Abstract
Objective
We examined the relationship of several cardiovascular risk factors (CVRF) to brachial artery flow-mediated dilatation (FMD) in Chinese subjects.
Methods
This was a cross-sectional study. In 2,511 Chinese adults (age 46.86±9.52 years, 1,891 men and 620 women) recruited from people who underwent health screening at The Third Xiangya Hospital, patients’ CVRF [age, body mass index (BMI), waist circumference (WC), blood pressure (BP), cholesterol parameters, creatinine (Cr), uric acid (UA), glucose level and smoking] and prevalence of present disease (hypertension, diabetes mellitus, coronary heart disease and hyperlipidemia) were investigated.
Results
Multivariate analysis revealed that FMD negative correlated with age (β=–0.29, P<0.001), gender (β=–0.12, P<0.001), BMI (β=–0.12, P=0.001), WC (β=–0.10, P=0.011), systolic BP (SBP) (β=–0.12, P<0.001), fasting glucose (β=–0.04, P=0.009), total cholesterol (TC) (β=–0.04, P=0.014), smoking (β=–0.05, P=0.003), and baseline brachial artery diameter (β=–0.35, P<0.001). FMD decreased with increasing age in both genders. In women, FMD was higher than men and age-related decline in FMD was steepest after age 40; FMD was similar in men above 55 years old.
Conclusions
In Chinese subjects, FMD may be a usefully marker of CVRF. Age, gender, BMI, WC, SBP, fasting glucose, TC, smoking, and baseline brachial artery diameter were independent variables related to the impairment of FMD. The influence of CVRF on endothelial function is more in women than men.
Keywords: Endothelial function, brachial artery flow-mediated dilatation (FMD), cardiovascular risk factors (CVRF)
Introduction
Vascular endothelial dysfunction is an early change during atherosclerosis, an initial step in the development of arteriosclerosis, and also an important sign of arteriosclerosis (1,2). In addition, the vascular endothelial dysfunction is also an independent predictor of cardiovascular events and all-cause mortality (3,4). In clinical practice, brachial artery flow-mediated dilatation (FMD) is used to reflect endothelial function, which can be evaluated by a noninvasive ultrasound technique (5). Compared with the ultrasound machine, this new device enables the automatic and rapid tracking of FMD over time in a reliable and repeatable way, avoiding the inaccuracy due to manual positioning.
The cardiovascular risk factors (CVRF) refer to the factors that may increase the occurrence and progression of cardiovascular diseases (CVD). Previous studies have demonstrated that FMD is associated with CVRF including smoking, body mass index (BMI), age, blood pressure (BP), blood glucose, and blood lipids. However, the conclusions remain controversial. Some investigators believed that such correlations were not statistically significant or even did not exit (6,7), while others argued that FMD was only associated with one or few of these risk factors (8,9). Such diversities may be explained by the differences in subjects, methodologies, sample sizes, measurement methods of FMD, and inclusion of vascular baseline diameters in multivariate analyses. In addition, racial differences have been found in FMD (10). While FMD has been found to be different between Asian populations and European populations, all the previous studies were conducted among European or Japanese population. Data from Chinese populations are still rare. Therefore, this study was designed to explore the potential correlations between FMD and CVRF and summarize their features.
Subjects and method
Study population
We retrospectively analyzed the clinical data of 2,511 participants who had undergone a health examination at The Third Xiangya Hospital of Central South University from March 1, 2013 to March 31, 2014. All participants received a general health questionnaire survey, anthropometric measurement, and routine blood sample tests. The questionnaire covered demographic background and medical history. Anthropometric measurements included height, weight, waist circumference (WC) and BP; both height and weight were measured with light clothing without shoes. BMI was calculated as body weight (kg) divided by the square of body height (m). BP was measured on the right upper arm in the sitting position after a 10-15-minute rest between 7 and 9 AM using a validated digital automatic BP monitor. Informed consent was obtained from all of the participants prior to entering the study. The study was conducted with the adherence to the Declaration of Helsinki and with the approval of the Ethics Guidelines Committee of the Central South University.
Measurements of laboratory
Blood samples were drawn after overnight fasting (>8 hours) for laboratory tests. All lab tests were conducted by certified experimental specialists using standard protocols at the hospital’s Department of Laboratory. The biochemical tests included assessments of fasting glucose, total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), creatinine (Cr) and uric acid (UA).
Measurements of FMD
We measured FMD according to the international guidelines (11) using a vascular ultrasound system equipped with an edge-tracking system for 2D imaging and pulsed Doppler flow velocimeter for automatic measurement (UNEXEF18G; UNEX Co. Ltd., Nagoya, Japan). Participants were measured between 8 and 12 AM by trained and experienced ultrasound doctors. In brief, the diameter of the brachial artery at rest was measured in the cubital region, and subsequently, the cuff was inflated to 50 mmHg above systolic BP (SBP) for 5 min and deflated. The diameter at the same point of the artery was monitored continuously, and the maximum dilatation from 45-60 s after deflation was recorded. This method has been validated previously (12).
FMD was calculated from the following equation: FMD (%) = (maximum diameter diameter at rest) ×100/diameter at rest.
CVRF and CVD definition
Hyperlipidemia was defined in accordance with the criteria set forth in the guidelines for dyslipidemia control in Chinese adults (2007 edition) (13). Total blood lipid level was defined as hyperlipidemia if TC, TG, or LDL-C were high (TC ≥6.22 mmol/L, TG ≥2.26 mmol/L, LDL-C ≥4.14 mmol/L).
Smoking status was defined as non-smoker, occasional smoker or regular smoker. The term non-smoker included patients who quit smoking >1 month before admission. The term occasional smoker included patients who smoking ≥4 times a week but less than one cigarette Average per day. The term regular smoker included patients who smoking >1 cigarette per day and last over 6 months.
Hypertension was diagnosed previously or newly, based on the 2010 Chinese guidelines for the management of hypertension (14).
Coronary artery disease. We included in the study patients with a history of acute coronary syndrome and/or confirmed by coronary angiography atherosclerosis of coronary vessels.
Diabetes mellitus. Patients with diabetes mellitus previously or newly diagnosed during hospitalization were taken into consideration.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD). Categorical variables were compared by means of χ2 test. Clinical and characteristics between sexes of the study population were compared with the t-test. Two-sided P<0.05 was considered statistically significant. One-way analysis of variance (ANOVA) followed by a post-hoc Student-Newman-Keul’s test was used to determine the differences among multiple groups. Relations between variables were determined by Spearman correlation coefficient analysis. Multivariate regression analyses were performed to identify factors associated with FMD in risk factors and laboratory data. All data analyses were performed using SPSS statistical software, version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline data
Table 1 summarizes the baseline data of the subjects. A total of 2,511 subjects [1,891 men (75.31%) and 620 women (24.69%)] aged 20-86 years (mean: 46.86±9.52 years) were enrolled in this study. Among them there were 554 hypertensive patients (22.06%), 231 diabetic patients (9.2%), 872 patients with hyperlipidemia (34.72%), 58 patients with coronary heart disease (2.31%), 221 occasional smokers (8.8%), and 776 frequent smokers (30.9%). Except for TC and LDL-C, the other indicators showed significant differences between men and women. The average FMD value was 6.80%±2.49%. Women had significantly higher FMD value than men (7.23%±2.860% vs. 6.65%±2.43%, P<0.001). Meanwhile, the baseline vascular diameter was significantly smaller in women than in men (3.52±0.49 vs. 4.33±0.52 mm, P<0.001).
Table 1. Demographics and clinical characteristics of the subjects (mean ± SD).
| Variables | Total (n=2,511) | Male (n=1,891) | Female (n=620) | P value |
|---|---|---|---|---|
| Age (year) | 46.86±9.52 | 46.43±8.98 | 48.15±10.91 | <0.001 |
| BMI (kg/m2) | 25.16±3.18 | 25.75±2.97 | 23.33±3.13 | <0.001 |
| WC (cm) | 85.95±9.29 | 88.60±8.02 | 77.88±8.19 | <0.001 |
| SBP (mmHg) | 125.83±16.60 | 126.67±15.24 | 123.26±19.98 | <0.001 |
| DBP (mmHg) | 80.15±11.77 | 81.50±11.53 | 76.02±11.54 | <0.001 |
| Fasting glucose (mmol/L) | 5.40±1.48 | 5.48±1.57 | 5.18±1.13 | <0.001 |
| TC (mmol/L) | 5.08±0.99 | 5.07±0.98 | 5.11±1.05 | 0.456 |
| TG (mmol/L) | 2.07±1.76 | 2.31±1.91 | 1.34±0.80 | <0.001 |
| LDL-C (mmol/L) | 2.70±0.87 | 2.68±0.86 | 2.76±0.92 | 0.08 |
| HDL-C (mmol/L) | 1.47±0.37 | 1.37±0.32 | 1.75±0.38 | <0.001 |
| Cr (μmol/L) | 72.83±15.93 | 78.33±13.13 | 56.07±11.35 | <0.001 |
| UA (μmol/L) | 324.71±89.24 | 353.58±76.97 | 236.68±62.45 | <0.001 |
| Hypertension, n (%) | 554 (22.06) | 413 (21.84) | 141 (22.74) | 0.638 |
| Diabtes mellitus, n (%) | 231 (9.20) | 189 (9.99) | 42 (6.77) | 0.016 |
| Hyperlipidaemia, n (%) | 872 (34.72) | 746 (39.45) | 126 (20.32) | <0.001 |
| CVD, n (%) | 58 (2.31) | 40 (2.11) | 18 (2.90) | 0.257 |
| Smoking, n (%) | ||||
| Non-smoker | 1,514 (60.29) | 908 (48.02) | 606 (97.74) | <0.001 |
| Occasional smoker | 221 (8.80) | 212 (11.21) | 9 (1.45) | <0.001 |
| Regular smoker | 776 (30.90) | 771 (40.77) | 5 (0.80) | <0.001 |
| FMD (%) | 6.80±2.49 | 6.65±2.43 | 7.23±2.860 | <0.001 |
| Baseline brachial artery diameter, mm | 4.12±0.62 | 4.33±0.52 | 3.52±0.49 | <0.001 |
Note: SD, standard deviation; BMI, body mass index; WC, waist circumference; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; Cr, creatinine; UA, uric acid; CVD, cardiovascular disease; FMD, flow-mediated dilatation. P<0.05 is considered statistically significant.
Associations between FMD and CVRF
Analysis of the associations between FMD and the reported CVRF showed that FMD was significantly correlated with age (r=–0.413, P<0.001), BMI (r=–0.164, P<0.001), WC (r=–0.184, P<0.001), SBP (r=–0.303, P<0.001), DBP (r=–0.223, P<0.001), fasting glucose (r=–0.189, P<0.001), TC (r=–0.104, P<0.001), TG (r=–0.413, P<0.001), LDL-C (r=–0.063, P=0.001), HDL-C (r=0.042, P=0.035), Cr (r=–0.055, P=0.006), UA (r=–0.102, P<0.001), and baseline brachial artery diameter (r=–0.336, P<0.001) (Table 2). Multivariate linear regression analysis showed that the following CVRF were entered into the stepwise regression equation: age (t=–16.068, P<0.001), gender (t=–5.090, P<0.001), BMI (t=3.408, P<0.001), WC (t=–2.554, P<0.001), SBP (t=–6.498, P<0.001), fasting glucose (t=–2.610, P=0.009), TC (t=–2.467, P=0.014), smoking (t=–2.943, P=0.003), and baseline brachial artery diameter (t=–15.946, P<0.001) (Table 3).
Table 2. Univariate correlations between FMD and clinical characteristics in the overall population and in different gender.
| Variables | All population (n=2,511) |
Male (n=1,891) |
Female (n=620) |
|||||
|---|---|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | |||
| Age (year) | –0.413 | <0.001 | –0.361 | <0.001 | –0.584 | <0.001 | ||
| BMI (kg/m2) | –0.164 | <0.001 | –0.093 | <0.001 | –0.304 | <0.001 | ||
| WC (cm) | –0.184 | <0.001 | –0.108 | <0.001 | –0.376 | <0.001 | ||
| SBP (mmHg) | –0.303 | <0.001 | –0.221 | <0.001 | –0.462 | <0.001 | ||
| DBP (mmHg) | –0.223 | <0.001 | –0.171 | <0.001 | –0.320 | <0.001 | ||
| Fasting glucose (mmol/L) | –0.189 | <0.001 | –0.160 | <0.001 | –0.239 | <0.001 | ||
| TC (mmol/L) | –0.104 | <0.001 | –0.052 | 0.023 | –0.247 | <0.001 | ||
| TG (mmol/L) | –0.139 | <0.001 | –0.062 | 0.007 | –0.272 | <0.001 | ||
| LDL-C (mmol/L) | –0.063 | 0.001 | –0.006 | 0.0797 | –0.230 | <0.001 | ||
| HDL-C (mmol/L) | 0.042 | 0.035 | –0.028 | 0.217 | 0.079 | 0.048 | ||
| Cr (μmol/L) | –0.055 | 0.006 | 0.038 | 0.098 | –0.069 | 0.085 | ||
| UA (μmol/L) | –0.102 | <0.001 | –0.015 | 0.511 | –0.213 | <0.001 | ||
| Baseline brachial artery diameter, mm | –0.336 | <0.001 | –0.311 | <0.001 | –0.421 | <0.001 | ||
Coefficients (r) and P values were calculated with the spearman correlation analysis for continuous variables. BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; Cr, creatinine; UA, uric acid; FMD, flow-mediated dilatation. P<0.05 is considered statistically significant.
Table 3. Multivariate analysis of the relation between flow-mediated vasodilation and variables.
| Variables | β | T value | P value |
|---|---|---|---|
| Age (year) | –0.298 | –16.068 | <0.001 |
| Gender | –0.122 | –5.090 | <0.001 |
| BMI (kg/m2) | –0.125 | –3.408 | 0.001 |
| WC (cm) | –0.100 | –2.554 | 0.011 |
| SBP (mmHg) | –0.124 | –6.498 | <0.001 |
| Fasting glucose (mmol/L) | –0.046 | –2.610 | 0.009 |
| TC (mmol/L) | –0.043 | –2.467 | 0.014 |
| Smoking | –0.056 | –2.943 | 0.003 |
| Baseline brachial artery diameter (mm) | –0.354 | –15.946 | <0.001 |
BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; TC, total cholesterol.
Relationship between FMD and gender
Analysis on the relationship between FMD and gender showed that: in men, age, BMI, WC, SBP, DBP, fasting glucose, TC, TG, and baseline brachial artery diameter were significantly correlated with FMD (all P<0.001); in women, age, BMI, WC, SBP, DBP, fasting glucose, TC, TG, LDL-c, HDL-c, UA, and baseline brachial artery diameter were significantly correlated with FMD (all P<0.001). The major five CVRF were defined as hypertension, diabetes, hyperlipidemia, coronary heart disease, and smoking. The subjects were grouped based on the number of their major CVRF (Figure 1). FMD gradually decreased with the increase of the number of major CVRF. Among subjects without any major cardiovascular risk factor, the FMD values were higher in women than in men; among subjects with one or more major CVRF, the FMD values were lower in women than in men. The differences between women and men increased along with the increase of the number of major CVRF.
Figure 1.
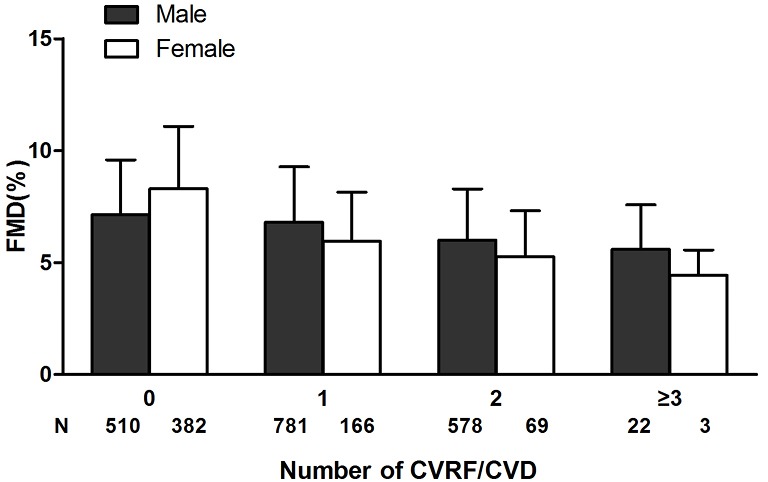
Illustration of FMD, flow-mediated dilatation depending on number of coexisting risk factors/diseases; 0, no risk factors/diseases; 1, have one risk factors/diseases; 2, have two risk factors/diseases; ≥3, have more than three factors/diseases. FMD, flow-mediated dilatation; CVRF, cardiovascular risk factors; CVD, cardiovascular disease.
Relationship between FMD and age
The 20-86-year-old subjects were grouped according to their ages (ten 5-year groups) (Figure 2). The potential relationship between FMD and age was evaluated in subjects with different number of major CVRF. Among all subjects, the FMD was significantly higher in women than in men in the 20 to 55 age groups. It significantly declined in the 40-45 age group in women; however, it showed no significant difference between women and men in the 55 and over age groups. Among subjects without major CVRF, the FMD values were significantly higher in women than in men in the 55 and under age groups, similar in the 55-59 age group, and significantly lower in the 60 and over age groups. Among subjects with major CVRF, the FMD values were higher in women than in men in the 45 and under age groups but showed no significant differences in the 45 and over age groups.
Figure 2.
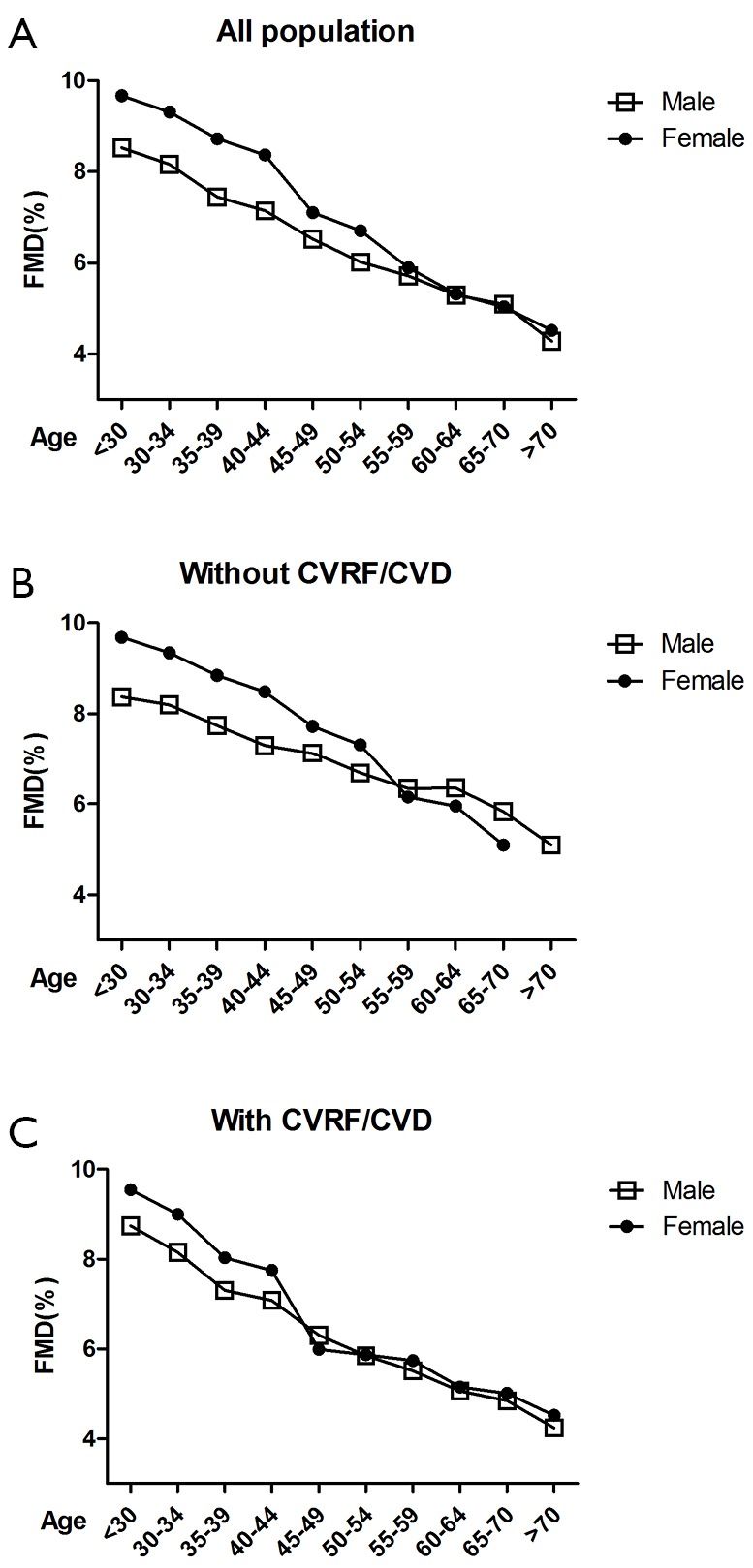
(A) Line graphs show FMD in all subjects classified into 10 groups based on 5-year of age; (B) line graphs show FMD in men and women classified into 10 groups based on 5-year of age in the no risk group; (C) line graphs show FMD in men and women classified into 10 groups based on 5-year of age in the cardiovascular risk factor and cardiovascular disease groups. CVRF, cardiovascular risk factors; CVD, cardiovascular disease; FMD, flow-mediated dilatation.
Discussion
It is well known that the CVRF can bring damage to vascular endothelium (15). As the biggest endocrine organ in human body, vascular endothelium maintains the normal systolic and diastolic conditions of blood vessels by secreting NO, endothelin-1, prostacyclin, bradykinin, and other factors. A decreased secretion of NO by vascular endothelium is manifested as endothelium-dependent diastolic dysfunction (16). In our current study, we determined the NO secretion to reflect the changes in vascular diastolic function, or by measuring the FMD value to reflect the endothelial dysfunction. In addition, we explored the potential relationships between the decline of FMD and the major CVRF.
We found that, among the various CVRF, age, gender, BMI, waist, SBP, fasting glucose, TC, smoking, and baseline brachial artery diameter are independent predictors of FMD. The relationships between FMD and CVRF have been reported in many literatures. Among them 18 studies are listed in Table 4. The included variables include age, sex, SBP, EPO, BMI, DBP, FPG, TC, HDL-c, Cr, UA, hsCRP, insulin, smoking, alcohol use, hypertension, dyslipidemia, diabetes mellitus, hyperuricemia, baseline brachial artery diameter, metabolic syndrome (MetS), and Framingham risk score. Multivariate analyses resulted in different conclusions. Schnabel et al. (22) believed FMD was associated with age, sex, BMI, SBP, DBP, TC, HDL-c, TG, C-reactive protein, hypertension, hypertension treatment, and dyslipidemia, whereas Philpott et al. (8) argued FMD was only associated with SBP. Although Mizia-Stec et al. (6) agreed FMD was associated with CVRF, he insisted that such correlations were limited among populations at high risk of CVD. Among these studies, disputes existed even in a single indicator. Maruhashi et al. (19) believed UA was an independent risk factor of impaired endothelial function in postmenopausal women, while Oikonen et al. (7) argued that UA was not correlated with the endothelial function. Thus, there is no definite conclusion on the associations between FMD and CVRF. As shown in our current study, FMD was correlated with the traditional CVRF including BP, blood sugar, blood lipid, obesity, and smoking, in particular the baseline brachial artery diameter; however, it was not correlated with Cr and UA. In our study, we further listed hyperlipidemia, hypertension, diabetes, coronary heart disease, and smoking as the five major risk factors. Grouping based on the number of risk factors showed that the FMD value declined along with the increase of the number of risk factors. Therefore, we can conclude that these CVRF can bring damage to vascular endothelium and then cause the decreased vasodilation capacity, resulting in the decline of FMD value.
Table 4. Summary of cross-sectional studies on relationship between FMD, flow-mediated dilatation and cardiovascular risk factors.
| No. | Author, year | Population | Age (years) | Country/ethnic/race | Indicators included in the multivariate analysis index for analysis | Multivariate analysis | Key findings |
|---|---|---|---|---|---|---|---|
| 1 | Mizia-Stec et al., 2014 (6) | 617 subjects (men: 349; women: 268) | Mean age: 50.1±14.9 | Polish | CVD, hyperlipidaemia, age, valve heart disease, Brachial artery diameter | Yes | FMD is related to the number of traditional CVRF/CVD. The value of FMD assessment in high risk patients is limited |
| 2 | Tilling et al., 2013 (17) | 317 subjects (men: 134; women: 183; premenopausal: 33) | Mean age: 55±6.8 | British | Age, sex, SBP, Hb, EPO, BMI, DBP, FPG, TC, HDL, hsCRP | Yes | FMD was independently negatively correlated with age, male sex and SBP |
| 3 | Maruhashi et al., 2013 (18) | 5,314 subjects (men: 4,135; women: 1,179) | Aged 17-86, mean age: 46±13 | Japanese | Age, sex, BMI, SBP, DBP, dyslipidemia, diabetes mellitus, smoking, baseline brachial artery diameter | Yes | FMD was correlated with age, sex, BMI, SBP, diabetes mellitus, smoking, and baseline brachial artery diameter |
| 4 | Maruhashi et al., 2013 (19) | 749 women (368 premenopausal and 381 postmenopausal) | Aged 30-74, mean age: 50.2±10.6 | Japanese | UA, age, HDL, FPG, smoking | Yes | UA was a significantly independent risk factor for endothelial dysfunction in postmenopausal women, but not in premenopausal women |
| 5 | Oikonen et al., 2012 (7) | 1,985 young adults (men: 923; women: 1,062) | Aged 30-45, women (37.9±4.9), men (37.6±5.0) | Finnish | BMI, GFR, SBP, TC, TG, Cr, UA, adiponectin, insulin, FPG, CRP, alcohol use | Yes | No associations were found between UA and FMD |
| 6 | Lunder et al., 2012 (20) | 100 healthy men | Aged 30-50, mean age: 41.9±6.4 | Slovenian | Age, BMI, SBP, DBP, HR, TC, HDL, FPG | Yes | FMD was associated with HR and HDL |
| 7 | Kawano et al., 2012 (12) | 181 T2DM patients (men: 108; women: 73) | Mean age: 64±10 | Japanese | Age, duration of diabetes, smoking, WC, SBP, HbAlc, TC, | Yes | FMD was associated with age, smoking, SBP, HbAlc and TC |
| Cr, statin, ACEI/ARB | |||||||
| 8 | Tomiyama et al., 2011 (21) | 2,732 healthy men | Mean age: 49±8 | Japanese | Hyperuricemia, MetS | No | Mild hyperuricemia may be a significant independent risk factor for endothelial dysfunction in subjects without MetS, whereas only severe hyperuricemia appeared to exacerbate endothelial dysfunction in similar subjects with MetS |
| 9 | Schnabel et al., 2011 (22) | 5,000 subjects (men: 2,540; women: 2,460) | Aged 34-74, mean age: 55.5±10.9 | German | Age, sex, BMI, current smoking, SBP, DBP, HR, height, TC, HDL, TG, FPG, CRP, diabetes, hypertension, hypertension treatment, dyslipidemia, CVD | Yes | FMD was associated with age, sex, BMI, SBP, DBP, TC, HDL, TG, CRP, hypertension, hypertension treatment and dyslipidemia |
| 10 | Hamburg et al., 2011 (23) | 7,031 subjects (men: 3,234; women: 3,797) | Aged 19-88, mean age: 48±13 | White | Age, sex, SBP, DBP, HR, BMI, TC, HDL, diabetes, current smoker, lipid-lowering medication, CVD | Yes | FMD was associated with age, sex, SBP and BMI |
| 11 | Philpott et al., 2009 (8) | 1,477 men without CVD | Mean age: 49.4±9.9 | Canadians | Age, SBP, FPG, BMI, LDL, HDL, current smoking, CRP | Yes | The only CVRF independently associated with FMD was SBP |
| 12 | Patel et al., 2009 (24) | 185 consecutive women | Mean age: 51±1 | Multi-Ethnic (White, black, Asian, Hispanic) | Age, BMI, CVD, current smoking, hypertension, diabetes, hypercholesterolemia, anti-hypertensive medication, lipid-lowering medication | Yes | FMD was associated with age, BMI and current smoking |
| 13 | Yeboah et al., 2008 (25) | 2,338 elderly | Aged 72-98, mean age: 78.3±4.2 | Multi-ethnic | Age, SBP, DBP, BMI, TC, LDL, HDL, TC, waist/hip ratio, Cr, number of CVRF, cigarette smoking | Yes | FMD was associated with age, HDL, waist/hip ratio, TC, number of CVRF |
| 14 | Tomiyama et al., 2008 (26) | 819 subjects free of CVD (men: 611; women: 208) | Mean age: 45±10 | Japanese | Age, gender, BMI, smoking, SBP, DBP, TC, HDL, TG, FPG | Yes | FMD was negative correlation with age, gender and smoking habit. In subjects ≥50 years of age, the FMD in men with one CVRF, excluding smoking, was similar to that in men with no CVRF. CVRF did not attenuate FMD in women |
| 15 | Suzuki et al., 2008 (27) | 819 subjects (men: 352; women: 467) | Mean age: 66.5±8.8 | 17% African-American, 66% Hispanic, 15% white | WC, TC, HDL, FPG, SBP | No | FMD was associated with WC, FPG and SBP |
| 16 | Gardin et al., 2008 (10) | 205 subjects (105 adult MA (42 men and 63 women) and 100 NHW (59 men and 41 women) | MA (age 46±14) and NHW (age 50±11) | MA NHW | Age, brachial artery diameter, BMI, SBP, LDL, HDL, TC, FPG, current smoking, urine albumin | Yes | MA men (BMI, SBP, urine albumin); MA women (BMI, urine albumin), NHW men (BMI, HDL), NHW women (age, BMI) |
| 17 | Yan et al., 2005 (9) | 1,578 men | Mean age: 49.37±9.92 | Canadian | Age, SBP, DBP, BMI, FPG, TC, TG, HDL, LDL, FRS | Yes | FMD was associated with SBP and DBP |
| 18 | Rodriguez et al., 2005 (28) | 579 subjects (men: 237; women: 342) | Mean age: 66±9 | Multi-ethnic (white, black, and Hispanic) | Age, gender, BMI, FPG, hypertensive status | Yes | After adjustment for age, gender, BMI, and hypertensive status, a higher FPG was significantly associated with a lower FMD |
CVD, cardiovascular disease; CVRF, cardiovascular risk factors; SBP, systolic blood pressure; Hb, haemoglobin concentration; EPO, erythropoietin; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; TC, total cholesterol; HDL, high density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; UA, uric acid; GFR, glomerular filtration rate; Cr, creatinine; CRP, C-reactive protein; TG, triglycerides; HR, heart rate; WC, waist circumference; HbAlc, glycosylated hemoglobin; MetS, Metabolic syndrome; LDL, low density lipoprotein cholesterol; MA, Mexican-American; NHW, non-Hispanic Whites; FRS, Framingham risk score.
FMD has shown certain racial differences (10). In their study on 5,000 German subjects aged 55.5±10.9 years, Schnabel et al. (22) found that the baseline FMD value was 8.08±4.88% among all the subjects, 6.53±3.73% in men, 9.69±5.39% in women; meanwhile, the baseline brachial artery diameter was 4.32±0.82 mm in all the subjects, 4.89±0.59 mm in men, and 3.74±0.59 mm in women. Oikonen et al. (7) studied 1,985 Finnish subjects aged 30-45 years and found that the FMD value was 7.64±3.79% in men and 9.88±4.84% in women. Maruhashi et al. (18) studied 5,314 Japanese subjects aged 46±13 years and found the average FMD value was 6.16±3.26% and the brachial artery diameter was 4.01±0.60 mm. In our current study, we found that, among the 2,511 Chinese subjects, the average FMD value was 6.80±2.49 mm and the brachial artery diameter was 4.12±0.62 mm. Thus, the FMD value is higher in the Caucasian populations than in East Asian populations but is basically the same between Chinese and Japanese. This can be explained both by the differences in the diastolic function and baseline brachial artery diameter among different populations and by the different measurement methods. Therefore, it is necessary to carry out studies on the CVRF that may affect the vascular endothelial dysfunction among different races and populations.
In our current study, women had higher FMD values than men, which was consistent with the previous study (29,30), suggesting the endothelial function is better in women than in men. However, further multivariate analysis showed that the difference in FMD between women and men might be due to the different baseline brachial artery diameter, which was smaller in women than in men; as a result, the vascular dilatation function differs between men and women. In our current study, we also explored the impacts of CVRF on FMD in men or women. Along with the increase of the number of CVRF, the FMD values declined in both men and women. Among subjects without any major cardiovascular risk factor, the FMD values were higher in women than in men; among subjects with one or more major CVRF, the FMD values were lower in women than in men; furthermore, the difference of the FMD values between women and men was even larger in subjects with more major CVRF. Obviously, these major CVRF have larger impacts on women. After the subjects were grouped according to age, comparisons of the FMD values among subjects with different age and gender showed that the vascular endothelial function dramatically declined in the 40 and over age groups; however, the differences in the FMD values between women and men were not statistically different in the 55 and over age groups. We then further divided our subjects into two groups: subjects with major CVRF and those without major CVRF. In subjects with major CVRF, the FMD value dramatically decreased in the 40 and over age groups; however, the differences in the FMD values between women and men were not statistically different in the 45 and over age groups. In subjects without major CVRF, the changes in the FMD value were basically consistent with the general populations. In women, the age-related endothelial dysfunction is associated with the decline of female hormones. Among them the estrogen level begins to fall in the 40-45 age group; during the perimenopause stage (45-55 years), the female hormone levels further decline. Thus, among women without major CVRF, the vascular endothelium is no longer protected by estrogen, and the FMD value, which reflects the vascular endothelial function, is no longer different between women and men. In contrast, in women with major CVRF, the decline of FMD value arrives early and becomes similar as that in men after the age of 45 years, suggesting that the protective effect of estrogen on endothelial dysfunction is offset by the CVRF. This is consistent with the previous reports. However, we divided the subjects on a 5-year basis, which was different from the 10-year grouping in other studies. Therefore, while Tomiyama et al. (26) and Maruhashi et al. (18) proposed that the cut-off age for the same FMD value between women and men was 50 years, we, on the basis of a smaller age interval, found it would be 55 years.
However, our study had some limitations. First, the sample size of women in our study was relatively small because our subjects were recruited from individuals who had received health check-ups and there were far more males receiving vascular disease screening in China mainly due to the income levels and the awareness about health and disease. Second, due to the lack of follow-up data, we were not able to elucidate the impacts of CVRF on FMD. Further follow-up of these subjects will be included in our future studies.
Conclusions
To conclude, FMD measurement is one of the useful methods for assessing the arterial endothelial function, which is correlated with multiple CVRF. FMD has shown certain racial differences. Our study, for the first time, explored the correlations of FMD with CVRF and its age- and gender-related specificities in a large Chinese population. We hope the findings of this study may shed a light on similar research on the endothelial dysfunction and cardiovascular events in Chinese populations and provide evidences for the establishing the FMD threshold values that suit the Chinese populations.
Acknowledgements
Funding: Supported by Foundation of Hunan Provincial Science & Technology Department (2014SK3062); Supported by Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-year Plan of China (2013BAI04B01).
Disclosure: The authors declare no conflict of interest.
References
- 1.Stoner L, Erickson ML, Young JM, et al. There’s more to flow-mediated dilation than nitric oxide. J Atheroscler Thromb 2012;19:589-600. [DOI] [PubMed] [Google Scholar]
- 2.Ruggiero D, Paolillo S, Ratta GD, et al. Endothelial function as a marker of pre-clinical atherosclerosis: assessment techniques and clinical implications. Monaldi Arch Chest Dis 2013;80:106-10. [DOI] [PubMed] [Google Scholar]
- 3.Xu Y, Arora RC, Hiebert BM, et al. Non-invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging 2014;15:736-46. [DOI] [PubMed] [Google Scholar]
- 4.Kaźmierski M, Michalewska-Wludarczyk A, Krzych LJ. Intima-media thickness and flow-mediated dilatation in the diagnosis of coronary artery disease in perimenopausal women. Pol Arch Med Wewn 2010;120:181-8. [PubMed] [Google Scholar]
- 5.Charakida M, de Groot E, Loukogeorgakis SP, et al. Variability and reproducibility of flow-mediated dilatation in a multicentre clinical trial. Eur Heart J 2013;34:3501-7. [DOI] [PubMed] [Google Scholar]
- 6.Mizia-Stec K, Wieczorek J, Orszulak M, et al. Flow-mediated dilatation (FMD) and prevalence of cardiovascular risk factors: the value of FMD assessment in high risk patients is limited. Kardiol Pol 2014;72:254-61. [DOI] [PubMed] [Google Scholar]
- 7.Oikonen M, Wendelin-Saarenhovi M, Lyytikainen LP, et al. Associations between serum uric acid and markers of subclinical atherosclerosis in young adults. The cardiovascular risk in Young Finns study. Atherosclerosis 2012;223:497-503. [DOI] [PubMed] [Google Scholar]
- 8.Philpott AC, Lonn E, Title LM, et al. Comparison of new measures of vascular function to flow mediated dilatation as a measure of cardiovascular risk factors. Am J Cardiol 2009;103:1610-5. [DOI] [PubMed] [Google Scholar]
- 9.Yan RT, Anderson TJ, Charbonneau F, et al. Relationship between carotid artery intima-media thickness and brachial artery flow-mediated dilation in middle-aged healthy men. J Am Coll Cardiol 2005;45:1980-6. [DOI] [PubMed] [Google Scholar]
- 10.Gardin JM, Allebban Z, Wong ND, et al. Endothelial function and urine albumin levels among asymptomatic Mexican-Americans and non-Hispanic whites. Cardiovasc Ultrasound 2008;6:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39:257-65. [DOI] [PubMed] [Google Scholar]
- 12.Kawano N, Emoto M, Mori K, et al. Association of endothelial and vascular smooth muscle dysfunction with cardiovascular risk factors, vascular complications, and subclinical carotid atherosclerosis in type 2 diabetic patients. J Atheroscler Thromb 2012;19:276-84. [DOI] [PubMed] [Google Scholar]
- 13.Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults. Chinese guidelines on prevention and treatment of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi 2007;35:390-419. [PubMed] [Google Scholar]
- 14.Liu LS, Writing Group of Chinese Guidelines for the Management of H. 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi 2011;39:579-615. [PubMed] [Google Scholar]
- 15.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation 2005;111:363-8. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson IB, Franklin SS, Cockcroft JR. Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension 2004;44:112-6. [DOI] [PubMed] [Google Scholar]
- 17.Tilling L, Hunt J, Jiang B, et al. Endothelial function does not relate to haemoglobin or serum erythropoietin concentrations and these do not explain the gender difference in endothelial function in healthy middle-aged men and women. Eur J Clin Invest 2013;43:225-30. [DOI] [PubMed] [Google Scholar]
- 18.Maruhashi T, Soga J, Fujimura N, et al. Relationship between flow-mediated vasodilation and cardiovascular risk factors in a large community-based study. Heart 2013;99:1837-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maruhashi T, Nakashima A, Soga J, et al. Hyperuricemia is independently associated with endothelial dysfunction in postmenopausal women but not in premenopausal women. BMJ Open 2013;3:e003659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lunder M, Janic M, Kejzar N, et al. Associations among different functional and structural arterial wall properties and their relations to traditional cardiovascular risk factors in healthy subjects: a cross-sectional study. BMC Cardiovasc Disord 2012;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomiyama H, Higashi Y, Takase B, et al. Relationships among hyperuricemia, metabolic syndrome, and endothelial function. Am J Hypertens 2011;24:770-4. [DOI] [PubMed] [Google Scholar]
- 22.Schnabel RB, Schulz A, Wild PS, et al. Noninvasive vascular function measurement in the community: cross-sectional relations and comparison of methods. Circ Cardiovasc Imaging 2011;4:371-80. [DOI] [PubMed] [Google Scholar]
- 23.Hamburg NM, Palmisano J, Larson MG, et al. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension 2011;57:390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel AR, Hui H, Kuvin JT, et al. Modestly overweight women have vascular endothelial dysfunction. Clin Cardiol 2009;32:269-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeboah J, Burke GL, Crouse JR, et al. Relationship between brachial flow-mediated dilation and carotid intima-media thickness in an elderly cohort: the Cardiovascular Health Study. Atherosclerosis 2008;197:840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomiyama H, Matsumoto C, Yamada J, et al. The relationships of cardiovascular disease risk factors to flow-mediated dilatation in Japanese subjects free of cardiovascular disease. Hypertens Res 2008;31:2019-25. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T, Hirata K, Elkind MS, et al. Metabolic syndrome, endothelial dysfunction, and risk of cardiovascular events: the Northern Manhattan Study (NOMAS). Am Heart J 2008;156:405-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez CJ, Miyake Y, Grahame-Clarke C, et al. Relation of plasma glucose and endothelial function in a population-based multiethnic sample of subjects without diabetes mellitus. Am J Cardiol 2005;96:1273-7. [DOI] [PubMed] [Google Scholar]
- 29.Skaug EA, Aspenes ST, Oldervoll L, et al. Age and gender differences of endothelial function in 4739 healthy adults: the HUNT3 Fitness Study. Eur J Prev Cardiol 2013;20:531-40. [DOI] [PubMed] [Google Scholar]
- 30.Virdis A, Taddei S.Endothelial aging and gender. Maturitas 2012;71:326-30. [DOI] [PubMed] [Google Scholar]


