cells in juvenile monkeys, since it was also evident among juvenile and adult monkeys with comparable frequencies of cytokine-producing cells (Figure 4B).
Figure 4.
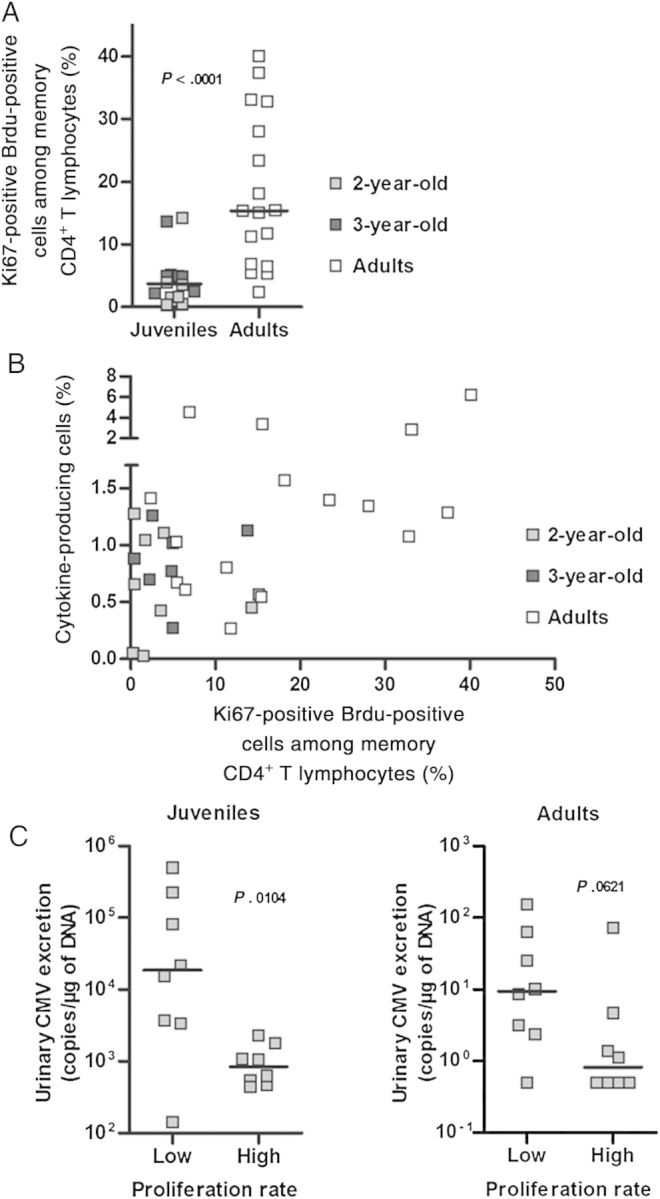
A, Cytomegalovirus (CMV)–specific CD4+ T-cell proliferative responses. Proliferating CD4+ T cells in response to whole CMV lysate were detected using the BrdU incorporation assay. Frequencies of Ki67+BrdU+ CD4+ T cells are corrected for the proportion of memory cells among the total CD4+ T lymphocyte population. Individual data and median values are shown. The Mann–Whitney test was used for group comparison (P < .0001). B, Relationship between cytokine-producing CD4+ T cells and proliferative response to whole CMV lysate. Data on individual monkeys are shown. C, Relationship between urinary CMV excretion and CMV-specific CD4+ T-cell proliferative responses. Animals have been stratified according to the CD4+ T-cell proliferative response (low: proportion below median; high: proportion above median). Viral excretion in urine specimens was compared between these subgroups, using the Mann–Whitney test. Individual data and median values are shown (P < .05 for juveniles, P < .07 for adults).
Similar to the relationship between viral excretion and polyfunctionality of CMV-specific CD4+ T lymphocytes, we observed higher levels of urinary but not salivary excretion of CMV in monkeys with low proliferation capacity (Figure 4C and data not shown). Interestingly, this relationship was observed in both juvenile and adult monkeys. These results indicate that CMV-specific CD4+ T cells have a lower proliferative capacity in animals with recent infection (juveniles) as compared to those with chronic infection (adults). Additionally, there is an age-independent inverse association between proliferative ability of CMV-specific CD4+ T cells and urinary CMV excretion.
Increased PD-1 Expression Contributes to Functional Exhaustion of CMV-Specific CD4+ T Lymphocytes
The association between lower polyfunctionality and reduced proliferative capacity of CD4+ T lymphocytes led us to evaluate the effect of exogenous IL-2 on CMV-specific CD4+ T-cell proliferation. A dose-dependent increase in CD4+ T-cell proliferative responses to rhesus CMV stimulation was observed in the presence of IL-2 in both juvenile and adult monkeys and resulted in abrogation of proliferative response differences between the 2 groups (Figure 5A). This supports the idea that a reduced IL-2 production capacity contributes to the defective proliferative ability of CMV-specific CD4+ T lymphocytes.
Figure 5.
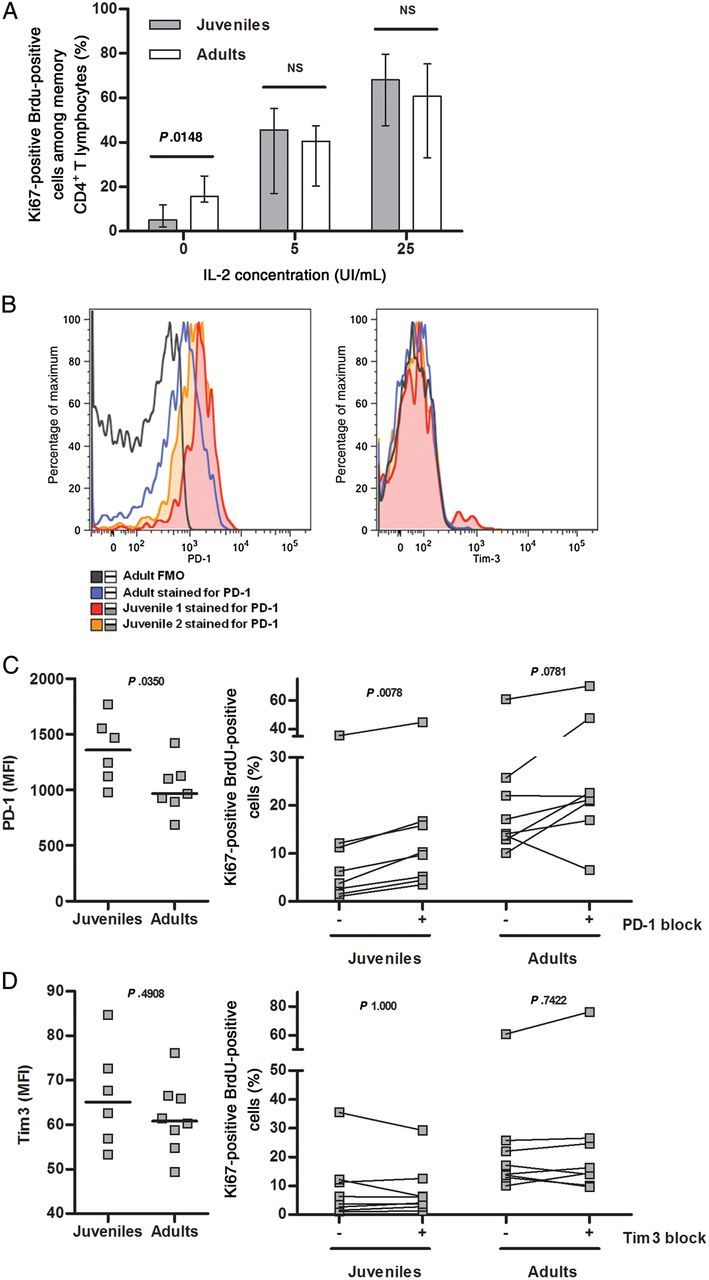
A, Effect of exogenous interleukin 2 (IL-2) on cytomegalovirus (CMV)–specific CD4+ T-cell proliferative responses. CD4+ T-lymphocyte proliferative responses to whole CMV lysate were measured in the absence or presence of exogenous IL-2. Median and interquartile ranges are shown. Values for juveniles and adults are compared at each concentration, using the Mann–Whitney test (P < .05 in the absence of exogenous IL-2). NS, not significant. B, PD-1 and Tim-3 expression on CMV-specific CD4+ T cells. Representative overlay histograms showing PD-1 (left) and Tim-3 (right) staining on interferon γ (IFN-γ)–producing CD4+ T cells stimulated by CMV lysate. Data are for 1 representative adult and 2 juvenile monkeys. Fluorescence minus one (FMO) staining–negative controls were used to establish background signal. C, Assessment of PD-1 impact on CMV-specific CD4+ T-cell function. PD-1 expression was evaluated by median fluorescence intensity (MFI) measurement on CMV-specific IFN-γ–producing CD4+ T cells (left; P < .05, by the Mann–Whitney test). The effect of PD-1 blockade on CD4+ T-cell proliferative responses to CMV lysate was assessed (right; P < .01 for juveniles and P > .05 for adults, by the Wilcoxon test). D, Assessment of Tim-3 impact on CMV-specific CD4+ T-cell function. Tim-3 expression was evaluated by MFI measurement on CMV-specific IFN-γ–producing CD4+ T cells (left panel, P > .05 using the Mann–Whitney test). The effect of Tim-3 blockade on CD4+ T-cell proliferative responses to CMV lysate was assessed (right; P > .05 for juveniles and adults, using the Wilcoxon test).
The inhibitory pathways PD-1 and Tim-3 play a central role in the functional exhaustion associated with chronic viral infections [25]. We recently showed that PD-1 but not Tim-3 controls proliferative responses of CD4+ T lymphocytes following primary human CMV infection [9]. Evaluation of CMV-responsive IFN-γ–producing cells revealed PD-1 but not Tim-3 expression on CMV-specific CD4+ T cells (Figure 5B). The median fluorescence intensity (MFI) of PD-1 expression was significantly higher in juvenile as compared to adult macaques (Figure 5C). No significant difference in the Tim-3 MFI was observed between the 2 groups (Figure 5D). Consistent with the described phenotype, PD-1–blocking antibodies significantly increased proliferative responses in all juvenile monkeys, but this effect was observed in only a subset of adults (Figure 5C). Tim-3 blockade had no effect on proliferative capacity (Figure 5D). These results indicate that the inhibitory pathway mediated by PD-1 is involved in the functional impairment of CMV-specific CD4+ T lymphocytes observed in the context of intense viral replication seen in recent CMV infection.
DISCUSSION
The aim of this cross-sectional study was to characterize the natural history of primary CMV infection in rhesus macaques and to analyze the relationship between viral replication and the quality of the CD4+ T-cell response. Primary human CMV infection is associated with functional exhaustion of virus-specific CD4+ T cells [1, 7–9], and the exhausted phenotype correlates with reduced viral control and clinical complications [10–13]. This study is the first to demonstrate that natural primary CMV infection in rhesus macaques recapitulates the virologic and immunologic features associated with human infection.
Viral excretion was observed in all animals >2 years of age but was absent in all but a single 1-year-old infant. Although urinary CMV excretion could not be examined soon after birth, the presence of congenital CMV infection appeared unlikely in the absence of CMV viremia, salivary CMV shedding, and CMV-specific T-cell responses. Thus, naturally acquired CMV infection appears to occur between 1 and 2 years of age in rhesus macaques, coinciding temporally with waning anti-CMV maternal antibodies and onset of seroconversion [15, 18]. Because group-housed rhesus macaques acquire CMV infection before sexual maturity, it is likely that horizontal transmission via exposure to CMV-positive mucosal secretions during play, biting, scratching, and grooming are the common modes of natural CMV acquisition in macaques.
The delayed postnatal acquisition of CMV in rhesus macaques as opposed to humans is particularly striking. In a prospective study of mother-to-child transmission of CMV in Gambia, 85% of children were infected by 1 year of age, CMV infection was commonly acquired during the early postnatal period even in infants born with high titers of anti-CMV maternal antibodies, and postnatal CMV acquisition was correlated with the magnitude and frequency of CMV excretion in colostrum and vaginal secretions [26]. Why a similar pattern is not observed in infant rhesus macaques is not clear. Macaque maternal factors contributing to delayed postnatal CMV acquisition could include differences in immunological factors and low magnitude or frequency of CMV shedding in breast milk and genital secretions. Because group-housed macaques are at risk for repeated CMV reinfections, it is also possible that preconceptual immunity is more robust in female rhesus macaques, and transplacental transfer of multiple strain-specific antibodies may provide broad protection to infants. Differences in mucosal defense mechanisms in infant macaques could also contribute to resistance against postnatal CMV infection. Elucidating mechanisms of protection against early CMV infection in rhesus macaques has important implications for CMV vaccine development to block mother-to-child transmission.
CMV was also commonly excreted in chronically infected adults, but the excretion pattern was heterogeneous. Consistent with previous studies in naturally or experimentally infected adult macaques [27, 28], we observed that >90% of adult monkeys excreted CMV in either saliva or urine or both but that a significant proportion of the animals excreted the virus at only one of the 2 sites. Not only was CMV shedding less common in adult macaques, it was also less intense as compared to that in juvenile animals. Indeed, viral loads detected in saliva or urine specimens from adult shedders were lower than viral loads measured in those of juvenile monkeys. Together, these results indicate that natural primary CMV infection in juvenile rhesus macaques is associated with intense viral excretion that persists at substantially lower levels in chronically infected adult animals. Of note, CMV viremia was rarely detected in the juvenile and adult macaques in this study. This is perhaps not surprising given that plasma viremia is only detected transiently in rhesus macaques following experimental CMV infection ([28, 29] and Kaur et al, unpublished data).
The detection of higher frequencies of CMV-specific CD4+ T lymphocytes in adult as compared to juvenile animals did not appear to be related to the intensity of viral excretion, since we did not detect a relationship between urine or saliva viral loads and the frequency of CMV-specific CD4+ T cells (data not shown). Chronic CMV infection in humans and mice is associated with the progressive accumulation of virus-specific CD4+ T cells, a phenomenon called memory inflation [30]. Although the phenomenon could not be explored in this cross-sectional study, it is plausible that the higher magnitude of CMV-specific CD4+ T cells in adult rhesus macaques may in part be explained by memory inflation. In contrast to frequency, the quality of CMV-specific CD4+ T cells was markedly affected by the intensity of viral replication. Indeed, the polyfunctionality and proliferative capacity of CMV-specific CD4+ T cells in juvenile monkeys was profoundly reduced, compared with that in adult monkeys. Furthermore, both polyfunctionality and proliferation were inversely associated with urinary excretion in juvenile animals. A similar inverse association in adults was only observed with CMV-specific CD4+ T-cell proliferation but not with polyfunctionality, suggesting that proliferative ability is a more sensitive marker of the functional regulation of CD4+ T cells, as observed in chronic viral infections in humans and animals [25, 31]. The association between urinary viral excretion and proliferative responses in adults suggests a role for ongoing viral replication in maintaining functional exhaustion of CD4+ T cells. However, these effects were more pronounced in juvenile macaques, and thus it is possible that age-related immune development mechanisms also contribute to the CD4+ T-cell exhaustion phenotype of primary rhesus CMV infection. Interestingly, no association was observed between CD4+ T-cell function and CMV shedding in saliva specimens, suggesting that different immune mechanisms control viral replication in the kidney and salivary glands. In support of this hypothesis, studies of CMV-infected children indicate that viral shedding decreases more rapidly with age in saliva than in urine and that cessation of viruria is associated with increased T-cell proliferative responses [6, 32]. The reduced proliferation of CMV-specific CD4+ T cells in juvenile monkeys was associated with a reduced proportion of cells producing IL-2. Addition of exogenous IL-2 increased the proliferative responses in both juvenile and adult animals and resulted in similar proliferative responses in both groups, suggesting that the defective proliferation at least partly involves a reduction in the production of IL-2. Juvenile CD4+ T cells also expressed higher levels of PD-1, and blockade of PD-1 improved cell proliferation. The findings of decreased polyfunctionality and decreased proliferative responses of CMV-specific CD4+ T cells that respond to exogenous IL-2 or PD-1 blockade replicate the results observed in human primary CMV infection [9, 33].
The function of CD4+ T cells is an established correlate of disease activity in chronic viral infections including those due to HIV, hepatitis B virus (HBV), and hepatitis C virus [34–43]. The association between intense viral replication and exhaustion of CD4+ T lymphocytes can be related to: 1) the functional regulation of T cells by prolonged exposure to high antigen loads; 2) reduced control of viral replication by exhausted T cells. In patients infected with HIV or HBV, T-cell exhaustion can be reversed by reducing viral load with antiviral treatments [40, 43, 44]. On the other hand, direct evidence that T-cell exhaustion limits viral control comes from the study of SIV-infected rhesus macaques in which PD-1 blockade reduces viral replication through a quantitative and qualitative improvement of B and CD8+ T-cell responses [45]. Targeting exhausted CD4+ T cells through PD-1 or other regulatory pathways during primary CMV infection may also improve protective immunity and viral control. Although this report does not provide information on anti-CMV CD8+ T lymphocytes, this subset could also be functionally regulated during primary CMV infection and therefore represent a therapeutic target. These therapeutic approaches will have to be evaluated carefully as PD-1 blockade was recently shown to increase immunopathology in a mouse model of persistent LCMV infection [46]. PD-1–deficient mice have also been shown to be highly susceptible to MHV-3 hepatitis [47]. In humans, PD-1 blockade has been used in cancer and was associated with autoimmune manifestations, such as pneumonitis, thyroiditis, and hepatitis [48, 49]. Rhesus macaques are a potentially valuable model to evaluate the safety and efficacy of therapeutic interventions relevant to primary human CMV infection. Thus, the effect of exogenous IL-2 or PD-1 blockade during primary CMV viremia and impaired CD4+ T-cell functionality could first be tested in the rhesus macaque CMV model.
In conclusion, this report demonstrates that primary CMV infection in rhesus macaques recapitulates virologic and immunologic features of human infection and therefore represents a relevant model to study the pathogenesis of primary CMV infection and to evaluate prophylactic and therapeutic interventions aimed at improving viral control.
Notes
Acknowledgments. We thank the staff of the NEPRC Veterinary Resources Department, for assistance in conducting this study; and Dr Martin Larsen (UPMC Université Paris 06, Paris, France), for the calculation of polyfunctionality indexes.
Financial support. This work was supported by the National Institutes of Health Public Health Services (grant P51OD011103 to the NEPRC), the Fonds de la Recherche Scientifique (to P. A. and A. M.), and the Communauté Française de Belgique (to P. A.).
Potential conflicts of interest. The Institute for Medical Immunology is cofunded by the Walloon Region and GlaxoSmithKline Vaccines. A. M. has served as a consultant for GlaxoSmithkline Vaccines and Hookipa Biotech. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gamadia LE, Remmerswaal EB, Weel JF, Bemelman F, van Lier RA, Ten Berge IJ. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood. 2003;101:2686–92. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- 2.Pourgheysari B, Piper KP, McLarnon A, et al. Early reconstitution of effector memory CD4+ CMV-specific T cells protects against CMV reactivation following allogeneic SCT. Bone Marrow Transplant. 2009;43:853–61. doi: 10.1038/bmt.2008.403. [DOI] [PubMed] [Google Scholar]
- 3.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4(+) T cells in immunity to viruses. Nat Rev Immunol. 2012;12:136–48. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casazza JP, Betts MR, Price DA, et al. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006;203:2865–77. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appay V, Zaunders JJ, Papagno L, et al. Characterization of CD4(+) CTLs ex vivo. J Immunol. 2002;168:5954–8. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 6.Tu W, Chen S, Sharp M, et al. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J Immunol. 2004;172:3260–7. doi: 10.4049/jimmunol.172.5.3260. [DOI] [PubMed] [Google Scholar]
- 7.Zanghellini F, Boppana SB, Emery VC, Griffiths PD, Pass RF. Asymptomatic primary cytomegalovirus infection: virologic and immunologic features. J Infect Dis. 1999;180:702–7. doi: 10.1086/314939. [DOI] [PubMed] [Google Scholar]
- 8.Levin MJ, Rinaldo CR, Jr, Leary PL, Zaia JA, Hirsch MS. Immune response to herpesvirus antigens in adults with acute cytomegaloviral mononucleosis. J Infect Dis. 1979;140:851–7. doi: 10.1093/infdis/140.6.851. [DOI] [PubMed] [Google Scholar]
- 9.Antoine P, Olislagers V, Huygens A, et al. Functional exhaustion of CD4+ T lymphocytes during primary cytomegalovirus infection. J Immunol. 2012;189:2665–72. doi: 10.4049/jimmunol.1101165. [DOI] [PubMed] [Google Scholar]
- 10.Stern H, Hannington G, Booth J, Moncrieff D. An early marker of fetal infection after primary cytomegalovirus infection in pregnancy. Br Med J (Clin Res Ed) 1986;292:718–20. doi: 10.1136/bmj.292.6522.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernando S, Pearce JM, Booth JC. Lymphocyte responses and virus excretion as risk factors for intrauterine infection with cytomegalovirus. J Med Virol. 1993;41:108–13. doi: 10.1002/jmv.1890410205. [DOI] [PubMed] [Google Scholar]
- 12.Revello MG, Lilleri D, Zavattoni M, et al. Lymphoproliferative response in primary human cytomegalovirus (HCMV) infection is delayed in HCMV transmitter mothers. J Infect Dis. 2006;193:269–76. doi: 10.1086/498872. [DOI] [PubMed] [Google Scholar]
- 13.Schrier RD, Freeman WR, Wiley CA, McCutchan JA group atH. Immune predispositions for cytomegalovirus retinitis in AIDS. J Clin Invest. 1995;95:1741–6. doi: 10.1172/JCI117851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yue Y, Barry PA. Rhesus cytomegalovirus a nonhuman primate model for the study of human cytomegalovirus. Adv Virus Res. 2008;72:207–26. doi: 10.1016/S0065-3527(08)00405-3. [DOI] [PubMed] [Google Scholar]
- 15.Vogel P, Weigler BJ, Kerr H, Hendrickx AG, Barry PA. Seroepidemiologic studies of cytomegalovirus infection in a breeding population of rhesus macaques. Lab Anim Sci. 1994;44:25–30. [PubMed] [Google Scholar]
- 16.Hansen SG, Strelow LI, Franchi DC, Anders DG, Wong SW. Complete sequence and genomic analysis of rhesus cytomegalovirus. J Virol. 2003;77:6620–36. doi: 10.1128/JVI.77.12.6620-6636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivailler P, Kaur A, Johnson RP, Wang F. Genomic sequence of rhesus cytomegalovirus 180.92: insights into the coding potential of rhesus cytomegalovirus. J Virol. 2006;80:4179–82. doi: 10.1128/JVI.80.8.4179-4182.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur A, Daniel MD, Hempel D, Lee-Parritz D, Hirsch MS, Johnson RP. Cytotoxic T-lymphocyte responses to cytomegalovirus in normal and simian immunodeficiency virus-infected rhesus macaques. J Virol. 1996;70:7725–33. doi: 10.1128/jvi.70.11.7725-7733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaur A, Hale CL, Noren B, Kassis N, Simon MA, Johnson RP. Decreased frequency of cytomegalovirus (CMV)-specific CD4+ T lymphocytes in simian immunodeficiency virus-infected rhesus macaques: inverse relationship with CMV viremia. J Virol. 2002;76:3646–58. doi: 10.1128/JVI.76.8.3646-3658.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaur A, Kassis N, Hale CL, et al. Direct relationship between suppression of virus-specific immunity and emergence of cytomegalovirus disease in simian AIDS. J Virol. 2003;77:5749–58. doi: 10.1128/JVI.77.10.5749-5758.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anonymous. The Institute of Laboratory Animal Resources, National Research Council: Guide for the Care and Use of Laboratory Animals. 1996:86–123. [Google Scholar]
- 22.Chan KS, Kaur A. Flow cytometric detection of degranulation reveals phenotypic heterogeneity of degranulating CMV-specific CD8+ T lymphocytes in rhesus macaques. J Immunol Methods. 2007;325:20–34. doi: 10.1016/j.jim.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen M, Sauce D, Arnaud L, Fastenackels S, Appay V, Gorochov G. Evaluating cellular polyfunctionality with a novel polyfunctionality index. PLoS One. 2012;7:e42403. doi: 10.1371/journal.pone.0042403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitcher CJ, Hagen SI, Walker JM, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 25.Frebel H, Richter K, Oxenius A. How chronic viral infections impact on antigen-specific T-cell responses. Eur J Immunol. 2010;40:654–63. doi: 10.1002/eji.200940102. [DOI] [PubMed] [Google Scholar]
- 26.Kaye S, Miles D, Antoine P, et al. Virological and immunological correlates of mother-to-child transmission of cytomegalovirus in the Gambia. J Infect Dis. 2008;197:1307–14. doi: 10.1086/586715. [DOI] [PubMed] [Google Scholar]
- 27.Huff JL, Eberle R, Capitanio J, Zhou SS, Barry PA. Differential detection of B virus and rhesus cytomegalovirus in rhesus macaques. J Gen Virol. 2003;84:83–92. doi: 10.1099/vir.0.18808-0. [DOI] [PubMed] [Google Scholar]
- 28.Price DA, Bitmansour AD, Edgar JB, et al. Induction and evolution of cytomegalovirus-specific CD4+ T cell clonotypes in rhesus macaques. J Immunol. 2008;180:269–80. doi: 10.4049/jimmunol.180.1.269. [DOI] [PubMed] [Google Scholar]
- 29.Yue Y, Kaur A, Eberhardt MK, et al. Immunogenicity and protective efficacy of DNA vaccines expressing rhesus cytomegalovirus glycoprotein B, phosphoprotein 65–2, and viral interleukin-10 in rhesus macaques. J Virol. 2007;81:1095–109. doi: 10.1128/JVI.01708-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pourgheysari B, Khan N, Best D, Bruton R, Nayak L, Moss PA. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol. 2007;81:7759–65. doi: 10.1128/JVI.01262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 32.Pass RF, Hutto SC, Reynolds DW, Polhill RB. Increased frequency of cytomegalovirus infection in children in group day care. Pediatrics. 1984;74:121–6. [PubMed] [Google Scholar]
- 33.Sester U, Presser D, Dirks J, Gärtner BC, Köhler H, Sester M. PD-1 expression and IL-2 loss of cytomegalovirus- specific T cells correlates with viremia and reversible functional anergy. Am J Transplant. 2008;8:1486–97. doi: 10.1111/j.1600-6143.2008.02279.x. [DOI] [PubMed] [Google Scholar]
- 34.Duvall MG, Jaye A, Dong T, et al. Maintenance of HIV-specific CD4+ T cell help distinguishes HIV-2 from HIV-1 infection. J Immunol. 2006;176:6973–81. doi: 10.4049/jimmunol.176.11.6973. [DOI] [PubMed] [Google Scholar]
- 35.Alatrakchi N, Damond F, Matheron S, et al. Proliferative, IFNgamma and IL-2-producing T-cell responses to HIV-2 in untreated HIV-2 infection. AIDS. 2006;20:29–34. doi: 10.1097/01.aids.0000198077.30421.bf. [DOI] [PubMed] [Google Scholar]
- 36.Boaz MJ, Waters A, Murad S, Easterbrook PJ, Vyakarnam A. Presence of HIV-1 Gag-specific IFN-gamma+IL-2+ and CD28+IL-2+ CD4 T cell responses is associated with nonprogression in HIV-1 infection. J Immunol. 2002;169:6376–85. doi: 10.4049/jimmunol.169.11.6376. [DOI] [PubMed] [Google Scholar]
- 37.Pereyra F, Addo MM, Kaufmann DE, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197:563–71. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 38.Ciuffreda D, Comte D, Cavassini M, et al. Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur J Immunol. 2008;38:2665–77. doi: 10.1002/eji.200838336. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg ES, Billingsley JM, Caliendo AM, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–50. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 40.Iyasere C, Tilton JC, Johnson AJ, et al. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J Virol. 2003;77:10900–9. doi: 10.1128/JVI.77.20.10900-10909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Younes SA, Yassine-Diab B, Dumont AR, et al. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J Exp Med. 2003;198:1909–22. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Folgori A, Spada E, Pezzanera M, et al. Early impairment of hepatitis C virus specific T cell proliferation during acute infection leads to failure of viral clearance. Gut. 2006;55:1012–9. doi: 10.1136/gut.2005.080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boni C, Bertoletti A, Penna A, et al. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J Clin Invest. 1998;102:968–75. doi: 10.1172/JCI3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tilton JC, Luskin MR, Johnson AJ, et al. Changes in paracrine interleukin-2 requirement, CCR7 expression, frequency, and cytokine secretion of human immunodeficiency virus-specific CD4+ T cells are a consequence of antigen load. J Virol. 2007;81:2713–25. doi: 10.1128/JVI.01830-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–10. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frebel H, Nindl V, Schuepbach RA, et al. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J Exp Med. 2012;209:2485–99. doi: 10.1084/jem.20121015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y, Wu S, Guo G, et al. Programmed death (PD)-1-deficient mice are extremely sensitive to murine hepatitis virus strain-3 (MHV-3) infection. PLoS Pathog. 2011;7:e1001347. doi: 10.1371/journal.ppat.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]