Abstract
Background and aims: In Japan the rise in the average life expectancy has caused an increase in the proportion of the population who are classed as geriatric. Accordingly, the number of elderly people being treated for cancer is increasing concomitantly. However, with the increase in age, the numbers of prior complications also increase. This is especially so in the advanced-aged patients, defined in Japan as those over the age of 85. Such complications may be too high risk for radical surgery and a less invasive treatment is warranted. Photodynamic therapy (PDT) is a noninvasive treatment approved by the Japanese National Health Insurance for the treatment of early stage superficial type esophageal and gastric cancers, early stage uterine cervical cancers and dysplasia, and early and advanced lung cancer. We report herein on the efficacy of palliative PDT using talaporfin sodium (Laserphyrin®) for a case of inoperable gastric cancer.
Material and methods: The patient was an 87-year-old-man, a diabetic with histories of diabetic nephropathy, cerebral infarction and myocardial infarction. This patient was first diagnosed as having gastric cancer in 2007 but surgery and chemotherapy were contraindicated due to his poor physical status and poor renal function, respectively, owing to the anticipated side effects. The patient was referred to our institution after hearing of PDT in 2009. He was treated with 1 course of porfimer sodium PDT and 3 courses of talaporfin sodium PDT with photodynamic diagnosis (PDD) during the period from September, 2009 to June, 2011.
Results: The massive gastric cancer located in the cardia was successfully treated with 4 PDT sessions without any serious complications; therefore the patient was able to orally ingest food until his death due to natural causes other than the cancer, in October, 2011.
Conclusion: Talaporfin sodium PDT is safe and effective treatment for advanced-aged patients suffering from inoperable gastric cancer.
Keywords: Talaporfin sodium (Laserphyrin®), photodynamic diagnosis (PDD), photodynamic therapy (PDT), gastric cancer, advanced-aged patient
Introduction
In Japan, a senior citizen is defined as a person over the age of 65. In medical terms those between ages 65 and 74 are termed younger-aged senior citizens, and those between ages 75 and 84 are termed older-aged senior citizens. People over the age of 85 are considered as advanced-aged senior citizens. The Japanese have the highest life expectancy in the world, averaging 79.44 years for men and 85.9 years for women, as of 2011. 1) As a result, Japan is quickly becoming an ageing society at a rate never seen in the world before. The population and proportion of the advanced-aged people is expected to increase significantly within the next decade or so. In the meantime, the primary cause of death as of 2011 according to actual mortality and mortality rate (per 10,000 population) is malignant neoplasms. 2)
Among the malignant neoplasms the mortality rate was greatest for lung cancer, followed by gastric cancer as second and colon cancer as third. From the demographic changes occurring, it can be easily said that developments in noninvasive treatments for the advanced-aged people with gastric, lung and colon cancers are warranted. 3)
The treatment of gastric cancer consists of curative surgery (conventional, laparoscopic and endoscopic), chemotherapy, radiotherapy and palliative treatments. However, when considering treating an advanced-aged patient for gastric cancer, most of the patients will show deterioration of cardiac, respiratory, renal and liver functions to some extent. In many cases, the low physical status of the patient will contraindicate radical surgery or even chemotherapy if the risk of deterioration of the patient's QOL is apparent.
Photodynamic therapy (PDT) is a treatment where tumor tissue incorporating a photosensitizer (PS) is irradiated with incident light at an appropriate wavelength, causing a photochemical reaction which releases free radicals bringing about necrosis of tumor cells through oxidative stress. This causes mitochondrial damage or nuclear damage, leading to cell apoptosis. In addition, a phenomenon known as the vascular shut down effect occurs where the endothelial cells of a tumoral neovascular blood vessel are destroyed resulting in ischemic necrosis of the tumor tissue. Such reactions are used to treat cancerous tumors and retinal proliferative neovascularization. PDT is a highly selective and noninvasive treatment and can be safely performed on even those advanced-aged patients with some extent of internal organ dysfunction. In Japan, PDT with a 630 nm wavelength excimer-dye laser, (EDL-1, -2, Hamamatsu Photonics K.K., Hamamatsu, Japan) using porfimer sodium (Photofrin®, Pfizer Co., Tokyo, Japan; P-PDT) as the PS, is approved by the Japanese National Health Insurance for the treatments of early stage lung cancers, superficial type esophageal cancers, superficial type early gastric cancers and early stage cancers and dysplasia of the uterine cervix. Also PDT with a 664 nm wavelength diode laser (PD laser, Panasonic Healthcare, Tokyo, Japan) using talaporfin sodium (Laserphyrin®, Meiji Seika Pharma, Tokyo, Japan; L-PDT) as the PS is approved for the treatments of both early and advanced stage lung cancers. L-PDT has advantages over P-PDT such that it has greater penetration in living tissue at longer exciting wavelengths, a greater vascular shut down effect (hemostatic effect), is metabolized much quicker and requires less light shielding time (shortening hospital stay), and can be also used for photodynamic diagnosis (PDD). L-PDT is considered an even less invasive treatment method compared to P-PDT and broadening of the indications to other cancers including gastric cancer is anticipated.
When PDT laser irradiation is performed using a field sequential method videoendoscope system, the system only acknowledges the strong white light during the laser irradiation and all color information is lost. Since 2003, we have been studying the compatibility between videoendoscopes and lasers. As a result, we have found that a simultaneous method videoendoscope system allows for a better dynamic color reproduction and a more accurate observation. 4) Some simultaneous method magnifying videoendoscopes with a high quality color CCD chip allows the visualization of red fluorescent light emitted by photosensitized tissues such as in P-PDT and L-PDT when excited by light from a shortwave purple diode laser (wavelength 405 nm). This confirms that PDD can be performed endoscopically. 5) A novel high resolution magnifying videoendoscope system, whereby both PDT and PDD can be performed, was successfully developed (XG-0001, Fujifilm, Tokyo, Japan). 6) The XG-0001 is a magnifying videoendoscope based on the commercially available EG-590ZW (Fujifilm), the magnification rate of which is up to 135 times, visualized on a 19 inch high-definition color monitor. It has a spectral image processing function known as flexible spectral imaging color enhancement (FICE) 7) which is regarded as image enhanced endoscopy. XG-0001 allows easy intra-procedural viewing of PS accumulation in the tumor before initial laser irradiation or prior to additional laser irradiation of L-PDT procedures, with a high resolution magnifying endoscopic observation using L-PDD.
Purpose
The purpose of this study was to investigate the clinical course and pathological findings of L-PDD and L-PDT for gastric cancer in an advanced-aged patient. The main themes of the investigation were the following:
The efficacy of endoscopic treatment focusing on PDT and to monitor procedural accidents, if any.
Comparison between P-PDT and L-PDT.
The efficacy of L-PDD
Materials and methods
During the period of 2009 and 2011, a single advanced-aged patient with unresectable gastric cancer of the cardia received 1 session of P-PDT and 3 sessions of L-PDT.
The apparatus used
Pre-treatment examinations were performed using the EG-590ZW videoendoscope. The XG-0001 and 405 nm diode laser (VDL-M1 with a spectroscope, M&M Co., Tokyo, Japan, presently out of production) were used for L-PDD. The EG-590ZW and 630 nm EDL-2 were used for P-PDT while the XG-0001 and 664 nm PD laser were used for L-PDT. The quartz fibers for laser irradiation were those commercially available in PDT for lung cancer.
To the best of our knowledge, this was the first attempt in the world to treat gastric cancer with L-PDT, therefore a written application “Treatment of upper digestive tract tumors with PDD and PDT with talaporfin sodium and diode lasers” was submitted to the ethical committee of our institution. The application (No. 21101) was approved on September 24th, 2009.
P-PDT
As a general rule, the treatment followed the clinical pathway set at the authors' institution for P-PDT for other cancers. 8) It is a 14 day protocol (with 2 continuous days of laser irradiation). On the afternoon of the first day of hospital admission, the patient receives an intravenous injection of the PS, porfimer sodium, at a dose of 2 mg/kg bodyweight. The patient's environment is light shielded and controlled so that the luminescence is less than 100 lux. At 48 hours after the injection, PDT with the EDL-2 is performed. The average pulse energy is 4 mJ and the repetition rate is 60 Hz (average power 240 mW). The total energy density of the irradiation delivered to the lesion and its surroundings is set to be greater than 60 J/cm2. If deemed necessary, laser irradiation is performed again at 72 hours after injection. Any necrotic debris present is removed using forceps. This is done so that the laser can be targeted to reach the deeper tissues invaded by the tumor thus making the PDT procedure even more effective. During the PDT procedure, midazolam hydrochloride (Dormicum, Astellas Pharma Inc., Tokyo, Japan) is administered to the patient as a sedative. Administration of 2.5∼3.5 mg is infused initially and, when required, additional dosage is added 1 mg at a time while observing the state of sedation and vital signs. After the procedure, flumazenil (Anexate, Astellas Pharma Inc.) is injected to arouse the patient. The restriction of light to the patient is gradually eased. On the 4th day of hospitalization, the limit of light shielding is eased from less than 100 lux to less than 300 lux. From the 5th day on, the limit is less than 500 lux (fluorescent lighting). On day 7, endoscopic examination of the treated lesion is performed with the EG-590ZW endoscope. If there is no bleeding from the laser ulcer then the patient is discharged from the hospital on day 14 (day 10 post PDT), after sunset. A post-treatment follow-up is planned at roughly 4 weeks from discharge. The patient is advised to avoid sunlight until the time of the follow-up appointment.
L-PDD and L-PDT
For L-PDT, only 8 days of hospitalization are required. On the morning of day 2, the patient receives an intravenous injection of talaporfin sodium at dose of 40 mg/m2 body surface. Light is shielded so that the luminescence of the patient environment is less than 100 lux. Six hours after the injection, PDD using the 405 nm diode laser is performed followed by PDT using the PD laser. The total energy density of the irradiation to be delivered is set at greater than 60 J/cm2 (average power 270∼500 mW, irradiation time 11 min 7 s), in accordance with P-PDT. The PD laser irradiation time is pre-set and locked at 11 min 7 sec and the average output power changed accordingly to the planned area of laser irradiation, so that the power density becomes 150 mW/cm2. 9) Calculations are performed to create a conversion table, which is used during treatment. After the initial PDT session, additional PDT sessions are planned in the morning or the afternoon of the next day. Additional PDT sessions are performed since it is known that a single PDT procedure may not suffice. L-PDD is performed when it is deemed necessary to search for any residual PS within the tumor cells. After two days from PS administration, the light shielding is eased to less than 300 lux, and after the 5th day the shielding is eased to less than 500 lux (fluorescent lighting). On the 6th PDT post-operative day, the lesion is observed using the EG-590ZW endoscope and if no bleeding or other complications from the laser ulcer are seen the patient is discharged after sunset on day 8 of hospitalization. The patient is instructed to avoid direct sunlight for 2 weeks and a follow-up appointment is planned 4 weeks after the discharge.
The current case
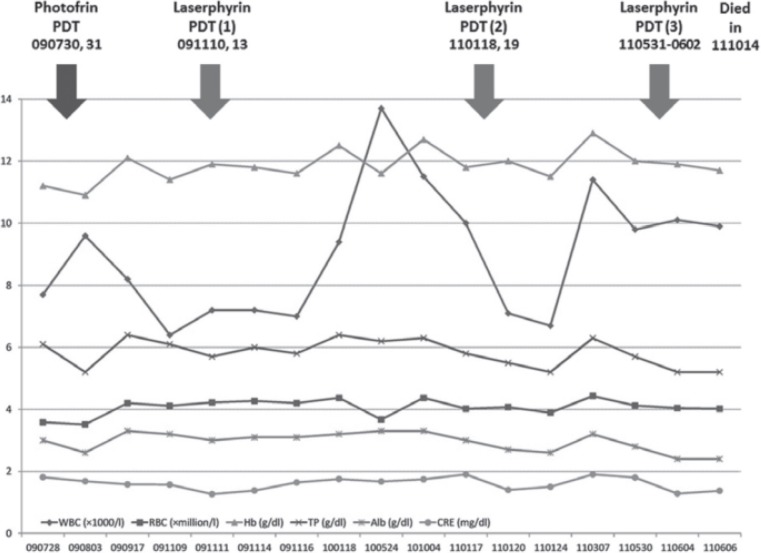
The case presented was an 87-year-old male diabetic complicated with renal dysfunction due to diabetic nephropathy and with histories of cerebral infarctions and myocardial infarction. This patient was being treated at a local medical facility when, in 2007, cancers of the gastric cardia and transverse colon were found. The latter was treated with a laparoscopic procedure and removed, however the gastric lesion was such that it could not be removed with endoscopic submucosal dissection (ESD). A total gastrectomy under general anesthesia would have been the only solution but the patient's poor physical status contraindicated such surgery. Chemotherapy was avoided due to his poor renal function and the anticipated side-effect morbidity. Observational follow-up without any treatment was chosen. The patient heard of the new procedure called PDT and was referred to the authors' hospital on February 26th, 2009. The initial endoscopic examination at our institution revealed a protruded mass measuring 3 cm along the major axis, which occupied the greater curvature of the cardia. Endoscopic ultrasonography (EUS) revealed that the depth of tumor invasion was at least into the submucosal (SM) layer, if not deeper. Imaging, such as CT among others, revealed no signs of distant or lymph node metastasis. However, since the efficacy of P-PDT alone was limited and a considerable amount of time would pass before the patient could be admitted to our hospital, we asked the referring medical facility to perform chemotherapy on the patient. The medical facility complied but chemotherapy resulted in leukocytopenia, lethargy and appetite loss and was suspended after 2 courses. The patient made a second request to be treated with PDT, and was admitted to our hospital on July 28th. The clinical course and laboratory data during the course of treatment are shown in Figure 1.
Figure 1:
Clinical course and laboratory data. WBC: white blood cell, RBC: red blood cell, Hb: hemoglobin, TP: total protein, Alb: albumin, CRE: creatinine
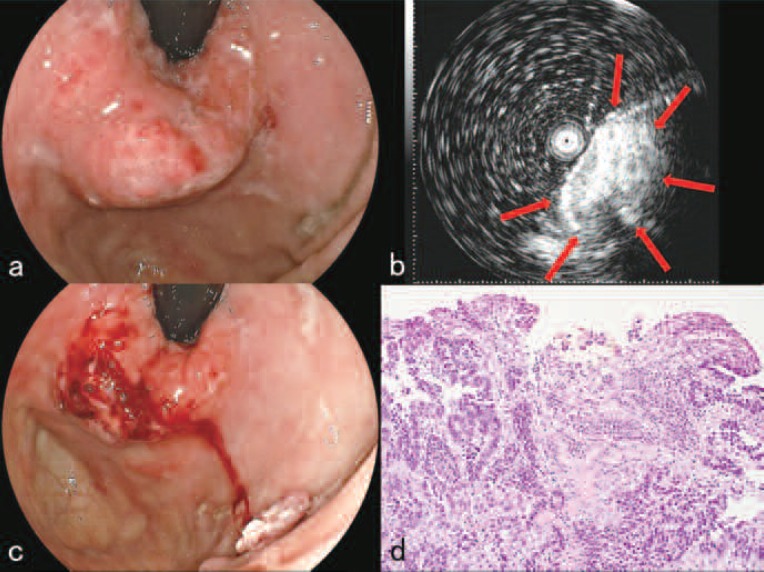
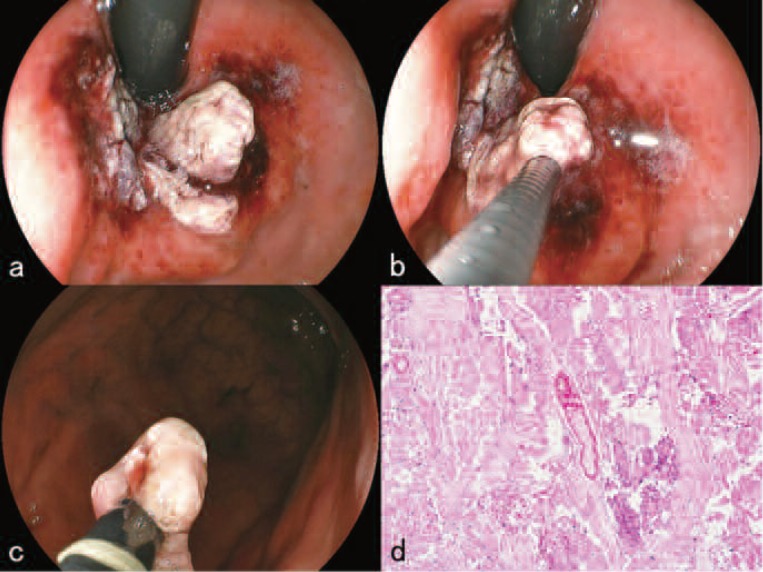
Endoscopic examination at the time of admission revealed a 30×30×25 mm protruded mass of the gastric cardia along the greater curvature (Figure 2a). EUS revealed that the depth of invasion of the tumor was at least into the SM if not deeper (Figure 2b) and that the tumor was showing a solid growth pattern. The patient and his family were informed using illustrations that P-PDT alone would have only a limited effect on the tumor and that an endoscopic combined therapy 10) with the endoscopic snare resection of the protruded mass using a high-frequency electric current device was required. The patient and his family were also informed that the series of treatments would be only palliative and have no curative effect and that reducing the size of the tumor and preventing the stenosis of the cardia were the only goals. A written informed consent by the patient and his family was obtained on a format approved by the ethical committee.
Figure 2:
Before treatments (July, 2009).
a. Endoscopic examination revealed a 30×30×25 mm protruded mass of the gastric cardia.
b. Endoscopic ultrasonography revealed that the depth of invasion of the tumor was at least into the submucosal layer (red arrows).
c. After the resection of portions of the protruded tumor.
d. High power magnified view (Hematoxylin-Eosin stain X 100) of the resected tumor. Histopathological finding showed vascular rich moderately differentiated tubular adenocarcinoma with infiltration of inflammatory cells.
The treatment course
P-PDT (Period of hospitalization July 28th ∼ August 10th, 2009):
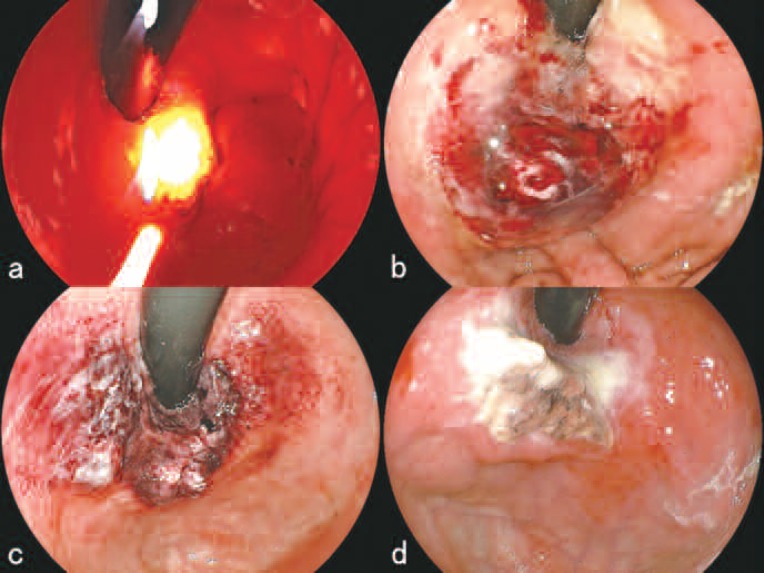
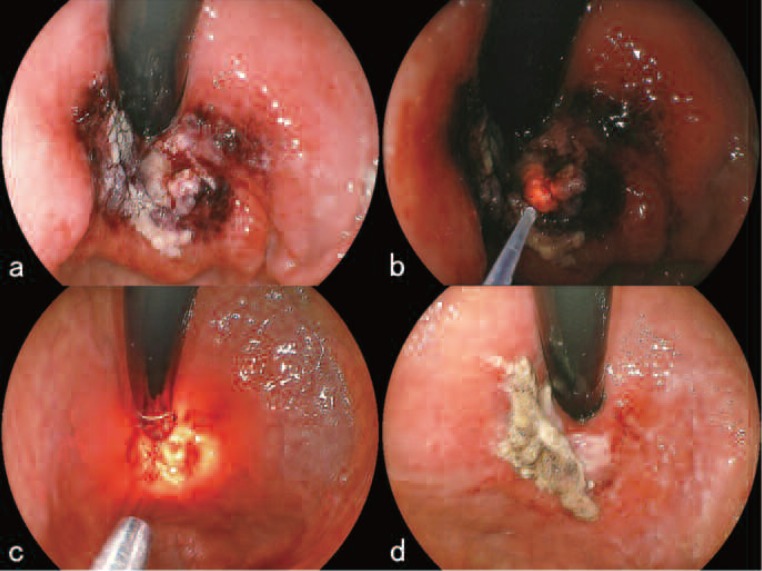
In the afternoon of July 28th, the day of hospital admission, 2 mg/kg of porfimer sodium was administered to the patient, and light shielding was started. On July 30th, after medication to prevent hemorrhage had been locally injected, portions of the protruded tumor were resected (Figure 2c). The histopathological findings are shown in Figure 2d. Following the partial resection of the tumor, 630 nm laser light from the EDL was directed toward the tumor through a free-cut quartz fiber that had been placed into position endoscopically. The parameters of the irradiation were 4 mJ per pulse at 60 Hz (average power: 240 mW) giving a total energy delivered of 800 J (Figure 3a). After the irradiation, the tumor and surrounding area became edematous (Figure 3b). On July 31st, the treated tumor on the side of the greater curvature of the cardia showed signs of general ischemic necrosis; the lesion was covered with dark red or white necrotic debris (Figure 3c). A hot biopsy forceps was used to remove the necrotic debris, and the lesion was irradiated with an additional 800 J of EDL. The examination on August 7th showed a pronounced reduction of the tumor size with necrotic debris adhering superficially (Figure 3d). No bleeding was seen and the general condition of the patient was good. The patient was discharged on August 10th as originally planned.
Figure 3:
Treatment course of PDT with porfimer sodium (July to August, 2009).
a. During irradiation with the 630 nm excimer-dye laser (EDL-2, Hamamatsu Photonics K.K., Hamamatsu, Japan) on July 30th.
b. After the irradiation, the tumor and surrounded area became edematous.
c. On July 30st, the treated tumor showed signs of general ischemic necrosis; the lesion was covered with dark red or white necrotic debris.
d. On August 7th, endoscopic examination showed a pronounced reduction of the tumor size with necrotic debris adhering superficially.
On the follow-up endoscopy on September 8th, although the size was substantially reduced, the tumor located at the cardia was still present and resembled a 0-IIa+IIc-like advanced cancer. The patient and family strongly requested another treatment, this time with L-PDT owing to its advantage of a shorter hospital stay. The second treatment was planned to take place in November of that year.
L-PDD, L-PDT 1st treatment (period of hospitalization: November 9∼17, 2009):
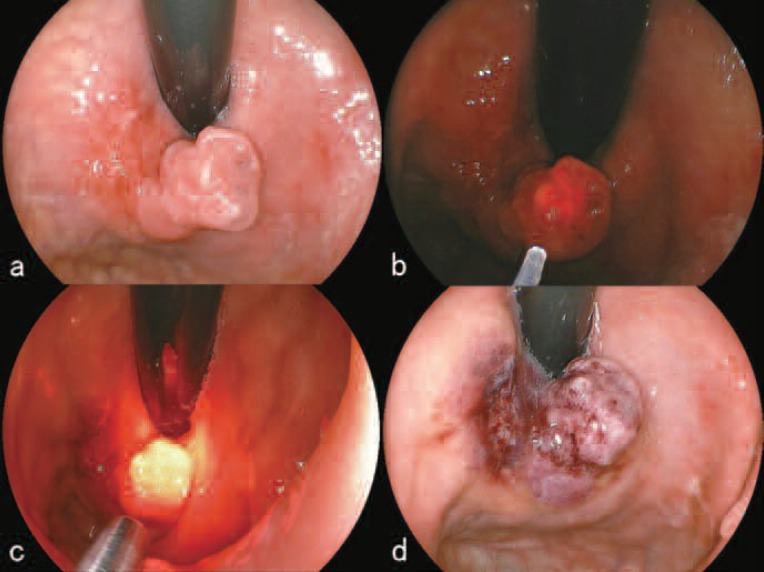
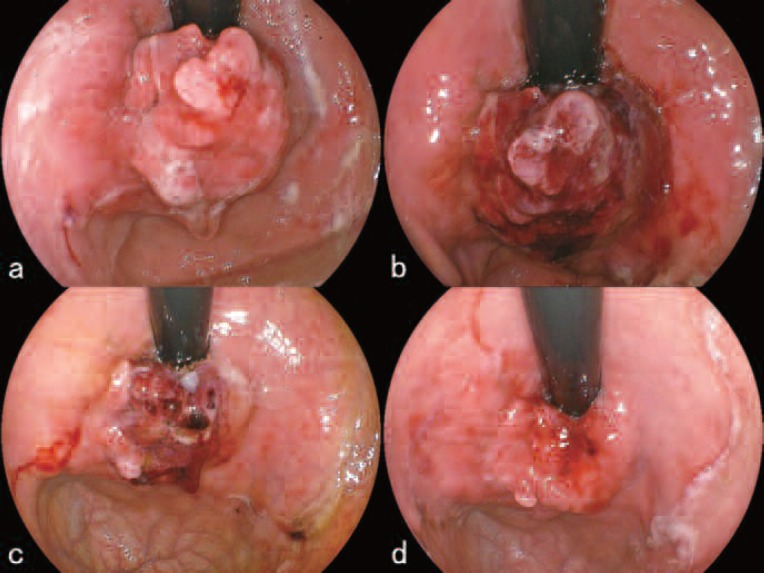
As of November 10th, the tumor located on the cardia near the greater curvature was ulcerative on the posterior portion. The anterior portion showed a polypoid growth measuring 15 mm in its major axis (Figure 4a). Six hours after talaporfin sodium was administered to the patient, L-PDD of the lesion was performed which revealed clear red fluorescence centering on the polypoid tumor (Figure 4b). Following PDD, the lesion was irradiated with the PD laser set at 270mW using a direct irradiation with a direct-type quartz fiber having an irradiation circumference of around 15 mm for 11 min 7sec (Figure 4c). After the irradiation, cessation of blood flow within the tumor was observed and the color of the irradiated area turned dark reddish (Figure 4d). Although only a single PDT session was originally planned, from the experience of the previous P-PDT session, the authors believed that a thorough treatment with an additional PDT session was necessary. The patient and his family were informed of the changes in plan and consent was obtained. The second L-PDT procedure was planned for November 13th. At the second procedure, the size of the polypoid portion of the tumor had decreased and the color of the tumor surface had become whitish (Figure 5a). After local injections to prevent intra-operative hemorrhage, parts of the tumor were excised with a high-frequency electric current device (Figure 5 b,c). The histopathological section of the excised tumor is shown in Figure 5d. During the excision, virtually no bleeding was seen (Figure 6a). PDD of the excised area revealed red fluorescence confirming that there was residual talaporfin sodium in the tissue and that cancerous tissue was therefore still there (Figure 6b). An additional PD laser irradiation was performed at 270 mW for 11 min 7 sec using the same direct-type quartz fiber as above (Figure 6c). On November 16, examination of the lesion showed a laser ulcer 25 mm in diameter, covered with necrotic debris but without any bleeding (Figure 6d). The patient was discharged from the hospital as planned.
Figure 4:
First treatment course of PDD and PDT using talaporfin sodium (November 10, 2009)
a. Before L-PDD and L-PDT. The anterior portion of the tumor showed a polypoid growth measuring 15 mm in its major axis.
b. L-PDD revealed clear red fluorescence centering on the polypoid tumor.
c. During irradiation with the 664 nm PD laser (Panasonic Healthcare, Tokyo, Japan) using a direct-type quartz fiber.
d. After the irradiation, cessation of blood flow within the tumor was observed and the color of the irradiated area turned dark reddish.
Figure 5:
Three days after the 1st treatment course of L-PDT (November 10, 2009)
a. The size of the polypoid portion of the tumor had decreased and the color of the tumor surface had become whitish.
b. Parts of the tumor were excised with endoscopic snare resection using a high-frequency electric current device.
c. Just after excision.
d. High power magnified view (Hematoxylin-Eosin stain X 100) of the excised tumor. Histopathological examination showed a fibrinoid degeneration of blood vessels surrounded by coagulation necrosis.
Figure 6:
First course of L-PDD and L-PDT (November 13, 16, 2009)
a. Just after excision. During the excision, virtually no bleeding was seen.
b. L-PDD of the excised area revealed red fluorescence confirming that there was residual talaporfin sodium in the tissue and that cancerous tissue was therefore still there.
c. Additional irradiation with the PD laser using the direct-type quartz fiber.
d. On November 16, endoscopic examination showed a laser ulcer 25 mm in diameter, covered with necrotic debris but without any bleeding.
A follow-up endoscopy on January 18th, 2010, revealed a tumor resembling a 0-IIc like advanced gastric cancer measuring 25 mm along the major axis, located on the side of the greater curvature of the gastric cardia. A biopsy of the lesion revealed a moderately differentiated tubular adenocarcinoma. On May 24th of the same year, endoscopy revealed no change in the size or classification of the tumor, but slight bleeding was seen. Chest and abdominal CT scans taken the same day showed no sign of lymph node or liver metastases. The tumor itself was not visible in this scan.
On October 4th, the tumor showed protuberant growth directing orally which easily bled on contact. The chest and abdominal CT scan taken the same day still did not show any signs of extra-luminal growth of tumor or lymph node or liver metastasis. The endoscopic findings could not deny the possibility of future stenosis of the cardia, and a 2nd session of L-PDD and L-PDT was planned.
L-PDD, L-PDT 2nd treatment (period of hospitalization: January 17∼25, 2011):
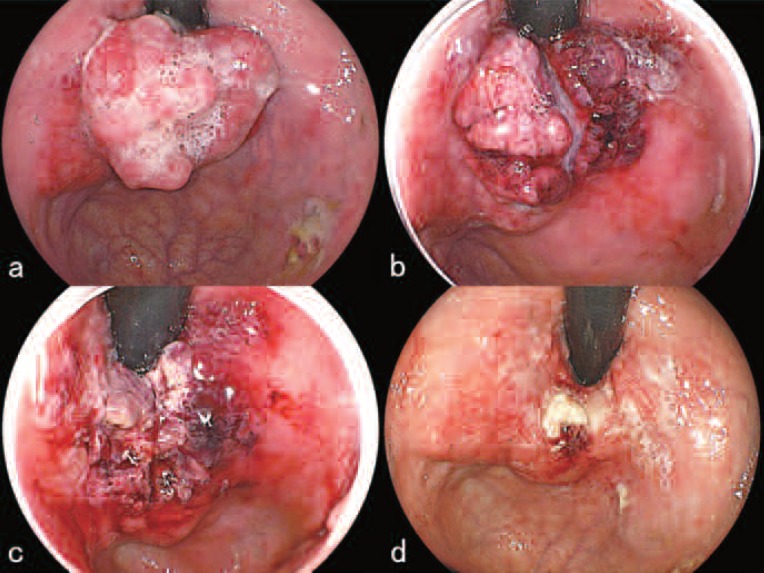
By January 18th, the tumor had grown even larger measuring 35×30 mm with some bleeding (Figure 7a). Since the area to be treated had enlarged, the PD laser power was set at 500 mW with the same irradiation time of 11 min 7 sec. Additional irradiation was stopped because the direct-type quartz fiber accidentally broke. The treatment was suspended for that day, at which time the whole tumor had become edematous and had turned dark red (Figure 7b). On the next day, January 19th, the protruded part of the tumor had reduced the most, showing signs of ischemic necrosis (Figure 7c). The necrotic debris was removed using a high-frequency electric current device and two sets of the PD laser irradiation were delivered at the same parameters as above. On January 24th, the protruded mass had significantly decreased compared to pre-treatment (Figure 7d), but slight bleeding was present and a hemostatic agent was applied. The endoscopy on March 7th revealed a large protruding tumor of the cardia measuring 40 mm along the major axis with contact bleeding at portions. The chest and abdominal CT scan still showed no sign of lymph node or liver metastasis. The tumor was visible on the scan for the first time. Another session of L-PDD and L-PDT was planned. For the 3rd session, the authors proposed 3 consecutive days of laser irradiation. This was due to the large tumor size and to the fact that only limited effects were noted following the 2-day irradiation method, where early regrowth of the tumor was seen after the previous two sessions. The patient and his family were informed of the proposed treatment method and gave their written consent.
Figure 7:
Second treatment of L-PDT (January 18, 19, 24, 2011)
a. Before treatment. The tumor had grown even larger measuring 35×30 mm with some bleeding.
b. Just after L-PDT, the whole tumor had become edematous and had turned dark red.
c. One the next day, January 19th, the protruded part of the tumor had reduced the most, showing signs of ischemic necrosis.
d. On January 24th, the protruded mass had significantly decreased compared to pre-treatment (Figure 7 a).
L-PDD and L-PDT 3rd treatment (period of hospitalization: May 30∼June 6, 2011):
The patient was re-admitted to the hospital on May 30th. Upon this re-admission the patient, who had histories of myocardial and cerebral infarctions, was allowed to take his anti-platelet medication, Bayaspirin (aspirin, Bayer Yakuhin, Ltd., Osaka, Japan) until the day of re-admission, considering the risk of cerebral or myocardial infarction. The endoscopic examination on May 31st revealed a solid protruding mass measuring 40 mm (Figure 8a). The tumor was irradiated with the PD laser for 2 sets at 500 mW for 11 min 7 sec using a direct-type quartz fiber, and was irradiated at 300 mW for the same exposure time using a cylindrical-type quartz fiber. The laser was aimed at the center of the massive tumor facing the anterior gastric wall. The next day, the tumor had shrunk dramatically and had turned dark red but another 20 mm tumor remained, located on the posterior side of the gastric cardia (Figure 8b). Necrotic tissue was excised using a high-frequency electric current device and was followed by L-PDD, confirming red fluorescence and the remnant tumor. Using the direct-type quartz fiber, the tumor was directly irradiated from within the stomach with the PD laser at a setting of 500 mW for 11 min 7 sec and from the esophagus at a setting of 330 mW for 8 minutes. Hemostatic agents were sprayed onto the lesion and the session was ended. On June 2nd, the tumor of the gastric cardia had shrunk substantially (Figure 8c) and again necrotic tissues were excised using a high-frequency electric current device. L-PDD was performed and then the target tumor was directly irradiated with the PD laser at a setting of 500 mW for 11 min 7 sec using the direct-type quartz fiber and at a setting of 354 mW for the same exposure time. Hemostatic agents were sprayed and the session was ended. Endoscopic examination on June 6th revealed almost complete resolution of the tumor with only small amounts of white necrotic tissue and blood clots (Figure 8d). The primary purposes of reducing the size of the tumor and preventing stenosis were achieved and the patient was discharged from the hospital.
Figure 8:
Third treatment of L-PDT (May to June, 2011)
a. On May 31st, endoscopic finding revealed a solid protruding mass measuring 40 mm.
b. The next day, the tumor had shrunk dramatically and had turned dark red but another 20 mm tumor remained, located on the posterior side of the gastric cardia
c. On June 2nd, the tumor of the gastric cardia had shrunk substantially
d. On June 6th, endoscopic findings showed almost complete resolution of the tumor with only small amounts of white necrotic tissue and blood clots.
The patient was followed-up at the referring medical facility before he passed away on October 14, 2011 due to a urinary tract infection. The authors have been told that the patient was able to eat, and was conscious right up until his death.
Results and Discussion
Treatment efficacy, procedural accidents and adverse effects:
Reducing the size of the tumor and preventing stenosis of the cardia were successful in both the endoscopic combined therapy where gross excision of the tumor using the high-frequency electric current device was followed by P-PDT, and successive L-PDT where additional L-PDT was performed after debridement. Also the fact that the patient was able to ingest food orally until immediately before his death denotes that this series of treatments was highly effective in preserving the QOL of the patient.
During the whole treatment course, only transient examples of slight anemia and hypoalbuminemia were seen and both resolved spontaneously within a month (Figure 1). Liver dysfunction was never seen nor was there any worsening of renal functions. Concerning hyperphotosensitivity, the patient experienced slight tanning after the P-PDT session, but none was seen after the L-PDT series. Following P-PDT, the patient also experienced slight abdominal pain and diarrhea but recovered in a few days. There were no complications associated with endoscopic treatments such as abdominal pain, bleeding or perforation.
Comparison between P-PDT and L-PDT:
From the perspective of the QOL of the patient, P-PDT usually requires 14 days of hospitalization followed by 4 more weeks of avoidance of direct sunlight. During the dormant 4 weeks where he/she must refrain from going out during the day, the patient may lose muscle tone or the strength to walk. L-PDT requires only 8 days of hospitalization and 2 weeks of sunlight avoidance after discharge from the hospital. The authors actually received a comment from the patient presented in this paper that following L-PDT he said he was “much better off”. If the treatment efficacy of the two PDTs is equal then L-PDT, with its shorter period of hospitalization and light shielding, is clearly superior to P-PDT, when treating the advanced-aged patient.
The authors have previously reported on a case of P-PDT in a patient with advanced gastric cancer resembling that of an early stage cancer 11). The patient was deemed inoperable due to liver cirrhosis. P-PDT was performed 9 times in the course of 6 years and during this period; PDT was successful in stopping the progression of the cancer. However, when the time between treatments was less than 6 months, liver dysfunction was seen and hyperphotosensitivity was aggravated. In the present report, the patient was treated once with P-PDT and three times with L-PDT. During the treatment course, no liver dysfunction was apparent and hyperphotosensitivity (slight tanning) was seen only after the initial P-PDT. It could not be said for certain, with only a single case, but the present experience suggests that successive L-PDT treatments may not be a problem. Therefore L-PDT may be effective not only for curative treatment of early gastric cancers, but also for palliative or complementary treatment for advanced gastric cancers without distant metastasis.
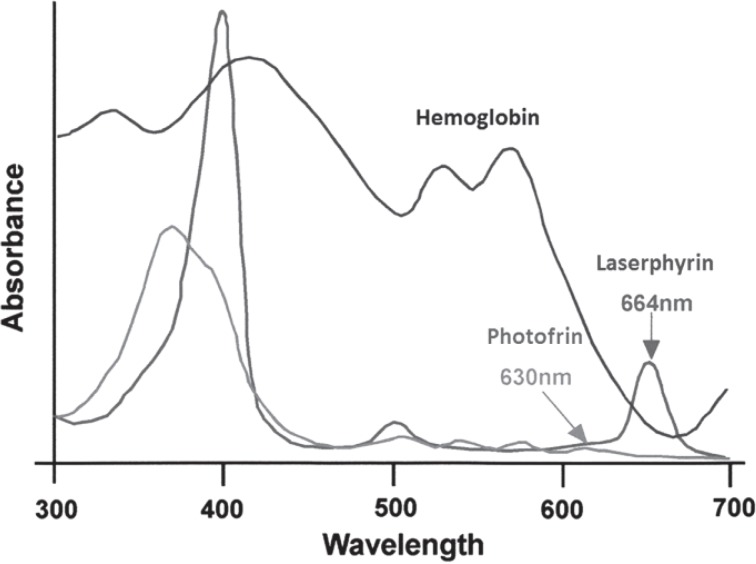
From endoscopic observations immediately after P-PDT, the surface of the gastric mucosa of both the tumor and surrounding normal tissue tended to be edematous and covered with watery mucous (Figure 3b). As for endoscopic findings after L-PDT, edematous changes were seen to a much lesser degree and dark red color changes in the center of the tumor were apparent (Figure 4b). From endoscopic magnifying observation, disruption of tumor vasculature was seen which denotes that L-PDT had a greater vascular shut down effect hence greater tumor necrosis due to ischemia compared to necrosis following P-PDT. The histopathology of the post-L-PDT necrotic tumor tissue revealed fibrinoid degeneration of the blood vessels of the tumor (Figure 5d), surrounded by tumor tissue featuring coagulation necrosis. The fibrinoid degeneration was similar to that seen in vasculitis, and it was hypothesized that this was an artifact related to the vascular shut down effect. As is shown in Figure 9, talaporfin sodium has an absorbance peak at the wavelength of 664 nm. At this wavelength hemoglobin has a relatively low absorbance meaning that hemoglobin is not a competing chromophore for L-PDT. The longer wavelength of 664 nm compared to 630 nm also means that L-PDT can penetrate target tissue more deeply than P-PDT. The clinical merit of L-PDT over PPDT is that, for the treatment of vascular rich tumors, the anti-tumor effect is very high and ischemia of the tumor caused by the vascular shut down effect also acts favorably for hemostasis. This means that the procedure could be performed safely even in those patients using anti-coagulant or anti-platelet medications or in those showing bleeding tendencies due to liver cirrhosis or renal failure.
Figure 9:
Absorption spectra of porfimer sodium (Photofrin) and talaporfin sodium (Laserphyrin).
The efficacy of L-PDD:
The L-PDD confirmed that talaporfin sodium (Laserphyrin®) was incorporated within the tumor by 6 hours after it was administered to the patient (Figure 4b).12) It also confirmed that this PS remained within the tumor for as long as 50 hours after the administration (Figure 6b).9) The primary advantage of talaporfin sodium over porfimer sodium (Photofrin®) was the shorter duration of light shielding due to the ability of the talaporfin sodium PS to be more readily metabolized. This was very important in the treatment of the advanced-aged patients regarding shortening the duration of hospitalization. However if talaporfin sodium in a tumor were to be metabolized and excreted at the same rate as normal tissue, planning for successive daily laser activation of the PS would be meaningless in the case of treating large tumors. However, the results in the present case have shown that talaporfin sodium remained within the tumor for over 50 hours from administration, and our experience has also shown that successive daily PD laser irradiations at such times were effective in reducing the size of the tumor. This experience has shown that L-PDT can be expected to be a mode of palliative treatment for advanced gastric cancer in advanced-aged patients. Additionally the talaporfin sodium related fluorescence became weaker after PDT. It is possible that L-PDD may further be applied to verify photobleaching.
Under initial circumstances in PDT method, a PS is selectively taken up by tumorous tissue and fluoresces when irradiated by laser energy at an appropriate wavelength and energy: this is PDD. However, if PDD fails to elicit any fluorescence following PDT, the phenomenon of photobleaching has occurred and the PDT has been successful, i.e., the PS has converted into porphyrins, and the porphyrins have been activated by the PDT process to cause selective destruction of the cancerous tissue. These finding were observed in the present study with the successive L-PDT treatments, and L-PDD was able to show when there was still some talaporfin sodium in remnant tumor which could then be treated again with L-PDT. L-PDT in the present case was therefore shown to be successful. Currently L-PDT for lung cancer and malignant brain tumors has been developed and is being applied in clinical practice in Japan.
Conclusion
L-PDT using talaporfin sodium as the PS and a 664 nm diode laser as the light source is a safe and effective, noninvasive treatment method for advanced-aged gastric cancer patients. Not only could it be used for curative treatment of early gastric cancers, but it also offers a method of palliative treatment of advanced gastric cancers.
Parts of this investigation are based on the study “I/II phase multi-institutional study of photodynamic therapy with talaporfin sodium (Laserphyrin) and diode laser (PD laser) for the treatment of remnant, locally recurring esophageal cancers following chemo-radiation therapy.” ; conducted as a part of the Project for the research for practical application of medical technology which was funded by a subsidy from the grants-in-aid for scientific research of the Japanese Ministry of Health, Labour and Welfare.
Acknowledgements
The authors thank Dr. Tomonori Yamada from the Department of Gastroenterology, Japanese Red Cross Nagoya Daini Hospital and Dr. Hiromi Katoka, Associate Professor of the Department of Gastroenterology, Assistant Director of the Department of Endoscopy of Nagoya City University Graduate School for their help with the patient; Dr. Yoshiki Ishii, Professor of the Department of Pulmonary Medicine and Clinical Immunology, Director of Respiratory Endoscopy Center Dokkyo Medical University for lending us the PD laser; Dr. Hideyuki Hiraishi, Professor of the Department of Gastroenterology, Director of the Gastrointestinal Endoscopy Center and Dr. Akira Terano, Chairman of the Dokkyo Group of Academic Institutions, for their support and cooperation.
Editor's Note: This paper was originally published in Japanese in The Journal of Japan Society for Laser Surgery and Medicine,Vol. 34.2:124–131, 2013, and has been specially translated for inclusion in Laser Therapy as an English Original Article.
Footnotes
* This article is based on a study first reported in The Journal of Japan Society for Laser Surgery and Medicine, Vol. 34.2:124–131, 2013
Disclosure of conflict of interest
No conflict of interest exists.
References
- 1: Cabinet Office, Government of Japan: Aged society white paper for 2013 (Available at http://www8.cao.go.jp/kourei/whitepaper/w-2013/zenbun/25pdf_index.html)
- 2: Ministry of Health, Labor and Welfare: The annual total of the general status of demographics retrieved from monthly reports for the year 2011. (http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/geppo/nengai11/kekka03.html)
- 3: Cancer information service, cancer countermeasures information center, National Cancer Center: The most recent cancer statistics. (http://ganjoho.jp/public/statistics/pub/statistics01.html)
- 4: Nakamura T. (2010). Establishing new methods of laser diagnosis and treatment for esophageal cancers. From the report on “I/II phase multicenter study of photodynamic therapy with talaporfin sodium (Laserphyrin) and diode laser (PD laser) for the treatment of remnant, locally recurred esophageal cancers after chemoradiation therapy.”; Project for the research for practical application of medical technology, Japanese Ministry of Health, Labor and Welfare. 18-20 [Google Scholar]
- 5: Nakamura T. (2011). Establishing new methods of laser diagnosis and treatment for esophageal cancers. From the report of I/II phase multicenter study of photodynamic therapy with talaporfin sodium (Laserphyrin) and diode laser (PD laser) for the treatment of remnant, locally recurred esophageal cancers after chemoradiation therapy.”; Project for the research for practical application of medical technology, Japanese Ministry of Health, Labor and Welfare. 18-19 [Google Scholar]
- 6: Nakamura T, Oinuma K, Terano K. (2013). The possibilities of photodynamic diagnosis (PDD). Naika, 111(3):504-508 [Google Scholar]
- 7: Miyake Y. (2007). The principle of FICE. In Portfolio of image processing of spectral endoscopes: supervised by Miyake Y. edited by Kouzu T, Fujinon Toshiba ES System Co, Ltd.: 6-14 [Google Scholar]
- 8: Oinuma K, Masuyama H, Nakamura T. (2013). Efficacy of two days method PDT using porfimer sodium for early gastric cancer unresectable for endoscopic submucosal dissection. Jour. Japan Soc. Laser Surg. and Med. 34(2): 118-123 [Google Scholar]
- 9: PD laser, package insert, 8th edition (authorization code: 21600BZZ00026000), 2012
- 10: Nakamura T, Shirakawa K, Yamagishi H, et al. (2006). Challenge to endoscopic treatment using PDT for advanced gastric cancer and rectal cancer. Jour. Japan Soc. Laser Surg. and Med. 27(1):42-50 [Google Scholar]
- 11: Nakamura T, Ejiri M. (2001). PDT for advanced cancers and its related treatments. In Medical treatment of digestive tract cancers in the 21st century. A summary and prospect of present problems., Fujimori T, Hoshihara Y. edit., Nagasako K. supervision, Shinkoh Igaku Pub.: 69-76 [Google Scholar]
- 12: Photofrin intravenous injection 75mg, package insert, 9th edition (authorization code: 22000 AMX00978), 2010