Abstract
Background
Vaccines consisting of allergen-derived peptides lacking IgE reactivity and allergen-specific T cell epitopes bound to allergen-unrelated carrier molecules have been suggested as candidates for allergen-specific immunotherapy.
Objective
To study whether prophylactic and therapeutic vaccination with carrier-bound peptides from the major birch pollen allergen Bet v 1 lacking allergen-specific T cell epitopes has influence on Bet v 1-specific T cell responses.
Methods
Three Bet v 1-derived peptides, devoid of Bet v 1-specific T cell epitopes, were coupled to KLH and adsorbed to aluminium hydroxide to obtain a Bet v 1-specific allergy vaccine. Groups of BALB/c mice were immunized with the peptide vaccine before or after sensitization to Bet v 1. Bet v 1- and peptide-specific antibody responses were analysed by ELISA. T cell and cytokine responses to Bet v 1, KLH, and the peptides were studied in proliferation assays. The effects of peptide-specific and allergen-specific antibodies on T cell responses and allergic lung inflammation were studied using specific antibodies.
Results
Prophylactic and therapeutic vaccination with carrier-bound Bet v 1 peptides induced a Bet v 1-specific IgG antibody response without priming/boosting of Bet v 1-specific T cells. Prophylactic and therapeutic vaccination of mice with the peptide vaccine induced Bet v 1-specific antibodies which suppressed Bet v 1-specific T cell responses and allergic lung inflammation.
Conclusion and Clinical Relevance
Vaccination with carrier-bound allergen-derived peptides lacking allergen-specific T cell epitopes induces allergen-specific IgG antibodies which suppress allergen-specific T cell responses and allergic lung inflammation.
Keywords: allergens, allergy vaccination, peptides, specific immunotherapy
Introduction
Recently, a strategy for the development of safe allergy vaccines has been developed (reviewed in 1), which is based on the hapten-carrier principle 2,3. According to this concept, allergen-specific IgG antibody responses can be obtained by immunization with allergen-derived peptides which are either chemically bound to a carrier molecule or linked to the carrier in the form of a recombinant fusion protein 1,4. The T cell help for the production of allergen-specific IgG antibodies is obtained from a carrier molecule. The allergen-derived peptides in the vaccines are derived from areas of allergens containing IgE binding sites, but themselves lack IgE reactivity, allergenic activity (i.e. ability to cross-link mast cell-or basophil-bound IgE) and allergen-specific T cell epitopes 5–12. Allergy vaccines that are based on such carrier-bound allergen-derived peptides should induce allergen-specific IgG antibodies which block IgE-mediated allergic inflammation and allow reducing IgE- as well as T cell-mediated side-effects during SIT 1.
It has been shown that immunization of animals (i.e. mice, rabbits) with carrier-bound allergen-derived peptides from several major allergens (i.e. timothy grass pollen allergen, Phl p 1, birch pollen allergen, Bet v 1, cat allergen, Fel d 1, olive pollen allergen, Ole e 1, house dust mite allergen, Der p 2, mould allergen, Alt a 1) which lacked IgE reactivity and allergenic activity induced allergen-specific IgG antibodies that inhibited allergic patients' IgE binding to the allergen and allergen-induced basophil activation 6–11. Thus, the major immunological mechanism underlying carrier-bound allergen-derived peptide vaccines seems to be the induction of allergen-specific IgG blocking antibodies which may inhibit IgE-mediated mast cell and basophil degranulation and thus immediate allergic inflammation 13,14. However, it is possible that allergen-specific IgG antibodies induced by SIT may also block boosts of allergen-specific IgE production 15–17 and eventually allergen-specific T cell activation. Support for the latter mechanism comes from the demonstration that SIT-induced allergen-specific IgG can block IgE-facilitated allergen presentation to T cells and thus T cell activation in vitro 18,19.
Here, we established an in vivo mouse model to investigate whether prophylactic and therapeutic vaccination with carrier-bound allergen-derived peptide vaccines lacking allergen-derived T cell epitopes can influence allergen-specific T cell responses. As model allergen, we used the major birch pollen allergen, Bet v 1 20, which contains most of the IgE epitopes present in tree pollens belonging to the order Fagales (birch, alder, hazel, oak) and is responsible for oral allergy syndrome to plant-derived food (e.g. apple, nuts, carrot) 21,22. A peptide vaccine was formulated with carrier-bound Bet v 1-derived peptides lacking allergenic activity and T cell epitopes, and it was studied whether prophylactic and/or therapeutic vaccination of mice influences allergen-specific T cell responses.
Methods
Allergens, peptides, KLH-coupled peptide vaccine
Purified recombinant Bet v 1 was purchased from Biomay AG (Vienna, Austria). Six Bet v 1-derived peptides of approximately 30 aa length spanning the Bet v 1 sequence (Fig.1) were synthesized using the 9-fluorenylmethoxycarbonyl (Fmoc) strategy with 2-(1H-Benzotriazol-1-yl) 1,1,3,3 tetramethyluronium hexafluorophosphat (HBTU) activation (0.1 mm small-scale cycles) on the Applied Biosystems peptide synthesizer Model 433A (Foster City, CA, USA). Each peptide contained one cysteine residue in addition to the original sequence to facilitate coupling to the carrier. Pre-loaded polyethylene glycol–polystyrene (PEG-PS) resins (0.15–0.2 mm/g loading) (Per Septive Biosystems, Warrington, UK) served as solid phase to build up the peptides. Chemicals were obtained from Applied Biosystems. Peptides were cleaved from the resins with a mixture of 250 μL distilled water, 250 μL triisopropylsilane (Fluka, Buchs, Switzerland), 9.5 mL trifluoroacetic acid for 2 h and precipitated in tert-Butylmethylether (Fluka, Buchs). The identity of the peptides was confirmed by mass spectrometry, and they were purified by preparative high-pressure liquid chromatography (HPLC) (PiChem, Graz, Austria).
Figure 1.
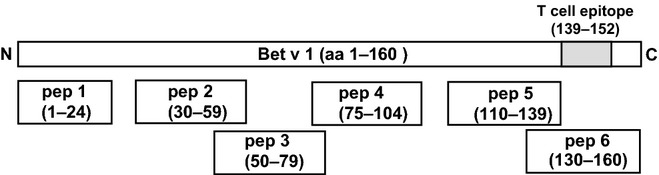
Overview of Bet v 1 peptides. Schematic representation of the Bet v 1 molecule from the N- to the C-terminus. Position and length of Bet v 1 peptides are indicated.
A peptide vaccine was prepared by coupling each of three peptides (pep 2, pep 3 and pep 4) to maleimide-activated keyhole limpet haemocyanin (KLH) via a cysteine residue according to the manufacturer's instructions (Pierce, Rockford, IL, USA) and by formulation of an equimolar mix of the conjugates. Peptides 2, 3 and 4 were selected because they have been shown to induce IgG antibodies which block birch pollen allergic patients' IgE binding to Bet v 1 6 and lack the major T cell epitope (i.e. aa 139-152) recognized by BALB/c mice 23. Furthermore, these peptides did not react with allergic patients' IgE, did not induce allergic inflammation in birch pollen allergic patients, and thus should represent safe vaccine candidates for SIT 6. The Phl p 5-derived peptide CAEEVKVIPAGELQVIEKVDAAFKVAATAANAAPANDK was coupled to KLH as described above to obtain a Bet v 1-unrelated peptide-KLH conjugate for control purposes.
Immunization of BALB/c mice
Six-week-old female BALB/c mice were purchased from Charles River (Germany). Animals were maintained in the animal care unit of the Department of Pathophysiology and Allergy Research, Medical University of Vienna, according to the local guidelines for animal care. The study was approved by the local ethics committee. The mouse model of birch pollen allergy obtained by sensitization to the major birch pollen allergen, Bet v 1, was established similar as described 23. In all experiments, groups of 5 mice were investigated. In the prophylactic peptide vaccination protocol, mice were immunized subcutaneously (s.c.) with a mix containing 10 μg of each of the three KLH-coupled Bet v 1 peptides adsorbed to aluminium hydroxide three times in 3-week intervals according to the immunization scheme (Fig.2) and then sensitized s.c. with aluminium hydroxide-adsorbed Bet v 1 (10 μg) (i.e. group P+/S+). For control purposes, groups were included which received only prophylactic immunization, but no sensitization (i.e. group P+/S−), neither prophylactic immunization nor sensitization (i.e. group PBS) or only sensitization (i.e. group P−/S+). In the therapeutic scheme, mice were sensitized s.c. with Bet v 1 (10 μg) adsorbed to aluminium hydroxide (three injections in 3 weeks intervals) followed by three s.c. immunizations with the peptide vaccine (i.e. group S+/T+). For control purposes, mice were not sensitized but received the peptide vaccine (i.e. group S−/T+) or were only sensitized (i.e. group S+/T−). In total, 7 groups of mice were studied (groups PBS, P+/S−, P+/S+, P−/S+, S+/T+, S+/T− and S−/T+). Blood samples were taken from the tail vein one day before each immunization and 3 weeks after the last immunization and were stored at −20°C until analysis. Mice were killed at day 120 to obtain spleen cells. Results were obtained in two independent sets of experiments. In the second experiment, two groups of mice were included which received a prophylactic or a therapeutic immunization with an irrelevant, Bet v 1-unrelated (i.e. grass pollen allergen Phl p 5-derived peptide) peptide coupled to KLH. In addition, groups of mice (n = 5) underwent the same prophylactic and therapeutic immunization protocol using either wild-type Bet v 1, or Bet v 1 fragments or a Bet v 1 trimer (10 μg/mouse) 24,25 as vaccines.
Figure 2.
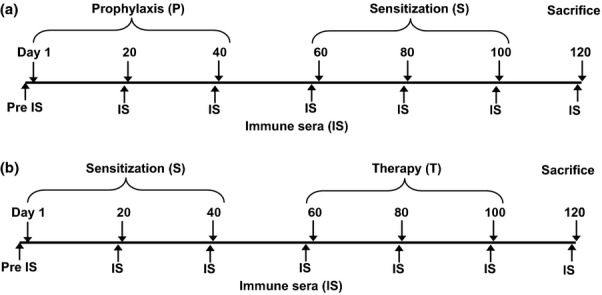
Scheme for prophylactic and therapeutic vaccination with KLH-coupled peptides. Timelines of the prophylactic (P) and therapeutic (T) vaccination with peptides. Time points of sensitization (S) with Bet v 1, bleeding, and killing are indicated.
To obtain Bet v 1 sensitized mice and Bet v 1 peptide-KLH-specific mouse antisera, two groups of mice (n = 8) were immunized with Bet v 1 or the Bet v 1 peptide-KLH-conjugates twice in three-week interval.
Analysis of allergen- and peptide-specific antibody responses
IgG1 antibody responses to Bet v 1 and the Bet v 1-derived peptides were measured by ELISA as described 26. Briefly, ELISA plates (Nunc Maxisorp, Rosklide, Denmark) were coated with rBet v 1 or Bet v 1-derived peptide 1, 2, 3, 4, 5 or 6 (5 μg/mL) and incubated with 1:1000 diluted mouse sera. Bound IgG1 antibodies were detected with a 1:1000 diluted monoclonal rat anti-mouse IgG1 antibody (Pharmingen, San Diego, CA, USA) and a 1:2000 diluted HRP-labelled sheep anti-rat antiserum (Amersham, Buckinghamshire, UK). Allergen-specific IgE, IgM, IgG2a, IgG3, IgA responses were measured as described in the online supporting information.
Bet v 1-specific rabbit IgG in mouse serum was detected by diluting mouse sera 1:20000 and a HRP-labelled donkey anti-rabbit Ig (Amersham) 1:2000.
Proliferation assays and rat basophil degranulation experiments
Spleens were removed under aseptic conditions, and a cell suspension was generated by passing spleens through a cell strainer. After lysis of erythrocytes, cells were washed and resuspended in complete medium (RPMI, 10% fetal calf serum, 0.1 mg/mL gentamicin, 2 mm glutamine). Splenocytes were plated into 96-well round-bottom plates at a concentration of 2 × 105 cells/well (200 μL) in triplicates and stimulated with rBet v 1 (2 μg/well), KLH (2 μg/well), a mixture of the three Bet v 1-derived peptides (0.3 μg of each peptide/well) or concanavalin A (0.5 μg/well) for 4 days. The cultures were pulsed with 0.5 μCi/well tritiated thymidine for 16 h and harvested. The proliferation responses were measured by scintillation counting. The ratio of the mean proliferation after antigen stimulation and medium control values, that is, the stimulation index (SI), was calculated 27.
In inhibition experiments, rabbit or mouse antisera specific for Bet v 1 peptides, Bet v 1, Bet v 1 fragments, Bet v 1 trimer or the unrelated grass pollen allergen Phl p 1 or normal mouse serum were added to the splenocyte cultures (1%, 5%, 10% v/v).
Experiments with rat basophil leukaemia cells are described in the online supporting information.
Murine model of allergic lung inflammation
Groups of mice (n = 5) were sensitized to Bet v 1 by one injection of alum-adsorbed Bet v 1 (10 μg). On day 28, rabbit anti-Bet v 1 peptide 2, 3 and 4 antisera or an irrelevant rabbit antiserum (anti-Phl p 1) were applied i.p. On three consecutive days, 5 μg Bet v 1 per mouse in 50 μL PBS was given intranasally to anesthetized mice. After killing, lung tissue was analysed for lymphocyte infiltration (Fig.8). Lung sections of 4 μm were stained with haematoxylin and eosin (H&E). The three most densely infiltrated areas in each H&E section were evaluated using an ocular grid with 100 small squares, each single square area 0.01 mm2. Percentages of inflammation area were calculated by counting the cells in the infiltrates within 3 single squares expressed as mean percentages.
Figure 8.
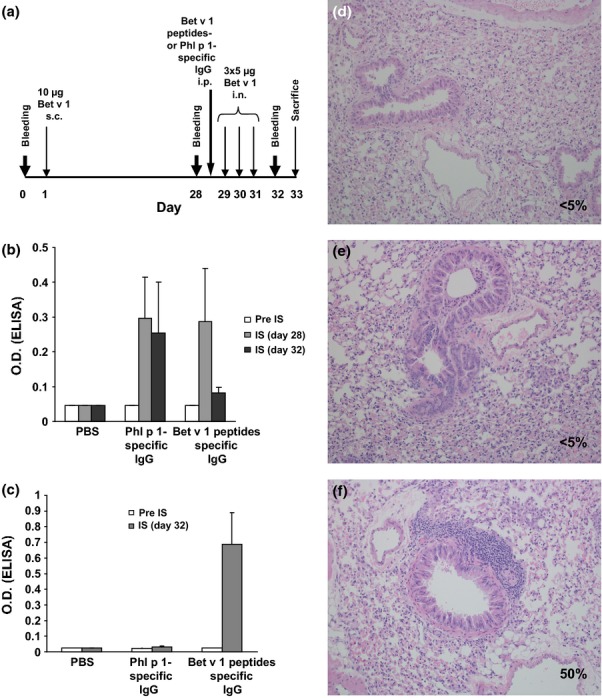
Adminstration of Bet v 1 peptide-specific antibodies prevents allergic lung inflammation. (a) Two groups of mice (n = 5) were sensitized with Bet v 1 and one group received PBS. One group of sensitized mice received Bet v 1 peptide-specific antibodies or Phl p 1-specific antibodies, and lung inflammation was induced by intranasal application of Bet v 1. (b) Mean OD values ± SD corresponding to Bet v 1-specific IgE levels (y-axis) are shown for the three mouse groups at different time points (x-axis). (c) Presence of Bet v 1-specific rabbit IgG antibodies in sera of the three groups of mice as in (b). H&E-stained lung sections from a representative mouse from the PBS group (d), the group treated with Bet v 1-peptide-specific antibodies (e), or with Phl p 1-specific antibodies (f). Percentages of infiltrated areas are shown in each section (d–f).
Statistical analysis
The reported P values are results of Wilcoxon Mann–Whitney U-test and exact significances. SPSS statistical software system 14.0 was used for calculations. Values of P < 0.05 were considered statistically significant. Error bars indicate SDs.
Results
Features of a Bet v 1-specific peptide vaccine lacking T cell epitopes for prophylactic and therapeutic vaccination
Three Bet v 1-derived synthetic peptides, designated pep 2, 3 and 4, are located in the major IgE-reactive area of the Bet v 1 allergen (Fig.1). These peptides neither react with IgE antibodies from birch pollen allergic patients nor exhibit allergenic activity 6. Pep 2, 3 and 4 do not contain T cell epitopes recognized by BALB/c mice 23. Furthermore, each of these peptides was shown to induce Bet v 1-specific IgG antibodies upon immunization, which strongly inhibited birch pollen allergic patients' IgE binding to the allergen (i.e. more than 50% inhibition) 6. Each of the peptides was coupled to the carrier molecule keyhole limpet haemocyanin (KLH) and adsorbed to the adjuvant aluminium hydroxide for prophylactic and therapeutic vaccination in a BALB/c model of Bet v 1 allergy. For prophylactic vaccination, three immunizations with the peptide vaccine were given before sensitization to Bet v 1, whereas for therapeutic vaccination, three immunizations were given after sensitization (Fig.2).
The peptide vaccine induces mainly Bet v 1-specific IgG1 antibodies receiving help from KLH-specific T cells
BALB/c mice were vaccinated 3 times with the peptide vaccine, Bet v 1, or PBS, and subsequently the induction of Bet v 1-specific T lymphocytes was analysed in splenocytes from the immunized animals. Bet v 1-specific proliferation of splenocytes was observed only in Bet v 1-immunized mice, while no lymphoproliferative responses could be detected in the peptide-vaccinated or PBS mice (Fig.3, left plot). None of the latter groups developed a peptide-specific T cell response, confirming that the peptides do not contain T cell epitopes (Fig.3, central plot). We found that the T cell response of mice immunized with the peptide vaccine was mainly directed to the carrier molecule KLH (Fig.3, right plot).
Figure 3.
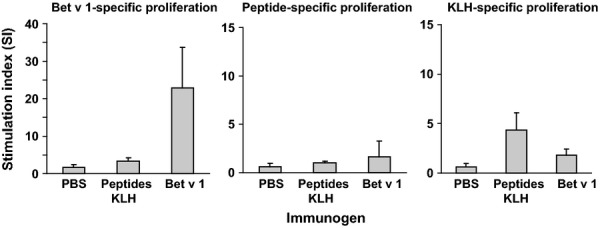
Specificities and magnitudes of splenocyte proliferations. Splenocytes from groups of mice which were immunized with the KLH-coupled peptides, Bet v 1, or PBS (x-axis) were stimulated with Bet v 1, a mixture of the 3 peptides, or KLH. Proliferation rates are shown as stimulation indices ±SD (y-axis).
Vaccination with the peptides was accompanied by the induction of a Bet v 1-specific IgG antibody response. Immunization with the complete Bet v 1 molecule led to higher Bet v 1-specific IgG levels than peptide vaccination (Fig.4). When we further analysed the specificity of these IgG antibodies, we found that the IgGs induced after peptide vaccination were exclusively directed to the three peptides (peptides 2, 3, and 4) included in the vaccine (Fig.4). In contrast, IgG antibodies induced by immunization with the complete Bet v 1 molecule recognized peptides 3, 4, 5, and 6 (Fig.4). Peptide 1 showed no IgG reactivity.
Figure 4.
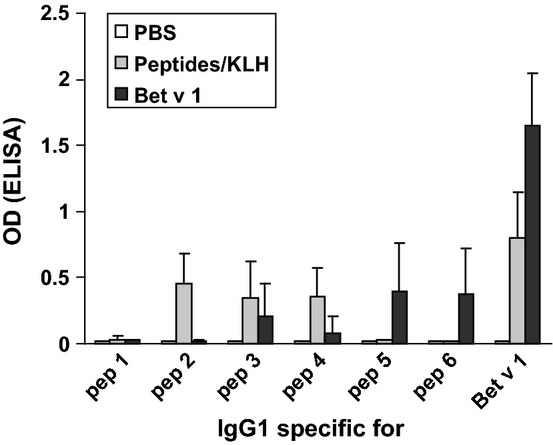
Specificities and magnitudes of IgG1 antibody responses. Sera from mice immunized with the peptide vaccine (grey bars), recombinant Bet v 1 (black bars), or PBS (white bars) were tested for IgG1 reactivities (optical density values ± SD: y-axis) to Bet v 1 and Bet v 1-derived peptides 1-6.
Prophylactic vaccination with the peptide vaccine prevents the development of allergen-specific T cell responses
Next, we investigated the influence of peptide vaccination on the development of allergen-specific T cell responses in the course of allergic sensitization. Compared with the unvaccinated but sensitized group (P−/S+), which developed a strong Bet v 1-specific T cell response with stimulation indices above 20 (Fig.5a, P−/S+), there was only low proliferation in response to the Bet v 1 allergen detectable in the mouse group which had received prophylactic peptide vaccination (Fig.5a, P+/S+). The latter response was significantly lower than that in mice without prophylactic vaccination (P < 0.05). No Bet v 1-specific T cell responses were observed in mice who had only received PBS or in mice who were vaccinated with the peptide vaccine but were not sensitized (P+/S−) (Fig.5a). None of the mouse groups showed any relevant proliferative responses to a mix of the three uncoupled Bet v 1 peptides, pep 2, 3, and 4 (Fig.5b). Mice that had received peptide vaccination but not unvaccinated or sensitized mice showed specific splenocyte proliferative responses to the carrier protein KLH (Fig.5c, P+/S−, P+/S+).
Figure 5.
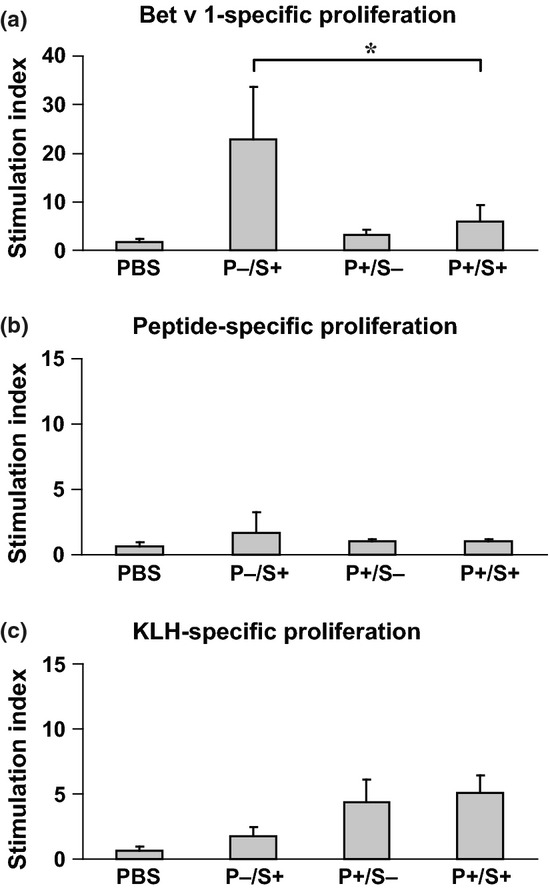
T cell responses of mice from the prophylactic scheme. The lymphoproliferative responses (y-axes: stimulation indices SI ± SD) of groups of mice, which were vaccinated according to a prophylactic protocol (Figure2) to rBet v 1 (a), a mixture of the three Bet v 1-derived peptides (b), or KLH (c) are displayed. Statistically significant differences are indicated (* P < 0.05). P: prophylaxis; S: sensitization.
Therapeutic vaccination with the peptide vaccine reduces Bet v 1-specific T cell proliferation
We furthermore tested the ability of the peptide vaccine to affect an already established allergen-specific T cell response in our mouse model. We found that after sensitization to Bet v 1, a robust Bet v 1-specific T cell response (i.e. SI's >10) was established (Fig.6a, S+/T−). This response was significantly reduced when mice were treated with the peptide vaccine (Fig.6a, S+/T+; P < 0.05). A relevant peptide-specific proliferation was observed in none of the mouse groups (Fig.6b). Mice treated with the peptide vaccine, but not mice who had not received the peptide vaccine, showed carrier-specific T cell responses (Fig.6c).
Figure 6.
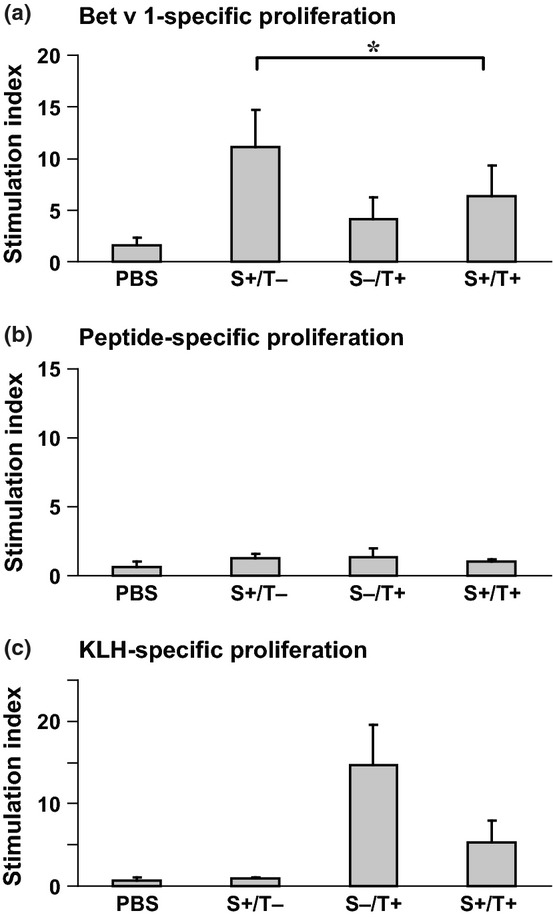
T cell responses of mice from the therapeutic scheme. Bet v 1- (a), peptide- (b), and KLH-specific (c) proliferations of splenocytes from mice of the therapeutic scheme (Fig.2) are displayed (y-axes: stimulation indices SI ± SD). Statistically significant differences are indicated (*P < 0.05). S: sensitization, T: therapy.
Peptide-induced antibodies inhibit Bet v 1-specific proliferation of splenocytes from Bet v 1-sensitized mice
In an initial set of experiments, we compared Bet v 1-specific IgM, IgG1, IgG2a, IgG3, IgA, and IgE responses in the different groups of mice (Online supporting information). There was no statistically significant difference between the various antibody levels in the sensitized mice with and without prophylactic vaccination (Figure S1a) and between the Bet v 1-sensitized mouse groups with and without treatment (Figure S1b). This result is somewhat in accordance with the cytokine pattern, as it also suggests that a modulation towards a Th1 response did not occur in the vaccinated mice (Table S1). We also investigated Bet v 1-specific IgE antibody levels. Again we found that there was no statistically significant difference between the sensitized mice with and without prophylactic vaccination regarding the IgE antibody levels (Figure S2a) and between the Bet v 1-sensitized mouse groups with and without treatment (Figure S2b). However, when the IgE antibodies were loaded onto RBL cells, we found that the vaccine did not induce anaphylactic responses and, most importantly, that vaccine-induced IgG prevented Bet v 1-induced basophil degranulation, which strongly suggests that these antibodies must have protective effects on allergic symptoms (Figure S3).
Next, we performed experiments and used a CSFE-like staining technology (i.e. VPD450-labelling) and gated on CD4+ cells to study T cell responses. For this purpose, groups of mice were sensitized to Bet v 1 and vaccinated with the peptide vaccine (S+/peptides-KLH), or an irrelevant peptide coupled to KLH (S+/irrelevant peptide-KLH) according to the vaccination protocol. For control purposes, groups of mice were only sensitized (S+) or only vaccinated with the peptide vaccine (S-/peptides-KLH). VPD450-labelled splenocytes were cultivated in the presence of Bet v 1, the Bet v 1-derived peptides, or KLH, stained for CD4, and analysed by flow cytometry. Results of the VPD450-staining are exemplified in Figure S4, whereby the results confirmed the data we obtained by proliferation assays. The measurement of cytokines in response to Bet v 1 in cell culture supernatants of splenocytes did not show a significant change in Th1/Th2/Treg-specific cytokine production in vaccinated mice compared to sensitized but untreated mice (Table S1), suggesting another mechanism than T cell modulation in the therapy group. In mice receiving prophylactic (i.e. preventive) vaccination, we noted a trend towards a decrease of cytokine secretion in the treated mice although this did not reach statistical significance.
As we could not find any evidence of direct modulation of Bet v 1-specific T cell function, we investigated whether an allergen-specific serum factor, that is, allergen-specific antibodies, could inhibit Bet v 1-specific proliferation.
Results obtained showed that serum from peptide-vaccinated mice suppressed Bet v 1-specific proliferation of splenocytes from Bet v 1-sensitized mice (Fig.7a). Moreover, we could show that only sera generated by vaccination of mice and rabbits with the Bet v 1-derived peptides, Bet v 1, recombinant Bet v 1 derivatives but not other control antigens had a suppressive effect on allergen-specific proliferation, suggesting that the proliferation of Bet v 1-specific T cells was indeed suppressed by Bet v 1-specific antibodies (Figs7b and c). Furthermore, we extended the study comparing the effect of the peptide vaccine to prophylactic and therapeutic vaccination with wild-type Bet v 1, with a mixture of two Bet v 1 fragments or with a Bet v 1 trimer, which have already been used for vaccination in patients in clinical trials 16,28. Using these molecules for prophylactic and therapeutic vaccination, we could again observe a suppressive effect on splenocyte proliferation (Figure S5).
Figure 7.
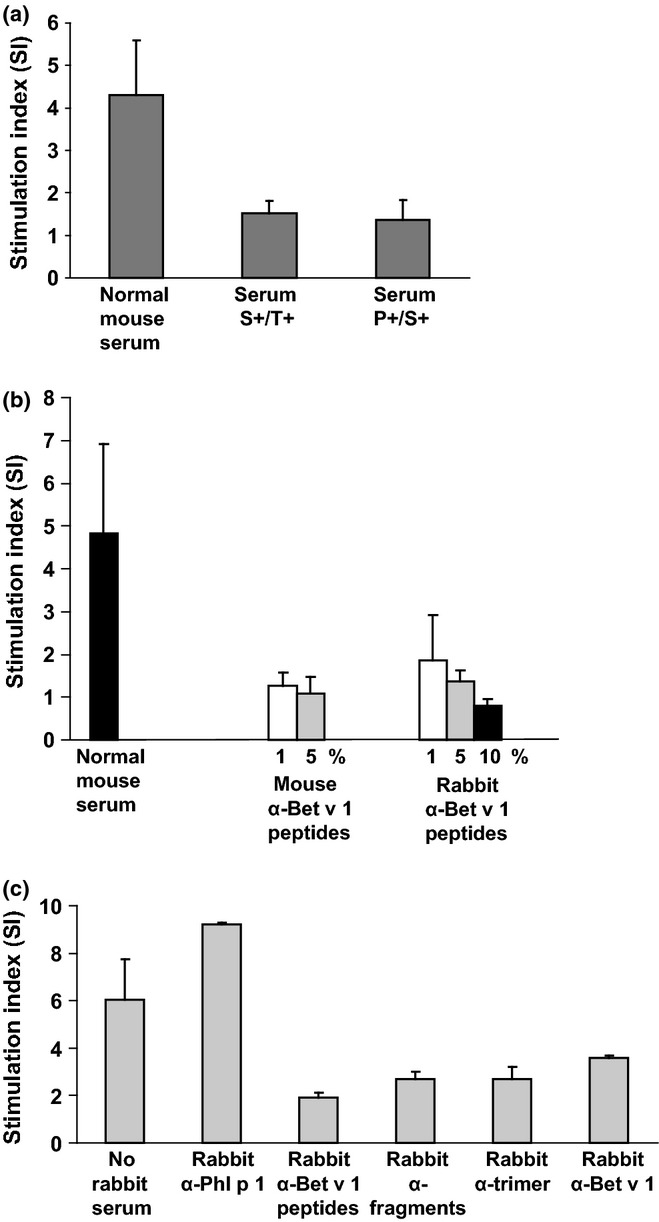
Inhibition of Bet v 1-specific T cell proliferation by Bet v 1-specific antibodies. (a) Splenocytes from four Bet v 1-sensitized mice were stimulated in the presence of normal mouse serum, serum from the S+/T+ group, or serum from the P+/S+ group from day 120 or (b) in the presence of normal mouse serum or different concentrations of mouse or rabbit anti-Bet v 1 peptide antisera. (c) Experiment performed as in (a, b) with cultured splenocytes without rabbit serum or with rabbit antisera specific for Phl p 1, Bet v 1, Bet v 1 fragments, Bet v 1 trimer, or Bet v 1 peptides. Stimulation indices ± SD are shown for triplicate cultures from 4 individual mice (y-axes).
Bet v 1 peptide-induced antibodies inhibit Bet v 1-induced lung inflammation
Next, we developed a mouse model for testing the impact of peptide vaccine-induced IgG antibodies on allergic lung inflammation. For this purpose, mice were sensitized to Bet v 1 and lung inflammation was induced by intranasal application of Bet v 1 (Fig.8a). When we applied Bet v 1 peptide-specific rabbit antibodies to these mice, IgE reactivity in their sera to Bet v 1 was suppressed due to their appearance in serum (Figs8b and c) and, most importantly, no allergic lung inflammation similar as in the only PBS treated mice was found (Figs8d and e). By contrast, mice that had not received Bet v 1 peptide-induced antibodies showed lung inflammation (Fig.8f).
Discussion
In this study, we investigated in a mouse model of birch pollen allergy the effects of a Bet v 1-specific peptide vaccine which consists of three carrier-bound non-allergenic Bet v 1 peptides lacking allergen-specific T cell epitopes on allergen-specific T cell responses. It has been demonstrated that immunization of mice and rabbits with the carrier-bound Bet v 1 peptides induced allergen-specific IgG antibodies which blocked allergic patients' IgE binding to the allergen as well as allergen-induced basophil activation 6. Furthermore, prophylactic immunization reduced the development of Bet v 1-specific IgE responses 6. These effects were explained by the induction of allergen-specific IgG antibodies, which block IgE recognition of the allergen. However, the question remained whether carrier-bound allergen peptides lacking allergen-specific T cell epitopes can influence allergen-specific T cell responses. Here, we show that immunization with the Bet v 1 peptide vaccine induces Bet v 1-specific IgG responses with T cell help specific for the carrier molecule KLH but without activation of Bet v 1-specific T cells.
This result (i.e. the induction of Bet v 1-specific IgG without inclusion of Bet v 1-specific T cell epitopes in the vaccine) can be explained according to the hapten-carrier principle described by the pivotal experiments of the Nobel laureate B. Benacerraf that led to the discovery of T cell-dependent antibody production 2,3. It also confirms that the Bet v 1 peptide vaccine does not stimulate Bet v 1-specific T cell responses which can be explained by the fact that the major T cell epitope recognized by BALB/c mice in the Bet v 1 allergen (i.e. peptide 139-152) was not included in the peptide vaccine 23.
It was therefore interesting to note that prophylactic peptide vaccination almost completely suppressed Bet v 1-specific T cell responses in mice which have been sensitized with Bet v 1, whereas non-vaccinated mice mounted strong Bet v 1-specific T cell responses (i.e. SI's > 20). There was no relevant Bet v 1-specific T cell response detectable in the mice which had received the prophylactic peptide vaccine, whereas a strong Bet v 1-specific IgG response was induced. We therefore suggest that the Bet v 1-specific IgG antibodies prevented the development of Bet v 1-specific T cell responses. This prevention may have been achieved by capturing of the allergen during sensitization and reduction in allergen presentation 29. In fact, it has been shown that allergic sensitization can be suppressed by allergen-specific IgG antibodies, which were either administered to naïve mice or even transferred from the mother to the offspring before sensitization 30.
Another finding, which may be important for therapeutic application of the peptide vaccine, was that sensitized mice which were treated with the peptide vaccine showed a significantly reduced Bet v 1-specific T cell response compared with mice which were not immunized. This result indicates that the peptide vaccine despite lacking the major T cell epitopes could reduce Bet v 1-specific T cell responses.
In this context, it has been demonstrated in mice as well as in allergic patients that IgE-facilitated allergen presentation by CD23 strongly augments allergen-specific T cell responses 31,32 and that this process can be inhibited by therapy-induced allergen-specific IgG antibodies 18,19. Based on the inhibition studies performed with allergen-specific antibodies showing that Bet v 1-specific T cell proliferation and allergic lung inflammation can be inhibited by the Bet v 1-specific antibodies, we suggest that the reduced allergen-specific proliferation is mainly caused by allergen-specific antibodies which can be induced by vaccination with the wild-type allergen, recombinant allergen derivatives, or allergen-derived peptides coupled to a carrier. Similar as has been reported for humans in clinical trials, this effect is most likely due to inhibition of IgE-facilitated antigen-presentation to T cells 18,33,34.
In summary, our study reveals a hitherto unknown and quite unexpected suppressive effect of allergy vaccines based on carrier-bound allergen peptides without allergenic activity and allergen-specific T cell epitopes on allergen-specific T cell responses. These vaccines may reduce therefore not only IgE-mediated but also T cell-mediated allergic inflammation.
Acknowledgments
This work was supported by Grants P23350-B11 and F4605 of the Austrian Science Fund (FWF) and by research grants from the Christian Doppler Research Association, Vienna, Austria, and BIOMAY AG, Vienna, Austria.
Conflict of interests
BL, MN, MF, FW and SV have no conflict of interest to declare. RV has received research grants from Biomay AG, Vienna, Austria, and Phadia, Uppsala, Sweden, and serves as a consultant for these companies.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Measurement of Bet v 1-specific immunoglobulin classes and subclasses by ELISA.
Figure S2. Development of Bet v 1-specific IgE responses in the course of prophylactic and therapeutic vaccination with Bet v 1-derived peptides.
Figure S3. Sera from peptide-vaccinated mice inhibit Bet v 1-induced effector cell degranulation in vitro.
Figure S4. VPD450-based detection of CD4+T cell proliferation in representative mice from each treatment group by FACS analysis.
Figure S5. Vaccination with hypoallergenic Bet v 1-derivatives containing T cell epitopes or wild-type Bet v 1 reduces Bet v 1-specific proliferation.
Table S1. Cytokine response of splenocytes from vaccinated mice in response to Bet v 1.
References
- Focke M, Swoboda I, Marth K, Valenta R. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy. 2010;40:385–97. doi: 10.1111/j.1365-2222.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- Katz DH, Paul WE, Goidl EA, Benacerraf B. Carrier function in anti-hapten immune responses. I. Enhancement of primary and secondary anti-hapten antibody responses by carrier preimmunization. J Exp Med. 1970;132:261–82. doi: 10.1084/jem.132.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul WE, Katz DH, Goidl EA, Benacerraf B. Carrier function in anti-hapten immune responses. II. Specific properties of carrier cells capable of enhancing anti-hapten antibody responses. J Exp Med. 1970;132:283–99. doi: 10.1084/jem.132.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta R, Niespodziana K, Focke-Tejkl M, et al. Recombinant allergens: what does the future hold? J Allergy Clin Immunol. 2011;127:860–4. doi: 10.1016/j.jaci.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Focke M, Mahler V, Ball T, et al. Nonanaphylactic synthetic peptides derived from B cell epitopes of the major grass pollen allergen, Phl p 1, for allergy vaccination. FASEB J. 2001;15:2042–4. doi: 10.1096/fj.01-0016fje. [DOI] [PubMed] [Google Scholar]
- Focke M, Linhart B, Hartl A, et al. Non-anaphylactic, surface-exposed peptides of the major birch pollen allergen, Bet v 1, for preventive vaccination. Clin Exp Allergy. 2004;34:1525–33. doi: 10.1111/j.1365-2222.2004.02081.x. [DOI] [PubMed] [Google Scholar]
- Edlmayr J, Niespodziana K, Linhart B, et al. A combination vaccine for allergy and rhinovirus infections based on rhinovirus-derived surface protein VP1 and a nonallergenic peptide of the major timothy grass pollen allergen Phl p 1. J Immunol. 2009;182:6298–306. doi: 10.4049/jimmunol.0713622. [DOI] [PubMed] [Google Scholar]
- Niespodziana K, Focke-Tejkl M, Linhart B, et al. A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J Allergy Clin Immunol. 2011;127:1562–70. doi: 10.1016/j.jaci.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twaroch T, Focke M, Civaj V, et al. Carrier-bound, non-allergenic Ole e 1 peptides for vaccination against olive pollen allergy. J Allergy Clin Immunol. 2011;128:178–84. doi: 10.1016/j.jaci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Chen KW, Focke-Tejkl M, Blatt K, et al. Carrier-bound nonallergenic Der p 2 peptides induce IgG antibodies blocking allergen-induced basophil activation in allergic patients. Allergy. 2012;67:609–21. doi: 10.1111/j.1398-9995.2012.02794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twaroch TE, Focke M, Fleischmann K, et al. Carrier-bound Alt a 1 peptides without allergenic activity for vaccination against Alternaria alternata allergy. Clin Exp Allergy. 2012;42:966–75. doi: 10.1111/j.1365-2222.2012.03996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhart B, Valenta R. Mechanisms underlying allergy vaccination with recombinant hypoallergenic allergen derivatives. Vaccine. 2012;30:4328–35. doi: 10.1016/j.vaccine.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- Valenta R, Ferreira F, Focke-Tejkl M, et al. From allergen genes to allergy vaccines. Annu Rev Immunol. 2010;28:211–41. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- Mothes N, Heinzkill M, Drachenberg KJ, et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy. 2003;33:1198–208. doi: 10.1046/j.1365-2222.2003.01699.x. [DOI] [PubMed] [Google Scholar]
- Niederberger V, Horak F, Vrtala S, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14677–82. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creticos PS, Schroeder JT, Hamilton RG, et al. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–55. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- van Neerven RJ, Wikborg T, Lund G, et al. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4 + T cells by inhibiting serum-IgE-facilitated allergen presentation. J Immunol. 1999;163:2944–52. [PubMed] [Google Scholar]
- Wachholz PA, Durham SR. Mechanisms of immunotherapy: IgG revisited. Curr Opin Allergy Clin Immunol. 2004;4:313–8. doi: 10.1097/01.all.0000136753.35948.c0. [DOI] [PubMed] [Google Scholar]
- Breiteneder H, Pettenburger K, Bito A, et al. The gene coding for the major birch pollen allergen Bet v 1, is highly homologous to a pea disease resistance response gene. EMBO J. 1989;8:1935–8. doi: 10.1002/j.1460-2075.1989.tb03597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederberger V, Pauli G, Grönlund H, et al. Recombinant birch pollen allergens (rBet v 1 and rBet v 2) contain most of the IgE epitopes present in birch, alder, hornbeam, hazel, and oak pollen: a quantitative IgE inhibition study with sera from different populations. J Allergy Clin Immunol. 1998;102:579–91. doi: 10.1016/s0091-6749(98)70273-8. [DOI] [PubMed] [Google Scholar]
- Kazemi-Shirazi L, Pauli G, Purohit A, et al. Quantitative IgE inhibition experiments with purified recombinant allergens indicate pollen-derived allergens as the sensitizing agents responsible for many forms of plant food allergy. J Allergy Clin Immunol. 2000;105:116–25. doi: 10.1016/s0091-6749(00)90186-6. [DOI] [PubMed] [Google Scholar]
- Bauer L, Bohle B, Jahn-Schmid B, et al. Modulation of the allergic immune response in BALB/c mice by subcutaneous injection of high doses of the dominant T cell epitope from the major birch pollen allergen Bet v 1. Clin Exp Immunol. 1997;107:536–41. doi: 10.1046/j.1365-2249.1997.d01-953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtala S, Hirtenlehner K, Vangelista L, et al. Conversion of the major birch pollen allergen, Bet v 1, into two nonanaphylactic T cell epitope-containing fragments: candidates for a novel form of specific immunotherapy. J Clin Invest. 1997;99:1673–81. doi: 10.1172/JCI119330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtala S, Hirtenlehner K, Susani M, et al. Genetic engineering of a hypoallergenic trimer of the major birch pollen allergen Bet v 1. FASEB J. 2001;15:2045–7. doi: 10.1096/fj.00-0767fje. [DOI] [PubMed] [Google Scholar]
- Vrtala S, Ball T, Spitzauer S, et al. Immunization with purified natural and recombinant allergens induces mouse IgG1 antibodies that recognize similar epitopes as human IgE and inhibit the human IgE-allergen interaction and allergen-induced basophil degranulation. J Immunol. 1998;160:6137–44. [PubMed] [Google Scholar]
- Linhart B, Bigenzahn S, Hartl A, et al. Costimulation blockade inhibits allergic sensitization but does not affect established allergy in a murine model of grass pollen allergy. J. Immunol. 2007;178:3924–31. doi: 10.4049/jimmunol.178.6.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli G, Larsen TH, Rak S, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–60. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Hjelm F, Carlsson F, Getahun A, Heyman B. Antibody-mediated regulation of the immune response. Scand J Immunol. 2006;64:177–84. doi: 10.1111/j.1365-3083.2006.01818.x. [DOI] [PubMed] [Google Scholar]
- Uthoff H, Spenner A, Reckelkamm W, et al. Critical role of preconceptional immunization for protective and nonpathological specific immunity in murine neonates. J Immunol. 2003;1:3485–92. doi: 10.4049/jimmunol.171.7.3485. [DOI] [PubMed] [Google Scholar]
- Carlsson F, Hjelm F, Conrad DH, Heyman B. IgE enhances specific antibody and T-cell responses in mice overexpressing CD23. Scand J Immunol. 2007;66:261–70. doi: 10.1111/j.1365-3083.2007.01953.x. [DOI] [PubMed] [Google Scholar]
- van der Heijden FL, van Neerven RJJ, van Katwijk M, Bos JD, Kapsenberg ML. Serum-IgE-facilitated allergen presentation in atopic disease. J Immunol. 1993;150:3643–50. [PubMed] [Google Scholar]
- Pree I, Shamji MH, Kimber I, Valenta R, Durham SR, Niederberger V. Inhibition of CD23-dependent facilitated allergen binding to B cells following vaccination with genetically modified hypoallergenic Bet v 1 molecules. Clin Exp Allergy. 2010;40:1346–52. doi: 10.1111/j.1365-2222.2010.03548.x. [DOI] [PubMed] [Google Scholar]
- Shamji MH, Francis JN, Würtzen PA, Lund K, Durham SR, Till SJ. Cell-free detection of allergen-IgE cross-linking with immobilized phase CD23: inhibition by blocking antibody responses after immunotherapy. J Allergy Clin Immunol. 2013;132:1003–5. doi: 10.1016/j.jaci.2013.05.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Measurement of Bet v 1-specific immunoglobulin classes and subclasses by ELISA.
Figure S2. Development of Bet v 1-specific IgE responses in the course of prophylactic and therapeutic vaccination with Bet v 1-derived peptides.
Figure S3. Sera from peptide-vaccinated mice inhibit Bet v 1-induced effector cell degranulation in vitro.
Figure S4. VPD450-based detection of CD4+T cell proliferation in representative mice from each treatment group by FACS analysis.
Figure S5. Vaccination with hypoallergenic Bet v 1-derivatives containing T cell epitopes or wild-type Bet v 1 reduces Bet v 1-specific proliferation.
Table S1. Cytokine response of splenocytes from vaccinated mice in response to Bet v 1.


