Abstract
Objectives:
Spinal manual therapy (SMT) is commonly used for treatment of musculoskeletal pain in the neck, upper back, or upper extremity. Some authors report a multi-system effect of SMT, including peripheral alterations in skin conductance and skin temperature, suggesting that SMT may initiate a sympathetic nervous system (SNS) response. The focus of this evidence-based review and meta-analysis is to evaluate the evidence of SNS responses and clinically relevant outcomes following SMT to the cervical or thoracic spine.
Methods:
A systematic search used the terms: ‘manual therapy’, ‘SMT’, ‘spinal manipulation’, ‘mobilization’, ‘SNS’, ‘autonomic nervous system’, ‘neurophysiology’, ‘hypoalgesia’, ‘pain pathophysiology’, ‘cervical vertebrae’, ‘thoracic vertebrae’, ‘upper extremity’, and ‘neurodynamic test’. Data were extracted and within-group and between-group effect sizes were calculated for outcomes of skin conductance, skin temperature, pain, and upper extremity range of motion (ROM) during upper limb neurodynamic tests (ULNTs).
Results:
Eleven studies were identified. Statistically significant changes were seen with increased skin conductance, decreased skin temperature, decreased pain, and increased upper extremity ROM during ULNT.
Discussion:
A mechanical stimulus at the cervical or thoracic spine can produce a SNS excitatory response (increased skin conductance and decreased skin temperature). Findings of reduced pain and increased ROM during ULNT provide support to the clinical relevance of SMT. This evidence points toward additional mechanisms underlying the therapeutic effect of SMT. The effect sizes are small to moderate and no long-term effects post-SMT were collected. Future research is needed to associate peripheral effects with a possible centrally-mediated response to SMT.
Keywords: Spinal manual therapy, Sympathetic nervous system, Cervical spine, Thoracic spine
Introduction
Pain resulting from disorders of the cervical spine, thoracic spine, and upper extremity is common, and is a frequent source of disability.1,2 In 2006, neck disorders accounted for 58% of health care visits, with 67% of these patients utilizing hospital-based outpatient care.3 Outpatient treatments for musculoskeletal pain in the neck, upper back, or upper extremity vary widely, and have included spinal manual therapy (SMT), or passive joint mobilization of the spine. Evidence of the multi-system effects of SMT can aid clinicians in selecting appropriate treatments individualized to each patient’s condition.
Pain in the upper quarter may result from local musculoskeletal dysfunction, peripheral nerve entrapment, or referral from the cervical or thoracic spine. Treatment often targets the local tissues believed to underlie the presenting signs and symptoms. However, targeting the spine may have additional benefits. Mobilizing the spine can result in local mechanical effects,4,5 and also decrease upper quarter local or referred pain via sensory input that inhibits transmission of peripheral pain signals.6–9 Changes peripheral to the spinal segment mobilized have included alterations in skin conductance and skin temperature, suggesting that SMT may initiate a sympathetic nervous system (SNS) response.10–12
A SNS response has been shown in animal studies with electrical stimulation of the dorsal periaqueductal gray, a midbrain structure linked with pain modulation.13,14 Parallel findings of a sympathetic response and analgesia have also been observed following lumbar SMT in animal models. The similarity in response has led authors to speculate that the effects of SMT might have a centrally-mediated component involving the SNS.11,12 In humans, lumbar SMT has been associated with changes in skin conductance15–17 and skin temperature,18,19 which are common measures of sympathetic activity. However, research support for the effects of cervical and thoracic SMT on the SNS is variable and no meta-analyses have been reported.
Assessing SNS responses typically includes measures of pain, skin conductance, and skin temperature. Clinically, hypoalgesic effects may be recorded using patient-reported numeric pain rating scales (NPRSs) or visual analog scales (VAS). Suppressing the transmission of painful stimuli may also decrease the mechanosensitivity of the nervous system. Decrease in mechanosensitivity may result in improved upper extremity range of motion (ROM), sometimes assessed during an upper limb neurodynamic test (ULNT).11,12 Activation of the SNS also results in activation of sweat glands, measured via skin conductance, and vascular smooth muscles resulting in cutaneous vasoconstriction, measured via skin temperature.10–12 Electrodermal sensors measure electrical conductance across the skin, frequently as part of a biofeedback unit, with electrodes placed on the index and fourth fingers of a participant’s hand. Thermistors on the fingertips measure peripheral skin temperature.
The focus of this evidence-based review and meta-analysis was to evaluate the evidence of SNS responses in the hands (increased skin conductance and decreased skin temperature) and clinically relevant outcomes (decreased pain and increased upper extremity ROM during ULNTs) following SMT to the cervical or thoracic spine in people with or without upper quarter dysfunction. Secondarily, this project examined whether SMT was more effective at producing changes in sympathetic responses and clinically relevant outcomes when compared to a control treatment.
Methods
Search strategy
Two individuals (JC and BS) performed independent systematic searches of the current literature in PubMed, CINAHL, PEDro, Hooked on Evidence, Cochrane, and Web of Science. The following terms were used alone and in combination: ‘manual therapy’, ‘spinal manual therapy’, ‘spinal manipulation’, ‘mobilization’, ‘sympathetic nervous system’, ‘autonomic nervous system’, ‘neurophysiology’, ‘hypoalgesia’, ‘pain pathophysiology’, ‘cervical vertebrae’, ‘thoracic vertebrae’, ‘upper extremity’, and ‘neurodynamic test’. Studies were first screened for duplicates then examined based on inclusion and exclusion criteria. A recursive search was performed from the references of those articles meeting the criteria.
Inclusion and exclusion criteria
Studies were included if interventions involved SMT to the cervical or thoracic spine segments in symptomatic or asymptomatic adults. In addition to SMT as the intervention, reported outcomes must include at least one of the following: skin conductance or skin temperature in the upper extremity, pain score at rest measured in the upper extremity, or mechanosensitivity measured through changes in upper extremity ROM during ULNTs as recorded at the elbow or shoulder.
The exclusion criteria for this review were studies that did not measure at least one of the following: skin conductance, skin temperature, pain score in the upper extremity at rest or during ULNT, or a measure of mechanosensitivity in the upper extremity. Studies were excluded if passive joint mobilization was performed to any joints other than the cervical or thoracic spine. Soft tissue mobilization could not be utilized exclusively or as part of the intervention process. Studies were excluded if pain scores were reported only during active movement as compared to pain at rest. Animal studies or studies written in a language other than English were not included.
Statistical analysis
Included studies were evaluated based on a hierarchy of evidence published by Jewell,20 where 1B is a single randomized controlled trial (RCT) and 4 is a case report. To assess the risk of bias in the individual studies, we evaluated RCTs further using the PEDro scale to identify methodological quality.21 The PEDro scale allows assessment of study quality according to 11 criteria, including documentation of blinding of participants, therapists, and outcome assessors.
Within group effect sizes were calculated from means and standard deviations for skin conductance, skin temperature, VAS, and upper extremity ROM during an ULNT before and following SMT for each individual study. Upper limb neurodynamic test effect sizes were calculated separately for the elbow and shoulder. Between group effect sizes were also calculated for all outcome measures to determine the post treatment effect between intervention and control groups. Thresholds for large, moderate, and small effect sizes were set at >0.8, 0.3–0.8, and <0.3, respectively. Ninety-five percent confidence intervals were also calculated for each effect size. The Q heterogeneity statistic was calculated when pooling data across studies to inform the decision on which model to utilize to pool data (fixed effect versus random effects). Individual studies were weighted by inverse variance alone or along with tau-squared depending on utilization of the fixed or random effects model.
Results
The two independent electronic searches yielded 55 relevant articles, out of which 44 articles were excluded because they did not meet the eligibility criteria, leaving 11 primary studies in this systematic review. The most common reasons for article exclusion were manual therapy performed at joints other than the cervical and thoracic spine, such as the shoulder or elbow, or not reporting pain at rest. Another reviewer confirmed that the 11 primary articles met the eligibility criteria. The process of study selection is shown in Fig. 1. The level of evidence was 1B for all 11 studies.20 Table 1 provides the PEDro score for each of the 11 studies; scores ranged from 7 to 9.21
Figure 1.
PRISMA diagram with results of literature search.
Table 1. PEDro scale.
| PEDro criteria | Chiu and Wright25 | Coppieters et al.22 | Jowsey and Perry23 | La Touche et al.24 | Moulson and Watson26 | Peterson et al.27 | Saranga et al.28 | Sterling et al.32 | Vicenzino et al.29 | Vicenzino et al.30 | Vicenzino et al.31 |
| Eligibility criteria specified | + | + | + | + | + | + | + | + | + | + | + |
| Random allocation | + | + | + | + | + | + | + | + | + | + | + |
| Concealed allocation | + | + | + | + | + | + | + | + | + | + | + |
| Groups similar at baseline | + | + | − | + | + | + | + | + | + | + | + |
| Subject blinding | − | − | + | + | − | − | − | + | + | + | + |
| Therapist blinding | − | − | − | − | − | − | − | − | − | − | − |
| Assessor blinding | + | + | + | + | − | + | + | + | + | + | + |
| Less than 15% dropouts | + | + | + | + | + | + | + | + | + | + | + |
| Intention-to-treat analysis | + | + | + | + | + | + | + | + | + | + | + |
| Between-group statistical comparisons | + | + | + | + | + | + | + | + | + | + | + |
| Point measures and variability data | + | + | + | + | + | + | + | + | + | + | + |
| PEDro score | 8 | 8 | 8 | 9 | 7 | 8 | 8 | 9 | 9 | 9 | 9 |
Table 2 summarizes the studies, including participants, treatment groups, interventions, measured outcomes, and results. In 3 out of the 11 studies22–24 participants were randomly assigned to groups in a parallel RCT design. The remaining 8 studies had crossover designs, with participants receiving each intervention in a random sequence over time. Out of 11 studies 623,25–29 explored the effects of SMT on asymptomatic individuals, while the remaining 5 studies observed SMT effects in participants with chronic lateral epicondylalgia,30,31 cervico-craniofacial pain,24 and nonacute cervicobrachial neurogenic pain.22,32
Table 2. Summary of studies.
| Study | Subjects | Experimental procedures | Measured outcomes | Results (within groups) |
| Chiu and Wright25 | 16 healthy subjects | C5 central PA, 2 Hz (3×1 minute bouts with intervening 1 minute rest periods) | Skin conductance | Skin conductance (P = 0.0003) |
| C5 central PA, 0.5 Hz (3×1 minute bouts with intervening 1 minute rest periods) | Skin temperature | Skin temperature (P = 0.655) | ||
| Control | ||||
| Coppieters et al.22 | 20 subjects with nonacute neurogenic cervicobrachial pain | Lateral glide at C5–T1 contralateral to the painful side (no duration specified) | Elbow extension ROM during ULNT 1 | Elbow ROM during ULNT 1 (P = 0.0005) |
| Therapeutic ultrasound (5 minutes at 0.5 W/cm2, 1 MHz, 20% duty cycle) | Pain intensity (VAS) during ULNT 1 | Pain (P = 0.0052) | ||
| Jowsey and Perry23 | 36 healthy subjects | T4 right rotary PA (3×1 minute bouts with intervening 1 minute rest periods) | Skin conductance | Skin conductance (P = 0.034) in R hand, (P = (0.052) in L hand |
| Placebo | ||||
| La Touche et al.24 | 32 subjects with cervico-craniofacial pain | AP upper cervical mobilization at 0.5 Hz (3×2 minutes bouts with intervening 30 seconds rest periods) | Skin conductance | Skin conductance (P<0.001) |
| Placebo | Skin temperature | Skin conductance (P = 0.08) | ||
| Moulson and Watson26 | 16 healthy subjects | C5/C6 Mulligan SNAG with right rotation (3 reps according to Mulligan’s Rule of 3) | Skin conductance | Skin conductance (P = 0.001) |
| Placebo | Skin temperature | Skin temperature (P = 0.088) | ||
| Control | ||||
| Peterson et al.27 | 16 healthy subjects | C5 central PA (3×1 minute bouts with intervening 1 minute rest periods) | Skin conductance | Skin conductance (P = 0.001) |
| Placebo | Skin temperature | Skin temperature (P = 0.02) | ||
| Control | ||||
| Saranga et al.28 | 20 healthy subjects | C5/C6 lateral glide (3×1 minute bouts with intervening 1 minute rest periods) | Elbow extension ROM during ULNT 1 | Elbow ROM during ULNT 1 (P = 0.001) |
| Placebo | ||||
| Control | ||||
| Sterling et al.32 | 30 subjects with mid to lower cervical pain lasting longer than 3 months and a dysfunction at C5/C6 | C5/C6 unilateral PA on symptomatic side (3×1 minute bouts with intervening 1 minute rest periods) | Skin conductance | Skin conductance (P = 0.002) |
| Placebo | Skin temperature | Skin temperature (P = 0.014) | ||
| Control | Pain intensity (VAS) at rest | VAS (P = 0.044) | ||
| Vicenzino et al.29 | 34 asymptomatic subjects | C5/C6 left lateral glide with upper limb in ULNT 1 (3×30 seconds bouts with intervening 1 minute rest periods) | Skin conductance | Skin conductance (P = 0.000) |
| C5/C6 left lateral glide with the upper limb in ULNT 2B | Skin temperature | Skin temperature (P>0.05) | ||
| Placebo | ||||
| Control | ||||
| Vicenzino et al. 30 | 15 subjects with lateral epicondylalgia | C5/C6 lateral glide contralateral to symptomatic side | Shoulder abduction ROM during ULNT 2B | Shoulder ROM during ULNT 2B (P = 0.049) |
| (3×30 seconds bouts with intervening 1 minute rest periods) | Pain intensity (VAS) at rest | VAS (P = 0.01) | ||
| Placebo | ||||
| Control | ||||
| Vicenzino et al. 31 | 24 subjects with chronic lateral epicondylalgia | C5/C6 lateral glide contralateral to symptomatic side | Skin conductance | Skin conductance (P = 0.0004) |
| (3×30 seconds bouts with intervening 1 minute rest periods) | Skin temperature | Skin temperature (P = 0.0004) | ||
| Placebo | Shoulder abduction ROM during ULNT 2B | Shoulder ROM during ULNT 2B (P = 0.028) | ||
| Control |
Abbreviations: PA: posterior–anterior mobilization; AP: anterior–posterior mobilization; VAS: visual analog scale; W/cm2: watts per centimeter squared; ROM: range of motion; SNAG: sustained natural apophyseal glide; ULNT: upper limb neurodynamic test; ULNT 1: biasing the median nerve; ULNT 2B: biasing the radial nerve.
Spinal manual therapy in most studies consisted of a Grade III mobilization technique (using Maitland classification), with the researcher applying oscillatory pressure at the designated vertebral segment. Three exceptions to this common application included a Mulligan sustained natural apophyseal glide (SNAG),26 an anterior–posterior upper cervical mobilization,24 and a lateral glide of an unspecified grade.22 In studies that included comparison groups, between-group calculations were performed using data from a group that received no treatment; the researcher stood motionless at the head of the plinth for an equivalent time to maintain constant experimental protocol (designated as control group). In La Touche et al.,24 the comparison group received manual contact at the same vertebral location but without pressure or oscillations (designated as a placebo group). Studies varied by level of spinal segment mobilized, ranging from C5 to T4 vertebral levels; the most common sites were C5–6.25–32
Data analysis
Effect sizes and 95% CI for all five outcomes were calculated for individual studies and across studies (Table 3). The Q statistics for within group effect sizes for skin temperature and skin conductance were significant (P<0.05), thus a random-effects model was used to pool data for those outcomes (Figs. 2 and 3). The Q heterogeneity statistic for within group and between group effect sizes for VAS and upper extremity ROM during ULNT outcomes was non-significant (P>0.05), indicating sufficient homogeneity among studies for use of a fixed-effect model to pool data (Figs. 4–6).
Table 3. Summary of within-group and between-group combined effect sizes and 95% CI.
| Outcome | Effect size (95% CI) |
| Within group | |
| Skin conductance24–27,29,31 | 0.94 (0.47, 1.41) |
| Skin temperature24–27,29,31 | −0.48 (−0.83, −0.12) |
| Pain on VAS22,30,32 | −0.66 (−1.00, −0.31) |
| Elbow extension ROM during ULNT 122,28 | 0.96 (0.50, 1.41) |
| Shoulder abduction ROM during ULNT 2B30,31 | 2.63 (2.02, 3.23) |
| Between group | |
| Skin conductance23–27,29,31 | 0.97 (0.73, 1.21) |
| Skin temperature24,26,27,29,31,32 | −0.77 (−1.02, −0.53) |
| Pain on VAS22,30,32 | −0.64 (−1.02, −0.26) |
| Elbow extension ROM during ULNT 122,28 | 1.53 (0.96, 2.11) |
| Shoulder abduction ROM during ULNT 2B30,31 | 0.69 (0.23, 1.15) |
VAS: visual analog scale, ROM: range of motion, ULNT: upper limb neurodynamic test; ULNT 1: biasing the median nerve; ULNT 2B: biasing the radial nerve.
Figure 2.
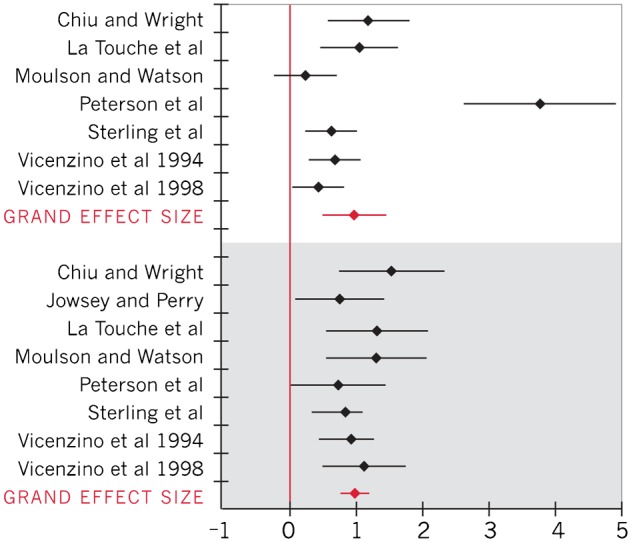
Forest plot of effect sizes for skin conductance (positive effect sizes represent an increase in skin conductance; within-group effect sizes represented in white; between-group effect sizes represented in gray)
Figure 3.
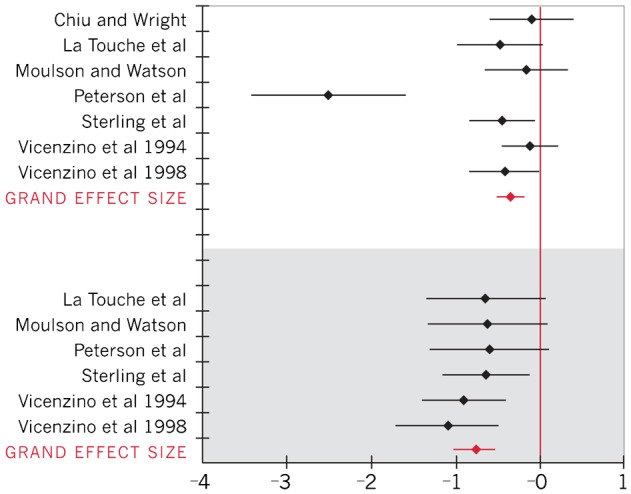
Forest plot of effect sizes for skin temperature (negative effect sizes represent a decrease in skin temperature; within-group effect sizes represented in white; between-group effect sizes represented in gray)
Figure 4.
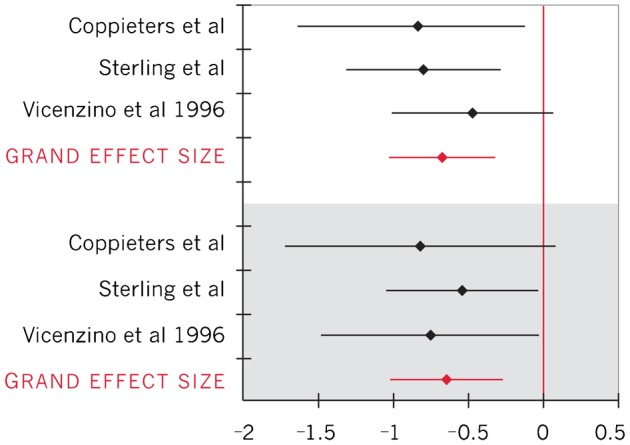
Forest plot of effect sizes for pain (pain was measured with the Visual Analog Scale (0–10); negative effect sizes represent a decrease in pain; within-group effect sizes represented in white; between-group effect sizes represented in gray)
Figure 6.
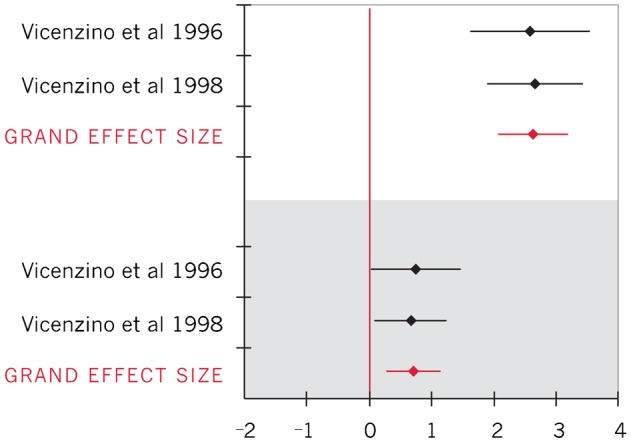
Forest plot of effect sizes for shoulder abduction ROM during upper limb neurodynamic test (ULNT) 2B (upper limb neurodynamic test 2B biasing the radial nerve; within-group effect sizes represented in white; between-group effect sizes represented in gray)
Figure 5.
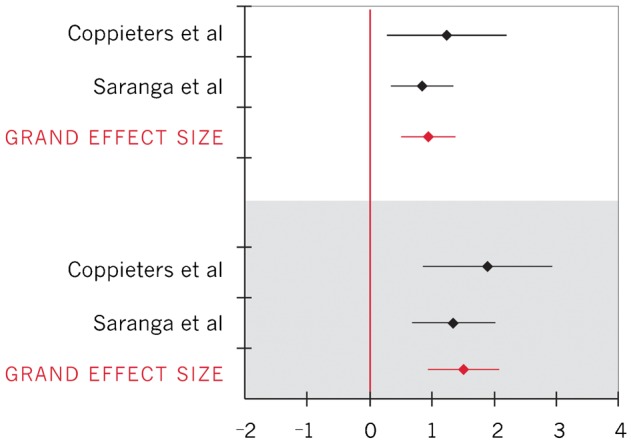
Forest plot of effect sizes for elbow extension ROM during upper limb neurodynamic test (ULNT) 1 (upper limb neurodynamic test 1 biasing the median nerve; positive effect sizes represent an increase in elbow extension ROM; within-group effect sizes represented in white; between-group effect sizes represented in gray)
Changes in clinical measures
To calculate importance of changes in clinical measures, between group effect sizes for pain and elbow extension ROM were converted back into units of clinical measures for the VAS (0–10 cm) and for degrees of ROM. Converting the 95% CI for the between-group VAS effect size to clinical units resulted in a pain decrease ranging from 0.51 to 2.0 cm among individuals with symptoms;32 the between-groups difference in elbow extension ROM ranged from 10.3° to 22.6° (average 16.4°) during ULNT 1 (symptomatic individuals).22 Insufficient data were reported to calculate within-group clinical importance of pain or ROM changes.
Discussion
The purpose of this evidence-based review was to gather the evidence for neurophysiologic effects in the upper extremity as a result of cervical or thoracic SMT. Analysis of the pooled data from 11 studies revealed statistically significant within-group and between-group effects, with increased skin conductance, decreased skin temperature, decreased pain at rest, and increased upper extremity ROM during ULNTs involving elbow extension and shoulder abduction.
These findings confirm that a mechanical stimulus at the cervical or thoracic spine in humans is capable of producing a SNS excitatory response. This response was noted in symptomatic and asymptomatic individuals. In addition, findings of reduced pain and reduced mechanosensitivity in symptomatic individuals provide support to the clinical relevance of peripheral responses to SMT. Saranga et al.28 found that asymptomatic individuals also noted reduced mechanosensitivity with an effect size similar to that of symptomatic individuals.22 Thus, even without symptoms of pain, individuals undergoing SMT may have improved ROM during neurodynamic tests compared to controls.
Findings from this evidence-based review are consistent with literature studying the effects of SMT in lumbar areas.16,17 Lumbar mobilization techniques, including lumbar SNAGs or a lumbar rotation Grade V manipulation, were applied to asymptomatic participants and compared to the effects of a placebo or control condition. Like the current results, lumbar SMT resulted in increased skin conductance and decreased skin temperature, with similar effect sizes16,17.
Statistically significant change, as noted in the current studies, may not reflect a minimal clinically important difference (MCID). The MCID is the smallest change in an outcome measure perceived as important and beneficial, sufficient to lead to a change in the patient’s management.33,34 For pain, the MCID has been documented as about 2 on the NPRS or 1.2 cm on the VAS.35 The confidence interval for the decrease in pain at rest for these studies22,30,32 was 0.51–2.0 cm. Only some of the individuals with symptoms experienced decreased pain that would be considered clinically important within the single session of the intervention. Long-term assessment of pain was reported for only one study; Vicenzino et al.30 reported that the worst pain in the 24 hours post SMT was 2.2 cm different from the control group, indicating that pain relief might persist following SMT. Regarding skin conductance, skin temperature, and upper extremity ROM during neurodynamic testing, no MCID values have been published. However, the ULNT measuring elbow extension ROM average change of 16.4° is more than the MCID for change in ROM (10°) when measuring via goniometer.36 This change suggests that SMT may have an important clinical effect on decreasing mechanosensitivity in the upper extremity.
Implications for clinical practice
An increased understanding of the peripheral responses to SMT promotes its acceptance as an evidence-based intervention. Findings that SMT to the cervical or thoracic spine results in immediate decreases in pain and mechanosensitivity support the utilization of SMT as an adjunct to other interventions.
Although manual therapy techniques are relatively safe and easy to apply, they have the potential to harm a patient if performed inappropriately. None of the included studies reported harm to their participants upon receiving mobilization. However, clinicians trained in mobilization techniques screen patients for contraindications to manual therapy, such as systemic disease or joint abnormalities that increase the risk of complications associated with SMT. This screening must include complete assessment of upper quarter disorders and clearing of the spine for the appropriateness of utilizing SMT for treatment of peripheral symptoms.
Evidence of peripheral responses to SMT adds to the discussion of therapeutic mechanisms for SMT in patients with upper quarter disorders, and assists with patient education when describing indications for SMT. Additionally, greater patient understanding may improve adherence to other treatment interventions.
Limitations
A number of limitations in this review warrant discussion. Only 322–24 of 11 studies were parallel RCTs. Patients receiving more than one intervention condition in succession may have persistent effects from a previous intervention when the next is applied. The length of time between intervention conditions in the cross-over trials ranged from 24 hours to a span of 2 weeks. Future studies could investigate the sufficiency of the wash-out period by comparing pre-intervention data across intervention conditions.
Although calculation of the Q statistic revealed statistical homogeneity for most outcomes, differences between primary studies indicate a heterogeneity that may affect the generalizability of these findings. For example, studying the effects of SMT in symptomatic and asymptomatic volunteers may affect findings, as could application of varying mobilization techniques and grades to the cervical or thoracic spine. Other differences between studies include varied blinding of participants, therapists, and outcome assessors (Table 1). For the included studies, the risk of bias toward change with intervention was mitigated by the objectivity of skin conductance and temperature testing, two measures that most people have minimal experience in controlling voluntarily. The risk of bias across studies appears low because of article location by two independent reviewers, confirmation by another reviewer that articles met the eligibility criteria, and the consistency of effect sizes across measures and individual articles. Peterson et al.27 had effect sizes for skin conductance and skin temperature that appeared to be outliers. Recalculation of the grand effect sizes with and without data from this study decreased the size of the grand effect, but the confidence intervals still did not cross zero, so the results of this review did not change.
The studies involving individuals with symptoms included those with non-acute cervical brachial pain, lateral epicondylalgia, cervico-craniofacial pain, and chronic mid to low cervical pain. Responses could have differed across these groups although the individual study effect sizes were fairly similar. In addition to this, the sample size of all 11 studies was relatively small, ranging from 16 to 36 total participants.
Studies included in this evidence-based review did not examine the long-term effects of SMT. While immediate responses post-SMT can indicate change, failure to assess long-term effects limits support of SMT in the clinical setting. Studies excluded from our review because they assessed pain or function in ways not comparable to included studies have reported changes measured at 48-hour follow up after SMT.37,38 One study examining effects of a thoracic manipulation in participants with neck pain found significantly higher scores on global rating of change scale at time of follow-up, 2–4 days post SMT.37 A thoracic manipulation was also applied to participants with shoulder impingement syndrome, with a significant reduction in numeric pain rating and shoulder pain and disability index scores at 48-hour follow up.38 Current research has not yet demonstrated the duration of sympathetic activation after SMT.
Lastly, some of the included studies failed to provide mean and standard deviation data to calculate effect sizes directly. Some effect sizes were calculated from the reported P value, which meant that the calculated effect sizes may have been unnecessarily conservative. Pre-intervention data for the intervention groups were rarely presented, and only one study presented pre and post data for the control groups.
Directions for future research
Despite showing a sympathoexcitatory effect, with skin conductance increasing and skin temperature decreasing, mechanisms for SMT are still relatively unclear. Future research should consider combining investigation of SNS effects and stimulation of different midbrain and cortical areas in humans to determine if areas similar to those in animals are involved. Such studies can help to determine if SMT responses have a centrally-mediated component.
No studies were excluded by diagnosis in this review. Future research to investigate possible differences in sympathetic response in different populations under different treatment conditions could prove informative. Surgical intervention in patients with thoracic outlet syndrome has been reported to increase skin temperature in the hand.39 Thus, in some diagnostic groups, skin temperature increase would be preferred. We have no evidence that SMT results in different directions of change in different groups of patients, such as those with a vascular component to their symptoms, but separating these groups may prevent dilution of effects.
The possibility of a dose-dependent response remains unexplored. The current studies all provided SMT within a single session. Future research should examine response to SMT as it might be applied clinically over multiple sessions. Additional studies to observe the long-term effects of SMT, SMT response in various clinical populations, and potential adverse effects of SMT need to be performed to determine the extent clinicians utilize SMT in the treatment of patients presenting with upper quarter symptoms and dysfunction.
Conclusion
Manual therapy directed to the cervical or thoracic spine produces a peripheral increase in skin conductance and a decrease in skin temperature, and also results in positive clinical outcome measures of decreased subjective pain reports at rest and improved upper extremity ROM during an ULNT. These findings are consistent with activation of the SNS in response to SMT. These findings may assist clinicians in educating their patients about the effects of SMT, and may also provide clinicians with evidence to guide them in considering SMT as a possible treatment for patients with appropriate upper quarter symptoms.
Conflict of Interest
Betty Smoot is partially supported by the BIRCWH K12, Grant Number K12HD052163 NICHD/NIH, and by the National Center for Advancing Translational Sciences, National Institute of Health, through UCSF-CTSI Grant Number KL2TR000143.
References
- 1.Huisstede B, Bierma-Zeinstra S, Koes B, Verhaar J. Incidence and prevalence of upper-extremity musculoskeletal disorders. A systematic appraisal of the literature. BMC Musculoskelet Disord. 2006;7:7. doi: 10.1186/1471-2474-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoe V, Urquhart D, Kelsall H, Sim M. Ergonomic design and training for preventing work-related musculoskeletal disorders of the upper limb and neck in adults. Cochrane Libr. 2012;8:1–107. doi: 10.1002/14651858.CD008570.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States, Second Edition. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011. [Google Scholar]
- 4.Evans D. Mechanisms and effects of spinal high-velocity low-amplitude thrust manipulation: previous theories. J Manipulative Physiol Ther. 2002;25:251–62. doi: 10.1067/mmt.2002.123166. [DOI] [PubMed] [Google Scholar]
- 5.Maigne JY, Vautravers P. Mechanism of action of spinal manipulative therapy. Joint Bone Spine. 2003;70:336–41. doi: 10.1016/s1297-319x(03)00074-5. [DOI] [PubMed] [Google Scholar]
- 6.Vernon H. Qualitative review of studies of manipulation-induced hypoalgesia. J Manipulative Physiol Ther. 2000;23:134–8. doi: 10.1016/s0161-4754(00)90084-8. [DOI] [PubMed] [Google Scholar]
- 7.Pickar J. Neurophysiological effects of spinal manipulation. Spine J. 2002;2:357–71. doi: 10.1016/s1529-9430(02)00400-x. [DOI] [PubMed] [Google Scholar]
- 8.Wright A. Recent concepts in the neurophysiology of pain. Man Ther. 1999;4:196–202. doi: 10.1054/math.1999.0207. [DOI] [PubMed] [Google Scholar]
- 9.Zusman M. Spinal manipulative therapy: review of some proposed mechanisms, and a new hypothesis. Aust J Physiother. 1986;32:89–99. doi: 10.1016/S0004-9514(14)60645-0. [DOI] [PubMed] [Google Scholar]
- 10.Schmid A, Brunner F, Wright A, Bachmann L. Paradigm shift in manual therapy? Evidence for a central nervous system component in the response to passive cervical joint mobilisation. Man Ther. 2008;13:387–96. doi: 10.1016/j.math.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Bialosky J, Bishop M, Price D, Robinson M, George S. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 2009;14:531–8. doi: 10.1016/j.math.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright A. Hypoalgesia post-manipulative therapy: a review of a potential neurophysiologic mechanism. Man Ther. 1995;1:11–6. doi: 10.1054/math.1995.0244. [DOI] [PubMed] [Google Scholar]
- 13.Lovick T. Interactions between descending pathways from the dorsal and ventrolateral periaqueductal gray matter in the rat. NATO ASI Ser. 1991;213:101–20. [Google Scholar]
- 14.Grayson J, Barton T, Cabot P, Souvlis T. Spinal manual therapy produces rapid onset analgesia in a rodent model. Man Ther. 2012;17:292–7. doi: 10.1016/j.math.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Perry J, Green A. An investigation into the effects of a unilaterally applied lumbar mobilisation technique on peripheral sympathetic nervous system activity in the lower limbs. Man Ther. 2008;13:492–9. doi: 10.1016/j.math.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Perry J, Green A, Singh S, Watson P. A preliminary investigation into the magnitude of effect of lumbar extension exercises and a segmental rotatory manipulation on sympathetic nervous system activity. Man Ther. 2011;16:190–5. doi: 10.1016/j.math.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Moutzouri M, Perry J, Billis E. Investigation of the effects of a centrally applied lumbar sustained natural apophyseal glide mobilization on lower limb sympathetic nervous system activity in asymptomatic subjects. J Manipulative Physiol Ther. 2012;35:286–94. doi: 10.1016/j.jmpt.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 18.George S, Bishop M, Bialosky J, Zeppieri G, Robinson M. Immediate effects of spinal manipulation on thermal pain sensitivity: an experimental study. BMC Musculoskelet Disord. 2006;7:68. doi: 10.1186/1471-2474-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bialosky J, Bishop M, Robinson M, Zeppieri G, George S. Spinal manipulative therapy has an immediate effect on thermal pain sensitivity in people with low back pain: a randomized controlled trial. Phys Ther. 2009;89:1292–303. doi: 10.2522/ptj.20090058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jewell D. Guide to evidence-based physical therapy practice. Sudbury, MA: Jones and Bartlett Publishers; 2008. [Google Scholar]
- 21.Maher C, Sherrington C, Herbert D, Moseley A, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–21. [PubMed] [Google Scholar]
- 22.Coppieters M, Stappaerts K, Wouters L, Janssens K. Aberrant protective force generation during neural provocation testing and the effect of treatment in patients with neurogenic cervicobrachial pain. J Manipulative Physiol Ther. 2003;26:99–106. doi: 10.1067/mmt.2003.16. [DOI] [PubMed] [Google Scholar]
- 23.Jowsey P, Perry J. Sympathetic nervous system effects in the hands following a grade III rotary posterior–anterior mobilisation technique applied to T4: a randomized, placebo-controlled trial. Man Ther. 2010;15:248–53. doi: 10.1016/j.math.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 24.La Touche R, Paris-Alemany A, Mannheimer J, Angulo-Diaz-Parreno S, Bishop M, Lopez-Valverde-Centeno A, et al. Does mobilization of the upper cervical spine affect pain sensitivity and autonomic nervous system function in patients with cervico-craniofacial pain? Clin J Pain. 2013;29:205–15. doi: 10.1097/AJP.0b013e318250f3cd. [DOI] [PubMed] [Google Scholar]
- 25.Chiu T, Wright A. To compare the effects of different rates of application of a cervical mobilisation technique on sympathetic outflow to the upper limb in normal subjects. Man Ther. 1996;1:198–203. doi: 10.1054/math.1996.0269. [DOI] [PubMed] [Google Scholar]
- 26.Moulson A, Watson T. A preliminary investigation into the relationship between cervical snags and sympathetic nervous system activity in the upper limbs of an asymptomatic population. Man Ther. 2006;11:214–24. doi: 10.1016/j.math.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Peterson N, Vicenzino B, Wright A. The effects of a cervical mobilisation technique on sympathetic outflow to the upper limb in normal subjects. Physiother Theory Pract. 1993;9:149–56. [Google Scholar]
- 28.Saranga J, Green A, Lewis J, Worsfold C. Effect of a cervical lateral glide on the upper limb neurodynamic test 1. Physiotherapy. 2003;89:678–84. [Google Scholar]
- 29.Vicenzino B, Collins D, Wright A. Sudomotor changes induced by neural mobilization techniques in asymptomatic subjects. J Man Manip Ther. 1994;2:66–74. [Google Scholar]
- 30.Vicenzino B, Collins D, Wright A. The initial effects of a cervical spine manipulative physiotherapy treatment on the pain and dysfunction of lateral epicondylalgia. Pain. 1996;68:69–74. doi: 10.1016/S0304-3959(96)03221-6. [DOI] [PubMed] [Google Scholar]
- 31.Vicenzino B, Collins D, Benson H, Wright A. An investigation of the interrelationship between manipulative therapy-induced hypoalgesia and sympathoexcitation. J Manipulative Physiol Ther. 1998;21:448–53. [PubMed] [Google Scholar]
- 32.Sterling M, Jull G, Wright A. Cervical mobilisation: concurrent effects on pain, sympathetic nervous system activity, and motor activity. Man Ther. 2001;6:72–81. doi: 10.1054/math.2000.0378. [DOI] [PubMed] [Google Scholar]
- 33.Wells G, Beaton D, Shea B, Boers M, Simon L, Strand V, et al. Minimal clinically important differences: review of methods. J Rheumatol. 2001;28:406–12. [PubMed] [Google Scholar]
- 34.Portney LG, Watkins MP. Foundations of clinical research, applications to practice, 3rd edn. Upper Saddle River, NJ: Pearson Prentice Hall; 2009. [Google Scholar]
- 35.Kelly A. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18:205–7. doi: 10.1136/emj.18.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstrong A, MacDermid J, Chinchalkar S, Stevens RS, King GJ. Reliability of range-of-motion measurement in the elbow and forearm. J Shoulder Elbow Surg. 1998;7:573–80. doi: 10.1016/s1058-2746(98)90003-9. [DOI] [PubMed] [Google Scholar]
- 37.Cleland J, Glynn P, Whitman J, Eberhart SL, MacDonald C, Childs JD. Short-term effects of thrust versus nonthrust mobilization/manipulation directed at the thoracic spine in patients with neck pain: a randomized clinical trial. Phys Ther. 2007;87:431–40. doi: 10.2522/ptj.20060217. [DOI] [PubMed] [Google Scholar]
- 38.Boyles RE, Ritland BM, Miracle BM, Barclay DM, Faul MS, Moore JH, et al. The short-term effects of thoracic spine thrust manipulation on patients with shoulder impingement syndrome. Man Ther. 2009;14:375–80. doi: 10.1016/j.math.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Ellis W, Cheng S. Intraoperative thermographic monitoring during neurogenic thoracic outlet decompressive surgery. Vasc Endovascular Surg. 2003;37:253–7. [PubMed] [Google Scholar]