Highlights
-
•
We developed a simple HPLC method to determine the oxidative status of human peroxiredoxin 2 (Prx2).
-
•
tert-Butyl hydroperoxide irreversibly hyperoxidizes cysteine residues of Prx2 more readily than H2O2.
-
•
Hyperoxidized Prx2 is more hydrophobic than the native form in red blood cells.
Abbreviations: t-BHP, tert-butyl hydroperoxide; DTPA, diethylenetriaminepentaacetic acid; DTT, dithiothreitol; HPLC, high-performance liquid chromatography; MALDI, matrix-assisted laser desorption/ionization; PMF, peptide mass fingerprinting; PBS, phosphate-buffered saline; RBC, red blood cell; ROS, reactive oxygen species; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; TOF, time-of-flight; MS, mass spectrometry; TFA, trifluoroacetic acid
Keywords: Peroxiredoxin 2, Oxidative stress, Red blood cell, tert-Butyl hydroperoxide, Hyperoxidation, Biomarker
Abstract
Peroxiredoxin 2 (Prx2) is the third most abundant protein in red blood cells (RBCs). In this study, we have succeeded in implementing the rapid and simultaneous detection of the hyperoxidized (Prx2-SO2/3) and reduced (Prx2-SH) forms of Prx2 in human RBCs using reverse phase high-performance liquid chromatography. The detection of a peak corresponding to Prx2-SO2/3 was clearly observed following treatment of tert-butyl hydroperoxide (t-BHP), but not H2O2, and was found to be dose-dependent. The identity of the peak was confirmed as Prx2 by immunoblotting and mass spectrometry analysis. Our results suggest that t-BHP hyperoxidizes cysteine residues in Prx2 more readily than H2O2, and that accumulation of hyperoxidized Prx2 might reflect disruption of redox homeostasis in RBCs.
1. Introduction
Peroxiredoxins (Prxs) constitute a group of redox enzymes that eliminate hydrogen peroxide using thioredoxin as the substrate; the isoforms Prx1–6 have been identified in recent studies [1]. Prxs contain cysteine residues that are highly sensitive to oxidation by peroxides [2]. It is also known that Prxs react with low level H2O2 at cysteine residues in the active site [2]. In cells, the oxidation state (disulfide form or reduced monomer) of cysteine residues is reversely regulated by the thioredoxin- and sulfiredoxin-dependent reductase systems [3]. However, the contribution of the reductase systems in human red blood cells (RBCs) remains u nclear [4,5]. Prxs are antioxidative proteins and Prx2 has been the focus of attention as a possible oxidative stress marker [6,7]. Indeed, certain studies have reported that hyperoxidized Prx2 can serve as an indicator of oxidation in blood preservation [8,9]. Additionally, the hyperoxidized forms of Prx family proteins have been found in neuronal cells [10] and the brains of patients with Alzheimer’s disease [11]. Although notable and interesting reports focusing on the oxidation of Prx2 are steadily increasing, the oxidation state and physiological formation process of irreversibly oxidized Prx2, termed the “hyperoxidized form”, are not well understood in human RBCs.
In this study, we assessed a possible method for detecting oxidation of Prx2, which is an abundant protein in RBCs. The production of hyperoxidized Prx2 in reactions with hydrogen peroxide (H2O2) and tert-butyl hydroperoxide (t-BHP) was investigated to establish a reverse-phase mode high performance liquid chromatography (HPLC)-based procedure for the separation and UV detection of the reduced and hyperoxidized forms of Prx2. Further, we conducted a more detailed analysis focusing on the oxidation state of susceptible thiol residues in hyperoxidized Prx2 produced by the treatment of human RBCs with t-BHP using specific antibody and mass spectrometry (MS) techniques.
2. Materials and methods
2.1. Chemicals
RIPA solution, tert-butyl hydroperoxide (t-BHP), and 3-amino-1,2,4-triazol (3-AT) were purchased from Sigma Aldrich (St. Louis, MO, USA). Dithiothreitol (DTT), mercaptosuccinate and hydrogen peroxide (H2O2) (atomic absorption grade) were obtained from Wako Pure Chemical Industries (Osaka, Japan). The protease inhibitor cocktail was obtained from Thermo Scientific (Waltham, MA, USA). HPLC grade water and acetonitrile were obtained from Nacalai Tesque (Kyoto, Japan). All other reagents were of the highest commercial grade available.
2.2. Preparation of RBCs and lysates
Blood samples were obtained from eight healthy subjects (21–51 years old; fasted for 12 h) with their informed consent. Blood was drawn into vacutainer tubes containing ethylenediamine tetraacetic acid (EDTA). RBCs were prepared by centrifugation at 800×g for 10 min at 4 °C and were washed in phosphate-buffered saline [PBS: 137 mM NaCl, 8.1 mM Na2HPO4, 2.68 mM KCl, 1.47 mM KH2PO4 (pH 7.4)] using the same centrifugation procedure. RBCs were resuspended in PBS (Hct. 0.40) containing 10 mM glucose and 1 mM diethylenetriaminepentaacetic acid (DTPA).
To avoid the artificial oxidation of Prx2 during the direct analysis of the hemolysate by reverse phase HPLC assay, RBCs were lysed in a hypotonic buffer [5 mM phosphate buffer (pH 7.4) containing 0.1% Triton-X 100, protease inhibitors] containing 5 mM DTT after incubation with or without each peroxide. The lysates were centrifuged at 15,000×g for 5 min at 4 °C and the supernatant was used as the sample for analysis of Prx2. All of the hemolysates were prepared within 60 min of collection and were subsequently rapidly separated using HPLC.
2.3. Hyperoxidation of Prx2 in RBCs
Irreversible oxidation of Prx2 was induced by H2O2 or t-BHP treatment. The RBC suspension (hematocrit adjusted to 40%) was incubated at 37 °C with 100–500 μM peroxides for 60 min, after which the RBCs were washed twice in PBS. Following hemolysis of the RBCs in a hypotonic buffer, the hemolysate was centrifuged to remove insoluble debris. The supernatant was immediately applied to a reverse phase HPLC system for protein separation.
2.4. HPLC system
Reverse phase HPLC was performed using a C18 column for protein (YMC-packed PROTEIN-RP; YMC Co., Tokyo, Japan) with a UV detector (280 nm). The RBC lysate was injected into the column, which was equilibrated with 40% acetonitrile, at a flow rate of 1.0 ml/min. HPLC was conducted using two mobile phases, A [water containing 0.1% trifluoroacetic acid (TFA)] and B (acetonitrile containing 0.1% TFA). The elution was conducted according to the following sequence: 40% B (0 min) – 40% B (20 min) – 45% B (50 min) – 90% B (50.1 min) – 90% B (60 min) – 40% B (60.1 min) – 40% B (70 min).
2.5. Western blot analysis and detection of Prx2 and Prx-SO2/3
Equal amounts of protein were subjected to SDS–PAGE (Any KD gel; Bio-Rad, Hercules, CA, USA) with (reducing condition) or without (non-reducing condition) DTT-reduction. For the immunoblot analyses of Prx2 and hyperoxidized Prx2 (Prx-SO2/3), the proteins were electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane; the membranes were blocked with 0.1% (w/v) skim milk containing TPBS [0.1% Tween (v/v) containing PBS (pH 7.4)], and were subsequently washed three times with TPBS. The washed PVDF membranes were then incubated for 1 h at room temperature with anti-Prx2 antibody (monoclonal; Abfrontier, South Korea) or an anti-Prx-SO2/3 antibody (polyclonal; Abfrontier). After extensive washing in TPBS, the blots were incubated at room temperature for 1 h with anti-mouse or anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody (Vector, Burlingame, CA, USA). The band images were captured using chemiluminescence reagents (Immobilon; Merck Millipore, Darmstadt, Germany) with an ImageQuant 400 (GE Healthcare, Japan Ltd., Tokyo, Japan).
2.6. Two-dimensional SDS–PAGE
Cell lysates or purified proteins were mixed with rehydration buffer (8 M urea, 2% CHAPS, 0.5% immobilized pH gradient buffer, 20 mM DTT, and 0.005% bromophenol blue) and loaded onto immobilized pH gradient strips (pH 4–7, 7 cm, Ready Strip IPG; Bio-Rad). Isoelectric focusing was carried out in four steps as follows: 250 V, 15 min; 4000 V, 1.0 h; 4000 V, 8–10,000 V-h, 500 V, 24 h. After reduction and alkylation, second dimensional electrophoresis was conducted on an SDS–PAGE with a 5–20% gradient gel (ATTO, Tokyo, Japan).
2.7. Protein identification
Identification of the proteins of interest was performed by in-gel digestion and peptide mass finger printing (PMF) using MS as described previously, with some modifications [12,13]. Briefly, gels were stained with a Silver Stain MS Kit (Wako Pure Chemical Industries) and the protein bands of interest were excised. The gel pieces were destained in a 1:1 solution of 100 mM sodium thiosulfate and 30 mM potassium ferricyanide and subsequently incubated in a reducing solution (25 mM NH4HCO3 and 25 mM DTT) for 20 min at 56 °C, followed by further incubation for 20 min at room temperature in an alkylation solution (25 mM NH4HCO3 and 55 mM iodoacetamide). The gel pieces were dehydrated with acetonitrile and incubated in 20 μl of digestion solution [50 mM NH4HCO3, 2 μg/ml trypsin (Trypsin Gold, mass spectrometry grade; Promega, Madison, WI, USA) and 0.01% ProteaseMax (Promega)] for 10 min at room temperature, followed by the addition of 10 μl digestion solution without trypsin. After incubation for 3 h at 42 °C, the peptides produced were extracted with 2.5% TFA and spectra were obtained using matrix-assisted laser desorption/ionization (MALDI)–time of flight (TOF)–TOF-MS (UltrafleXtreme; Bruker Daltonics, Bremen, Germany). The data set was entered into an in-house Mascot search engine (Matrix Sciences, London, UK) to find the closest match with known proteins registered in the Swiss-Prot database.
2.8. Other methods
Protein concentrations were determined using a BCA kit (Thermo Scientific) with bovine serum albumin as a standard.
3. Results
First, we investigated the separation conditions for Prx2 in RBC lysates using reverse-phase columns for protein separation. The eluted fractions were subjected to SDS–PAGE, followed by Western blotting detection with anti-Prx2 antibodies. Fig. 1A shows a chromatogram of lysates from native RBCs and the separation pattern of Prx2. Using the gradient program that we established in this study, Prx2 was detected at a retention time of 42 min (Peak-a, Fig. 1A). Additionally, a minor band was observed at a retention time of 46 min. We next examined the effect of oxidative stress on Prx2 elution using the commonly employed oxidant H2O2. Compared to control (Fig. 1A), changes in the chromatogram and separation pattern of Prx2 were not prominent in H2O2-treated RBCs (Fig. 1B), although the low intensity Prx2 band at a retention time of 46 min tended to increase. Following pretreatment with inhibitors (10 mM 3-AT for catalase, 7 mM succinate for GPx1) before exposure of RBC suspensions to 500 μM H2O2, no significant hyperoxidation of Prx2 was observed (data not shown).
Fig. 1.
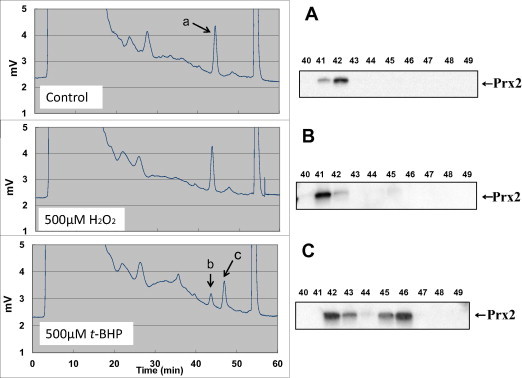
Chromatograms of human RBC lysate as analyzed by reverse phase chromatography and Western blot analysis. Reverse phase HPLC was performed using an YMC-pack PROTEIN-RP column (4.6 × 250 mm) as described in Section 2. The separated proteins were collected in 1.0 ml fractions and concentrated using a centrifugation concentrator system (Sakuma, Tokyo, Japan) for further analysis. After concentration, all fractions (3–50 min) were subjected to Western blot analysis with anti-Prx2 antibody after 12% SDS–PAGE and transfer to a PVDF membrane. RBC lysates were prepared after treatment with 500 μM H2O2 (B), t-BHP (C) or without peroxide (A). A typical result from the other three samples providing similar results is presented.
In contrast, the intensity of the peak corresponding to native Prx2 (Peak-b, Fig. 1C) decreased following treatment with t-BHP, which is more hydrophobic than H2O2. Concomitant with the decrease in native Prx2 (Peak-b), a new peak appeared at a retention time of 46 min (Peak-c, Fig. 1C). Quantitative analysis of the Prx2 peaks showed that these alterations occurred in a t-BHP concentration-dependent manner (Fig. 2). Although it is likely that t-BHP-induced hyperoxidation is more obvious at high concentrations, a low concentration (∼100 μM) of t-BHP significantly hyperoxidized Prx2 in human RBCs (Fig. 2).
Fig. 2.
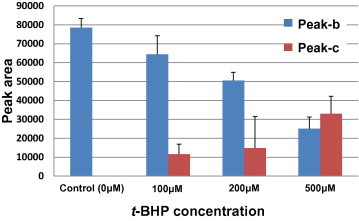
Dose dependent alterations in peak areas obtained by reverse phase chromatography. HPLC was performed using a reverse phase column for proteins, as described in Section 2. RBC lysates were prepared after treatment with 0–500 μM t-BHP. Peak b and Peak c are indicated on the chromatogram as shown in Fig. 1B.
Second, for a more detailed assessment of the separation capacity of Prx2 in the present HPLC system, protein composition in the Prx2-detected fractions (Peak-a and Peak-c) was evaluated by SDS–PAGE followed by silver staining. As shown in Fig. 3, the most prominent bands detected in both fractions (bands No. 1 and No. 2) were proteins of ∼23 kDa. Using in-gel digestion and PMF (MALDI–TOF–TOF-MS), proteins derived from bands No. 1 and No. 2 were both identified as human Prx2 (Accession; UniprotKB:P32119) with sequence coverages of 51.5% and 65.7%, respectively.
Fig. 3.
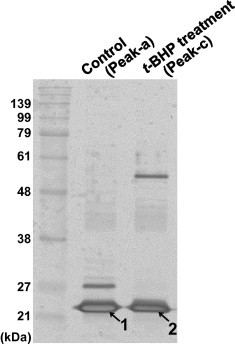
Protein identification by MS analysis. RBCs were treated with or without 500 μM t-BHP and proteins contained in fractions corresponding to peaks a and c in Fig. 1 were prepared. The proteins were separated using 12% SDS–PAGE and detected by silver staining. Protein bands No. 1 and No. 2 were excised and subjected to in-gel digestion and PMF using MALDI–TOF–TOF-MS. Proteins derived from bands No. 1 and No. 2 were identified as human Prx2 (Accession; P32119).
To clarify the oxidative status of Prx2, two-dimensional (2D) electrophoresis of the t-BHP-treated sample was performed, followed by immunoblot detection of Prx2. The results showed two spots of equal molecular weight with pI values of 5.8 and 5.4; the more acidic spot (pI 5.4) is more clearly observed in the t-BHP-treated sample compared to the control sample (Fig. 4). Furthermore, the two peaks obtained from the oxidized samples by reverse phase HPLC were also examined by immunoblotting. While similar band intensities (23 kDa) were observed for both peaks detected with the anti-Prx2 antibody, anti-PrxSO2/3 immunoreactivity was markedly increased in Peak-c compared to Peak-b (Fig. 5). These results indicated that the native and hyperoxidized forms of Prx2 were separated by our HPLC system.
Fig. 4.
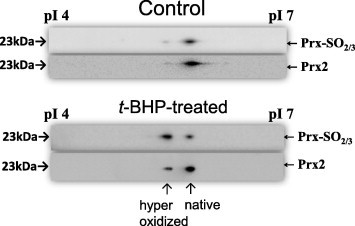
Oxidative status of Prx2 in human RBCs analyzed by 2D-PAGE and Western blot analysis. Prx2 from freshly obtained RBCs that were untreated (A) or treated (B) with 500 μM t-BHP was subjected to 2D-PAGE as described in Section 2. The separated and transferred proteins were detected by Western blotting with anti-Prx2 (upper image) or anti-Prx-SO2/3 (lower image). A typical result from two other samples exhibiting similar responses is presented.
Fig. 5.
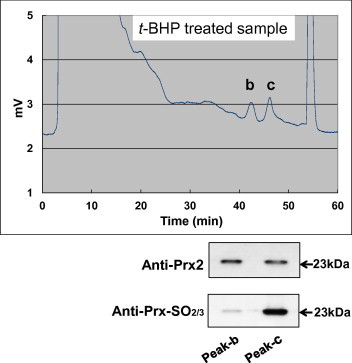
Oxidative status of Prx2 in human RBCs following t-BHP treatment. An RBC lysate was prepared after treatment with 500 μM t-BHP. After concentration, each fraction was subjected to Western blot analysis with anti-Prx2 antibody (A; upper image) and reprobed with anti-Prx-SO2/3 antibody (B; lower image). Peaks b and c are indicated on the chromatogram. A typical result from two other samples exhibiting similar responses is presented.
4. Discussion
Prxs are a widespread family of cysteine-dependent peroxidases, and their oxidation state is presumed to be involved in other functions such as chaperone activity [14]. Using antibodies specific for sulfinic and sulfonic acids (Prx-SO2/3), various examples have been found of Prx hyperoxidation in physiologically relevant events. Given the need to better understand the biological roles of Prx hyperoxidation, it is important to be able to characterize this property in the Prx family. We have recently focused our attention on the physiological significance of Prx2, which is abundant in RBCs, and have found that the oligomers consisting of the reduced form of Prx2 in human RBCs exhibit chaperone activity nearly equal to that of the hyperoxidized form [15]. Thus, real-time monitoring of the hyperoxidation status was suggested to be significant for studying the physiological roles of Prxs.
In this study, we developed a reverse-phase HPLC-based method for the rapid separation of reduced and hyperoxidized Prx2 using human RBCs as the sample, in which the activity of the reversible redox system is low. In the present method, the sample preparation and assay procedures were shown to easily separate and detect the reduced and hyperoxidized forms of Prx2. Therefore, this method is more useful than 2D-PAGE with Western blot analysis in yielding accurate results that reflect the hyperoxidative status of human RBCs. Indeed, the present study demonstrated that the detection of Prx2 hyperoxidation and estimation of the protein oxidation state can occur within 2 h after blood sampling. To verify the production and accumulation of the hyperoxidized form of Prx2 in RBCs, we also estimated the oxidative status using 2D electrophoresis/Western blot analysis.
As shown in Fig. 4, significant spots that have maintained the pI of native Prx2 were detected with anti-Prx-SO2/3 antibody. It is likely that these spots observed are artificially hyperoxidized proteins produced after the separation by isoelectric focusing followed by PAGE and Western blot analysis, as very little peak corresponding to hyperoxidized Prx2 was observed in the HPLC results for the same untreated sample (Fig. 1B), and a faint band was observed with anti-Prx-SO2/3 antibody for the same t-BHP treated sample (Fig. 5). Concurrently, good separation between the reduced and hyperoxidized forms of Prx2 was achieved when reversed phase HPLC was utilized to assess Prx2 in RBC samples. The peaks on the chromatogram were identified using MALDI–TOF-MS. Observation of hyperoxidized Prx2, formed upon exposure to cytotoxic ROS, revealed that Prx2 in RBCs might be refractory to oxidation by peroxides, since the hyperoxidized form was produced upon exposure to t-BHP (100–500 μM) but not to H2O2 (500 μM). In addition to Prx2, protein bands of ∼27 kDa and 53 kDa were detected in control and t-BHP treated fractions, respectively. These proteins are likely to be Prx2-binding proteins whose interaction is affected by the hyperoxidative status of Prx2, although there are other possible explanations, such as accidental coincidence due to equivalent retention times.
Rhee and co-workers provided the first LC–Q–TOF-MS evidence for reversible conversion of cysteine residues of Prx1, 2, and 3 to sulfinic acid (–SO2−) in a rat macrophage-derived cell line [16,17]. Moreover, they reported that Cys residues in yeast Prx1 are hyperoxidized to sulfonic acid (–SO3−), based on TOF-MS analysis [18]. Rabilloud et al. reported that a sensitive cysteine, Cys-51, was shown to be oxidized to cysteine sulfonic acid by t-BHP, leading to the formation of an inactivated form of Prx2 in human T-lymphoma cells [19]. However, there are insufficient studies dealing with the oxidation status of freshly prepared Prx2 from human RBCs, since detailed analysis requires long periods of time for 2D electrophoresis following by Western blot analysis. Our HPLC-based method is not only rapid, but also more useful for quantitation in comparison with Western blot analysis after separation by 2D-PAGE, which provides qualitative rather than quantitative data. Indeed, it is indicated that the antibodies available typically recognize sulfonic acid forms more strongly than native forms [20].
Yang et al. reported that H2O2-hyperoxidized recombinant human Prx1 eluted earlier than native Prx1 from a reverse-phase HPLC system [21]. The hyperoxidized Prx2 in human RBCs treated by t-BHP was retained more strongly by the reverse-phase HPLC column than native Prx2. Thus, we propose that the hydrophobicity is not only dependent on the oxidation state of the Cys-51 residue, but also on the steric conformation of oxidized Prx2. Our results appear to indicate that a form of hyperoxidized Prx2 was altered to become more hydrophobic than native Prx2.
In conclusion, the present study suggests that the oxidation-susceptible Cys residue of Prx2 from human RBC was oxidized into a hyperoxidized (sulfinic and sulfonic acids) derivative by t-BHP. This purification procedure might be applied to real-time assays for detecting reduced and hyperoxidized Prx2. Consequently, this method may enable determination of the oxidative status and redox balance in Prx2 in vivo. Further investigations using this method into the physiological function of Prx2 and its application to clinical specimens are expected in the near future.
Author contributions
Y.O. and M.N. designed and performed research; Y.I., M.T. and T.S. acquired and analyzed data; Y.I. and Y.O. wrote the paper.
Acknowledgement
This work was partially supported by JSPS KAKENHI Grant Number 24590766.
References
- 1.Hall A., Karplus P.A., Poole L.B. Typical 2-Cys peroxiredoxins – structures, mechanisms and functions. FEBS J. 2009;276:2469–2477. doi: 10.1111/j.1742-4658.2009.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Low F.M., Hampton M.B., Winterbourn C.C. Peroxiredoxin 2 and peroxide metabolism in the erythrocyte. Antioxid. Redox Signal. 2008;10:1621–1630. doi: 10.1089/ars.2008.2081. [DOI] [PubMed] [Google Scholar]
- 3.Rhee S.G., Woo H.A. Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H2O2, and protein chaperones. Antioxid. Redox Signal. 2011;15:781–794. doi: 10.1089/ars.2010.3393. [DOI] [PubMed] [Google Scholar]
- 4.Cha M.K., Kim I.H. Thioredoxin-linked peroxidase from human red blood cell: evidence for the existence of thioredoxin and thioredoxin reductase in human red blood cell. Biochem. Biophys. Res. Commun. 1995;217:900–907. doi: 10.1006/bbrc.1995.2856. [DOI] [PubMed] [Google Scholar]
- 5.Low F.M., Hampton M.B., Peskin A.V., Winterbourn C.C. Peroxiredoxin 2 functions as a noncatalytic scavenger of low-level hydrogen peroxide in the erythrocyte. Blood. 2007;109:2611–2617. doi: 10.1182/blood-2006-09-048728. [DOI] [PubMed] [Google Scholar]
- 6.Poynton R.A., Hampton M.B. Peroxiredoxins as biomarkers of oxidative stress. Biochim. Biophys. Acta. 2014;1840:906–912. doi: 10.1016/j.bbagen.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Matte A., Low P.S., Turrini F., Bertoldi M., Campanella M.E., Spano D., Pantaleo A., Siciliano A., De Franceschi L. Peroxiredoxin-2 expression is increased in beta-thalassemic mouse red cells but is displaced from the membrane as a marker of oxidative stress. Free Radic. Biol. Med. 2010;49:457–466. doi: 10.1016/j.freeradbiomed.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rinalducci S., D’Amici G.M., Blasi B., Vaglio S., Grazzini G., Zolla L. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion. 2011;51:1439–1449. doi: 10.1111/j.1537-2995.2010.03032.x. [DOI] [PubMed] [Google Scholar]
- 9.Rinalducci S., D’Amici G.M., Blasi B., Zolla L. Oxidative stress-dependent oligomeric status of erythrocyte peroxiredoxin II (PrxII) during storage under standard blood banking conditions. Biochimie. 2011;93:845–853. doi: 10.1016/j.biochi.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Cumming R.C., Dargusch R., Fischer W.H., Schubert D. Increase in expression levels and resistance to sulfhydryl oxidation of peroxiredoxin isoforms in amyloid beta-resistant nerve cells. J. Biol. Chem. 2007;282:30523–30534. doi: 10.1074/jbc.M700869200. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida Y., Yoshikawa A., Kinumi T., Ogawa Y., Saito Y., Ohara K., Yamamoto H., Imai Y., Niki E. Hydroxyoctadecadienoic acid and oxidatively modified peroxiredoxins in the blood of Alzheimer’s disease patients and their potential as biomarkers. Neurobiol. Aging. 2009;30:174–185. doi: 10.1016/j.neurobiolaging.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Ishida Y.I., Yamasaki M., Yukizaki C., Nishiyama K., Tsubouchi H., Okayama A., Kataoka H. Carnosol, rosemary ingredient, induces apoptosis in adult T-cell leukemia/lymphoma cells via glutathione depletion: proteomic approach using fluorescent two-dimensional differential gel electrophoresis. Hum. Cell. 2014;27:68–77. doi: 10.1007/s13577-013-0083-6. [DOI] [PubMed] [Google Scholar]
- 13.Takeshita M., Ishida Y.I., Akamatsu E., Ohmori Y., Sudoh M., Uto H., Tsubouchi H., Kataoka H. Proanthocyanidin from blueberry leaves suppresses expression of subgenomic hepatitis C virus RNA. J. Biol. Chem. 2009;284:21165–21176. doi: 10.1074/jbc.M109.004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang H.H., Lee K.O., Chi Y.H., Jung B.G., Park S.K., Park J.H., Lee J.R., Lee S.S., Moon J.C., Yun J.W., Choi Y.O., Kim W.Y., Kang J.S., Cheong G.W., Yun D.J., Rhee S.G., Cho M.J., Lee S.Y. Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell. 2004;117:625–635. doi: 10.1016/j.cell.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Ogasawara Y., Ohminato T., Nakamura Y., Ishii K. Structural and functional analysis of native peroxiredoxin 2 in human red blood cells. Int. J. Biochem. Cell Biol. 2012;44:1072–1077. doi: 10.1016/j.biocel.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Woo H.A., Chae H.Z., Hwang S.C., Yang K.S., Kang S.W., Kim K., Rhee S.G. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science. 2003;300:653–656. doi: 10.1126/science.1080273. [DOI] [PubMed] [Google Scholar]
- 17.Woo H.A., Kang S.W., Kim H.K., Yang K.S., Chae H.Z., Rhee S.G. Reversible oxidation of the active site cysteine of peroxiredoxins to cysteine sulfinic acid. Immunoblot detection with antibodies specific for the hyperoxidized cysteine-containing sequence. J. Biol. Chem. 2003;278:47361–47364. doi: 10.1074/jbc.C300428200. [DOI] [PubMed] [Google Scholar]
- 18.Lim J.C., Choi H.I., Park Y.S., Nam H.W., Woo H.A., Kwon K.S., Kim Y.S., Rhee S.G., Kim K., Chae H.Z. Irreversible oxidation of the active-site cysteine of peroxiredoxin to cysteine sulfonic acid for enhanced molecular chaperone activity. J. Biol. Chem. 2008;283:28873–28880. doi: 10.1074/jbc.M804087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabilloud T., Heller M., Gasnier F., Luche S., Rey C., Aebersold R., Benahmed M., Louisot P., Lunardi J. Proteomics analysis of cellular response to oxidative stress. Evidence for in vivo overoxidation of peroxiredoxins at their active site. J. Biol. Chem. 2002;277:19396–19401. doi: 10.1074/jbc.M106585200. [DOI] [PubMed] [Google Scholar]
- 20.Nelson K.J., Parsonage D., Karplus P.A., Poole L.B. Evaluating peroxiredoxin sensitivity toward inactivation by peroxide substrates. Methods Enzymol. 2013;527:21–40. doi: 10.1016/B978-0-12-405882-8.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang K.S., Kang S.W., Woo H.A., Hwang S.C., Chae H.Z., Kim K., Rhee S.G. Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site cysteine to cysteine-sulfinic acid. J. Biol. Chem. 2002;277:38029–38036. doi: 10.1074/jbc.M206626200. [DOI] [PubMed] [Google Scholar]


