Abstract
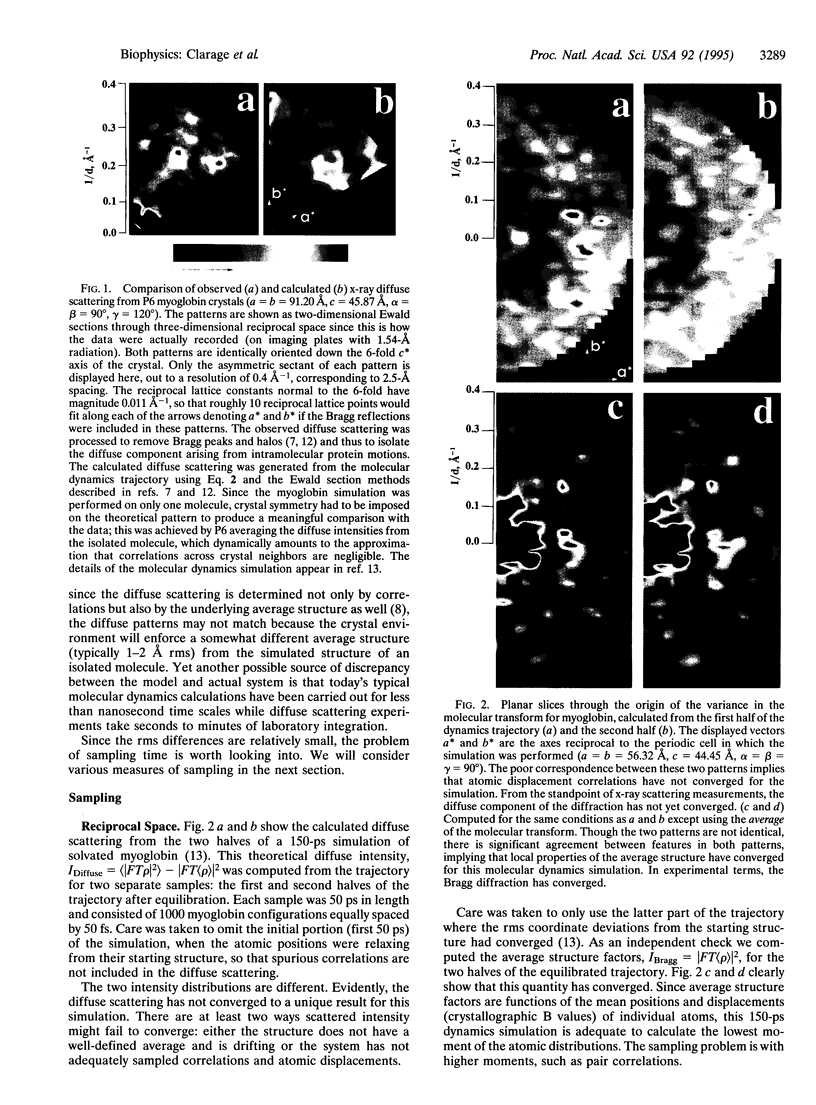
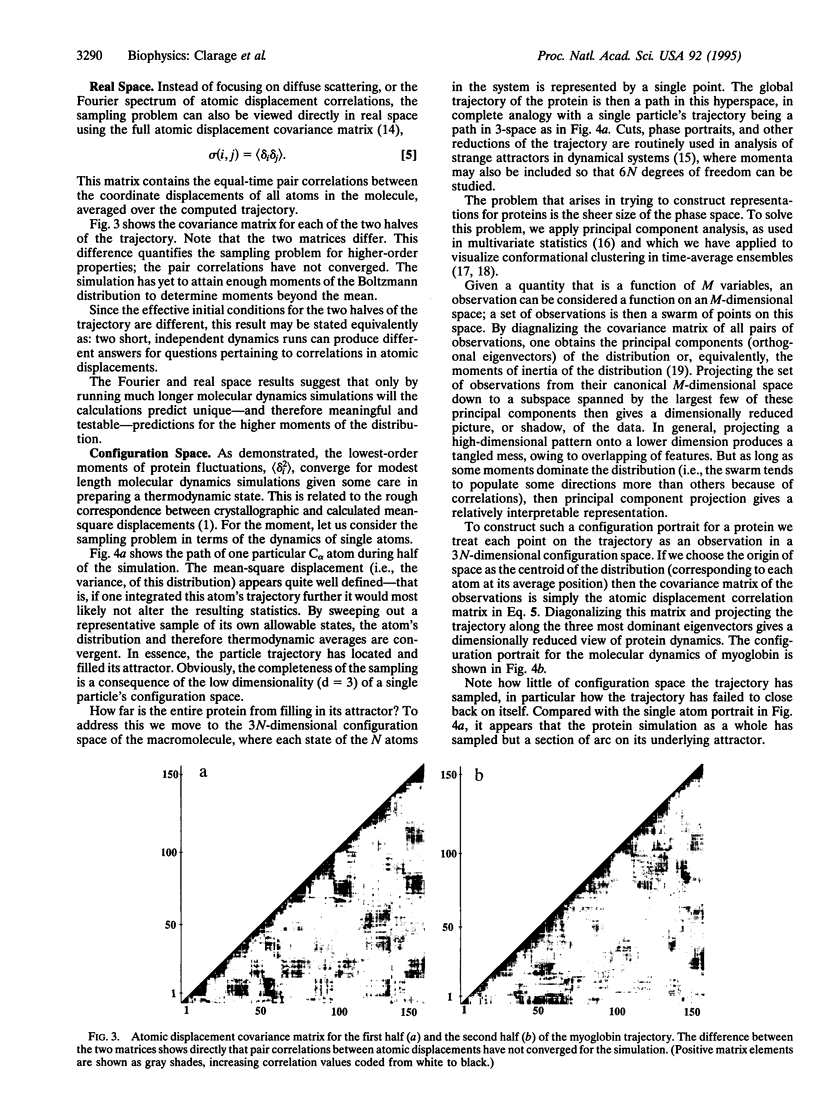
Correlations in low-frequency atomic displacements predicted by molecular dynamics simulations on the order of 1 ns are undersampled for the time scales currently accessible by the technique. This is shown with three different representations of the fluctuations in a macromolecule: the reciprocal space of crystallography using diffuse x-ray scattering data, real three-dimensional Cartesian space using covariance matrices of the atomic displacements, and the 3N-dimensional configuration space of the protein using dimensionally reduced projections to visualize the extent to which phase space is sampled.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amadei A., Linssen A. B., Berendsen H. J. Essential dynamics of proteins. Proteins. 1993 Dec;17(4):412–425. doi: 10.1002/prot.340170408. [DOI] [PubMed] [Google Scholar]
- Boylan D., Phillips G. N. Motions of tropomyosin: characterization of anisotropic motions and coupled displacements in crystals. Biophys J. 1986 Jan;49(1):76–78. doi: 10.1016/s0006-3495(86)83599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar D. L., Clarage J., Salunke D. M., Clarage M. Liquid-like movements in crystalline insulin. Nature. 1988 Apr 14;332(6165):659–662. doi: 10.1038/332659a0. [DOI] [PubMed] [Google Scholar]
- Chacko S., Phillips G. N., Jr Diffuse x-ray scattering from tropomyosin crystals. Biophys J. 1992 May;61(5):1256–1266. doi: 10.1016/S0006-3495(92)81934-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarage J. B., Clarage M. S., Phillips W. C., Sweet R. M., Caspar D. L. Correlations of atomic movements in lysozyme crystals. Proteins. 1992 Feb;12(2):145–157. doi: 10.1002/prot.340120208. [DOI] [PubMed] [Google Scholar]
- Clarage J. B., Phillips G. N., Jr Cross-validation tests of time-averaged molecular dynamics refinements for determination of protein structures by X-ray crystallography. Acta Crystallogr D Biol Crystallogr. 1994 Jan 1;50(Pt 1):24–36. doi: 10.1107/S0907444993009515. [DOI] [PubMed] [Google Scholar]
- Diamond R. On the use of normal modes in thermal parameter refinement: theory and application to the bovine pancreatic trypsin inhibitor. Acta Crystallogr A. 1990 Jun 1;46(Pt 6):425–435. doi: 10.1107/s0108767390002082. [DOI] [PubMed] [Google Scholar]
- Doster W., Cusack S., Petry W. Dynamical transition of myoglobin revealed by inelastic neutron scattering. Nature. 1989 Feb 23;337(6209):754–756. doi: 10.1038/337754a0. [DOI] [PubMed] [Google Scholar]
- Eriksson M. A., Berglund H., Härd T., Nilsson L. A comparison of 15N NMR relaxation measurements with a molecular dynamics simulation: backbone dynamics of the glucocorticoid receptor DNA-binding domain. Proteins. 1993 Dec;17(4):375–390. doi: 10.1002/prot.340170406. [DOI] [PubMed] [Google Scholar]
- Faure P., Micu A., Pérahia D., Doucet J., Smith J. C., Benoit J. P. Correlated intramolecular motions and diffuse X-ray scattering in lysozyme. Nat Struct Biol. 1994 Feb;1(2):124–128. doi: 10.1038/nsb0294-124. [DOI] [PubMed] [Google Scholar]
- García AE. Large-amplitude nonlinear motions in proteins. Phys Rev Lett. 1992 Apr 27;68(17):2696–2699. doi: 10.1103/PhysRevLett.68.2696. [DOI] [PubMed] [Google Scholar]
- Ichiye T., Karplus M. Collective motions in proteins: a covariance analysis of atomic fluctuations in molecular dynamics and normal mode simulations. Proteins. 1991;11(3):205–217. doi: 10.1002/prot.340110305. [DOI] [PubMed] [Google Scholar]
- Kolatkar A. R., Clarage J. B., Phillips G. N., Jr Analysis of diffuse scattering from yeast initiator tRNA crystals. Acta Crystallogr D Biol Crystallogr. 1994 Mar 1;50(Pt 2):210–218. doi: 10.1107/S0907444993011692. [DOI] [PubMed] [Google Scholar]
- Levitt M., Sander C., Stern P. S. Protein normal-mode dynamics: trypsin inhibitor, crambin, ribonuclease and lysozyme. J Mol Biol. 1985 Feb 5;181(3):423–447. doi: 10.1016/0022-2836(85)90230-x. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr Comparison of the dynamics of myoglobin in different crystal forms. Biophys J. 1990 Feb;57(2):381–383. doi: 10.1016/S0006-3495(90)82540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]