Abstract
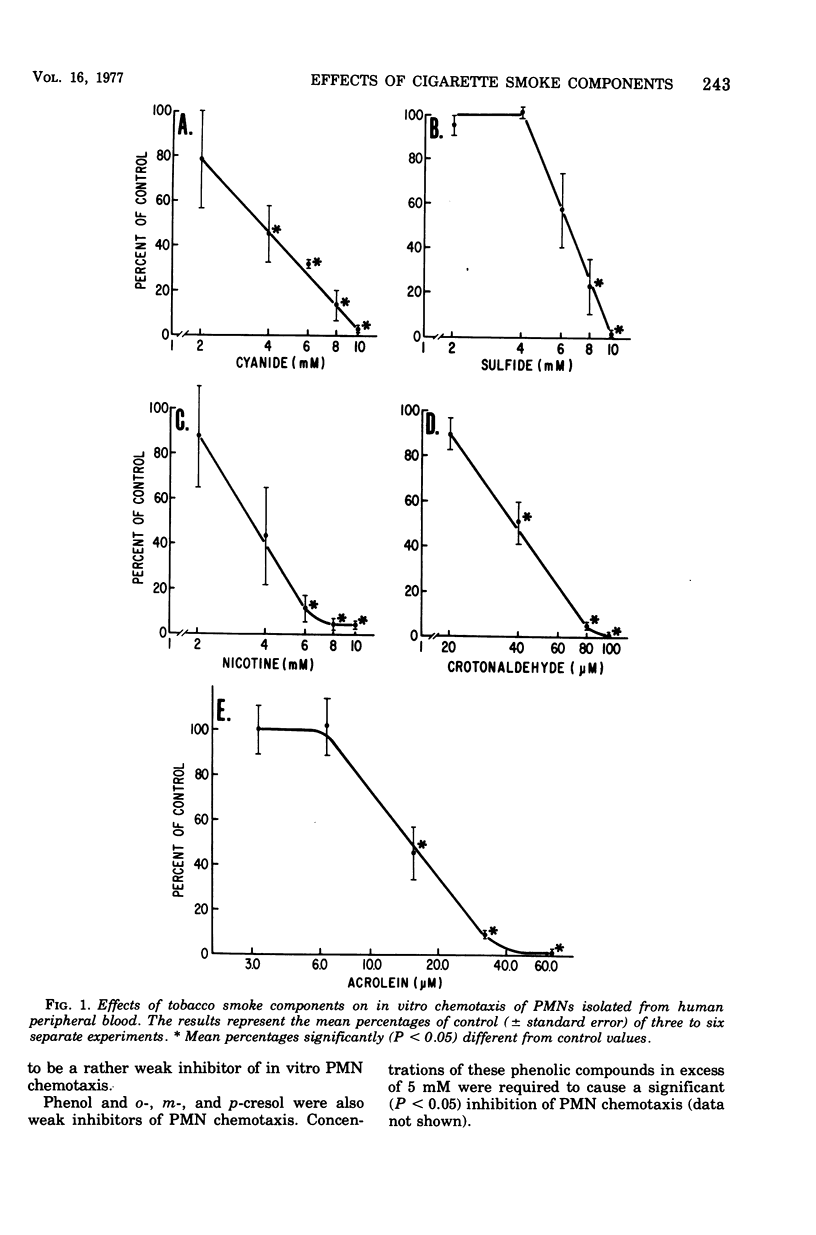
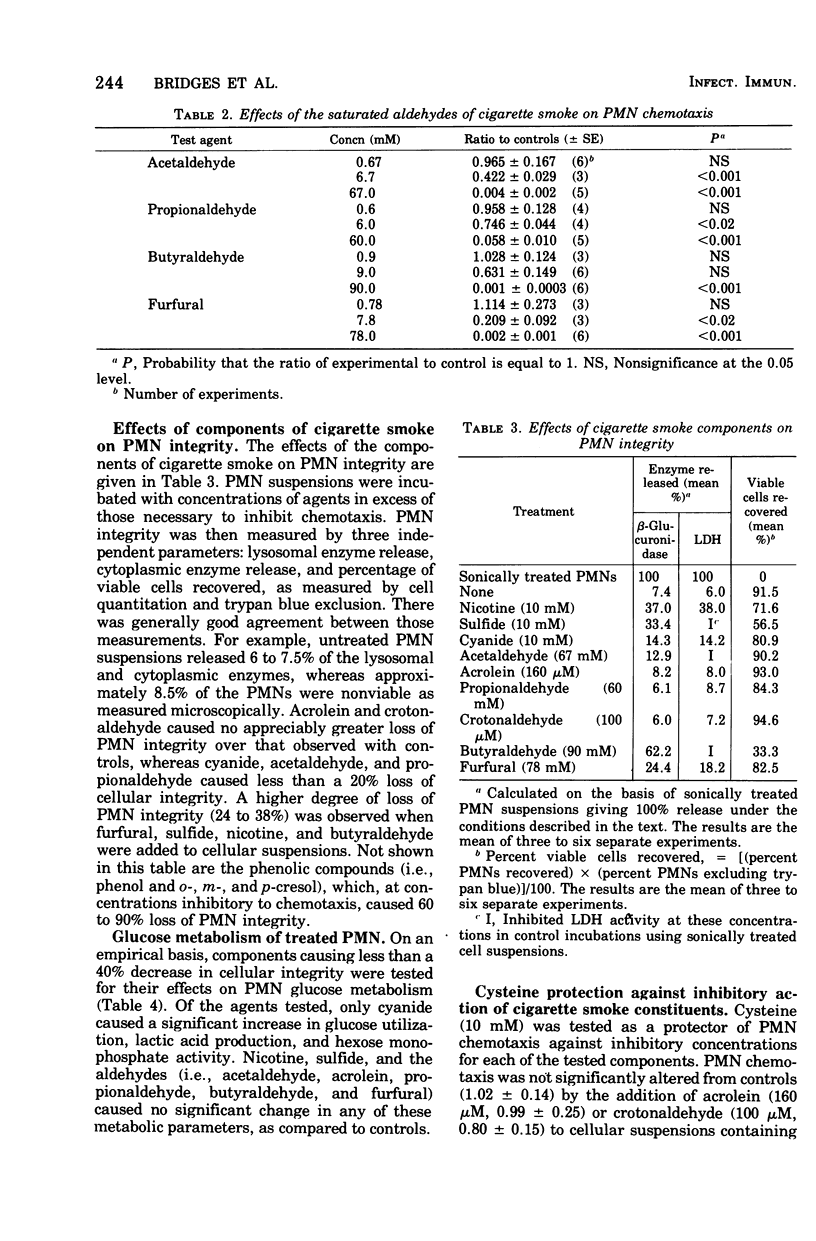
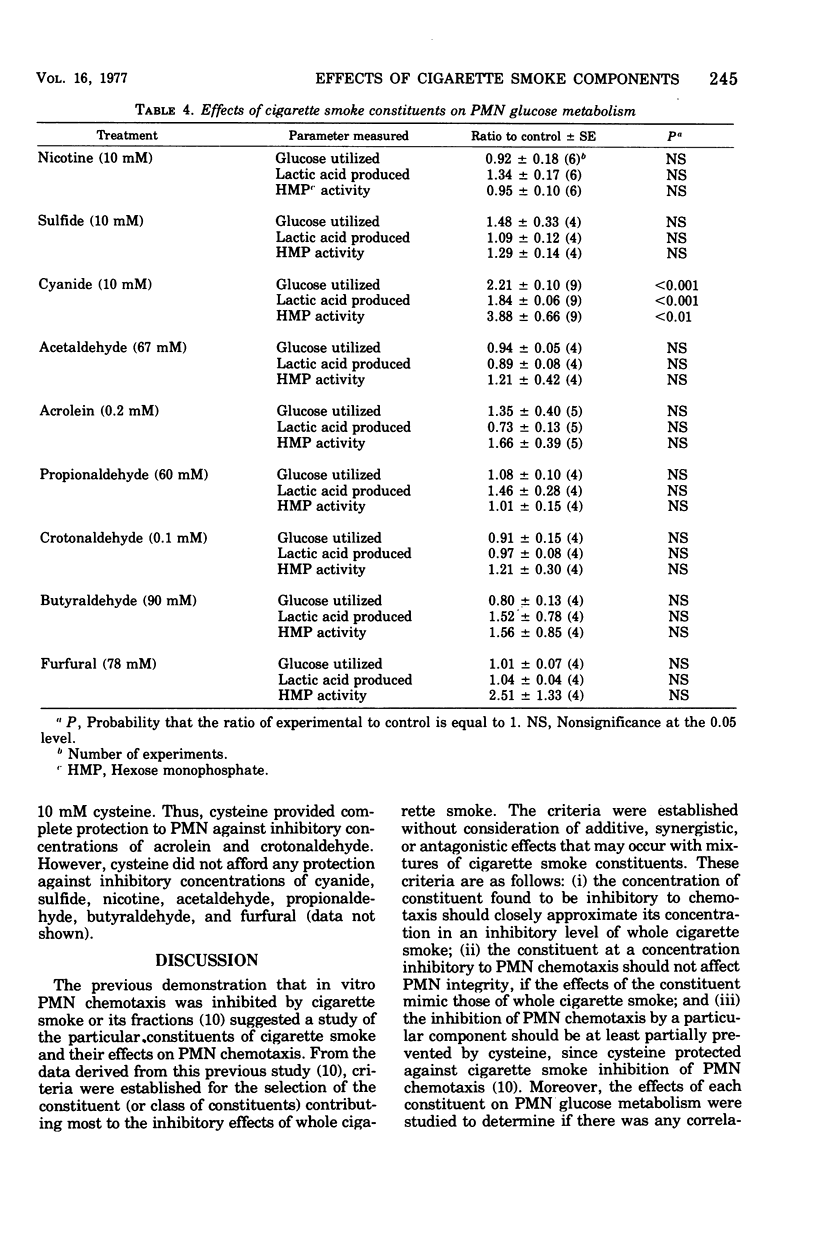
Some ciliostatic components of cigarette smoke were studied as inhibitors of in vitro chemotaxis of human polymorphonuclear leukocytes (PMNs). In comparison to their concentration in an inhibitory level of cigarette smoke, the unsaturated aldehydes acrolein and crotonaldehyde were the most potent inhibitors, whereas nicotine, cyanide, acetaldehyde, and furfural were the next strongest inhibitors. In contrast, sulfide, propionaldehyde, butyraldehyde, and the phenols (phenol and o-, m-, and p-cresol) were relatively weak inhibitors of PMN chemotaxis. Acrolein and crotonaldehyde mimicked whole cigarette smoke in their effects on PMNs by not causing loss of PMN viability, yet their effects were prevented by the addition of cysteine. On the other hand, addition of nicotine, cyanide, acetaldehyde, and furfural to PMN suspensions resulted in a limited loss of cellular viabilities, and their effects on PMNs were not prevented by cysteine. Of the tested components, only cyanide significantly altered PMN glucose metabolism by increasing carbon flow via the glycolytic and hexose monophosphate pathways in a manner similar to that observed with whole cigarette smoke. The results of this study suggest that the unsaturated aldehydes, including acrolein and crotonaldehyde, are major contributors to the inhibitory properties of cigarette smoke. The inhibitory effects of these unsaturated aldehydes are probably due to a direct interaction of these oxidants and/or thiol-alkylating agents with PMNs, yet the glucose metabolism of these cells is unaffected. One interpretation of these data is that PMN chemotaxis is dependent upon particular cellular proteins containing one or more essential thiol group(s) but that these proteins are unrelated to glucose metabolism.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Stowring L. Glycolytic and gluconeogenic enzyme activities in renal cortex of diabetic rats. Am J Physiol. 1973 Apr;224(4):930–936. doi: 10.1152/ajplegacy.1973.224.4.930. [DOI] [PubMed] [Google Scholar]
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandmann U., Rydgren L., Norberg B. The difference between random movement and chemotaxis. Effects of antitubulins on neutrophil granulocyte locomotion. Exp Cell Res. 1974 Sep;88(1):63–73. doi: 10.1016/0014-4827(74)90618-1. [DOI] [PubMed] [Google Scholar]
- Borel J. F. Effect of some drugs on the chemotaxis of rabbit neutrophils in vitro. Experientia. 1973 Jun 15;29(6):676–678. doi: 10.1007/BF01944767. [DOI] [PubMed] [Google Scholar]
- Boxer L. A., Hedley-Whyte E. T., Stossel T. P. Neutrophil action dysfunction and abnormal neutrophil behavior. N Engl J Med. 1974 Nov 21;291(21):1093–1099. doi: 10.1056/NEJM197411212912101. [DOI] [PubMed] [Google Scholar]
- Bridges R. B., Kraal J. H., Huang L. J., Chancellor B. M. Effects of tobacco smoke on chemotaxis and glucose metabolism of polymorphonuclear leukocytes. Infect Immun. 1977 Jan;15(1):115–123. doi: 10.1128/iai.15.1.115-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Clark R. A., Kimball H. R. Defective granulocyte chemotaxis in the Chediak-Higashi syndrome. J Clin Invest. 1971 Dec;50(12):2645–2652. doi: 10.1172/JCI106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELAUNAY A., LEBRUN J., BARBER M. Factors involved in 'chemotactism' of leucocytes in vitro. Nature. 1951 May 12;167(4254):774–775. doi: 10.1038/167774a0. [DOI] [PubMed] [Google Scholar]
- Eichel B., Shahrik H. A. Tobacco smoke toxicity: loss of human oral leukocyte function and fluid-cell metabolism. Science. 1969 Dec 12;166(3911):1424–1428. doi: 10.1126/science.166.3911.1424. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Rosenthal A. S. The regulatory role of divalent cations in human granulocyte chemotaxis. Evidence for an association between calcium exchanges and microtubule assembly. J Cell Biol. 1974 Sep;62(3):594–609. doi: 10.1083/jcb.62.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. Stimulation of human neutrophil leukocyte aerobic glucose metabolism by purified chemotactic factors. J Clin Invest. 1974 Feb;53(2):591–599. doi: 10.1172/JCI107594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS H., LOTZ M. Factors influencing chemotaxis of the polymorphonuclear leucocyte. Br J Exp Pathol. 1956 Oct;37(5):477–480. [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Pincus S. H. Hydrogen peroxide utilization in myeloperoxidase-deficient leukocytes: a possible microbicidal control mechanism. J Clin Invest. 1971 Oct;50(10):2226–2229. doi: 10.1172/JCI106718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble R. C., Penny B. B. Comparison of leukocyte count and function in smoking and nonsmoking young men. Infect Immun. 1975 Sep;12(3):550–555. doi: 10.1128/iai.12.3.550-555.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotti A., Ancker K., Arrhenius E., Enzell C. Effects of tobacco and tobacco smoke constituents on cell multiplication in vitro. Toxicology. 1975 Sep;5(1):49–62. doi: 10.1016/0300-483x(75)90069-4. [DOI] [PubMed] [Google Scholar]
- TENNANT J. R. EVALUATION OF THE TRYPAN BLUE TECHNIQUE FOR DETERMINATION OF CELL VIABILITY. Transplantation. 1964 Nov;2:685–694. doi: 10.1097/00007890-196411000-00001. [DOI] [PubMed] [Google Scholar]


